Abstract
Sensorineural deafness and balance dysfunction are common impairments in humans frequently caused by defects in the sensory epithelium of the inner ear, composed of hair cells and supporting cells. Lineage studies have shown that hair cells and supporting cells arise from a common progenitor, but how these progenitors are generated remains unknown. Although various molecules have been implicated in the development of the sensory progenitors, none has been shown to be required for the specification of these progenitors in the mammalian inner ear. Here, using both loss-of-function and gain-of-function approaches, we show that Jagged1 (JAG1)-mediated Notch signaling is both required and sufficient for the generation of the sensory progenitors. Specifically, we find that loss of JAG1 signaling leads to smaller sensory progenitor regions without initial effects on proliferation or cell death, indicating that JAG1 is involved in initial specification events. To further test whether Notch signaling is involved in specification of the sensory progenitors, we transiently expressed an activated form of the Notch1 receptor (NICD) using a combined Tet-On/Cre induction system in the mouse. NICD expression resulted in ectopic hair cells and supporting cells in the nonsensory regions of the cochlea and vestibule. These data indicate that Notch specifies sensory progenitors in the inner ear, and that induction of Notch may be important for regenerating or replacing hair cells and supporting cells in the mammalian inner ear.
Keywords: development, JAG1, SOX2, Tet-On, NICD
The mammalian inner ear contains six separate sensory regions required for hearing and balance. Each sensory organ is composed of two basic cell types, sensory hair cells and associated supporting cells. Lineage studies in the chick have shown that both cell types arise from a common sensory progenitor (1, 2) that differentiates into a hair cell or a supporting cell via lateral inhibition mediated by the Notch signaling pathway (3–7). Lineage studies in the chick and fate-mapping studies in the mouse also have demonstrated that hair cells and supporting cells can share a common lineage with neurons of the statoacoustic ganglion (SAG), which derive from the otocyst and innervate the hair cells in the ear (2, 8).
At present, the factors that specify the sensory progenitors are not known, although various molecules have been implicated. One major candidate is the high-mobility group (HMG) transcription factor SOX2. SOX2 is expressed in the sensory regions, and loss of SOX2 results in inner ears that develop without any hair cells or supporting cells, likely due to loss of the sensory progenitors (9). However, ectopic expression of SOX2 in cochlear explants does not lead to new sensory regions (10), raising the question as to whether SOX2 is sufficient for sensory specification. Other factors shown to be involved in prosensory development in the inner ear include Wnt, Bmp, Fgf, and Notch signaling pathways (11–19). Which of these signaling pathways is required for sensory progenitor specification, rather than for sensory progenitor maintenance and/or other aspects of sensory development, is not known.
Notch signaling plays multiple roles in inner ear development, including roles in determining the size of the otic placode (20), in development of the neural and sensory components of the inner ear (16–19), and in differentiation of the hair cells and supporting cells via lateral inhibition (3–7). In the mouse, lateral inhibition is mediated by the Delta-like1 (DLL1) and Jagged2 ligands (5, 6), which are expressed in the hair cells during differentiation. However, the Notch ligand Jagged1 (JAG1) is expressed earlier during ear development and is associated with the sensory regions (21). Inactivation of JAG1 in the otocyst leads to smaller or missing sensory regions (16, 18, 19), indicating that JAG1 is required for the development of the sensory progenitors. Whether JAG1 is required for specification or for sensory progenitor cell maintenance is not clear, however. To address this question in the mammalian system, we took two approaches: (i) a loss-of-function approach examining the initial consequences of loss of JAG1 function on sensory progenitor development and (ii) a gain-of-function approach to determine whether activated Notch can initiate sensory development. Our experiments demonstrate that loss of JAG1 function leads to fewer cells expressing sensory markers without initial changes in cell proliferation or cell death. In addition, forced Notch signaling leads to ectopic hair cells and supporting cells, indicating that Notch can induce sensory development de novo in nonsensory portions of the inner ear. Taken together, our findings support a role for JAG1-mediated Notch signaling in specifying the sensory progenitors in the mammalian inner ear.
Results
Sensory Markers Are Altered in the Jag1-cko Early Otocyst.
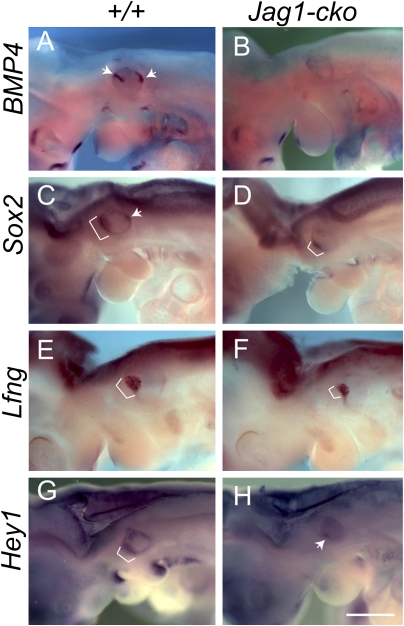
We crossed Jag1flox/flox mice with Foxg1-Cre;Jag1del1/+ double heterozygotes to generate Jag1 conditional knockout (Jag1-cko) embryos (19). Our previous data showed that loss of JAG1 function in the inner ear leads to fewer sensory progenitors at embryonic day (E) 12.5 (19). To examine when altered sensory establishment begins in JAG1-deficient inner ears, we assessed the expression of several early sensory markers, including Bmp4, Sox2, Lfng, and Hey1, before E12.5. We found expression defects as early as E10.25 (Fig. 1). At E9.5, Bmp4 showed diffuse expression in the posterior otic cup that was unchanged in Jag1-cko mutants (Fig. S1 A and B; n = 6/6). In contrast, at E10.25, Bmp4 showed an anterior streak and a posterior spot of expression in the WT otocyst, which was either absent or severely reduced in the mutant otocyst (Fig. 1 A and B; n = 6/6). Sox2, which has been shown to mark all of the sensory regions in the inner ear and is normally expressed in a large anterior domain and a smaller posterior region (9), showed a smaller anterior region and a missing posterior region at E10.25 (Fig. 1 C and D; n = 5/6). We further quantified SOX2 expression in sections (Fig. S1 C–F and H) and found that its expression domain occupied just 56% of the volume of the Jag1-cko otocyst, compared with 73% of the volume of the WT otocyst (n = 6 WT and 5 Jag1-cko ears; P < 0.01). Lfng, a modulator of the Notch pathway that marks the early maculae and cochlea (22), was expressed in a large anterior domain in the WT otocysts but demonstrated a smaller domain in the Jag1-cko otocysts (Fig. 1 E and F; n = 6/7). Hey1, a potential downstream target of the Notch pathway (23), also showed a large anterior domain of expression that was reduced in Jag1-cko otocysts (Fig. 1 G and H; n = 9/11). To ensure that the expression domains did not appear smaller because the mutant otocysts were smaller, the volumes of the WT and Jag1-cko otocysts were calculated at E10.25 in sections. The volumes were not statistically significantly different at this stage [mean WT volume, 4.99 ± 0.34 nL (n = 15 ears); mean Jag1-cko volume, 4.51 ± 0.39 nL (n = 14 ears); P = 0.39)]. These data indicate that sensory markers are altered in Jag1-cko otocytes by E10.25.
Fig. 1.
Sensory markers are absent or down-regulated in Jag1-cko inner ears by E10.25. (A–H) The expression pattern of Bmp4, Sox2, Lfng, and Hey1 by whole mount in situ hybridization. Embryos are shown in a lateral view, with anterior to the left. Brackets and arrows point to the expression domains of indicated genes in the inner ear. (Scale bar: 1 mm.)
Cell Proliferation and Cell Death Are Not Initially Altered in Jag1-cko Inner Ears.
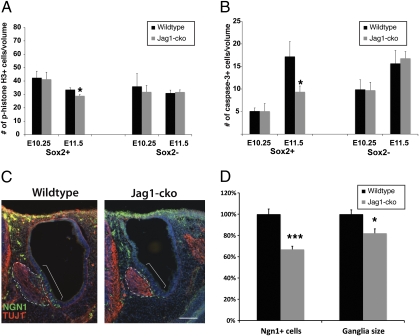
Notch has been shown to be an important pathway for maintaining cell proliferation and survival (24–26). Thus, JAG1 might be important for the proliferation and/or survival of the sensory progenitors, which could explain the smaller sensory domains observed by marker analysis. To determine whether there were differences within the sensory domain or within the nonsensory domain, we colabeled sections with SOX2 and either phospho-histone-H3 (pHistone H3) to detect proliferation (Fig. 2A) or activated caspase 3 to detect dying cells (Fig. 2B). To control for differences in otocyst size and SOX2 expression, we normalized the data by volume. We quantified proliferating or apoptotic cells in the early otocyst within SOX2-positive and -negative regions. This analysis revealed no significant difference in either proliferation or cell death in the E10.25 otocyst, but a significant decrease in both proliferation and cell death within the SOX2-positive region at E11.5 (Fig. 2 A and B). These results demonstrate that cell proliferation and cell death are not affected at E10.25, when sensory markers are altered, supporting a role for JAG1 in specification of the sensory progenitors. However, a later reduction in proliferation and cell death within the sensory domain at E11.5 suggests that JAG1 may be important for later sensory maintenance as well.
Fig. 2.
Reduction in progenitor cell number is not caused by increased death, decreased proliferation, or adoption of a neural fate. (A) Proliferating cells were quantified per nanoliter of SOX2-positive or -negative regions at the indicated ages using pHistone H3 (E10.25: n = 6 WT, 5 Jag1-cko; E11.5: n = 10 WT, 7 Jag1-cko). (B) Cell death was quantified per nanoliter of SOX2-positive or -negative regions using activated caspase-3 as an apoptotic marker (E10.25: n = 6 WT, 5 Jag1-cko; E11.5: n = 5 WT, 6 Jag1-cko). (C) Representative sections showing the TUJ1-positive facioacoustic ganglion complex (dotted lines) and NGN1-positive region (brackets). (D) The total number of NGN1+ cells in the otic epithelium was quantified, and the total volume of the facioacoustic ganglion complex was calculated at E10.5. Error bars represent SEM. *P < 0.05; ***P < 0.001.
Missing Sensory Progenitors Do Not Adopt a Neuronal Cell Fate.
Because we found no differences in cell death or cell proliferation at early otocyst stages, we hypothesized that the missing progenitors instead adopt a neuronal fate. This would be similar to the role of DLL1 in the developing SAG, where it mediates a choice between neuronal and sensory progenitor fate (18). JAG1 expression in the neuronal domain of the anteroventral otocyst is consistent with the possibility that JAG1 plays a role in development of the otic ganglion (Fig. S1G). To test for the presence of increased numbers of otic neuroblasts, we quantified Neurogenin 1 (NGN1)-positive cells within the otocyst at E10.5 (Fig. 2 C and D). NGN1, one of the earliest markers of the prospective otic neurons, is expressed before neuroblast delamination and migration (Fig. 2C and Fig. S1G) (27). The number of NGN1-positive cells was decreased by ≈30% in Jag1-cko otocysts (Fig. 2D). Moreover, the volume of the facioacoustic ganglion complex in Jag1-cko mutants at E10.5 (quantified using TUJ1, a neuron-specific class III β-tubulin) was slightly reduced, consistent with the finding of fewer NGN1-positive cells (Fig. 2D). These data show that the missing sensory progenitors are not adopting a neuronal fate, and that loss of JAG1-mediated Notch signaling leads to a decrease in the number of cells adopting a neuronal fate.
Notch Activation via a Combined Tet-On/Cre Induction System Leads to Ectopic Sensory Regions in the Inner Ear.
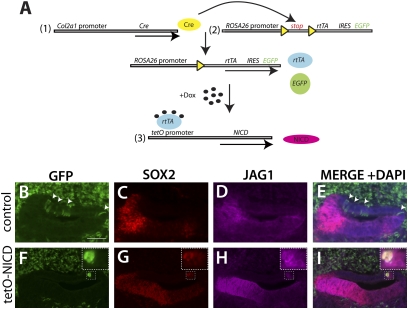
To test whether Notch can initiate sensory development in the inner ear, we used an activated form of the Notch1 receptor, the Notch intracellular domain (NICD). We used a strategy in which NICD would be turned on but then turned off, so that subsequent actions of Notch (such as in lateral inhibition) could take place normally. To achieve this type of regulated expression, we used the tetracycline-on (Tet-On) system in combination with the Cre-recombinase system (Fig. 3A). To induce expression of NICD in the nonsensory regions of the ear, we used a Col2a1-Cre allele (28). Beginning at E10.5, the Col2a1-Cre demonstrated patchy expression in the nonsensory regions of the otic vesicle as well as in the surrounding mesenchymal populations (Fig. S2 A–C).
Fig. 3.
Prosensory markers are up-regulated in E12.5 ears via a trigenic Tet-On system to activate Notch. (A) Three alleles are required in this system: (i) Cre recombinase, driven by the Col2a1 promoter; (ii) reverse tetracycline transactivator (rtTA), under control of the ubiquitously expressed ROSA26 promoter; and (iii) NICD allele, driven by the tetracycline inducible promoter (tetO). A floxed stop cassette is present between the ROSA26 promoter and rtTA, thus confining rtTA expression to the cells in which Cre recombinase is present. An internal ribosome entry site (IRES) followed by the EGFP gene is located downstream of rtTA, thereby allowing tracking of rtTA-expressing cells by EGFP expression. rtTA will bind to tetO in the presence of doxycycline, thereby activating expression of NICD. (B–I) SOX2, JAG1, and EGFP expression in the E12.5 cochlea from the Col2a1-Cre;ROSA-rtTA;tetO-NICD cross. (B–E) Control ears (Col2a1-Cre;ROSA-rtTA bigenic) showing EGFP expression in the dorsal nonsensory regions of the cochlea (arrowheads) that do not express SOX2 and JAG1. (F–I) Trigenic cochlea demonstrating an EGFP-positive cluster that also expresses SOX2 and JAG1. A high-power view of the boxed area is shown in the upper right corner. Note that some SOX2+ cells lie outside the EGFP-positive cluster. (Scale bar: 50 μm.)
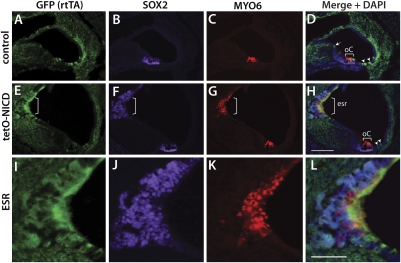
To achieve Notch overexpression, we generated trigenic embryos expressing Col2a1-Cre, ROSA-rtTA, and tetO-NICD (Fig. 3A). The ROSA-rtTA transgene contained an IRES-EGFP reporter, so that cells in which Cre was expressed were also permanently marked by EGFP. Thus, we could follow the normal fate of Col2a1-Cre expression in Col2a1-Cre;ROSA-rtTA littermate controls. Pregnant females were injected with doxycycline at E10.5 or were fed doxycycline in the food and water between E9.5 and E11.5. At E12.5, prosensory markers (SOX2 and JAG1) were up-regulated in Col2a1-expressing cells (marked by EGFP) in the trigenic animals. At this stage, EGFP-expressing cells were seen primarily in the dorsal nonsensory regions of the cochlea (Fig. 3 B and F) and the nonsensory regions of the vestibule between the saccule and posterior crista (Fig. S3 A and E). In the presence of tetO-NICD, EGFP-expressing cells also were positive for SOX2 and JAG1, suggesting that these cells had adopted a sensory progenitor fate (n = 5 mutants, 4 controls; Fig. 3 F–I and Fig. S3 E–H). Interestingly, the SOX2- and JAG1-positive regions were slightly larger than the EGFP-positive regions (Fig. 3 F–I and Fig. S3 E–H, Insets), suggesting the possibility of some nonautonomous effects on the surrounding cells. To determine whether the E12.5 ectopic prosensory cells could differentiate into hair cells and supporting cells, we harvested embryos at E18.5 and examined them using sensory markers including MYO6 (hair cells), SOX2 (supporting cells), and EGFP, which indicated previous rtTA/NICD expression. We found that ectopic hair cells and supporting cells formed in the nonsensory regions of the cochlea and saccule and were coexpressed with EGFP (Fig. 4 E–L and Fig. S4 E–L), indicating that Notch can induce ectopic sensory regions (ESRs).
Fig. 4.
Ectopic Notch activation induces the generation of hair cells and supporting cells in the cochlea at E18.5. SOX2 is a marker for supporting cells, Myosin VI (MYO6) is a marker for hair cells, and EGFP reflects expression of rtTA. (A–D) Control inner ear from a Col2a1-Cre/rtTA bigenic mouse demonstrates EGFP+ cells in the nonsensory regions of the cochlea (arrow in D) but does not show SOX2 or MYO6 expression. (E–H) Large EGFP-positive regions are observed in the nonsensory regions of the Col2a1-Cre/rtTA/tetO-NICD (tetO-NICD) trigenic inner ear that are also positive for SOX2 and MYO6. Arrowheads in D and H indicate EGFP-positive regions that likely arose after doxycycline administration. (I–L) High-power views of the ESR. Brackets indicate the sensory patches. esr, ectopic sensory region; Oc, organ of Corti. (Scale bars: 100 μm for A–H and 50 μm for I–L.
In some sections, the ESRs appeared to be contained within their own compartment, similar to each of the endogenous sensory regions. Analysis of whole mount cochleae revealed the presence of ectopic compartments in the basal regions of the cochlea (n = 3; Fig. S5). Interestingly, the EGFP-positive region was confined to the sensory region and did not extend throughout the entire ectopic compartment (dotted area in Fig. S5 D and F), suggesting that the nonsensory region of the compartment was induced by the ESR. The ESR locations and doxycycline administration routes are summarized in Table S1.
All Cells Within the Otic Epithelium May Be Competent to Become Sensory Progenitors.
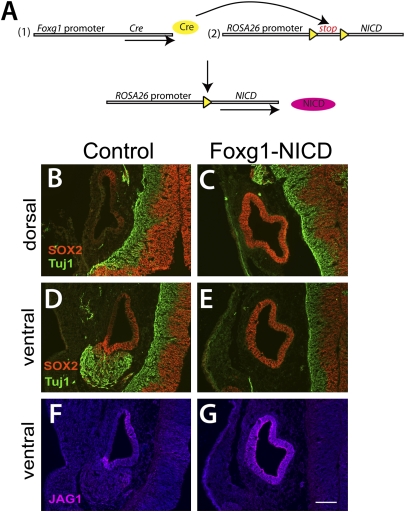
Whether all cells within the early otocyst are competent to respond to Notch activation and become sensory progenitors is unclear. In the Col2a1-Cre;ROSA-rtTA;tetO-NICD experiments, the regions that could become sensory are limited to those in which Col2a1-Cre is expressed. To investigate which cells in the otocyst are capable of responding to Notch, we crossed a more widely expressed Cre allele (Foxg1-Cre) with the ROSA-NICD allele to achieve widespread Notch induction in the otic vesicle (Fig. 5A). Foxg1-Cre is expressed throughout the otic vesicle before E10 (29). Unfortunately, the embryos from this cross do not survive beyond E11.5–E13.5 and exhibit failure of neural tube closure. Nonetheless, the expression of sensory progenitor markers can be assessed to determine whether or not the cells were adopting a sensory fate. We examined Foxg1-Cre;ROSA-NICD embryos at E10.5 (n = 5 mutants and 3 ROSA-NICD+/−controls), and found that SOX2 and JAG1 were up-regulated throughout the otic vesicle, indicating that all cells might be adopting a sensory progenitor fate (Fig. 5 C, E, and G). The epithelium in the mutant otocysts had a uniform thickened appearance, consistent with the appearance of the ventral region of the otocyst, from which the sensory areas are thought to derive. In addition, the otocysts did not close completely, exhibiting regions that were still continuous with the ectoderm. The neural markers TUJ1, NEUROD, and ISL1 were severely reduced or absent in the Foxg1-Cre;ROSA-NICD inner ears (n = 5 mutants and 4 controls; Fig. 5E and Fig. S6 B and D). PAX2, a regional marker expressed in the medial otocyst at E10.5, was expressed normally in the Foxg1-Cre;ROSA-NICD otocysts (Fig. S6B), indicating that these otocysts were not simply delayed in their development. Overall, these data suggest that the activation of Notch in the early otocyst can induce cells to adopt a sensory progenitor cell fate at the expense of all other cell types, including neurons.
Fig. 5.
All cells in the early otic vesicle may be competent to adopt a sensory fate. (A) Strategy to activate Notch signaling constitutively in all otic epithelial cells in the mouse inner ear. The Cre recombinase is under the control of Foxg1 promoter, which is expressed throughout the otic epithelium. Cre recombination removes the floxed stop cassette that is present between ROSA26 promoter and NICD, thereby allowing expression of NICD. (B, D, and F) In the control, SOX2 and JAG1 are expressed in the anterior and posterior regions of the otocyst at E10.5. The SAG, which is marked by TUJ1, is associated with the anterior region of the inner ear. (C, E, and G) In the double-transgenic inner ear, SOX2 and JAG1 are expressed throughout the otic epithelium, and the SAG is not present. Anterior is down, and lateral is to the left. (Scale bar: 100 μm.)
Discussion
Our results demonstrate that JAG1-mediated Notch signaling specifies the sensory progenitors in the inner ear. Specifically, our data show that loss of JAG1-mediated Notch signaling leads to absent or smaller sensory marker domains at early otocyst stages, and that this change is not caused by cell death or reduced cell proliferation. However, the detection of later changes in cell proliferation and cell death leaves open the possibility that JAG1 also plays a role in sensory progenitor maintenance. We also found that activated Notch can induce de novo sensory development in nonsensory regions of the inner ear, consistent with a role in specification. Taken together, the combined loss and gain-of-function data indicate that Notch is both required and sufficient for the initiation of sensory development in the developing otocyst.
Marker analysis in the loss-of-function allele Jag1-cko shows that although sensory specification is altered, it is not completely abolished. For example, at E10.25, Bmp4 expression was absent or severely reduced, whereas other markers showed smaller domains of expression. This continued (albeit smaller) expression domain of some sensory markers is consistent with the fact that some sensory regions still form in the Jag1-cko inner ears (19), including the saccular macula, some cells in the utricular macula, and some cells in the middle and apical regions of the cochlea. This might be due to a redundancy with the Dll1 gene, which is also expressed at otic vesicle stages (21). Loss of DLL1 function leads to defects in the sensory maculae, the regions least affected in Jag1-cko inner ears (18, 19). Thus, the remaining sensory domains in the Jag1-cko inner ears may be due to Notch activation via DLL1.
Our results indicate that missing sensory progenitors are not adopting a neuronal fate, as would be expected if JAG1 was acting via lateral inhibition to specify a sensory cell fate versus a neuronal fate. JAG1 is expressed in the neurosensory domain (Fig. S1G), suggesting a possible role in determining sensory components versus neural components of the ear. However, instead of a larger otic ganglion as seen in the Dll1 mutant (18), the ganglion is actually smaller, with significantly fewer neuroblasts (NGN1-positive cells) within the otic vesicle (Fig. 2D). Lineage experiments indicate that sensory cells in the maculae and neuronal cells can share a common progenitor (2). In addition, fate-mapping experiments have demonstrated that not all NGN1-positive cells become neuroblasts, with some becoming sensory and nonsensory cells in the macular regions (8). These data support the existence of a neurosensory progenitor, although at present there is no unique marker for these progenitors. Thus, our data suggest that JAG1 may be important for the specification or maintenance of the neurosensory progenitors, given the decrease in both sensory and neural progenitors in Jag1-cko inner ears.
Our gain-of-function data demonstrate that activation of Notch leads to the formation of ESRs in the cochlea and vestibule. Interestingly, the EGFP-positive regions associated with ESRs were generally larger than the EGFP-positive regions observed in the Col2a1-Cre;ROSA26-rtTA controls. This suggests that along with altering the fate of these cells, Notch also may increase their proliferation. In other systems, Notch is known to affect proliferation (24–26), and it might play a similar role in the inner ear via JAG1 signaling. This interpretation is also supported by the loss-of-function results in the Jag1-cko inner ear, showing significantly reduced proliferation in the SOX2-positive region at E11.5 (Fig. 2A).
We also found that ESRs can lead to ectopic compartment formation in some cases (Fig. S5 A and B). The EGFP-positive region was confined to the sensory area (Fig. S5 D and F), demonstrating that the development of the nonsensory region of the compartment is non–cell-autonomous and is likely initiated by the ESR. In the inner ear, each sensory region is housed within a compartment, although the exact relationship between the sensory organ and the nonsensory portion of the compartment is not well understood. Previous studies have suggested that the sensory regions may direct the formation of the associated nonsensory regions of the compartment. For example, in the SOX2 and JAG1 loss-of-function inner ears, nonsensory compartments that are associated with sensory regions are missing or malformed, whereas regions that do not contain sensory regions (e.g., the common crus, endolymphatic duct) develop relatively normally (9, 18, 19). In addition, fibroblast growth factors expressed in the cristae can direct canal outgrowth by regulating Bmp2 (30), demonstrating a direct molecular connection between sensory development and nonsensory development. Taken together, these data suggest that sensory regions can drive the development of the nonsensory portion of the compartment in which they reside.
The results from the Foxg1-Cre;ROSA-NICD cross suggest that all regions of the early otocyst are responsive to Notch and can adopt a prosensory phenotype, even at the expense of neuronal cell types. The loss of otic neurons is somewhat surprising, given that the loss of JAG1 leads to a reduction of neuronal progenitors (possibly neurosensory progenitors). Based on these results, one might expect activation of Notch to lead to excess neurosensory progenitors. However, when considering other outcomes from manipulations of Notch signaling, this result is not entirely unexpected, given that deletion of DLL1 in mouse or a mutation in the zebrafish mind bomb gene leads to excess neuronal production (18, 31). Thus, it is likely that this aspect of the phenotype better reflects the function of the DLL1 ligand.
In mammals, genetic and/or environmental insults to the inner ear lead to hearing loss and/or vestibular dysfunction, often due to permanent loss or dysfunction of the sensory regions of the inner ear. Our results show that induction of Notch signaling can generate both hair cells and supporting cells from nonsensory cells in a mammalian system. Thus, Notch might be an important pathway to manipulate when designing therapies to treat deafness and balance disorders caused by loss or dysfunction of hair cells and/or supporting cells.
Methods
Mice.
The following mouse strains were used: Col2a1-Cre (28), Foxg1-Cre (29), Jag1del1 (32), Jag1flox (19), ROSA26R-LacZ reporter (33), ROSA-NICD (34), ROSA26-rtTA (35), and tetO-NICD (36). Timed matings were determined by checking for vaginal plugs, and the day of the plug was considered E0.5. All mouse experiments were approved by the University of Rochester’s Committee on Animal Resources.
Whole Mount in Situ Hybridization.
Embryos were fixed in 4% PFA in PBS buffer at 4 °C for 6–12 h and then washed with PBT (PBS with 0.1% Tween-20). Embryos were dehydrated in graded methanol/PBT series (25%, 50%, 75% and 100%) and stored at −20 °C. Digoxygenin-labeled RNA probes for Bmp4, Hey1, Lfng and Sox2 were synthesized from linearized plasmids according to the manufacturer's instructions (Roche). Hybridization was performed as described previously (37). To reduce variation, whole litters, including WT and mutant embryos, were hybridized with the same probe and reacted for the same length of time in the same reaction tube, and then subsequently genotyped.
Immunohistochemistry.
For frozen sections, embryos were fixed in 4% PFA in PBS buffer at 4 °C for 2–12 h, cryoprotected, and then sectioned at a thickness of 14–18 μm. The sections were incubated with primary antibodies overnight at 4 °C and then incubated with secondary antibodies for 2 h at room temperature. For cochlear whole-mounts, immunohistochemistry was performed as described previously (38). The antibodies used are listed in Table S2.
Quantification of Cell Death, Cell Proliferation, and Volume of the Ganglia and Otocyst.
pHistone H3 and activated caspase 3 were used to mark dividing and dying cells, respectively. Immunohistochemistry on cryosections with antibodies to both markers was performed in conjunction with SOX2 staining, and the number of immunopositive cells in the entire inner ear was counted. SOX2-positive and -negative areas in the epithelium were quantified using Zeiss Axiovision software. For volume measurements, the area (in μm2) of each section was measured and converted to a volume by multiplying by the thickness of the section (14–18 μm). Area measurements of the entire otic epithelium were obtained by subtracting the inner area from the outer area. Volumes are expressed in nL, because measurements in μm3 resulted in unwieldy numbers. Counts are expressed per nL of SOX2-positive or -negative regions. TUJ1 was used to mark the SAG. However, because the SAG was difficult to distinguish from the facial ganglion at E10.5, both ganglia were included in the volume measurements. The area of the ganglia was measured as described above.
Administration of Doxycycline.
Two methods were used to deliver doxycycline. The pregnant females were given doxycycline-containing food (400 mg of doxycycline was dissolved in 50 mL of drinking water containing 10% sucrose and then mixed with 100 g of mouse chow) and water (containing 2 mg/mL of doxycycline and 5% sucrose) between E9.5 and E11.5. Alternatively, doxycycline was dissolved in PBS (pH 7.4) at 10 mg/mL and injected i.p. into pregnant females at a dose of 35 μg/g body weight on E10.5.
Supplementary Material
Acknowledgments
We thank Thurma McDaniel for the frozen sectioning and Dr. Rick Libby, Dr. Patricia White, and Kim Fernandes for discussions and careful reading of the manuscript. This work was supported by a Basil O’Connor Starter Scholar Award from the March of Dimes (to A.E.K.), a career development award from Research to Prevent Blindness (to A.E.K.), and a grant from the National Institutes of Health (DC009250, to A.E.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003089107/-/DCSupplemental.
References
- 1.Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- 3.Lewis J. Rules for the production of sensory cells. Ciba Found Symp. 1991;160:25–39. discussion 40–53. [PubMed] [Google Scholar]
- 4.Corwin JT, Jones JE, Katayama A, Kelley MW, Warchol ME. Hair cell regeneration: The identities of progenitor cells, potential triggers and instructive cues. Ciba Found Symp. 1991;160:103–120. doi: 10.1002/9780470514122.ch6. discussion 120–130. [DOI] [PubMed] [Google Scholar]
- 5.Lanford PJ, et al. Notch signaling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 6.Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- 7.Riley BB, Chiang M, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- 8.Raft S, et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- 9.Kiernan AE, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 10.Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens CB, Davies AL, Battista S, Lewis JH, Fekete DM. Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev Biol. 2003;261:149–164. doi: 10.1016/s0012-1606(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 12.Chang W, et al. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirvola U, et al. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 14.Pauley S, et al. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- 17.Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: Specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- 18.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison A, Hodgetts C, Gossler A, Hrabé de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 22.Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leimeister C, Externbrink A, Klamt B, Gessler M. Hey genes: A novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 24.Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Müller glia. J Neurosci. 2010;30:3101–3112. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gering M, Patient R. Notch signalling and haematopoietic stem cell formation during embryogenesis. J Cell Physiol. 2010;222:11–16. doi: 10.1002/jcp.21905. [DOI] [PubMed] [Google Scholar]
- 26.Purow BW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 27.Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 28.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- 29.Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 30.Chang W, Brigande JV, Fekete DM, Wu DK. The development of semicircular canals in the inner ear: Role of FGFs in sensory cristae. Development. 2004;131:4201–4211. doi: 10.1242/dev.01292. [DOI] [PubMed] [Google Scholar]
- 31.Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: Evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- 32.Xue Y, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 33.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belteki G, et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev Biol. 1998;198:277–285. [PubMed] [Google Scholar]
- 38.Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.