Abstract
Sleep is homeostatically regulated in all animal species that have been carefully studied so far. The best characterized marker of sleep homeostasis is slow wave activity (SWA), the EEG power between 0.5 and 4 Hz during nonrapid eye movement (NREM) sleep. SWA reflects the accumulation of sleep pressure as a function of duration and/or intensity of prior wake: it increases after spontaneous wake and short-term (3–24 h) sleep deprivation and decreases during sleep. However, recent evidence suggests that during chronic sleep restriction (SR) sleep may be regulated by both allostatic and homeostatic mechanisms. Here, we performed continuous, almost completely artifact-free EEG recordings from frontal, parietal, and occipital cortex in freely moving rats (n = 11) during and after 5 d of SR. During SR, rats were allowed to sleep during the first 4 h of the light period (4S+) but not during the following 20 h (20S−). During the daily 20S− most sleep was prevented, whereas the number of short (<20 s) sleep attempts increased. Low-frequency EEG power (1–6 Hz) in both sleep and wake also increased during 20S−, most notably in the occipital cortex. In all animals NREM SWA increased above baseline levels during the 4S+ periods and in post-SR recovery. The SWA increase was more pronounced in frontal cortex, and its magnitude was determined by the efficiency of SR. Analysis of cumulative slow wave energy demonstrated that the loss of SWA during SR was compensated by the end of the second recovery day. Thus, the homeostatic regulation of sleep is preserved under conditions of chronic SR.
Keywords: cortex, EEG, slow wave activity, slow wave energy, theta activity
Sleep is homeostatically regulated in all mammalian and nonmammalian species that have been carefully studied so far: in general, the longer an animal stays awake, the longer and/or deeper it sleeps (1–4). The best characterized marker of sleep pressure in mammals and birds is slow wave activity (SWA), defined as the electroencephalogram (EEG) power between 0.5 and 4 Hz during nonrapid eye movement (NREM) sleep. SWA peaks at sleep onset and decreases with time spent asleep (3). Staying awake from ∼3 to ∼24 h results in progressively higher SWA levels at sleep onset, and naps during the day reduce SWA the following night (3). Slow waves reflect the synchronous firing of large groups of cortical neurons coordinated by an underlying slow oscillation, the fundamental cellular phenomenon of NREM sleep (5). Increasing evidence suggests that slow waves can mediate some of sleep's beneficial effects, from the prevention of cognitive impairment to memory consolidation (6–9). Thus, SWA may be more than just an epiphenomenon of NREM sleep and may be related to its functions.
Numerous studies have shown that SWA increases after periods of spontaneous wake or following a few hours of sleep deprivation (10–12). However, fewer experiments have measured SWA after >1 d of sleep deprivation or after several days of sleep restriction (SR), during which sleep is only allowed for a few hours every day (13–18). One study found that SWA did not increase above baseline level after 4 d of total sleep deprivation (16), and 5 d of SR resulted in a progressively smaller SWA increase in one case (15) and in no change except after the first day of SR in another (14). These results have raised doubt about the ability of SWA to reflect chronic sleep need and, more generally, have suggested that under conditions of chronic SR sleep may be regulated by both allostatic and homeostatic mechanisms (14).
Experiments to chronically enforce wake are inherently difficult, because sleep pressure increases rapidly and some sleep cannot be avoided, irrespective of stimulation. Even during experiments of short-term (<24 h) sleep deprivation some portion of baseline sleep (usually 5–10%) is always maintained (see refs. in ref. 4), and during several days of “total” sleep deprivation rats still sleep at least 10% of the time, due to “microsleep” episodes (19). Perhaps more importantly, spectral EEG analysis reveals that even during acute sleep deprivation slower activity, including SWA (0.5–4 Hz) or low theta (5–7 Hz) activity, leaks into periods during which the subject may be moving around with eyes open, and which are conventionally scored as wake (e.g., rats) (20, 21). Of note, when wake is prolonged beyond its physiological duration cortical neuronal activity changes (22), and brain metabolism tends to decrease, rather than increase (23, 24), suggesting that the brain may switch into a sleep-like mode. Unfortunately in previous studies that measured SWA after chronic sleep deprivation or SR (13–18) either the wake EEG could not be recorded continuously or a detailed analysis could not be performed, due to the presence of EEG artifacts.
Chronic sleep loss is increasingly frequent in our society and has detrimental effects on both cognitive function (25) and general health (26, 27). Thus, the issue of whether sleep remains homeostatically regulated under chronic SR is important not only for the study of sleep regulation per se, but also to understand its negative consequences. Here we performed continuous, almost completely artifact-free EEG recordings from frontal, parietal, and occipital cortex in freely moving rats during and after 5 d of SR, in which sleep was allowed for only 4 h/d starting at light onset. To achieve the highest possible efficiency of SR, the automated method of sleep deprivation (disk over water) was supplemented by continuous (24 h/d × 5 d) visual observation by the experimenter. We found that in all animals SWA showed a significant homeostatic increase on each day of SR and for at least 2 recovery d after SR. The SWA increase was more pronounced in the frontal regions, consistent with previous studies of acute sleep deprivation (12, 28, 29), and its magnitude was strongly determined by the efficiency of SR. Moreover, analysis of cumulative slow wave energy (SWE = SWA × time) demonstrated that the loss of SWA during SR was compensated by the end of the second recovery day after SR. Thus, in rats, sleep homeostasis and SWA regulation appear intact under chronic SR conditions.
Results
SR Decreases Sleep Latency and Increases Sleep Consolidation.
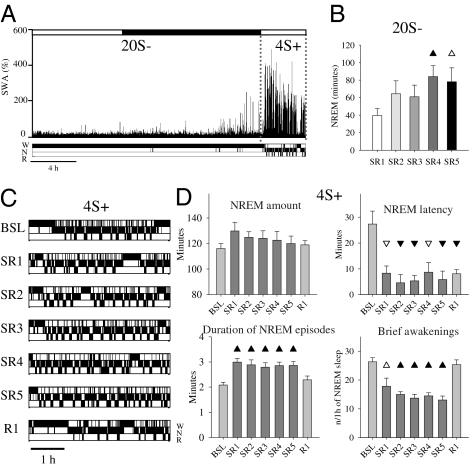
We first tested whether during baseline all major sleep parameters, including the daily profile of SWA, did not differ in rats housed on the disk-over-water as compared with rats recorded in their home cages and found that this was the case (SI Materials and Methods and Fig. S1). Each of the 5 d of SR included 20 h of total sleep deprivation (20S−) followed by 4 h of sleep opportunity starting at light onset (4S+; Fig. S2A). During 20S− the disk-over-water method, supplemented by continuous visual observation by the experimenter (Materials and Methods), was effective in preventing most, although not all, sleep in all rats (Fig. 1A). Specifically, there were almost no consolidated sleep bouts during 20S−, and >90% of all sleep attempts lasted <20 s (mean duration: 10.3 ± 0.1 s). NREM sleep was reduced by ∼80% relative to baseline values during the same 20-h period (from 6.2 ± 0.3 to 1.1 ± 0.2 h; from 8.2 ± 0.3 to 3.2 ± 0.2 h in 24 h; Fig. S2A). In other words, only ∼5% of time during 20S− was spent asleep (Fig. 1B), resulting in a cumulative loss of ∼25 h of sleep across the 5 SR d). REM sleep was almost completely eliminated (<0.5% of baseline, see below).
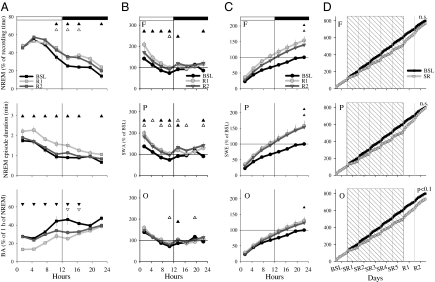
Fig. 1.
(A) Hypnogram and SWA in frontal cortex (percentage of 24-h mean value in NREM sleep) during 1 of the 5 SR days in one representative rat. W, wake; N, NREM sleep; R, REM sleep. (B) Daily amount of NREM sleep during 20S− (mean ± SEM, n = 11): the amount of sleep increases across days [rANOVA, factor “day,” F(4,40) = 4.97, P = 0.002]. Triangles indicate differences relative to the first SR day (black, P < 0.05; white, P < 0.1). (C) 4S+ hypnograms in a representative rat during baseline (BSL), 5 SR days, and first recovery day (R1). Note also that the sleep latency in R1 is similar to that in BSL, because rats had already slept ad libitum for 24 h before R1 (Fig. S2A). (D) NREM sleep amount, NREM sleep latency, NREM episode duration and number of brief awakenings during 4S+ (mean ± SEM, n = 11). Triangles show differences from baseline (black, P < 0.05; white, P < 0.1, after Bonferroni correction).
Total sleep time during 20S− was higher during the last 2 d of SR relative to the first 3 d, suggesting increased sleep pressure (Fig. 1B). Changes in sleep architecture during 4S+ were also indicative of increased sleep propensity (Fig. 1C). Specifically, whereas the amount of NREM sleep did not change markedly, the latency to the first NREM sleep episode decreased from ∼25 min to <10 min on all SR days (Fig. 1D). Moreover, sleep became more consolidated, as indicated by a ∼30% increase in the duration of NREM sleep episodes and by a ∼50% decrease in the number of brief awakenings (Fig. 1D). Thus, when allowed to sleep during 4S+, rats did so in a more consolidated way.
SR Increases NREM SWA and Does So Mostly in the Frontal Cortex.
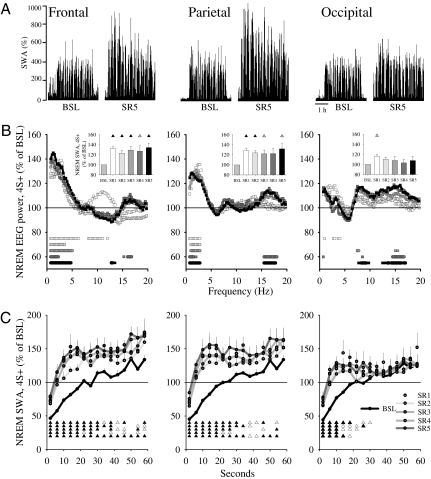
Acute sleep deprivation is followed not only by shorter sleep latency, longer sleep episodes, and fewer brief awakenings, but also by increased NREM SWA. Thus, we asked whether SR also increases sleep pressure as measured by SWA. Indeed, on most SR days NREM SWA during 4S+ was significantly above baseline levels (by ∼20%) in frontal and parietal cortex (Fig. 2 A and B). SWA in occipital cortex, by contrast, showed a minor increase only on the first day of SR. Changes in sleep EEG power were largely restricted to SWA in the frontal and parietal derivation, whereas in the occipital area, where changes in SWA were minor, higher frequencies (13–18 Hz) were also enhanced (Fig. 2B).
Fig. 2.
(A) SWA (% of 24-h mean values in NREM sleep) during 4S+ in baseline and the last day of SR in one representative rat. (B) NREM sleep EEG power during 4S+ in the 5 d of SR plotted as percentage of the corresponding baseline period (mean, n = 8). Squares depict frequency bins that differed significantly from baseline (P < 0.05, paired t test). (Insets) Four-hour mean values of NREM SWA. Triangles show differences from baseline [paired t test; black, P < 0.05; white, P < 0.1; rANOVA, factor “day”: frontal, F(5,35) = 6.06, P = 0.0004; parietal, F(5,35) = 5.49, P = 0.0008; occipital, F(5,35) = 1.97, P = 0.1]. (C) Time course of NREM SWA during individual NREM sleep episodes in baseline and during SR (4S+). Triangles show differences from baseline (black, P < 0.05; white, P < 0.1; paired t test; mean ± SEM, n = 8).
We next measured SWA changes within each NREM episode, because the intraepisode buildup of SWA is another sensitive measure of sleep pressure, becoming faster after short-term sleep deprivation in rats, mice, and humans (28, 30, 31). On all 5 SR d, the buildup of SWA during 4S+ was faster, suggesting a more rapid transition to the deep stages of sleep. This was the case also in the occipital cortex, despite less pronounced changes in mean SWA (Fig. 2C). Moreover, again consistent with a homeostatic process and in line with previous studies of total sleep deprivation (20), SWA was highest at the beginning of each 4S+ period and its decline was faster than during baseline (Fig. S3).
We also tested other sensitive markers of sleep pressure related to SWA, namely the amplitude and slope of slow waves, because they are known to increase at the beginning of the sleep period or after acute sleep deprivation (32–34). We found that during 4S+ both markers were higher than in baseline on all SR days and in all three cortical areas, again indicating higher sleep pressure during SR (Fig. S4 A and B). As for SWA, the differences were more pronounced in the anterior derivations.
Because we observed regional differences in the response to SR, we analyzed the origin and propagation of slow waves. As expected, we found that during baseline most (>30%) slow waves originated in the frontal cortex, whereas fewer started in more posterior areas (Fig. S4C). Interestingly, on the first day of SR the number of slow waves with frontal origin increased, while slow waves with parietal and occipital origin became less frequent, a trend then maintained across the entire duration of SR (Fig. S4D). Thus, SR increases NREM SWA and slow waves and does so more in the frontal regions.
SR Slows the Wake EEG Mostly in the Occipital Cortex.
In rodents, sustained wake also affects the wake EEG, with increases in the low-frequency range that spans SWA and low theta (5–7 Hz) activity (20, 21, 29). Such changes may reflect the buildup of sleep pressure during wake and may even partially compensate for sleep need. Thus, we next investigated how SR affects the wake EEG.
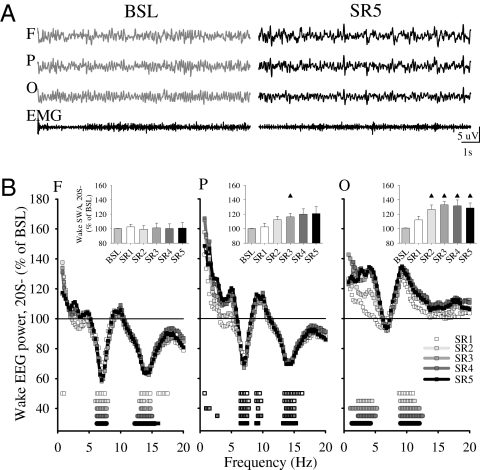
During 20S− we frequently observed that the high theta (7–9 Hz) activity typical of baseline wake (21) was reduced in the frontal and parietal cortex, consistent with our behavioral observation that rats on the disk do not often engage in active exploratory behavior (see below). On the other hand, EEG power in the SWA range increased mostly in the occipital derivation (Fig. 3). Thus, changes in the wake EEG during 20S− seemed somehow complementary to those in NREM SWA during 4S+, which were largest in the frontal derivation (Fig. 2B), and mean (averaged across all rats) wake SWA for each SR day was negatively correlated with mean NREM SWA (r = −0.81, P < 0.001). In other words, the frontal cortex, which showed the highest sleep SWA during 4S+, had the lowest wake SWA during the preceding 20S−, whereas the occipital cortex showed the highest wake SWA and the lowest sleep SWA. Similar results were also observed when SWA during 20S− was calculated across all three vigilance states, rather than only in wake.
Fig. 3.
(A) 12-s wake EEG traces from frontal (F), parietal (P), and occipital (O) cortex and EMG in a representative rat during BSL and day 5 of SR. (B) Wake EEG power during 20S− in the 5 d of SR plotted as percentage of the corresponding 20-h baseline period (mean, n = 8). Squares indicate frequency bins that differed from baseline (P < 0.05, paired t test). (Insets) Mean 20-h values of wake SWA in baseline and SR days 1–5 (n = 8). Triangles show differences from baseline [paired t test, P < 0.05; rANOVA, factor “day”: frontal, F(5,35) = 0.49, NS; parietal, F(5,35) = 3.36, P = 0.01; occipital, F(5,35) = 3.79, P = 0.008].
Efficiency of Sleep Loss Determines the SWA Rebound During SR.
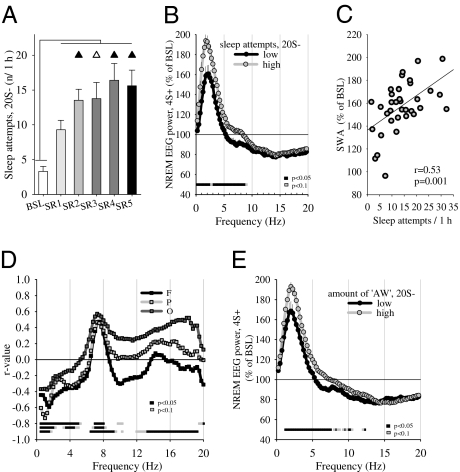
How does the efficiency of sleep deprivation affect recovery sleep? To address this question we first investigated whether the level of NREM SWA during 4S+ could be predicted by the sleep/wake history during the preceding 20S− episodes. Although rats were awake >90% of the time during 20S−, short (<20 s) sleep attempts occurred sporadically (Fig. S5 A–C). Their number was negligible during baseline (3.3 attempts/1 h), increased significantly within each SR day, and was highest in the last 2 d of SR (Fig. 4A). Moreover, within each SR day, their number increased during the light period and remained high during the dark phase (Fig. S6). Thus, we subdivided all 20S− phases into those with few (lowest 33%: average 6.3 attempts/1 h) and those with many (top 33%: average 22.3 attempts/1 h) sleep attempts. We found that during 4S+ the EEG power in the low frequencies (<9 Hz) was higher when it was preceded by a 20S− phase with many sleep attempts, and lower when 20S− included fewer sleep attempts (Fig. 4B). In other words, the sleepier the rats were during 20S−, as indicated by their repeated attempts to fall asleep, the higher was the sleep pressure during subsequent 4S+, as measured by NREM SWA, and the two parameters were positively correlated (Fig. 4C). Mean SWA during the sleep attempts was low (84.35 ± 4.8% of the mean 24-h baseline value in NREM sleep), consistent with their short duration and their inability to significantly decrease sleep pressure.
Fig. 4.
(A, Left) mean number of sleep attempts (<20 s) per hour during 20S−. Triangles show differences from day 1 of SR (black, P < 0.05; white, P < 0.1). (B) NREM EEG power spectra (average of all derivations) during 4S+ plotted as percentage of baseline, shown separately for days with high (highest 33%, twelve 4S+ periods) or low (lowest 33%, twelve 4S+ periods) number of sleep attempts during 20S− (mean ± SEM, n = 8). (C) Relationship between the number of sleep attempts per hour during 20S− intervals and SWA in subsequent 4S+ periods (n = 8). (D) Correlations (Pearson) between wake EEG power during 20S− and NREM SWA in subsequent 4S+. R-values are plotted for each frequency bin for F, P, and O cortex. The strong correlation in O between sleep SWA and wake beta activity may be due to the fact that the latter includes frequencies (∼14–18 Hz) that are influenced significantly by the high harmonic of theta activity (∼7–9 Hz), which in rats is strongest in O due to the proximity to the hippocampus. Because SWA correlates with theta activity itself, one could also expect high correlation with its harmonics. (E) NREM EEG power spectra (average of the F, P, and O) during the 4S+ periods plotted as percentage of baseline, shown separately for days with high (highest 33%) and low (lowest 33%) number of AW epochs during the preceding 20S− periods (mean ± SEM, n = 8).
Next, we asked whether the occurrence of wake SWA during 20S− affects the NREM SWA during the following 4S+ periods. Indeed, we found that the two parameters were negatively correlated in all three cortical areas, and the effect was largely specific for the SWA range (Fig. 4D). Thus, the higher the wake SWA was during 20S−, the lower the sleep SWA in the following 4S+. To confirm this result, we subdivided the SR days into two groups of high or low SWA during 20S− (calculated over all three vigilance states or NREM only) and computed the corresponding NREM spectra during the following 4S+ phase. We found that NREM SWA during 4S+ was significantly lower when preceded by a 20S− phase with more slow waves (P < 0.05). Interestingly, we found that during each 4S+ phase and the 2 post-SR recovery days SWA increased also during wake and REM sleep, suggesting that the “leakage” of slow waves into other vigilance states may be another sensitive marker of increased NREM sleep pressure.
Wake with High Theta Activity Affects NREM SWA During SR.
During SR we did not systematically score the rats’ behavior, as it was highly fragmented. However, we observed that rats on the disk tried to minimize their locomotor activity by moving just enough to adjust to the slowly rotating disk and tended to be less engaged in active exploration than during baseline. Active exploratory behavior in rodents is associated with high theta activity (35), and we confirmed this fact by analyzing video recordings during baseline in a subset of animals (Fig. S5 D–F). Moreover, in all rats we compared active wake (AW) and quiet wake (QW) EEG during baseline (∼5 min of recording for each state) and found that AW was reliably associated with a faster and higher theta peak, consistent with data in mice (11) and rats (21, 36). Specifically, during baseline the EEG power between 1–6 Hz was higher in QW compared with AW, whereas the opposite was true for the EEG power between 6 and 9 Hz, which was at least 50% higher in AW (Fig. S5E). Thus, during SR we used the power ratio 6.25–9 Hz/1.25–6 Hz to distinguish AW from QW. Overall, rats spent more time in AW during SR than during baseline (Fig. S7), as expected because they were awake longer, but every day the relative amount of AW (percentage of total wake) did not differ from baseline (Fig. S7). Moreover, the average ratio 6.25–9 Hz/1.25–6 Hz within AW was actually lower during each SR day relative to baseline (Fig. S7) in frontal and parietal cortex, suggesting that when the disk rotates rats move more but may explore less.
Previous studies (11, 12, 36) showed that AW leads to more intense sleep as measured by SWA. Thus, we asked whether the amount of high theta activity during 20S− could affect subsequent sleep quality during 4S+. Indeed, the theta power between 7 and 8 Hz during 20S− was positively correlated with increased sleep intensity as measured by SWA during the following 4S+ (Fig. 4D). Moreover, after dividing the 20S− periods on the basis of the number of AW epochs, we found that those with more AW were followed by a 4S+ phase with higher NREM SWA (Fig. 4E). Thus, AW, presumably dominated by exploratory behavior (35), leads to more intense sleep also during SR, consistent with the idea that homeostatic changes in sleep SWA are determined by both duration and quality of wake.
Lost SWA Is Fully Recovered After SR in Frontal and Parietal Cortex.
Signs of higher sleep pressure persisted after the end of SR, because sleep was longer and more consolidated (with longer bouts and fewer brief awakenings) throughout the first day of recovery (Fig. 5A). In frontal and parietal cortex NREM SWA was also increased, compared with baseline during the entire light phase of the first post-SR day (Fig. 5B), resulting in higher cumulative slow wave energy by the end of 24 h (SWE, SWA × time; Fig. 5C). More limited increases in NREM SWA and higher SWE were still seen during the second day after SR (Fig. 5C), suggesting that the homeostatic sleep pressure cumulated during 5 SR d took at least 2 d to dissipate.
Fig. 5.
(A) Time course of NREM sleep, NREM episode duration, and number of brief awakenings (BA) during BSL and 2 post-SR recovery days (mean ± SEM, n = 11). Triangles show differences from baseline [Upper, BSL vs. R1; Lower, BSL vs. R2, black, P < 0.05; white, P < 0.1 after significance in rANOVA; NREM sleep, “day”: F(14,210) = 8.87, P = 9.3625e-004, “day” × “time interval”: F(14,210) = 1.73, P = 0.05; NREM episode duration, day: F(14,210) = 5.87, P = 0.0071, day × time interval: F(14,210) = 1.44, P = 0.13; number of BA, day: F(14,210) = 16.5, P = 1.4416e-005, day × time interval: F(14,210) = 1.97, P = 0.0216]. (B) Time course of SWA during BSL and 2 recovery days in F, P, and O (mean ± SEM, n = 8). Triangles show differences from baseline [black, P < 0.05; white, P < 0.1 after significance in rANOVA, interaction factors day × time interval: frontal, F(14,147) = 5.25, P = 5.3376e-008; parietal, F(14,147) = 3.9, P = 1.1684e-005; occipital, F(14,147) = 2.82, P = 9.3817e-004]. (C) Time course of slow wave energy (SWE) in NREM sleep during baseline and 2 recovery days (mean ± SEM, n = 8; symbols as in A). (D) Time course of total (cumulative) SWE computed over all three vigilance states during baseline, SR (shaded area), and 2 recovery days. Mean values (n = 8).
Finally, we computed cumulative SWE throughout the entire experiment (before, during, and after SR) and compared it to the values that SWE would have reached if undisturbed sleep had been permitted during the same period. A small SWE deficit was still present by the second day after SR when only NREM SWE was included in the computation (P < 0.1, frontal and parietal derivation; P < 0.01, occipital derivation, Fig. S2C). Such deficit, however, disappeared in the frontal and parietal cortex when SWA during wake and REM sleep were also included in the computation (Fig. 5D). SWE in the occipital derivation approached baseline values but was still lower at a tendency level (P < 0.1). Thus, frontal and parietal cortex recovered all SWA within 2 d after SR, mostly during NREM sleep, but to some extent also during wake and REM sleep.
Changes in REM Sleep During and After SR.
REM sleep was almost completely suppressed during 20S− and showed a large rebound on each of the five 4S+ periods, when REM sleep latency decreased and number and duration of REM sleep episodes increased (Fig. S8A). REM sleep EEG spectra during the five 4S+ periods showed a large increase in power in most frequencies including SWA, especially in frontal and parietal cortex, whereas EEG power in the alpha/theta range (6.25–10 Hz) increased less or not at all (occipital cortex; Fig. S8B). In previous studies (37, 38) an increased ratio SWA to alpha/theta during REM sleep was suggested to reflect the buildup of REM pressure, but during our SR experiment this ratio either did not change (frontal and occipital cortex) or increased, but not progressively (parietal cortex). Large increases in REM sleep were also present after SR and as a result, almost all REM sleep lost during SR was recovered by the end of the second post-SR day (Fig. S8C).
Discussion
We show here that all of the established markers of sleep pressure that we measured responded to chronic SR as they do after acute sleep loss, namely with an increase in sleep attempts, a decrease in sleep latency and brief awakenings, and an increase in the duration of sleep episode, SWA, and the amplitude and slope of NREM slow waves. It is worth pointing out that constant visual observation of the rats for 5 consecutive days was required to prevent sleep as successfully as possible. This may explain why an SWA rebound (and other measures of homeostatic sleep pressure) was not observed in some previous studies that used similar SR regimes, but in which these somewhat “extreme” measures were not taken. Indeed, in the most recent study (14) the overall average SWA during the 20S− phases was not significantly lower that during the same time period during baseline, when rats could sleep ad libitum, and in a previous total sleep deprivation study SWA during 4 d of presumed “total waking” was 65.5% of the total 24-h SWA during baseline, when rats slept at least for 12–14 h (16). In contrast, a recent human study (13) where subjects were allowed to spend time outdoors (which may have helped them to stay awake more effectively), showed a sleep rebound on each of the 5 d of SR. Another difference between our study and that of Kim et al. (14), who used the same SR protocol, is that we analyzed the wake EEG for the entire duration of the experiment. This is crucial to interpret changes in SWA, because the latter is exquisitely sensitive to the sleep/waking history of the previous several hours (e.g., ref. 12), and it declines quickly during sleep (39).
Do the observed changes in SWA suggest that the homeostatic mechanisms of sleep regulation remain intact during SR? One could argue that if so, the increasing sleep pressure should have resulted in progressively larger SWA rebounds during the five 4S+ phases. However, this should not necessarily be the case. Already after the first SR day rats were asleep ∼3 of the 4 allowed hours, with almost a third of total sleep consisting of REM sleep, and spent the remaining time mostly eating and grooming in preparation for sleep. In our experience, even rats sleep deprived for 1–2 wk will not forgo these activities in the first 1–2 h of recovery. Moreover, both the amplitude and the incidence of slow waves, and thus SWA, cannot increase indefinitely, as they are limited by the extent of the recruitment of local neural populations in the slow oscillation (33, 34). On the other hand, if SWA during SR is homeostatically regulated its levels during each sleep opportunity window should be determined by the sleep pressure accumulated during the preceding wake interval. We found that this was the case: the more effective we were in enforcing wake during each 20S−, the larger was the sleep SWA rebound during the following 4S+. More stringently, SWE analysis demonstrated that all SWA lost during SR was recovered within the first 2 d of recovery. Importantly, although most (∼90%) of the recovery occurred during NREM sleep, some also took place in REM sleep and wake, indicating that sleep pressure during SR is high enough to force the leakage of slow waves into other behavioral states. Thus, it appears that homeostatic mechanisms of sleep regulation as measured by SWA remain intact during and after 5 d of SR, although we cannot rule out that longer periods of sleep loss would have produced different results. A period of 5 d was chosen to follow as closely as possible the design used by Kim and colleagues (14), and because it mimics what often happens in real life, where sleep is restricted during the week days and recovered during the weekend.
We found that the SWA rebound after chronic SR was largest in the frontal cortex and lowest in the occipital cortex, consistent with the results of a recent study of chronic SR in humans (18) and of several studies of total sleep deprivation (29, 40, 41). The frontal predominance in SWA is also observed in baseline conditions (29, 40–42) and may result from stronger cortico–cortical connections in the anterior as compared with posterior cortex (41), leading to stronger neuronal synchronization and a preferential origin of sleep slow waves in frontal areas (43, 44). Recent evidence suggests that SWA reflects the extent of neuronal activity and plasticity that occurred during the prior wake period (45–48). Thus, higher frontal SWA may also reflect heavier neuronal use/plasticity in the anterior as compared with posterior cortex, although this remains to be demonstrated. In summary, several not mutually exclusive mechanisms may underlie the frontal predominance of SWA, but their respective role remains to be tested experimentally.
Our results show that the intrusion of slow waves during wake slows down the build up of sleep pressure and does so in a region-specific manner, a finding never reported before, to our knowledge. From a practical point of view this means that, especially in experiments in which wake is prolonged for many hours or days, the analysis of the wake EEG in different cortical areas is important to understand the sleep homeostatic response. From a functional point of view, an intriguing possibility is that “wake slow waves” may decrease sleep need because they provide, at the cellular level, at least some of the benefits of sleep slow waves. Although highly speculative at this time, this hypothesis can be tested experimentally, for instance by measuring whether molecular and electrophysiological markers of synaptic strength, which decrease during sleep (49), are also decreased during wake with sustained increase in SWA. It is worth remembering, however, that an increase in wake SWA, whether caused by prolonged wake or by certain drugs (e.g., anticholinergic agents) (50), consistently results in cognitive deficits (4).
Materials and Methods
Animals.
Male WHY rats (n = 11) were implanted for EEG recordings as described (36). Animal procedures followed National Institutes of Health guidelines and facilities were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison. SR (20S−) was enforced using the disk-over-water method (16) (SI Materials and Methods).
EEG Scoring.
Behavioral states were scored as described (36) (Fig. S5A) using three EEG signals (frontal, F; parietal, P; occipital, O) and muscle (EMG) signal. Because one of the main goals of the study was to assess the effects of SR on the wake EEG, we took extreme care in excluding from the latter even very short episodes (<2 s) containing slow waves, and epochs were scored as NREM sleep if the EEG amplitude was >2-fold higher than wake (Fig. S5B). Sleep attempts were defined as sleep episodes <20 s preceded and followed by wake episodes >20 s (Fig. S5C). Brief awakenings were defined as short episodes of arousal <16 s (20). Sleep latency was defined as the time elapsed between the onset of the 4S+ period and the first NREM sleep episode >16 s. Epochs with movement or technical artifacts were excluded from spectral analysis (baseline: 4.6 ± 1.2%, of which 95.6 ± 1.3% occurred in wake; SR: 5.9 ± 1.6%, 96.7 ± 1.5% in wake). Spectral analysis was performed on a subset of rats (n = 8), in which EEG signals remained stable throughout the entire 8-d experimental period.
Signal Processing and Statistical Analysis.
Data acquisition and analysis was performed as described (34, 36) using MATLAB (Math Works). Detection of individual slow waves was performed according to ref. 34 and SI Materials and Methods). Changes in sleep variables, EEG power in selected frequency bands, or EEG spectra were tested with one-way ANOVA for repeated measures (rANOVA) with factor “day,” or with two-way rANOVA with factors day and “time interval.” Contrasts were tested by two-tailed paired t tests for those cases where rANOVA was significant. The relationships between the efficiency of 20S− and subsequent sleep during 4S+ were assessed with linear correlation analysis.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grant P20 MH077967 (to C.C.), a National Institutes of Health Director's Pioneer award (to G.T.), and Air Force Office of Scientific Research Grant FA9550-08-1-0244 (to G.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002570107/-/DCSupplemental.
References
- 1.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2005. pp. 405–417. [Google Scholar]
- 3.Tobler I. Phylogeny of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2005. [Google Scholar]
- 4.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: A view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 6.Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landsness EC, et al. Sleep-dependent improvement in visuomotor learning: A causal role for slow waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 9.Walsh JK. Insights into the public health burden of insomnia. Sleep. 2006;29:142–143. doi: 10.1093/sleep/29.2.142. [DOI] [PubMed] [Google Scholar]
- 10.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber R, Deboer T, Tobler I. Prion protein: A role in sleep regulation? J Sleep Res. 1999;8(Suppl 1):30–36. doi: 10.1046/j.1365-2869.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 12.Vyazovskiy VV, Ruijgrok G, Deboer T, Tobler I. Running wheel accessibility affects the regional electroencephalogram during sleep in mice. Cereb Cortex. 2006;16:328–336. doi: 10.1093/cercor/bhi110. [DOI] [PubMed] [Google Scholar]
- 13.Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: Support from EEG dynamics. Sleep. 2009;32:217–222. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci USA. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancel M, Kerkhof GA. Effects of repeated sleep deprivation in the dark- or light-period on sleep in rats. Physiol Behav. 1989;45:289–297. doi: 10.1016/0031-9384(89)90130-3. [DOI] [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 18.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS ONE. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat: An update of the 1989 paper. Sleep. 2002;25:18–24. doi: 10.1093/sleep/25.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Franken P, Dijk DJ, Tobler I, Borbély AA. Sleep deprivation in rats: Effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everson CA, Smith CB, Sokoloff L. Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J Neurosci. 1994;14:6769–6778. doi: 10.1523/JNEUROSCI.14-11-06769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JC, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 25.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 26.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–69. [PubMed] [Google Scholar]
- 29.Huber R, Deboer T, Tobler I. Topography of EEG dynamics after sleep deprivation in mice. J Neurophysiol. 2000;84:1888–1893. doi: 10.1152/jn.2000.84.4.1888. [DOI] [PubMed] [Google Scholar]
- 30.Deboer T, de Boer T. Sleep and sleep homeostasis in constant darkness in the rat. J Sleep Res. 2009;18:357–364. doi: 10.1111/j.1365-2869.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 31.Huber R, Deboer T, Tobler I. Sleep deprivation in prion protein deficient mice sleep deprivation in prion protein deficient mice and control mice: Genotype dependent regional rebound. Neuroreport. 2002;13:1–4. doi: 10.1097/00001756-200201210-00005. [DOI] [PubMed] [Google Scholar]
- 32.Bersagliere A, Achermann P. Slow oscillations in human non-rapid eye movement sleep electroencephalogram: Effects of increased sleep pressure. J Sleep Res. 2010;19:228–237. doi: 10.1111/j.1365-2869.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- 33.Riedner BA, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 36.Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- 37.Brunner DP, Dijk DJ, Borbély AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–113. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 38.Shea JL, et al. Rapid eye movement (REM) sleep homeostatic regulatory processes in the rat: Changes in the sleep-wake stages and electroencephalographic power spectra. Brain Res. 2008;1213:48–56. doi: 10.1016/j.brainres.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobler I, Borbély AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 40.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: Correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 41.Vyazovskiy VV, Tobler I. Regional differences in NREM sleep slow-wave activity in mice with congenital callosal dysgenesis. J Sleep Res. 2005;14:299–304. doi: 10.1111/j.1365-2869.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 42.Werth E, Achermann P, Borbély AA. Brain topography of the human sleep EEG: Antero-posterior shifts of spectral power. Neuroreport. 1996;8:123–127. doi: 10.1097/00001756-199612200-00025. [DOI] [PubMed] [Google Scholar]
- 43.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci USA. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28:4088–4095. doi: 10.1523/JNEUROSCI.5510-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32:719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyazovskiy V, Borbély AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 48.Vyazovskiy VV, Tobler I. Handedness leads to interhemispheric EEG asymmetry during sleep in the rat. J Neurophysiol. 2008;99:969–975. doi: 10.1152/jn.01154.2007. [DOI] [PubMed] [Google Scholar]
- 49.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 50.Bradley PB, Fink M Collegium Internationale Neuro-Psycho-Pharmacologicum [from old catalog] Anticholinergic Drugs and Brain Functions in Animals and Man. New York: Elsevier; 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.