Abstract
Background
Phase I trials offer advanced cancer patients the opportunity to pursue life-prolonging cancer treatments. In this study, we compared the timing of referral and symptom burden between patients referred to palliative care by Phase I oncologists and those referred by non-Phase I oncologists.
Methods
All 57 patients with advanced solid tumors referred by Phase I to our palliative care outpatient clinic in 2007/2008 were included. The comparison cohort consisted of 114 non-Phase I patients stratified by age, sex and cancer diagnosis in a 1:2 ratio. We retrieved information regarding patient characteristics, Edmonton Symptom Assessment Scale (ESAS), timing of referral and survival.
Results
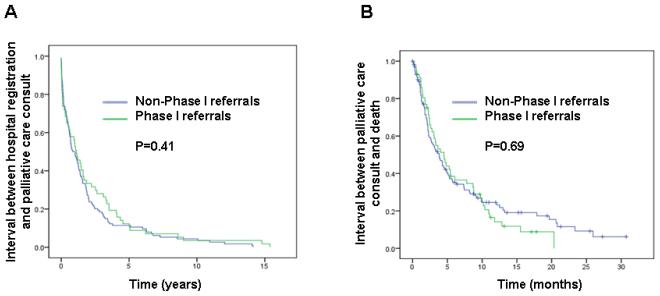
Both cohorts had the following matched characteristics: average age 57, female 44% and gastrointestinal cancers 47%. At the time of palliative care consultation, Phase I patients were more likely than non-Phase I patients to have a better performance status (ECOG 0-1, 61% vs. 36%, P=0.003). ESAS was not different except for better well-being in the Phase I cohort (mean 4.5 vs. 5.5, p=0.03). No difference was found for the duration between M.D. Anderson registration and palliative care consult (13 vs. 11 months, P=0.41) and overall survival from time of palliative care consult (5 vs. 4 months, P=0.69).
Conclusions
Phase I outpatients referred to palliative care had a better performance status but similar symptom burden as non-Phase I patients. Phase I involvement did not delay palliative care referral compared to non-Phase I. This supports the development of a simultaneous care model.
Keywords: Advanced cancer, Palliative Care, Phase I, Referral timing, Simultaneous care, Symptoms
INTRODUCTION
Advanced cancer patients usually develop higher symptom burden with disease progression, and have fewer standard treatment options available.1,2 A small proportion of these patients enroll onto Phase I clinical trials, fueled by the hopes of better disease control and improved survival.3 While Phase I agents may result in symptom benefit through disease stabilization or shrinkage, they can also be associated with various toxicities and logistical burden, including frequent hospital visits and investigations.4,5 These, coupled with cancer related symptoms and complications, can significantly compromise patients’ quality of life.
Phase I patients generally have a poor prognosis, with median survival of 6-9 months.6-8 Given the significant morbidity and mortality among Phase I patients, the involvement of palliative care can be potentially beneficial. Palliative care has evolved as a discipline that focuses on improvement of the quality of life of cancer patients and families through early identification, assessment and treatment of symptoms.9 Timely referral to palliative care is an important indicator of quality of care, as patients gain access to multi-dimensional care early in the trajectory of illness.10,11 However, studies support that Phase I patients are less likely to consider palliative care, home health aide, counselors and chaplains.12 Thus, the pursuit of Phase I therapy may potentially delay referral to palliative care.
To date, there is only one study on the symptom burden of Phase I patients compared to non-Phase I patients,12 and no information on the impact of Phase I involvement on the timing of referral to palliative care. A better understanding of the timing of palliative care referral and symptom profile can provide the foundation for optimizing care for these individuals. Using a retrospective cohort design, we compared the timing of referral and symptom burden between patients referred to palliative care by Phase I oncologists and those referred by non-Phase I oncologists.
PATIENTS AND METHODS
Subjects
The Institutional Review Board at M. D. Anderson Cancer Center approved this study and waived the requirement for informed consent. All patients with advanced solid tumor referred to the outpatient supportive care clinic at M.D. Anderson Cancer Center between January 1, 2007 and Dec 31, 2008 as the first palliative care consultation were identified. Fifty-seven patients were referred by Phase I, and were designated as the Phase I cohort. Among the remaining patients, we randomly selected 114 as the non-Phase I cohort, stratified by age, (<60 or ≥60), sex (male or female) and cancer diagnosis (breast, gastrointestinal, genitourinary, gynecologic, head and neck, lung and other) in a 1:2 ratio.
Patient Characteristics, Symptoms and Timing of Referral
We retrospectively retrieved patient demographics (age, sex, race), cancer diagnosis, timing of diagnosis, encounters with medical oncology, Phase I and palliative care, treatment history, and survival from institutional databases, electronic health records, and Tumor Registry Vital Statistics Database. We also collected information on the Edmonton Symptom Assessment Scale (ESAS), the Memorial Delirium Assessment Scale (MDAS), and Eastern Cooperative Oncology Group (ECOG) performance status at the time of palliative care outpatient consultation.
Statistical Analysis
We summarized the baseline demographics and symptom profile using descriptive statistics, including medians, means, standard deviations, ranges, and frequencies together with 95% confidence intervals (CI).
We compared the baseline characteristics and symptom profile between the Phase I and non-Phase I cohorts. Comparisons were made using the Student’s t-test for continuous variables that were normally distributed (i.e. ESAS), the Mann-Whitney test for continuous, non-parametric variables (e.g. prior systemic therapy courses), and the Chi-square test or Fisher’s exact test for categorical variables (e.g. MDAS). A two-sided P-value of less than 0.05 was considered to be statistically significant.
Timing of palliative care referral was defined a priori based on two intervals, including time from M.D. Anderson registration to palliative care consultation, and overall survival from time of palliative care consult. All time-event analyses were plotted by using the Kaplan-Meier method, and survival curves were compared by the log-rank test.13,14 Overall survival was calculated from the date of palliative care referral to the date of death from any cause or the date at which the patient was last known alive. Multivariate analysis was performed by using the Cox proportional hazards model with backward elimination.
The Statistical Package for the Social Sciences (SPSS version 16.0, SPSS Inc., Chicago, Illinois) software was used for statistical analysis.
RESULTS
Baseline Characteristics
Table 1 summarizes patient demographics at first palliative care outpatient clinic consultation. Patients referred by Phase I were less likely to present with metastatic disease, and to have more prior chemotherapy regimens compared to those referred by non-Phase I oncologists. Among the Phase I cohort, 6 (11%), 13 (23%), 13 (23%) and 25 (43%) patients completed 1, 2, 3 and ≥4 lines of chemotherapy prior to palliative care consultation, respectively, compared to 33 (29%), 25 (22%), 14 (12%) and 19 (17%) for the non-Phase I group. A small proportion (26%) of patients in the Phase I cohort were seen directly by Phase I without medical oncology involvement from our institution. Among the non-Phase I cohort, 12/114 (11%) had also been seen Phase I, but were referred by non-oncologists to palliative care.
Table 1. Baseline Characteristics at Palliative Care Clinic Consultation.
| Phase I Referrals (N=57) |
Non-Phase I Referrals (N=114) |
P-Value | |
|---|---|---|---|
| Age (SD) | 55 (13) | 57 (15) | 0.50 |
| Female | 25 (44%) | 50 (44%) | >0.99 |
| Ethnicity | |||
| Caucasian | 40 (70%) | 73 (64%) | 0.39 |
| African American | 9 (16%) | 18 (16%) | |
| Hispanics | 4 (7%) | 18 (16%) | |
| Other | 4 (7%) | 5 (4%) | |
| Cancer diagnosis | |||
| Breast | 5 (9%) | 10 (9%) | >0.99 |
| Gastrointestinal | 27 (47%) | 54 (47%) | |
| Genitourinary | 6 (11%) | 12 (11%) | |
| Gynecologic | 3 (5%) | 6 (5%) | |
| Head and neck | 3 (5%) | 6 (5%) | |
| Lung | 1 (2%) | 2 (2%) | |
| Other | 12 (21%) | 24 (21%) | |
| Stage | |||
| Recurrent | 0 (0%) | 3 (3%) | 0.046 |
| Locally advanced | 2 (4%) | 16 (14%) | |
| Metastatic | 55 (97%) | 95 (83%) | |
|
Number of lines of chemotherapy
regimens prior to palliative care consultation 1 |
3 (2-5) | 2 (1-3) | <0.001 |
| Treated with Phase I therapy | 43 (75%) | 4 (4%) | <0.001 |
| Sequence of team involvement 2 | |||
| Oncology, Phase I, Palliative care | 42 (74%) | 2 (2%) | <0.001 |
| Phase I, Palliative care | 15 (26%) | 0 (0%) | |
| Oncology, Palliative care | 0 (0%) | 98 (86%) | |
| Oncology, Palliative care, Phase I | 0 (0%) | 10 (9%) | |
| Palliative care, Oncology | 0 (0%) | 4 (3%) | |
| Median interval from diagnosis of advanced cancer to palliative care consultation, months (95% confidence interval) |
28 (19-37) | 12 (9-14) | <0.001 |
|
| |||
| Deaths | 49 (86%) | 89 (78%) | 0.22 |
Abbreviations: SD, standard deviation
Between the time of advanced cancer diagnosis and palliative care consultation
Denotes the time sequence of patient encounters with oncology, Phase I and palliative care, ordered by the date of first visit to each service
Symptom Profile
The symptom burden at first palliative care outpatient clinic consultation is shown in Table 2. Patients referred by Phase I had better performance status, and were less likely to be delirious (MDAS≥8) at the time of presentation. The two cohorts had similar physical and psychosocial symptoms as assessed by ESAS, although the Phase I cohort had a better overall well being (mean 4.5 vs. 5.5, P=0.03) compared to the non-Phase I cohort.
Table 2. Symptom Burden at Palliative Care Clinic Consultation.
| Phase I Referrals (N=57) |
Non-Phase I Referrals (N=114) |
P-Value | |
|---|---|---|---|
| Mean Edmonton Symptom Assessment | |||
| Scale (SD) | |||
| Pain | 5.3 (2.8) | 5.1 (3.0) | 0.70 |
| Fatigue | 5.7 (2.6) | 6.4 (2.6) | 0.09 |
| Nausea | 2.7 (3.2) | 2.4 (2.8) | 0.48 |
| Depression | 2.6 (3.0) | 3.5 (2.9) | 0.07 |
| Anxiety | 2.8 (3.0) | 3.5 (2.9) | 0.17 |
| Drowsiness | 3.7 (3.2) | 4.3 (3.2) | 0.27 |
| Appetite | 5.0 (3.2) | 5.7 (3.3) | 0.21 |
| Well being | 4.5 (2.4) | 5.5 (2.8) | 0.03 |
| Dyspnea | 3.4 (3.2) | 3.1 (2.9) | 0.52 |
| Sleep | 4.8 (2.6) | 4.6 (3.2) | 0.71 |
| Performance status | |||
| 0-1 | 35 (61%) | 41 (36%) | 0.003 |
| 2-3 | 19 (34%) | 68 (60%) | |
| 4 | 3 (5%) | 5 (4%) | |
| Delirium (MDAS ≥8) | 0 (0%) | 10 (10%) | 0.032 |
Abbreviations: SD, standard deviation
Medical Oncology and Phase I Involvement
Compared to the non-Phase I cohort, patients referred by Phase I had significantly longer survival from diagnosis of advanced cancer (Table 3). Both cohorts were followed by medical oncology for a similar duration (17 vs. 14 months, p=0.36). Patients in the Phase I cohort were referred to Phase I a median of 23 months (95% confidence interval (CI) 11-45 months) from time of diagnosis of advanced cancer, and a median of 6.5 months (95% CI 4.4-8.6 months) before death.
Table 3. Timing of Palliative Care and Phase I Referrals.
| Phase I Referrals (N=57) |
Non-Phase I Referrals (N=114) |
P-Value | |
|---|---|---|---|
| Median interval in months (95% confidence interval) | |||
| Diagnosis of advanced cancer to death | 36 (25-47) | 23 (17-29) | 0.01 |
| Diagnosis of advanced cancer to palliative care consultation |
28 (19-37) | 12 (9-14) | <0.001 |
| M.D. Anderson registration to palliative care consultation |
13 (8-20) | 11 (5-15) | 0.41 |
| Palliative care consultation to death | 5 (3-6) | 4 (3-5) | 0.69 |
| First medical oncology contact to palliative care consultation |
19 (13-25) | 8 (4-12) | 0.019 |
| First referring team contact to palliative care consultation |
1 (0.6-1.5) | 9 (5-13) | <0.001 |
| Median number of clinic visits (interquartile range) | |||
| Medical oncology visits before palliative care consultation |
12.5 (4.8-24) | 10 (4.5-21.5) | 0.45 |
| Palliative care visits until last followup/death | 4 (3-7) | 4 (2-6) | 0.39 |
No difference was found in survival from referring team’s last visit (median 28 vs. 41 days, p=0.84); however, the last medical oncology visit happened earlier in the Phase I cohort (200 vs. 41 days, P<0.001), suggesting that Phase I oncologists, rather than the referring oncologists, were primarily responsible for the care of these patients in the last few months of life.
Quality of End-of-Life Care
Among all the patients who died, those referred by Phase I were more likely than the non-Phase I cohort to receive chemotherapy within the last 30 days of life (31% vs. 13%, P=0.014). The interval between last chemotherapy and death also appeared to be shorter for the Phase I cohort (median 60 days vs. 81 days, P=0.06), although this did not reach statistical significance.
Only a small proportion of patients in both cohort were admitted to the intensive care unit within the last 30 days of life (4% vs. 6%, P>0.99). We did not detect any differences in the proportion of in-hospital deaths between the two groups (18% vs. 28%, P=0.21).
Palliative Care Referral
Patients referred by Phase I had a significantly longer interval from diagnosis of advanced cancer to palliative care consultation, as well as longer interval between first oncology contact and palliative care consultation (Table 3). However, the two key markers of timing of palliative care referral, time from M.D. Anderson registration to palliative care consultation and overall survival from time of palliative care consult, did not differ between the two groups (Table 3). In Cox regression multivariate analysis, only cancer diagnosis was significantly associated with the timing of palliative care referral (Table 4).
Table 4.
Cox Regression Multivariate Analysis for Survival from Palliative Care Consultationa
| Hazard ratio (95% confidence interval) |
P-value | |
|---|---|---|
| Cancer diagnosis | 0.05 | |
| Breast | 0.52 (0.26-1.06) | 0.07 |
| Gastrointestinal | 1.0 | Reference |
| Genitourinary | 0.87 (0.48-1.59) | 0.65 |
| Gynecologic | 0.94 (0.43-2.06) | 0.88 |
| Head and neck | 0.66 (0.31-1.39) | 0.27 |
| Lung | 0.60 (0.15-2.47) | 0.48 |
| Othersb | 0.43 (0.26-0.71) | 0.001 |
| Performance status | 1.17 (0.98-1.40) | 0.09 |
Variables included in this model were age, sex, race, cancer diagnosis, stage, referring team, performance status at presentation, number of medical oncology visits before palliative care consultation, and number of chemotherapy courses before palliative care consultation. Since this model aimed to identify factors that affect the timing of referral rather than factors that confer a poor prognosis, we specifically excluded symptoms from the analysis as some are known to be prognostic factors (e.g. delirium and dyspnea) that could potentially confound this analysis.
Include all cancer types other than breast, gastrointestinal, genitourinary, gynecologyic, and head and neck cancers. Examples included sarcoma, neurologic, and endocrine tumors.
The duration between referring team’s initial contact and palliative care referral was shorter for patients in the Phase I cohort, suggesting that these patients were referred promptly to palliative care (Table 3). No difference was found in the duration of overlap between palliative care consult and referring team’s last visit (median 63 vs. 44 days, P=0.84).
Consistent with the above findings, the number of medical oncology clinic visits before palliative care consultation, and the number of palliative care clinic visits until death were similar between the two cohorts (Table 3). Patients in the Phase I cohort had a median of 4 (interquartile range 1.5-10.5) Phase I visits before palliative care consult.
DISCUSSION
In this retrospective cohort study, we found that patients referred to palliative care by Phase I oncologists had a better performance status but similar symptom burden as those referred by non-Phase I oncologists, and were more likely to receive chemotherapy close to the end-of-life. Survival from time of palliative care referral was approximately 4 months for both cohorts, suggesting that Phase I involvement did not delay palliative care referral. This supports the development of a successful simultaneous care model.
One interesting though not completely surprising finding from this study is that the Phase I cohort had a longer overall survival from diagnosis of advanced cancer compared to non-Phase I cohort, suggesting that these patients generally have a less aggressive disease course relative to patients who were not referred.15 In contrast, a number of patients in the non-Phase I cohort deteriorated rapidly, limiting their ability to enroll onto clinical trials.
We found that patients from the Phase I cohort had a better performance status and were less likely to be delirious at presentation to palliative care; this finding is not surprising given that patients need to be well enough to be referred for Phase I treatments.15,16 We found that Phase I patients otherwise had similar symptom expression compared to non-Phase I patients. Our findings are consistent with a recent prospective cohort study from a different institution using the Memorial Symptom Assessment Scale.12 Similar to other studies on palliative care populations, fatigue, pain and anorexia were the most common and severe symptoms in both cohorts.17 Importantly, our results support that both Phase I and non-Phase I patients have significant physical and psychological distress at the time of presentation to palliative care outpatient clinic, pointing to the need for intensive interventions.
We had a number of reasons to suspect that Phase I patients could be referred to palliative care later than non-Phase I patients. Phase I treatments were typically offered late in the disease trajectory, when patients have already exhausted all standard treatments. Thus, while the non-Phase I oncologists had ample of opportunities to make palliative care referrals early in the disease course, the timing of referral by Phase I physicians is dependent on when the Phase I consultations take place, which are generally within the last few months of life.
In addition to logistical challenges, the literature suggests that patients on Phase I trials are not psychologically prepared for transition to end-of-life.12,18 Agrawal et al. surveyed 163 patients on Phase I protocol regarding their decision making process. While over 80% of patients stated that they were aware of palliative care and hospice as alternatives to Phase I trial, less than 10% seriously considered these options for themselves.18 Potential explanations include lack of understanding of palliative care services, lack of self-perceived need for support services due to better performance status, and the desire to focus only on cancer treatments. Indeed, patients embarking on Phase I trials generally have heightened expectations in regard to survival and treatment outcomes.19,20 This, coupled with a sense of denial, makes them much less likely to desire palliative care services. Finally, the misconception by some oncologists that a palliative care referral could destroy hope presents another barrier to the referral process.21,22
Despite the many factors that could potentially limit palliative care referral among Phase I patients, we were encouraged to find that Phase I involvement did not delay palliative care referral. The median duration between first Phase I contact and palliative care referral was only 31 days, suggesting that Phase I physicians made their referral relatively quickly without significant delays. Since Phase I physicians work closely with patients with advanced cancer near the end-of-life, they may have a better understanding of the palliative care needs of their patients, and the potential benefits related to a timely referral. For individuals too sick to enroll onto experimental protocol, they may be referred to palliative care for transition to the end-of-life. For other patients participating in Phase I trials, palliative care helps to support them through treatment by optimizing their functional status, symptoms and support systems.
Consistent with this model of integrated care, patients referred by Phase I were more likely to receive chemotherapy within the last 30 days of life. This finding is not unexpected given that these patients were actively seeking further treatments. The administration of chemotherapy close to the end-of-life has been used as an indicator of poor quality of cancer care.23-25 However, this marker is of limited value as an outcome measure since the timing of death cannot be accurately predicted. There are always patients who desire aggressive treatments despite understanding the unfavourable risk:benefit ratio. Finally, the emergence of targeted therapies with lower toxicity profile compared to traditional cytotoxic therapies has allowed sicker patients to receive anti-cancer treatments later in the disease trajectory. Thus, the 30 day criterion may not be appropriate for Phase I cancer patients.
Close collaborations between Phase I and palliative care under a simultaneous care model can help optimize both quantity and quality of life, while tailoring care to the individuals’ needs.26-28 Clinicians, patients and families should understand that they do not have to choose between Phase I and Palliative care, but could take advantage of both services synergistically. Under this integrated care approach, Phase I oncologists can deliver the latest cancer treatments with the aim of cancer control, while the palliative care team focuses on symptom management, psychosocial interventions, family counseling, and transition of care. Given the significant symptom burden, emotional distress and poor prognosis of Phase I patients, almost all of them may benefit from this simultaneous care approach.
The optimal timing for palliative care referral has not been defined, although the general understanding is that patients were referred late.29-31 The American Society of Clinical Oncology (ASCO) recently published a position statement supporting the vision of integrating palliative care into oncology practice from the time of cancer diagnosis.11 In our study of patients who were first referred as outpatients, palliative care involvement was limited to the last 4 months of life, with a median of 4 clinical encounters. Thus, much work remains to be done to bring palliative care earlier in the disease trajectory, ideally before the Phase I visit. Studies from our group and others have demonstrated that palliative care referral is dependent on various factors, including oncologists’ perception and attitudes,21,32 patient characteristics and preferences33,34 and healthcare infrastructure and policies.35 Consistent with the literature,36 we found that cancer diagnosis, which dictates which team of oncologists the patient sees, is a key determinant on the timing of referral to palliative care. While not examined in this study, factors associated with referral to Phase I may include institutional resources, oncologists’ attitudes, patient education and preferences, as well as cancer site and stage.15,37,38
Our study has a number of limitations. First, data were collected retrospectively. Second, despite the fact that our Phase I and palliative care programs represent a few of the largest programs in the United States, the sample size was small. This is partly because we elected to focus on outpatient referrals as a model of integration. Third, this study only examined the timing of referral, as we did not have access to data of patients seen by Phase I and non-Phase I services, but who were not subsequently referred to palliative care. Fourth, 11% of patients in the non-Phase I cohort had consulted Phase I either before or after palliative care consultation, although they were not referred by Phase I. This “contamination” may have reduced any observed differences in our outcomes. Finally, findings from our study are institution-specific and are not generalizable to other programs. Nevertheless, it showcases how two healthcare teams with seemingly opposing objectives can care for patients collaboratively.
To our knowledge, this is the first study to examine the timing of referral between Phase I and non-Phase I patients. We found that Phase I involvement did not delay palliative care referral in our institution. Despite a better performance status, patients referred by Phase I had significant symptom burden similar to those referred by non-Phase I services, and were more likely to be receiving chemotherapy closer to death. These findings point to the need for interdisciplinary palliative care interventions concurrent with Phase I under a simultaneous care model. While not every patient requires intensive symptom management up front, timely referral can facilitate rapport building, longitudinal psychosocial support, early recognition and treatment of symptoms, and advance care planning. To ensure timely access to comprehensive cancer care for all patients, improvements in reimbursement policy, palliative care resources, education of oncologists, patients and families, and research on novel integration models are urgently needed.
Condensed abstract.
In this retrospective cohort study, we found that Phase I outpatients referred to palliative care had a better performance status but similar symptom burden as non-Phase I patients. Phase I involvement did not delay palliative care referral compared to non-Phase I, supporting the development of a simultaneous care model.
Figure 1. Timing of Palliative Care Referral.
Kaplan-Meir curves for (A) Interval between M.D. Anderson registration and palliative care consultation, and (B) Interval between palliative care consultation and death
ACKNOWLEDGEMENTS
We would like to thank Ray Chacko for assistance with database management.
Funding: Supported in part by the National Institutes of Health grants, RO1NR010162-01A1, RO1CA122292-01 and RO1CA124481-01 (EB), and the Clinician Investigator Program, Royal College of Physicians and Surgeons of Canada (DH)
REFERENCES
- 1.Tishelman C, Petersson LM, Degner LF, et al. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381–5389. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 2.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 3.Cox K. Informed consent and decision-making: Patients’ experiences of the process of recruitment to phases I and II anti-cancer drug trials. Patient Educ Couns. 2002;46:31–38. doi: 10.1016/s0738-3991(01)00147-1. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MZ, Slomka J, Pentz RD, et al. Phase I participants’ views of quality of life and trial participation burdens. Support Care Cancer. 2007;15:885–890. doi: 10.1007/s00520-007-0216-0. [DOI] [PubMed] [Google Scholar]
- 5.Cox K. Enhancing cancer clinical trial management: Recommendations from a qualitative study of trial participants’ experiences. Psychooncology. 2000;9:314–322. doi: 10.1002/1099-1611(200007/08)9:4<314::aid-pon464>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Janisch L, Mick R, Schilsky RL, et al. Prognostic factors for survival in patients treated in phase I clinical trials. Cancer. 1994;74:1965–1973. doi: 10.1002/1097-0142(19941001)74:7<1965::aid-cncr2820740723>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Wheler J, Tsimberidou AM, Hong D, et al. Survival of patients in a phase 1 clinic: The M. D. anderson cancer center experience. Cancer. 2009;115:1091–1099. doi: 10.1002/cncr.24018. [DOI] [PubMed] [Google Scholar]
- 8.Penel N, Delord JP, Bonneterre ME, et al. Development and validation of a model that predicts early death among cancer patients participating in phase I clinical trials investigating cytotoxics. Invest New Drugs. 2009 doi: 10.1007/s10637-009-9224-x. [DOI] [PubMed] [Google Scholar]
- 9.Pain relief and palliative care . National Cancer Control Programmes: Policies and Managerial Guidelines. ed 2 World Health Organization; Geneva: 2002. pp. 83–91. [Google Scholar]
- 10.Smith TJ, Schnipper LJ. The american society of clinical oncology program to improve end-of-life care. J Palliat Med. 1998;1:221–230. doi: 10.1089/jpm.1998.1.221. [DOI] [PubMed] [Google Scholar]
- 11.Ferris FD, Bruera E, Cherny N, et al. Palliative cancer care a decade later: Accomplishments, the need, next steps -- from the american society of clinical oncology. J Clin Oncol. 2009;27:3052–3058. doi: 10.1200/JCO.2008.20.1558. [DOI] [PubMed] [Google Scholar]
- 12.Finlay E, Lu HL, Henderson H, et al. Do phase 1 patients have greater needs for palliative care compared with other cancer patients? Cancer. 2009;115:446–453. doi: 10.1002/cncr.24025. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 15.Ho J, Pond GR, Newman C, et al. Barriers in phase I cancer clinical trials referrals and enrollment: Five-year experience at the princess margaret hospital. BMC Cancer. 2006;6:263. doi: 10.1186/1471-2407-6-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 17.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal M, Grady C, Fairclough DL, et al. Patients’ decision-making process regarding participation in phase I oncology research. J Clin Oncol. 2006;24:4479–4484. doi: 10.1200/JCO.2006.06.0269. [DOI] [PubMed] [Google Scholar]
- 19.Weinfurt KP, Castel LD, Li Y, et al. The correlation between patient characteristics and expectations of benefit from phase I clinical trials. Cancer. 2003;98:166–175. doi: 10.1002/cncr.11483. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JD, Hitt J, Koczwara B, et al. Impact of quality of life on patient expectations regarding phase I clinical trials. J Clin Oncol. 2000;18:421–428. doi: 10.1200/JCO.2000.18.2.421. [DOI] [PubMed] [Google Scholar]
- 21.Fadul NA, El Osta B, Dalal S, et al. Comparison of symptom burden among patients referred to palliative care with hematologic malignancies versus those with solid tumors. J Palliat Med. 2008;11:422–427. doi: 10.1089/jpm.2007.0184. [DOI] [PubMed] [Google Scholar]
- 22.Freireich EJ, Kurzrock R. The role of investigational therapy in management of patients with advanced metastatic malignancy. J Clin Oncol. 2009;27:304–306. doi: 10.1200/JCO.2008.19.6543. [DOI] [PubMed] [Google Scholar]
- 23.Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 24.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 25.Tang ST, Wu SC, Hung YN, et al. Determinants of aggressive end-of-life care for taiwanese cancer decedents, 2001 to 2006. J Clin Oncol. 2009;27:4613–4618. doi: 10.1200/JCO.2008.20.5096. [DOI] [PubMed] [Google Scholar]
- 26.Meyers FJ, Linder J, Beckett L, et al. Simultaneous care: A model approach to the perceived conflict between investigational therapy and palliative care. J Pain Symptom Manage. 2004;28:548–556. doi: 10.1016/j.jpainsymman.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Meyers FJ, Linder J. Simultaneous care: Disease treatment and palliative care throughout illness. J Clin Oncol. 2003;21:1412–1415. doi: 10.1200/JCO.2003.01.104. [DOI] [PubMed] [Google Scholar]
- 28.Hui D, Elsayem A, Li Z, et al. Antineoplastic therapy use in patients with advanced cancer admitted to an acute palliative care unit at a comprehensive cancer center: A simultaneous care model. Cancer. 2010 doi: 10.1002/cncr.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita T, Akechi T, Ikenaga M, et al. Late referrals to specialized palliative care service in japan. J Clin Oncol. 2005;23:2637–2644. doi: 10.1200/JCO.2005.12.107. [DOI] [PubMed] [Google Scholar]
- 30.IOM National Cancer Policy Board: Improving Palliative Care for Cancer. ed 1st Institute of Medicine; Washington, DC: 2001. [Google Scholar]
- 31.Hui D, Elsayem AF, De la Cruz M, et al. Availability and integration of palliative care in US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadul N, Elsayem A, Palmer JL, et al. Predictors of access to palliative care services among patients who died at a comprehensive cancer center. J Palliat Med. 2007;10:1146–1152. doi: 10.1089/jpm.2006.0259. [DOI] [PubMed] [Google Scholar]
- 33.Miyashita M, Hirai K, Morita T, et al. Barriers to referral to inpatient palliative care units in japan: A qualitative survey with content analysis. Support Care Cancer. 2008;16:217–222. doi: 10.1007/s00520-007-0215-1. [DOI] [PubMed] [Google Scholar]
- 34.Beccaro M, Costantini M, Merlo DF, et al. Inequity in the provision of and access to palliative care for cancer patients. results from the italian survey of the dying of cancer (ISDOC) BMC Public Health. 2007;7:66. doi: 10.1186/1471-2458-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassel CK, Ludden JM, Moon GM. Perceptions of barriers to high-quality palliative care in hospitals. Health Aff (Millwood) 2000;19:166–172. doi: 10.1377/hlthaff.19.5.166. [DOI] [PubMed] [Google Scholar]
- 36.Cheng WW, Willey J, Palmer JL, et al. Interval between palliative care referral and death among patients treated at a comprehensive cancer center. J Palliat Med. 2005;8:1025–1032. doi: 10.1089/jpm.2005.8.1025. [DOI] [PubMed] [Google Scholar]
- 37.Castel P, Negrier S, Boissel JP, et al. Why don’t cancer patients enter clinical trials? A review. Eur J Cancer. 2006;42:1744–1748. doi: 10.1016/j.ejca.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 38.Daugherty C, Ratain MJ, Grochowski E, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol. 1995;13:1062–1072. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]