Abstract
Hagfish, which lack both jaws and vertebrae, are considered the most primitive vertebrate known, living or extinct. Hagfish have long been the enigma of vertebrate evolution not only because of their evolutionary position, but also because of our lack of knowledge on fundamental processes. Key elements of the reproductive endocrine system in hagfish have yet to be elucidated. Here, the presence and identity of a functional glycoprotein hormone (GPH) have been elucidated from the brown hagfish Paramyxine atami. The hagfish GPH consists of two subunits, α and β, which are synthesized and colocalized in the same cells of the adenohypophysis. The cellular and transcriptional activities of hagfish GPHα and -β were significantly correlated with the developmental stages of the gonad. The purified native GPH induced the release of gonadal sex steroids in vitro. From our phylogenetic analysis, we propose that ancestral glycoprotein α-subunit 2 (GPA2) and β-subunit 5 (GPB5) gave rise to GPHα and GPHβ of the vertebrate glycoprotein hormone family, respectively. The identified hagfish GPHα and -β subunits appear to be the typical gnathostome GPHα and -β subunits based on the sequence and phylogenetic analyses. We hypothesize that the identity of a single functional GPH of the hagfish, hagfish GTH, provides critical evidence for the existence of a pituitary-gonadal system in the earliest divergent vertebrate that likely evolved from an ancestral, prevertebrate exclusively neuroendocrine mechanism by gradual emergence of a previously undescribed control level, the pituitary, which is not found in the Protochordates.
Keywords: glycoprotein hormone, reproduction, basal vertebrate, agnathan, hypothalamic-pituitary axis
The hypothalamic-pituitary axis, which is specific to vertebrates, is considered to be an evolutionary innovation and seminal event that emerged before or during the differentiation of the ancestral jawless vertebrates (agnathans) (1). Such an evolutionary innovation is one of the key elements leading to physiological divergence, including reproduction, growth, metabolism, stress, and osmoregulation in subsequent evolution of jawed vertebrates (gnathostomes). Reproduction in gnathostomes is controlled by a hierarchically organized endocrine system called the hypothalamic-pituitary-gonadal (HPG) axis. Despite the diverged patterns of reproductive strategies and behaviors, this endocrine network is remarkably conserved throughout gnathostomes. Gonadotropins (GTHs), in response to hypothalamic gonadotropin-releasing hormone (GnRH), are secreted from the pituitary and stimulate the gonads, inducing the synthesis and release of sex steroid hormones, which in turn elicit growth and maturation of the gonads.
Two functional GTHs, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), together with thyroid-stimulating hormone (TSH) form the glycoprotein hormone (GPH) family consisting of two noncovalently bound subunits, α and β. The α-subunit of LH, FSH, and TSH within a single species has an identical primary amino acid sequence, whereas the β-subunits are diverse and convey hormone specificity (2). These GPHs are believed to have evolved from a common ancestral GPH through duplication of β-subunit genes and subsequent divergence (2). Two GTHs and TSH have been identified in all taxonomic groups of gnathostomes, including the three divergent lineages of gnathostome fishes, the Actinopterygii (ray-finned fishes), the Sarcopterygii (lung fishes), and the Chondrichthyes (cartilaginous fishes) (2). However, until this study and the one in lampreys in 2006 (3), there had been no clear evidence for pituitary glycoprotein hormones in agnathans. In lampreys, one of two living members of agnathans, only one GPH subunit, a GTHβ-like molecule, has been identified (3). Although an α-subunit of lamprey GPH has not yet been reported (1), the presence of a heterodimeric GTH in lampreys is strongly supported by substantial physiological and immunohistochemical studies (2, 3).
The other extant representative of the agnathans, hagfish, mostly inhabit a deep marine environment that is relatively free of circadian, or even seasonal changes. Because hagfish represent the most basal and primitive vertebrate that diverged more than 550 million years ago (4, 5), they are of particular importance in understanding the evolution of the HPG axis related to vertebrate reproduction. Nevertheless, our knowledge of the endocrine regulation of reproduction in the hagfish has been poorly understood. The adenohypophysis of hagfish reflects a primitive state and consists of only a series of mass of cells separated from the neurohypophysis by connective tissue (6, 7). There is no clear cytological differentiation between the pars distalis and the pars intermedia (6, 7) and it had not been established whether the hagfish pituitary contains tropic hormones of any kind (8, 9). However, recent studies have revealed immunoreactive (ir)-GTH in the adenohypophysis of both the Atlantic and the Pacific hagfish, and further demonstrated that ir-GTH cells predominated in adults with developing gonads (10–12). These studies strongly suggested the presence of GTH in the hagfish.
Recently, a fourth heterodimeric GPH has been discovered in the human genome and termed “thyrostimulin” because of its thyroid-stimulating activity (13). The thyrostimulin-α subunit, called glycoprotein-α subunit 2 (GPA2), is homologous but not identical to the common α-subunit (GPHα or GPA1). With the discovery of GPA2 and glycoprotein-β subunit 5 (GPB5, thyrostimulin-β) homologs not only in other vertebrates but invertebrates—including fly, nematode, and sea urchin (14, 15)—it is proposed that an ancestral glycoprotein (GP) existed before the divergence of vertebrates and invertebrates, and that later gene-duplication events in vertebrates produced the thyrostimulin (GPA2 and GPB5) and GTH/TSH [GPHα and GPHβ (LHβ/FSHβ/TSHβ)] (Fig. S1) (14). The basal lineage of chordates, such as tunicates and amphioxus, contains GPA2 and GPA5 in their genome but not GPHα and GPHβ (16–19). Lamprey may also have GPA2 and GPB5, in addition to the canonical GTHβ-like protein (1, 3). At present, no information is available as to the presence of TSH or GPA2/GPB5 in the hagfish.
The objective of this study was to identify a pituitary GPH and to examine its possible functions for gonadal activities in the most primitive vertebrate, the hagfish. We hypothesize that the identity of a single, functional GPH, a GTH-like molecule, in hagfish, provides critical evidence for the existence of a HPG system in the most basal vertebrate. Furthermore, we propose that this HPG system likely evolved from an ancestral, prevertebrate exclusively neuroendocrine mechanism by gradual emergence of components of a previously undescribed control level, the pituitary, which is not present in the Protochordates (1).
Results
Identification of Hagfish GPH Molecules.
From the cDNA library, 2,304 (96-hole well plate × 24) clones were subjected to sequence analysis from the 5′ end, of which only 3 clones corresponded to pituitary hormones. Two of three clones showed sequence similarity to GPHβ subunits, whereas the remaining one showed similarity to the GPHα subunit (Fig. 1). The putative hagfish GPHα cDNA consists of 545 nucleotides encoding a prehormone of 118 amino acids (aa), with a putative signal peptide of 24-aa residues and a mature protein of 94-aa residues (Fig. S2). By aligning cysteine (Cys) residues and introducing deletions to obtain the maximal identity, the amino acid sequence was comparable to that of α-subunits of gnathostome GPHs, (Fig. 1A); 8 of 10 Cys were conserved, and two possible N-glycosylation sites were at position 77 and 102, respectively (Fig. 1A and Fig. S3).
Fig. 1.
Molecular characteristics of hagfish GPH. Alignment of the amino acid sequences of vertebrate GPHα (A) and GPHβ (B) subunits. Gaps are inserted to minimize alignment of Cys (C) residues among GPHα subunits, and between hagfish GPHβ and lamprey GTHβ, and FSHβ, LHβ, TSHβ subunits of fish species. The two sequences of hagfish GPH are indicated with blue letters and Cys residues that are conserved for all of these sequences are shown with red letters. Putative N-glycosylation sites are indicated by asterisks. The DDBJ/EMBL/GenBank accession numbers of amino acid sequences used for the alignment are as follows: (A) Shark GPHα (AJ310342), Sturgeon GPHα (AJ310343), and Lungfish GPHα (AB050093); (B) Lamprey GTHβ (AY730276), Shark FSHβ (SCA310344), LHβ (AJ310345), Sturgeon FSHβ (AST251658), LHβ (AJ251656), TSHβ (AJ251659), and Lungfish FSHβ (AJ578040), LHβ (AJ578038), and TSHβ (AJ578039).
The putative hagfish GPHβ cDNA consists of 639 nucleotides encoding a prehormone of 126 aa, including a signal peptide of 15-aa residues and a mature protein of 110-aa residues (Fig. S3). By aligning Cys residues, the amino acid sequence was comparable to gnathostome GPHβ subunits; 12 Cys were entirely conserved (Fig. 1B and Fig. S3). The amino acid sequence also has two possible N-glycosylation sites at position 25 and 42 (Fig. 1B and Fig. S3).
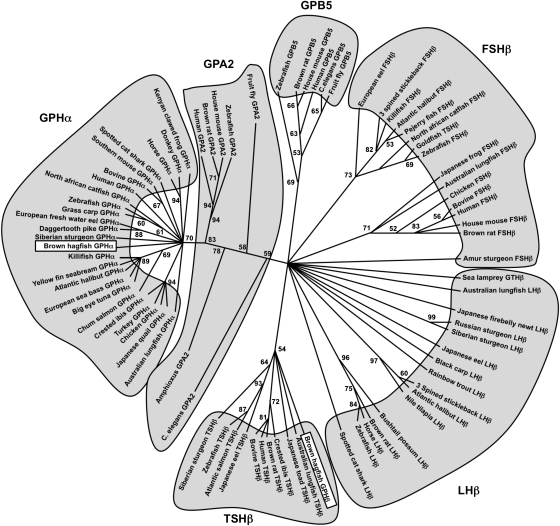
As shown in Fig. 2 (Fig. S1), we propose that an ancestral GP through gene duplication gave rise to ancestral GPA2 and GPB5. Likely through another gene duplication or whole-genome duplication, an ancestral GPA2 then gave rise to the vertebrate GPHα and amphioxus/vertebrate GPA2 (19). Interestingly, the hagfish GPHα forms a clade with the gnathostome GPHαs. For the β-subunits, we propose that an ancestral GPB5 further diverged into vertebrate GPHβ (LHβ, FSHβ, and TSHβ) and GPB5 (18). The hagfish GPHβ forms a clade with the TSHβs; however, the bootstrap values are low and hagfishes evolved before the gnathostomes (Fig. 2). The sea lamprey GTHβ also groups with the GPHβs but appears to be one of the outgroups of the LHβs (Fig. 2). This molecular and phylogenic result shows that the cloned two subunits in the hagfish pituitary are related to gnathostome GPHα and -β subunits and are the earliest diverged vertebrate GPH subunits that have been identified.
Fig. 2.
The molecular phylogenetic analysis was constructed using Maximum-likelihood method using 80 deduced amino acid sequences of the α- and β-subunits of FSH, TSH, and LH; GPB5 and GPA2. Sequences were aligned using the Mafft (33) sequence-alignment method in conjunction with Seaview version 4.2 (34). To complete the bootstrap analyses we used GARLI (genetic algorithm for rapid-likelihood inference) (35) and Grid computing (36) through The Lattice Project (37), which includes clusters and desktops in one encompassing system (38). Numbers on the branches indicate bootstrap probabilities following 1,000 replications in constructing the tree. The DDBJ/EMBL/GenBank accession numbers of sequences used for analysis are listed in Table S1. Note that the goldfish TSHβ forms a clade with FSHβs, not with TSHβs of other species.
Cellular Characterization of GPH-Producing Cells in Hagfish Pituitary.
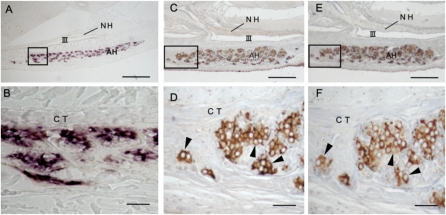
Hagfish GPHα mRNAs were expressed in many cells in most clusters of the adenohypophysis, whereas no hybridization signals were detected in other tissues, including the brain (Fig. 3 A and B). The distributional patterns of cells expressing the GPHα gene were quite similar to those stained with anti-hagfish GPHα (Fig. 3). An intense immunoreaction to anti-hagfish GPHα was observed in ≈30 to 60% of cells of the adenohypophysis (Fig. 3 C and D). Adjacent sections stained with anti-hagfish GPHβ indicated that the same cells were immunopositive (Fig. 3 E and F). These observations are clearly suggestive that both hagfish GPHα and -β subunits are synthesized and colocalized in the same cells of the adenohypophysis as well-known in gnathostomes.
Fig. 3.
Histochemical characteristics of GPH producing cells in the adenohypophysis of mature hagfish. (A and B) Cellular expression of GPHα gene detected by in situ hybridization with labeled hagfish GPHα riboprobe. (C–F) Immunohistochemical detection of GPHα- and β-producing cells on adjacent pituitary sections detected with specific antisera: (C and D) anti-hagfish GPHα; (E and F) anti-hagfish GPHβ. Note that GPHα-producing cells are well in accordance with GPHβ-producing cells (arrowheads). B, D, and F show magnified views of regions shown by rectangles in A, C, and E, respectively. AH, adenohypophysis; CT, connective tissue; NH, neurohypophysis; III, third ventricle. (Scale bars: A, C, and E, 100 μm; B, D, and F, 20 μm.)
Positive Correlation Between Pituitary GPH Activity and Gonadal Development.
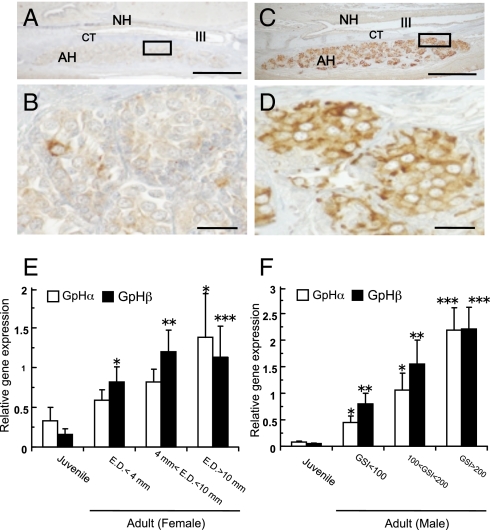
No accumulation or a faint accumulation of ir-GPHβ was found in a few adenohypophysial cells in juveniles (Fig. 4 A and B). On the other hand, a heavy accumulation of ir-GPHβ was found in many adenohypophysial cells in adults with developing gonads (Fig. 4 C and D). These results were quite similar to those obtained with anti-hagfish GPHα (Fig. S4). A highly significant difference was found in the accumulation of ir-GPHα between two experimental groups, adults with developing gonads and immature adults, whereas no difference between groups of the juveniles and immature adults (Table S2). Similar to their cellular activities, in both females and males, GPHα and -β transcripts were the lowest in the juveniles (Fig. 4 E and F). In the adults, both transcripts increased significantly in accordance with the developing condition of gonads, and they reached the highest expression in the adults having well-developed gonads. However, there were no significant differences between GPHα and -β mRNA expression in the pituitary of any one individual. As a result, hagfish GPHα and -β activities were closely related with developmental stages of the gonad.
Fig. 4.
Correlation between pituitary GPH activities and gonadal development in hagfish. (A–D) Cellular activities of GPHβ cells in the hagfish pituitary. Note that intense immunoreactions are observed in mature female (C and D), but faint reactions presented in juvenile (A and B). AH, adenohypophysis; CT, connective tissue; NH, neurohypophysis; III, third ventricle. (Scale bars: A and C, 100 μm; B and D, 20 μm.) (E and F) Relative GPHα and GPHβ gene expressions in the pituitary of female (E) and male (F) hagfish. Open bars represent GPHα gene expressions and filled bars represent GPHβ gene expressions. The two GPH mRNA levels were normalized by β-actin mRNA levels. Relative values are expressed as mean ± SE (n = 8–18). Significant differences from the juvenile are indicated by *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Note that two GPH transcripts in both sexes increase in accordance with the developmental stage of the gonad.
Native GPH Stimulates the Releases of Sex Steroids from Organ-Cultured Testis.
Hagfish GPH was isolated from pituitaries by reversed-phase HPLC (Fig. S5A). The protein eluted at 31 min contained over 28-KDa protein (Fig. S5B, lane 1), but two bands of 14 and 16 KDa appeared under reducing conditions (Fig. S5B, lane 2). A 14-KDa protein was immunoreactive with anti-hagfish GPHα and 16-KDa protein was immunoreactive with anti-hagfish GPHβ, respectively (Fig. S5B, lanes 3 and 4). N-terminal sequences of 14-KDa and 16-KDa proteins were determined to be DFQDSSRXTLRTS and QASQACDL, corresponding to positions 25 to 37 of deduced amino acid sequence of hagfish pre-GPHα and positions 16 to 23 to that of hagfish pre-GPHβ, respectively.
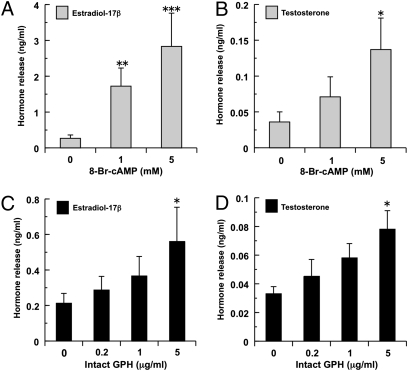
To examine whether the native GPH is functional and has a tropic action, we performed an in vitro bioassay and examined its biological potency by measuring the release of estradiol-17β and testosterone from organ-cultured testis. As a positive control, cell-permeable cAMP analog, 8-Bromoadenosine (8-Br) cAMP was used and led to dose-dependent increases in the releases of both sex steroids in vitro (Fig. 5 A and B). Similarly, intact hagfish GPH fractions increased hormone release in a dose-dependent manner and, at highest concentration (5 μg/mL), induced significant increase in hormone secretion of both sex steroids (Fig. 5 C and D). These results provide evidence that the hagfish GPH identified here has stimulatory effects on steroidogenesis and is thus considered a “GTH-like hormone” that can regulate gonadal functions.
Fig. 5.
Biological activity of native GPH from hagfish pituitary with in vitro bioassay. In vitro effects of 8-Br-cAMP (A and B, gray columns) and native GPH (C and D, black columns) on the releases of estradiol-17β (A and C) and testosterone (B and D) from organ-cultured testis. Values are expressed as mean ± SE (n = 6–8). Significant differences from the control groups (0 mM in A and B, 0 μg/mL in C and D) are indicated by *, P < 0.05; **, P < 0.01; and ***, P < 0.001. It is notable that intact GPH stimulates the release of sex steroids from cultured testis in vitro, indicating “gonadotropic action” of hagfish GPH.
Discussion
In the present study, the presence and identity of a functional GPH have been elucidated from the brown hagfish, Paramyxine atami (one of the species of the Pacific hagfish). Our data show high sequence similarity to gnathostome GPHα- and GPHβ-subunit families and not to the GPA2 and GPB5. Both subunits of GPH are produced in the same cells of the adenohypophysis, providing definite evidence for the presence of a heterodimeric GPH in the hagfish. GPH increases at both the gene and protein levels, corresponding to the reproductive stages of the hagfish. Moreover, purified native GPH induces the concentrations of sex steroids from cultured testis in a dose-dependent manner. These results clearly show that the GPH identified here acts as a functional “gonadotropic hormone” and is the earliest evolved pituitary GPH that has been identified in a basal vertebrate leading to the gnathostome GTH and TSH lineages.
The mature protein of hagfish GPHα contains 8 of 10 Cys residues and two N-glycosylation sites at the homologous positions to GPHα subunits of jawed vertebrates. In comparison, 12 Cys residues of hagfish GPHβ are exactly conserved at homologous position to three kinds of gnathostome GPHβ subunits, LHβ, FSHβ, and TSHβ. These Cys residues do not match the 10-Cys residue alignment found in GPB5 (18). Two putative N-glycosylation sites are homologous to FSHβ, the first one to LHβ and the second to TSHβ. As examined in human GTHs (20), well-conserved Cys residues found in hagfish GPH seem to be quite important for its predicted Cys-knot structure, comprised of disulfide bridges, and for specific binding to its putative receptors. From these biochemical characteristics, hagfish GPH clearly belongs to the pituitary TSH, FSH, and LH family in vertebrates.
Because the identified hagfish GPHα and -β subunits appear to be the typical GPHα and GPHβ subunits found in gnathostomes based on the sequence and phylogenetic analyses, we propose the following evolutionary scenario of the glycoprotein family (Fig. S1). An ancestral GP likely existed in the common ancestor of protostomes and deuterostomes, and diverged then into two subunits (GPA and GPB) in both lineages. After the split of protostomes and deuterostomes, these two subunits evolved as ancestral GPA2 and GPB5 in both lineages. As currently reported, two rounds of whole-genome duplications have been proposed to occur before the agnathan-gnathostome split in part based on the establishment of gene repertories (21). Ancestral GPA2/GPB5 molecules may have provided two diverged units, GPA2/GPB5 and GPHα/GPHβ, during the early phase of agnathan divergence. The latter, which is specific to the vertebrate pituitary, may have further diverged into three functional units, GPHα and LHβ/FSHβ/TSHβ after the agnathan-gnathostome split, but the former unit may also have independently evolved in the vertebrate lineages.
To date, there has been no evidence to support the presence of TSH in agnathans (2). Currently, it is proposed that one GTH-like molecule in lampreys stimulates both a GTH-like receptor located in the gonad and a TSH-like receptor in the thyroid tissue based on the identity and functional studies of these receptors (22, 23). Whether there are two GPHs in the lamprey consisting of a single GTH and perhaps a TSH remains to be determined. In the present study, only one type of GPH-producing cells, containing both GPHα and -β subunits, has been identified in the hagfish adenohypophysis. If the second form of GPH (GTH or TSH) is present in the hagfish pituitary, differences in the occurrence or intensity of immunoreactivities between the two subunits would be evident. Moreover, the two genes encoding their subunits show equivalent expression during reproductive development in both sexes. Thus, it seems most likely that hagfish GPH (hereafter referred to as hagfish GTH), the only GPH that has been identified, is a direct descendent of a common ancestor of the gnathostome GPH family and that this molecule may act both as a GTH- and TSH-like hormone, as proposed in the lamprey (1).
In jawed vertebrates, GTHs, in response to hypothalamic GnRH, are released from the pituitary and act on the gonads to regulate steroidgenesis and gametogenesis. Lampreys have a functional HPG axis, which is supported by a vast number of biochemical, molecular, histochemical, and functional studies (1). In contrast, our knowledge of the HPG axis of the hagfish is almost completely lacking. The lack of information is mostly a result of the fact that hagfish pituitary function is difficult to assess from cytological and physiological studies (6, 7). For example, complete hypophysectomy in Eptatretus stoutii has not provided any clear evidence for pituitary gonadotropic activity (8). On the other hand, in Eptatretus burgeri, there have been some indications that gonadal development and spermatogenesis may be retarded after hypophysectomy (9). Thus, the pituitary-gonadal axis of hagfish has been considered to be minimally, if at all, functional for a long time (7). However, our recent studies have revealed that ir-GTH-like material was demonstrable in the pituitary of the Pacific hagfish, Paramyxine atami, and further that accumulation of ir-GTH-like material was strongly correlated to the degrees of the developmental conditions of gonads (10–12). Furthermore, in the Atlantic hagfish, Myxine glutinosa, seasonal changes in GnRH and gonadal steroids, such as estradiol and progesterone, were demonstrated corresponding to gonadal reproductive stages (24, 25). In support of these studies, our current findings show that molecular expression of hagfish GTH is significantly correlated with their gonadal stages, and functionally active, as demonstrated by induced release of sex steroids from the testis (Fig. 5, Fig. S4, and Table S2). Thus, our data revealed that hagfish has a functional pituitary-gonadal system that is highly conserved across all vertebrates.
The control and integration of reproductive processes is generally regarded as probably the oldest and original function of the vertebrate hypothalamic-pituitary system. If this is indeed the case, a key point of interest would be to elucidate the early evolution of the HPG axis, or more simply, the GnRH-GTH-gonadal axis. Indeed, GnRHs, including GnRH-like peptides, have been found not only in chordates including amphioxus (26) and tunicates but also in multiple invertebrate phyla (27). Moreover, amphioxus has a complete set of genes for sex steroid hormone-synthesizing enzymes (17), as well as two genes for steroid hormone receptors, one more closely related to vertebrate estrogen receptors (17). In contrast, Ciona has only a few genes for enzymes that synthesize steroid hormones, suggesting tunicate-specific losses (28). However, neither amphioxus nor tunicates have homologs of adenohypophysial hormones (i.e., GTH, growth hormone, adrenocorticotropic hormone). Genes of adenohypophysial hormone receptors are also missing in the amphioxus and tunicate genome. Thus, adenohypophysial hormones appear to have evolved in the vertebrates. Before or during the early evolution of agnathans, two functional units have evolved for establishing the complex functional unit of the brain-pituitary axis: (i) the neurohypophysis, which originates from the ventral hypothalamus and (ii) the adenohypophysis, which originates from endoderm in hagfish or ectoderm in all other vertebrates (29). Involvement of hypothalamic GnRH in the reproductive system seems to be established through its ability to promote GTH release from the adenohypophysis, thus the vertebrate pituitary has evolved as a transducer of neural information to hemal information to the gonad. It is most likely that, concomitantly with the emergence of the pituitary gland at the early phase of vertebrate evolution, a vertebrate ancestor had already acquired the fundamental pituitary-gonadal axis regulated by a glycoprotein hormone.
In conclusion, we report identification of a unique glycoprotein hormone (GPH) in the pituitary of hagfish, the earliest divergent extant lineage and most primitive vertebrate. It seems most likely that an ancestral GPH gave rise to only one GTH in hagfish and that multiplicity of GPHs arose later during the early evolution of gnathostomes. We hypothesize that this functional pituitary GPH found in hagfish helps to delineate the evolution of the complex neuro/endocrine axis of reproduction in vertebrates. Furthermore, we propose that this HPG system likely evolved from an ancestral, prevertebrate exclusively neuroendocrine mechanism by gradual emergence of components of a previously undescribed control level, the pituitary gland.
Materials and Methods
Animals, cDNA cloning, molecular phylogenetic and statistical analyses, in situ hybridization and immunohistochemistry, and quantitative real-time PCR analysis are described in SI Materials and Methods. Pituitary cDNA library was prepared as reported in ref. 30. The ligated cDNA was transformed into Electro Max DH12S competent cells (Invitrogen). Sequences of primers used for full-length hagfish GTHα and -β cDNAs are shown in Table S3. PCR was conducted as described ref. 3 and all PCR products were cloned into pT7 Blue T-Vector (Novagen) and transformed into JM109 competent cells (Nippon Gene). Establishment of hagfish steroid hormone assay by time-resolved fluoroimmunoassay (TR-FIA) is also described in SI Materials and Methods (Fig. S6).
Purification of Native GPH and in Vitro Bioassay.
Purification and characterization of native GPH from hagfish pituitaries was performed as described in SI Materials and Methods. Maturing testis showing over 200 gonadosomatic index (GSI = testicular weight/body weight × 105) was removed from hagfish (n = 20), and was divided uniformly sized pieces (about 20 mg per piece). Each piece of testis was preincubated with 500 μL of the culture medium containing 75% Herbst's artificial seawater with 9.8 mg/mL Medium 199 (31) and 1× antibiotic-antimycotic (Invitrogen) at 15 °C for 24 h. The tissues were then cultured in 500 μL of the culture medium with or without native hagfish GPH at the same conditions. The tissues were also incubated with cell-permeable cAMP analog, 8-Br-cAMP (Sigma), as a positive steroidgenic inducer. The medium was collected and concentrations of estradiol-17β (E2) and testosterone (T) were measured by TR-FIA as described in ref. 32.
Supplementary Material
Acknowledgments
We thank Allisan Aquilina-Beck (University of New Hampshire, Durham, NH) and Dr. Adam Bazinet (University of Maryland, College Park, MD) for phylogenetic analysis. We also thank our anonymous reviewers for their constructive comments. This work was supported in part by Grant-in Aid for Basic Research from the Ministry of Education, Japan (to K.U. and M.N.), a Grant for Promotion of Niigata University Research Project (to K.U.), the Japan Gasoline Company-Saneyoshi Scholarship Foundation 2005 (to K.U.), and National Science Foundation Grant IOS-0849569 (to S.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AB546740 and AB546741 for hagfish GPHα and hagfish GPHβ, respectively, and AB546742 for hagfish β-actin).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002208107/-/DCSupplemental.
References
- 1.Sower SA, Freamat M, Kavanaugh SI. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: New insights from lampreys. Gen Comp Endocrinol. 2009;161:20–29. doi: 10.1016/j.ygcen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Kawauchi H, Sower SA. The dawn and evolution of hormones in the adenohypophysis. Gen Comp Endocrinol. 2006;148:3–14. doi: 10.1016/j.ygcen.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Sower SA, et al. Identification of sea lamprey GTHbeta-like cDNA and its evolutionary implications. Gen Comp Endocrinol. 2006;148:22–32. doi: 10.1016/j.ygcen.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Hall BK. Evolutionary Developmental Biology. London: Chapman & Hall; 1998. [Google Scholar]
- 5.Janvier P. Early Vertebrates. Oxford: Clarendon Press; 1996. [Google Scholar]
- 6.Holmes RL, Ball JN. The Pituitary Gland, a Comparative Account. London: Cambridge University Press; 1974. [Google Scholar]
- 7.Gorbman A. Reproduction in Cyclostome Fishes and Its Regulation. New York, London: Academic Press; 1983. pp. 1–28. [Google Scholar]
- 8.Matty AJ, Tsuneki K, Dickhoff WW, Gorbman A. Thyroid and gonadal function in hypophysectomized hagfish, Eptatretus stouti. Gen Comp Endocrinol. 1976;30:500–516. doi: 10.1016/0016-6480(76)90120-9. [DOI] [PubMed] [Google Scholar]
- 9.Patzner RA, Ichikawa T. Effect of hypophysectomy on the testis of the hagfish, Eptatretus burgeri, Girard (Cyclostomata) Zool Anz. 1977;199:371–380. [Google Scholar]
- 10.Miki M, Shimotani T, Uchida K, Hirano S, Nozaki M. Immunohistochemical detection of gonadotropin-like material in the pituitary of brown hagfish (Paramyxine atami) correlated with their gonadal functions and effect of estrogen treatment. Gen Comp Endocrinol. 2006;148:15–21. doi: 10.1016/j.ygcen.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Nozaki M, Shimotani T, Uchida K. Gonadotropin-like and adrenocorticotropin-like cells in the pituitary gland of hagfish, Paramyxine atami; immunohistochemistry in combination with lectin histochemistry. Cell Tissue Res. 2007;328:563–572. doi: 10.1007/s00441-006-0349-3. [DOI] [PubMed] [Google Scholar]
- 12.Nozaki M. The hagfish pituitary gland and its putative adenohypophysial hormones. Zoolog Sci. 2008;25:1028–1036. doi: 10.2108/zsj.25.1028. [DOI] [PubMed] [Google Scholar]
- 13.Nakabayashi K, et al. Thyrostimulin, a heterodimer of two new human glycoprotein hormone subunits, activates the thyroid-stimulating hormone receptor. J Clin Invest. 2002;109:1445–1452. doi: 10.1172/JCI14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudo S, Kuwabara Y, Park JI, Hsu SY, Hsueh AJ. Heterodimeric fly glycoprotein hormone-alpha2 (GPA2) and glycoprotein hormone-beta5 (GPB5) activate fly leucine-rich repeat-containing G protein-coupled receptor-1 (DLGR1) and stimulation of human thyrotropin receptors by chimeric fly GPA2 and human GPB5. Endocrinology. 2005;146:3596–3604. doi: 10.1210/en.2005-0317. [DOI] [PubMed] [Google Scholar]
- 15.Park J, II, Semeyonov J, Cheng CL, Hsu SYT. Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine. 2005;26:267–276. doi: 10.1385/ENDO:26:3:267. [DOI] [PubMed] [Google Scholar]
- 16.Dos Santos S, et al. Distinct expression patterns of glycoprotein hormone-α2 (GPA2) and -β5 (GPB5) in a basal chordate suggest independent developmental functions. Endocrinology. 2009;150:3815–3822. doi: 10.1210/en.2008-1743. [DOI] [PubMed] [Google Scholar]
- 17.Holland LZ, et al. The amphoioxus genome illustrates vertebrate origins and cephalochordare biology. Genome Res. 2008;18:1100–1111. doi: 10.1101/gr.073676.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tando Y, Kubokawa K. Expression of the gene for ancestral glycoprotein hormone beta subunit in the nerve cord of amphioxus. Gen Comp Endocrinol. 2009;162:329–339. doi: 10.1016/j.ygcen.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Tando Y, Kubokawa K. A homolog of the vertebrate thyrostimulin glycoprotein hormone α subunit (GPA2) is expressed in amphioxus neurons. Zoolog Sci. 2009;26:409–414. doi: 10.2108/zsj.26.409. [DOI] [PubMed] [Google Scholar]
- 20.Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuraku S, Meyer A, Kuratani S. Timing of genome duplications relative to the origin of the vertebrates: Did cyclostomes diverge before or after? Mol Biol Evol. 2009;26:47–59. doi: 10.1093/molbev/msn222. [DOI] [PubMed] [Google Scholar]
- 22.Freamat M, Kawauchi H, Nozaki M, Sower SA. Identification and cloning of a glycoprotein hormone receptor from sea lamprey, Petromyzon marinus. J Mol Endocrinol. 2006;37:135–146. doi: 10.1677/jme.1.02064. [DOI] [PubMed] [Google Scholar]
- 23.Freamat M, Sower SA. A sea lamprey glycoprotein hormone receptor similar with gnathostome thyrotropin hormone receptor. J Mol Endocrinol. 2008;41:219–228. doi: 10.1677/JME-08-0030. [DOI] [PubMed] [Google Scholar]
- 24.Kavanaugh SI, Powell ML, Sower SA. Seasonal changes of gonadotropin-releasing hormone in the Atlantic hagfish Myxine glutinosa. Gen Comp Endocrinol. 2005;140:136–143. doi: 10.1016/j.ygcen.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Powell ML, Kavanaugh SI, Sower SA. Seasonal concentrations of reproductive steroids in the gonads of the Atlantic hagfish, Myxine glutinosa. J Exp Zoology A Comp Exp Biol. 2004;301:352–360. doi: 10.1002/jez.a.20043. [DOI] [PubMed] [Google Scholar]
- 26.Chambery A, et al. Characterization and putative role of a type 1 gonadotropin-releasing hormone in the cephalochordate amphioxus. Endocrinology. 2009;150:812–820. doi: 10.1210/en.2008-1066. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Tello JA, Zhang W, Tsai P-S. Molecular cloning, expression pattern, and immunocytochemical localization of a gonadotropin-releasing hormone-like molecule in the gastropod mollusk, Aplysia californica. Gen Comp Endocrinol. 2008;156:201–209. doi: 10.1016/j.ygcen.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell RK, Satoh N, Degnan BM. Piecing together evolution of the vertebrate endocrine system. Trends Genet. 2004;20:359–366. doi: 10.1016/j.tig.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Gorbman A. Early development of the hagfish pituitary gland: Evidence for the endodermal origin of the adenohypophysis. Am Zool. 1983;23:639–654. [Google Scholar]
- 30.Kato S, Ohtoko K, Ohtake H, Kimura T. Vector-capping: A simple method for preparing a high-quality full-length cDNA library. DNA Res. 2005;12:53–62. doi: 10.1093/dnares/12.1.53. [DOI] [PubMed] [Google Scholar]
- 31.Osada M, Harata M, Kishida M, Kijima A. Molecular cloning and expression analysis of vitellogenin in scallop, Patinopecten yessoensis (Bivalvia, Mollusca) Mol Reprod Dev. 2004;67:273–281. doi: 10.1002/mrd.20020. [DOI] [PubMed] [Google Scholar]
- 32.Yamada H, Satoh R, Yamashita T, Kambegawa A, Iwata M. Development of a time-resolved fluoroimmunoassay (TR-FIA) for testosterone: Measurement of serum testosterone concentrations after testosterone treatment in the rainbow trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 1997;106:181–188. doi: 10.1006/gcen.1996.6861. [DOI] [PubMed] [Google Scholar]
- 33.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 35.Zwickl DJ. PhD dissertation. Austin, TX: University of Texas; 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. [Google Scholar]
- 36.Cummings MP, Huskamp JC. Grid computing. EDUCAUSE Rev. 2005;40:116–117. [Google Scholar]
- 37.Bazinet AL, Cummings MP. The Lattice Project: A grid research and production environment combining multiple grid computing models. In: Weber MHW, editor. Distributed and Grid Computing: Science Made Transparent for Everyone. Principles, Applications and Supporting Communities. Marburg: Rechenkraft.net; 2008. [Google Scholar]
- 38.Myers DS, Bazinet AL, Cummings MP. Expanding the reach of Grid computing: combining Globus- and BOINC-based systems. In: Talbi E-G, Zomaya A, editors. Grids for Bioinformatics and Computational Biology, Wiley Book Series on Parallel and Distributed Computing. New York: John Wiley & Sons; 2008. pp. 71–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.