Abstract
Objective
To measure body composition in patients with thalassemia and explore its relationship to abnormal growth and bone mass.
Study design
Cross-sectional, multi-center study. Fat, lean, and bone mineral density (BMD) were assessed by DXA. Medical history, food frequency and physical activity questionnaires were conducted in 257 transfused thalassemia patients (23.7 ± 11 yr, Mean±SD, 51% male) compared with 113 non-transfused patients (21.3 ± 13 yr, 44% male).
Results
Subjects with thalassemia were leaner compared with healthy Americans from NHANES III data. Transfused subjects had higher percentage body fat compared with non- transfused after controlling for age, sex and ethnicity; 11.8% of non-transfused pediatric subjects were considered underweight, significantly lower than NHANES data (p=0.03). Hemoglobin level was positively related to lean mass (p=0.008). Body fat and lean mass were positive predictors for both height and BMD Z-scores after adjustment for transfusion status, age, sex, ethnicity, calcium intake and physical activity (all p<0.001).
Conclusions
Though the majority of adult patients with thalassemia had healthy body composition with rare obesity, young, non-transfused patients appear at risk for being underweight. Optimizing physical activity and appropriate use of transfusion therapy may improve growth and bone health in these at risk patients.
Keywords: DXA, lean mass, fat mass, body mass index (BMI), calcium
Optimal nutritional status is imperative for achieving the genetic potential for growth and pubertal development in children as well as for robust immune function and bone health in adults. For children, growth is most commonly used as a gross assessment of an individual’s overall nutritional status. Children with short stature, or those who “fall off their growth centile,” raise a red flag for any pediatrician. For adults, body composition is a gross marker of an individual’s overall nutritional health. Those patients with significantly decreased fat and lean body mass show clear signs of malnutrition from either decreased energy intake or elevated expenditure. On the other hand, obesity, or an elevation of total body fat also reveals altered nutritional status. In both forms of malnutrition, increased inflammatory and oxidative stress markers have been observed (1,2,3). Additionally, inflammation and oxidative stress have been linked to reduced bone mineral density and increased fracture risk (4,5).
Patients with thalassemia (Thal) are known to have abnormal growth, altered pubertal development and immune function as well as deficits in bone mineral acquisition. The etiology of these co-morbidities is typically ascribed to the toxic effects of transfusion related iron-overload. Though the relationship of nutritional status to abnormal growth, pubertal development, immune status and bone health has been shown in other chronic pediatric diseases (6,7,8), few studies have specifically focused on aspects of nutritional status and health in patients with Thal. The majority of the published work has summarized observations in small numbers of subjects primarily conducted outside North America (9,10,11,12,13).
The objective of this study was to assess body composition in a large population of subjects with Thal in North America using one of the most robust, non-invasive assessment tools available, dual energy x-ray absorptiometry or DXA. The cross-sectional study was conducted by the Thalassemia Clinical Research Network (TCRN), comprised of five Thal centers (and eight satellites) in North America with access to all Thal syndromes. Particular emphasis was placed on the relationship between body composition, growth, development and bone mass.
The specific hypotheses for this study were as follows: (a) patients with Thal have lower body mass index (BMI) in comparison with age and sex-matched published normative values, (b) calcium intake and physical activity are related to body composition, and finally (c) insufficient body fat is associated with poor growth, pubertal development and bone health.
Methods
This study was part of the TCRN cross-sectional low bone mass study. Inclusion criteria were diagnosis of Thal regardless of genotype and age 6 and older. Exclusion criteria included pregnancy and any known pre-existing medical condition that was known to affect bone mass or require chronic systemic administration of steroids. The protocol was approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all TCRN institutions. A signed informed consent, and assent in case of a minor, was obtained. Details regarding the recruitment and enrollment procedures have been published (14).
All subjects had body composition and bone mineral density assessed by dual energy x- ray absorptiometry (DXA, Hologic, Bedford MA, QDR 4500 with Delphi software upgrades). Spine, proximal femur and whole body scans were performed at individual centers and unanalyzed scans sent to a central facility for analysis by a blinded technologist using the manufacturers software and reference data. Analyzed whole body DXA scans report a 3 compartment model to differentiate body composition: fat, lean mass and bone mineral content. Height and weight were measured and medical history was obtained by interview and review of medical records. Dietary calcium intake was estimated from a 46-item self-completed food frequency questionnaire which included questions regarding calcium and vitamin D supplementation. A fasting morning blood sample was obtained for measurement of ferritin and transferrin receptor concentrations. Samples were batched and analyzed at a central facility as previously described (14).
Statistical Analysis
Subjects were categorized as transfused (TxThal) if they were currently receiving transfusion therapy on a routine basis, typically 7 or more transfusions during the 12 months prior to entering the study. They were categorized as non-transfused (NTxThal), if they were not currently receiving transfusion therapy, which included 8 beta Thal intermedia and 18 E-beta Thal subjects who had previously been treated with transfusion therapy. Body mass index (BMI) was calculated as kg/m2. Anthropometric Z-scores were calculated relative to age- and sex-specific norms produced by the CDC (15). Presence of an endocrinopathy (i.e. hypogonadism, hypothyroidism, diabetes mellitus, growth hormone deficiency, hypoparathyroidism) was defined as having an identified clinical history or prescribed treatment (as described previously, 14). Daily calcium intake (dietary + supplements) was considered adequate if it was above 800 mg/d for the age group of 6-8y, 1300 mg/d for 9-18y, 1000 mg/d for 19- 50y and 1200 mg/d for 51y and older.
Adults were defined as individuals > 20 yrs and pediatric subjects ≤ 20 years (to coincide with CDC data for BMI percentiles). Adult subjects were also categorized as underweight by BMI if <18.5, normal weight: 18.5 to <25, overweight: 25.0 to <30 and obese ≥ 30.0 (16). For pediatric subjects, a BMI of <5th% for age and sex was considered underweight, between the 5th and 85th, normal weight, between the 85th% and 95th% at risk for overweight and above the 95th was defined as overweight (15). Adult BMI data were also compared with data from the US National Health and Nutrition Examination Survey (17). Body composition data obtained by DXA was compared with the recently published reference data from NHANES (18).
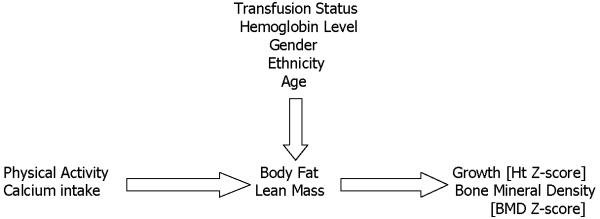
Continuous variables were summarized as means with standard deviations and categorical variables were summarized as simple percentages. General linear models were used to model the effect of calcium intake, physical activity, hemoglobin, and serum transferrin receptor on the body composition measures after controlling for age, sex, and transfusion status. Additional models examined the relationship of body composition to growth and co-morbidity measures such as BMD ZEscore, controlling for similar covariates. Logistic regression was used to model the effect of body composition on the presence of binary outcomes such as the presence of hypogonadism (Figure 1; available at www.jpeds.com). Subgroup analyses were also performed in the NTxThal group alone. All inferences are based on two-tailed tests with a threshold of alpha = 0.05 for declaring significance. All analyses were conducted using SAS (version 9.1.3, SAS Institute, Cary NC).
Figure 1.
Simplified Model of the Predictors of Body Composition and its Effect on Growth and Bone Health in Patients with Thalassemia
Results
A total of 386 subjects with Thal were recruited and consented to participate. Sixteen subjects were excluded from this analysis due to insufficient body composition data. The total sample (n=370) was divided into 2 groups: (TxThal) 257 subjects with Thal major and E-beta Thal who were chronically transfused (age range 6.1 to 75.4 years); and 113 never or not currently transfused subjects (NTxThal) with other Thal syndromes (age range: 6.1 to 53.1 yrs). The genotypes of the transfused and non-transfused sub-groups are provided (Table I). There was a similar distribution of males and females in the Thal subgroups, as well as age distribution.
Table 1. Characteristics of Transfused and Non-Transfused Subjects with Thalassemia^.
| Transfused Thalassemia (n=257) |
Non-Transfused Thalassemia (n=113) |
p-value | |
|---|---|---|---|
| Age, years | 23.7 ± 11.4 | 21.3 ± 13.4 | NS |
| % Pediatric, ≤ 20 yrs | 44.7% | 60.2% | |
| % Adult, > 20 yrs | 55.3% | 39.8% | |
| Sex, % male | 51% | 44% | NS |
| Ethnicity | Asian, 37.0% Caucasian, 60.3% Other, 2.7% |
Asian, 55.8% Caucasian, 14.2% Other, 14.2% |
0.001 |
| Thalassemia Genotype ~ | ß-thalassemia (94.0%) E beta Thal (5.1%) Hb H/CS (0.8%) |
ß-thalassemia (39.8%) E Beta Thal (27.4%) Hb H & Hb H/CS (32.7%) |
|
| Hemoglobin, g/dL | 10.1 ± 1.2 | 8.9 ± 1.6 | 0.001 |
| Serum ferritin, ng/mL (range) |
2026 ± 1666 (94 to 11,995) |
833 ± 1638 (10 to 12,280) |
0.001 |
| Serum transferrin receptor, ug/mL | 19.8 ± 11.2 | 40.3 ± 21.1 | 0.001 |
| Height Z-score, pediatrics | −1.2 ± 1.2 | −1.1 ± 1.1 | NS |
|
Body Mass Index, kg/m2 all adult subjects (n=187) |
23.3 ± 3.6 (n=136) |
21.4 ± 2.4 (n=42) |
0.0001 |
| Underweight, BMI <18.5 | 5.2 % | 9.5 % | NS, 0.009#1,0.005#2 |
| Overweight/Obese >25.0 | 26.5 % | 11.9 % | NS, <0.001#1,<0.00 1#2 |
|
Body Mass Index, kg/m2 ^^ all pediatric subjects (n=183) |
18.9 ± 4.1 (n=115) |
18.0 ± 3.3 (n=68) |
NS |
| Underweight, <5th centile | 7.8 % | 11.8 % | NS, NS#1 , 0.03#2 |
| Overweight, >95th centile | 3.5 % | 1.5 % | NS, NS#1, NS#2 |
| AP L1-L4 Spine Z-score | −2.0 ± 1.2 | −1.6 ± 1.1 | 0.002 |
| Age at Menarche, years | 15.5 ± 4.1 (88) |
13.9 ± 1.9 (38) |
0.004 |
| Calcium intake, mg/day (range) * |
1713 ± 920 (79 to 5,700) |
1353 ± 844 (50 to 3,743) |
NS |
| Calcium / Vitamin D | |||
| Supplementation** | 68.4% | 55.8% | NS |
| Current Activity Level, % | |||
| Very Light | 6.0% | 10.0% | |
| Light | 17.3% | 13.6% | NS |
| Moderate | 27.4% | 30.9% | |
| Active | 30.6% | 27.3% | |
| Very Active | 18.5% | 18.2% | |
| Co-morbidities | |||
| Hypogonadism, % | 54.0% | 13.3% | <0.001 |
| Growth hormone deficiency, % |
14.0% | 2.7% | 0.001 |
| Diabetes, % | 14.2% | 1.8% | 0.003 |
| Hypothyroidism, % | 11.0% | 2.7% | 0.008 |
| Low bone mass, % | 52.1% | 43.4% | NS |
Continuous variables were summarized as mean ± SD and compared using t-tests. Categorical variables were summarized as percents and compared using chi-square tests except for comparison with NHANES III data as noted below
Transfused group of alpha thalassemia were all Hb H/Constant spring
Calcium intake: dietary intake plus supplementation
Calcium / Vitamin D supplementation: percentage of subjects who took calcium and/or vitamin D supplements.
p-values from Multi-testing for BMI categories compared to NHANES III data for healthy adult and pediatric Americans (17). #1: Tx compared to NHANES III data, #2: NTx compared with NHANES III data
Percentage of pediatric patients with BMI values below the 5th or above the 95th percentiles
Hemoglobin level, an indicator of chronic transfusion therapy, was significantly greater in the transfused subEgroup, as expected. Similarly, serum ferritin, an indicator of iron toxicity, was higher in the transfused group, but elevated in both the transfused and non-transfused subgroups above what is considered a normal value (10-300 ng/mL). Whereas, serum transferrin receptor concentration, an indicator of erythropoietic activity, was higher in non-transfused compared with the transfused subjects with Thal. As has been observed previously, the overall prevalence of growth deficits and endocrine abnormalities was higher in the transfused compared with the non-transfused group (Table I).
As expected, in the group as a whole there was a high correlation between the calculated value of BMI and the direct assessment of fat mass (r=0.86, p<0.001) and lean mass by DXA (r=0.70, p<0.001). There was also a strong correlation between total body mass as assessed by DXA and measured body weight (r=0.99, p<0.0001).
Body Composition for the Group as a Whole
Average percentage body fat by DXA in adult males was 23% and adult females 33%. Average BMI for the group as a whole was 22.9 ± 3.6 for males and 22.8 ± 3.4 for females. Using currently accepted definitions, only 11 adults (6.2%), 7 of whom were male, were classified as underweight by BMI criteria. A very small percentage of adults with Thal were considered obese, 3.4% (1 male, 5 females). Compared with median data from NHANES 1999-2000, adult patients with Thal are much leaner compared with contemporary adult Americans (BMI: 28.4±6.2, p<0.0001). Additionally, a significantly smaller proportion of adults in the group as a whole are categorized as overweight or obese (n=41, 23% vs. NHANES 68%, p<0.0001). Using recently published body composition reference values from NHANES, Thal subjects have significantly lower percentage body fat (Figure 2).
Figure 2.
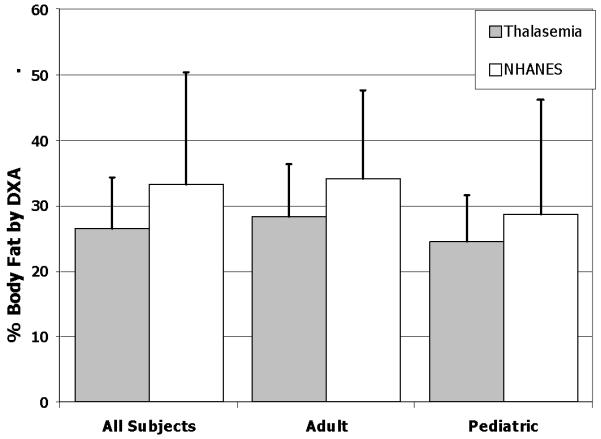
Percentage Body Fat (Mean ± SD) determined by DXA in the group, as well as in adult (≥20 years) and pediatric subjects (<20 years) compared with NHANES reference data (Reference #18)
For the younger patients (≤20 yrs), 9% (n=17) were considered underweight by BMI criteria and only 3% of children were considered overweight or obese. Using the CDC definitions, both NTx and TxThal pediatric subjects were significantly leaner than the average U.S. pediatric population (both <0.001).
Overall, as is typically observed in healthy populations, adult females had a higher percentage of body fat compared with males (33 ± 5% vs. 23 ± 6%, p<0.001); similarly pediatric females had higher body fat compared with pediatric males (29 ± 6% vs. 21 ± 7%, p<0.001). Additionally as a group, adult subjects with Thal had a higher percentage body fat as compared with all pediatric subjects (28±8% vs. 25±8%, p<0.001).
The relationship between categorical levels of body mass index (BMI) and body fatness as assessed by DXA is shown in Table II (available at www.jpeds.com). We observed BMI to be an imperfect tool to categorize individuals with Thal according to body fatness. This was found to be the case particularly in adult male subjects. For example one very physically active male was categorized as overweight (BMI=25.8), yet had a body fat by DXA of 17.8%. More commonly, however, subjects had higher than expected body fat for a given BMI category: 15 of the 22 male subjects with BMI between 25 and 30 kg/m2 had body fat by DXA between 26.1 and 38.2%, values which are in contrast to published ethnic specific equations which predict body fat to be 21 to 25% in adult males according to overweight BMI criteria [19].
Table 2. Range of Percentage Body Fat measured by DXA in Adult and Pediatric Subjects with Thalassemia According to Body Mass Index (BMI) cutoff criteria.
| Males | Females | ||||
|---|---|---|---|---|---|
| Adult Subjects, >20 yrs (n=187) |
BMI Criteria | n | % Body Fat (range) |
n | % Body Fat (range) |
| Underweight | < 18.5 | 7 | 10.5% - 25.5% | 4 | 27.1% - 32.4% |
| Normal Weight | 18.5 – 24.9 | 52 | 10.8% - 35.5% | 70 | 24.6% - 43.9% |
| Overweight | 25.0 – 29.9 | 22 | 17.8% - 38.2% | 12 | 30.5% - 46.4% |
| Obese | > 30.0 | 1 | 38.1% | 5 | 39.3% - 46.6% |
|
Pediatric Subjects, ≤ 20 yrs
(n=183) |
|||||
| Underweight | <5th | 11 | 12.8% - 25.8% | 6 | 15.4% - 32.7% |
| Normal Weight | 5th – 85th | 65 | 10.6% - 37.9% | 79 | 16.7% - 38.7% |
| At risk for Overweight | >85th – 95th | 10 | 8.5% - 35.2% | 5 | 29.7% - 47.3% |
| Overweight | > 95th | 4 | 21.3% - 42.5% | 1 | 40.5% |
Body Composition: SubEGroup Comparisons
TxThal had more total body fat mass (14.3±7.4 vs. 11.4±5.4 kgs, p<0.0001), and percentage body fat (27.3±7% vs. 24.9±8% p=0.007, Figure 3) compared with NTxThal. However, absolute lean mass (34.6 ±11.1 vs. 32.7±12.8, p=0.16) was not significantly different in Tx vs. NTxThal. After adjustment for age, sex and ethnicity, total body fat mass and percentage body fat remained significantly higher in TxThal vs. NTxThal (both p<0.003).
Figure 3.
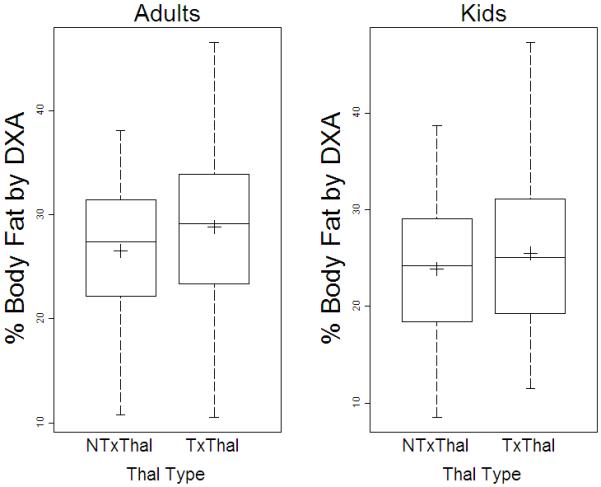
Percentage Body Fat determined by DXA in A, adults (≥ 20 years) and B, pediatric subjects (< 20 years) with transfusion (TxThal) and non-transfusion dependent thalassemia (NTxThal)
Predictors of Body Composition in Thalassemia
Calcium intake, as a percentage of recommended intake, was inversely related to fat mass (95%CI: -1.47, E0.21; p=0.009), lean mass (95%CI: -2.29, -0.39; p=0.006) as well as overall body weight (95%CI: -3.54, -0.70; p=0.004) after controlling for age, sex, height Z- score and transfusion status. In adults, for every 100 mg of calcium intake, body weight decreased by 0.16 kg. Current physical activity level was also a strong predictor of reduced body fat (95%CI: -5.32, -0.14; p=0.001) and lower body weight (p=0.001). The group of subjects with Thal who were most physically active had the lowest percentage of total body fat (23.3 ± 6.8% vs. sedentary 29.1 ± 7.4%) (Figure 4; available at www.jpeds.com).
Figure 4.
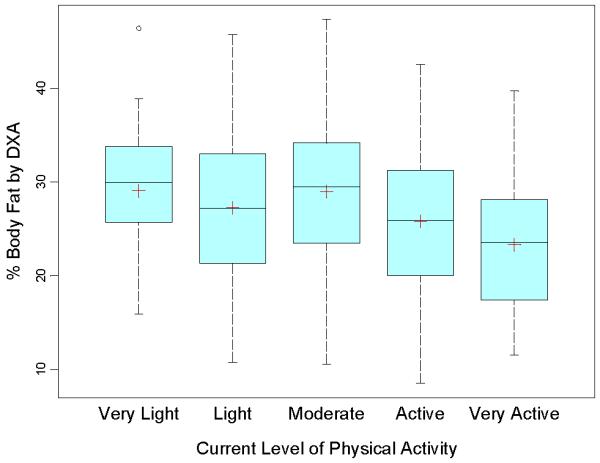
Percentage Body Fat determined by DXA according to Current Self-Assessed Physical Activity Level in Subjects with Thalassemia
Variables related to iron load and transfusion therapy were also related to body composition. Hemoglobin level was positively related to lean mass (95%CI: 0.23, 1.53; p=0.008) as well as overall body weight (95%CI: 0.35, 2.27; p=0.008) after controlling for age, sex, ethnicity, height Z-score and transfusion status. For every one g/dL increase in hemoglobin, lean body mass increased by 0.9 kg. This effect was primarily driven by the pediatric group, as when the analyses were separated by age group, the relationship between hemoglobin and lean body mass was no longer observed in the adult group. In univariate models, serum transferrin receptor and smoking status were related to fat mass, but were no longer significant after adjustment for age, sex, height Z-score and transfusion status. Serum ferritin and nucleated RBCs were not related to any body composition measures in this sample.
Relationship of Body Composition to Co-Morbidities
Short stature (Height Z-score <-2.0) was present in 28% of the entire sample. Body fat and lean mass were positive predictors for height Z-score (p<0.001) after controlling for age, sex, ethnicity, calcium intake, physical activity and transfusion status (Table III). Low bone mass (AP L1EL4 spine Z-Score <E2.0) was also a common observation in this population of subjects (previously published, ref 14). Height (p<0.0001) and weight Z-score (p<0.0001) were both positively correlated with spine BMD Z-score, r=0.34 and 0.41, respectively. After controlling for age, sex, ethnicity, height Z-score and transfusion status, only fat mass was positively associated with whole body bone mineral density Z-score (95%CI: 0.001, 0.04; p=0.04). For the group as a whole, body composition, fat and lean mass, were not related to the presence of hypogonadism, diabetes, or hypothyroidism. However, in the younger group (< 20 years) hypogonadism was marginally associated with a decreased BMI Z-score (p=0.045) after controlling for age, sex, ethnicity and transfusion status. When similar analyses were performed on the NTxThal group alone, body composition (lean and fat mass) were only predictive of growth status (height Z-score) but not BMD Z-score (data not shown).
Table 3. Association of Body Fat (Model 1) and Lean Mass (Model 2) with Growth and Bone Health, adjusting for Age, Sex, Ethnicity, Transfusion status, Calcium intake and Physical activity in Patients with Thalassemia (n=370).
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Predictor | Slope | Standard Error | P-value^^ | Slope | Standard Error | P-value ^^ |
| Height Z-score | Age, years | −0.03 | 0.01 | <0.001 | −0.03 | 0.01 | <0.001 |
| Sex (F vs. M)* | −0.24 | 0.13 | 0.058 | 0.27 | 0.13 | 0.04 | |
| Transfusion Status (Tx vs NTx)** | −0.71 | 0.14 | <0.001 | −0.56 | 0.14 | <0.001 | |
| Asian Ethnicity*** | −0.46 | 0.14 | 0.004 | −0.42 | 0.14 | 0.01 | |
| Calcium Dietary Reference Intake (one unit is 100%) |
−0.01 | 0.07 | NS | 0.01 | 0.07 | NS | |
| Very Active Physical Activity Level# | 0.44 | 0.29 | NS | 0.42 | 0.29 | NS | |
| Body Fat, kg ^ | 0.06 | 0.01 | <0.001 | -- | -- | -- | |
| Lean Mass, kg ^ | -- | -- | -- | 0.04 | 0.01 | <0.001 | |
|
| |||||||
|
Bone Mineral
Density Z-score |
Age, years | −0.05 | 0.01 | <0.001 | −0.05 | 0.01 | <0.001 |
| Gender (F vs M)* | −0.06 | 0.12 | NS | 0.21 | 0.13 | NS | |
| Transfusion Status (Tx vs NTx)** | −0.47 | 0.14 | <0.001 | −0.36 | 0.14 | 0.009 | |
| Asian Ethnicity*** | −0.16 | 0.13 | NS | −0.16 | 0.13 | NS | |
| Calcium Dietary Reference Intake (one unit is 100%) |
−0.12 | 0.06 | NS | −0.13 | 0.07 | 0.052 | |
| Very Active Physical Activity Level# | 0.72 | 0.27 | 0.01 | 0.68 | 0.28 | 0.04 | |
| Body Fat, kg ^ | 0.04 | 0.01 | <0.001 | -- | -- | -- | |
| Lean Mass, kg ^ | -- | -- | -- | 0.02 | 0.01 | 0.01 | |
Sex: Female vs. Male (reference). Male sex is negatively associated with height z-score when body fat is in the model, but was positively associated with height when lean mass is in the model. This inverse relationship is likely a reflection of the relative amount of fat mass vs. lean in females compared with males.
Transfusion status: transfused vs. non-transfused (reference) subjects with thalassemia
Ethnicity: Asian, Other, Caucasian (reference)
Physical Activity estimated by self assessment Likert scale: Very Lightly (reference), Lightly, Moderately, Active, Very Active
Body fat and lean mass determined by whole body DXA
Multiple linear regression analysis was used to examine the association of variables of interest with growth and with bone health. Even after adjusting for other covariates, the two body composition variables were each significantly (p-value<0.05) associated with the outcome variable. For example, as predicted in the model above, Height Z-score is expected to increase by 0.04 units for every 1 kg increase in lean mass in the subjects with thalassemia studied.
Discussion
This study explored body composition and its relationship to growth and bone health in a large sample of contemporary patients with Thal using modern techniques. Though the majority of subjects were classified as having a healthy body composition with very few obese patients, non-transfused adult and pediatric subjects with Thal in this cohort had significantly lower BMI as well as percentage body fat by DXA compared with published reference data on healthy American. Additionally, though these data are associative in nature, we observed significant positive relationships between fat mass and increased height and improved bone health after controlling for age, sex, ethnicity, calcium intake, physical activity and transfusion status.
These data are corroborated by others in the field who have provided more direct evidence of the link between altered growth and nutritional status in small groups of pediatric patients with Thal. Fuchs et al provided intensive nutrition support for one month in 12 young children (1 to 3 years) with Thal from Thailand and observed improvements in weight, height and body fat, which declined when nutrition support was removed [11,20]. Another group provided a high calorie diet to 15 children with Thal major from Egypt for 8 weeks [12]. They observed an increase in BMI, skinfolds, insulin like growth factor-1 and albumin compared with a group of non-supplemented Thals. Tienboon et al went one step further and provided additional liquid formula to 12 toddlers with Thal for one month [10]. They observed significant improvements in various markers of immune function after this short intense supplementation.
There is a known strong positive correlation between body size, composition and bone mass. In our study, a significant positive association was observed between fat mass and whole body BMD Z-score after controlling for age, sex, height Z-score and transfusion status. Others have reported bone mass to be dependent upon lean mass after correction for height in healthy female populations [21] with the effects on bone mass primarily explained by increased mechanical stress on the skeleton [22]. More recent literature has focused on the relationship between energy metabolism and bone turnover with the origin linked to adipocyte and osteoblast differentiation [23]. Other large prospective studies in adults and children have corroborated this hypothesis: fat mass is a strong independent determinant of total body bone mass and bone area [24]. Similar effects were observed in the upper extremities of subjects, therefore negating the reasoning that the connection is due simply to increased mechanical stress. Though previous work from our group [14] and others has focused primarily on the relationship between endocrinopathy and reduced bone mass in patients with thalassemia, the findings from this study support the role that whole body nutritional status (fat mass) may play, particularly in the non-transfused subject.
Though we failed to show an association between current levels of body fat and hypogonadism or delayed pubertal development in the group as a whole, others have observed associations between body mass index and pubertal development. Filosa et al observed that delays in pubertal development in six young males with Thal were linked to a stable body mass index or lack of an increase in body fat [9]. On the other hand, females within the same study who had significant increases in BMI progressed more normally through puberty. There may be an important physiological relationship between nutritional status and adrenarche which we were unable to identify in this cross-sectional study design; this needs to be explored more carefully particularly in non-transfused patients with Thal.
As might be expected, we report that a subjects’ current level of physical activity is associated with body composition; moreover that higher levels of physical activity have positive effects on lean mass and bone health. Though this may be considered logical, there is a paucity of published data which address levels of physical activity in Thalassemia. Literature from healthy cohorts which links increased physical activity and intensity to reduced body fat is quite robust [25,26], and validated tools are available to ascertain level of physical activity. In this study, a brief, 5 question Likert scale, with detailed definitions of types of activities which may be considered “very active” compared with “active” was used. This simple tool proved to be discriminatory with regard to body fatness and may be considered in future interventional trials in which activity is considered a covariate.
In addition to physical activity, we also observed an association between total calcium intake and body fat, lean and total weight. High calcium diets have also been shown to attenuate body fat accumulation and weight gain and to increase fat breakdown and preserve metabolism during caloric restriction in non-thalassemic populations [27]. Though not all meta-analyses agree, there does appear to be a relationship between dietary or supplemental calcium intake and body composition, particularly for obese or overweight individuals. What is interesting is that a relationship between calcium intake and body composition was observed in this rather lean thalassemic population. Of note, calcium intake (dietary plus supplementation) was negatively associated not only with body size but bone mass. It is common practice for Thal patients diagnosed with low bone mass to be placed on a rigid supplementation regimen, therefore in this cohort, total calcium intake may actually be a proxy measure for severity of skeletal deficit.
In this study, body mass index was used as a categorical tool to compare this population of patients with Thal with published reports from large contemporary samples of individuals without Thal. BMI has been shown to be a robust tool to categorize populations; however it can be an inaccurate tool to predict body fatness in individuals. As was observed for some subjects in this cohort, BMI overestimated the true incidence of overweight. For example, a very high BMI can be observed in an extremely lean individual (e.g. body builders with high body weight but low body fat). Fewer categorical errors are typically made in the reverse, as a low BMI could be observed in an individual with muscle wasting and increased body fat for size. But an uncharacteristically high percentage body fat in an individual with low weight should be identified and monitored.
Body composition by DXA is a more robust tool to assess specific body composition compartments, fat, lean and mineral mass. Though ranges have been suggested [28], and new reference ranges have been published [18], there are no universally established body fat percentage criteria to categorize groups as underweight or overweight [29]. In these subjects with Thal, we observed similar age and sex body fat patterns to what is observed in non-thalassemic populations. Older subjects were fatter than younger, and females had greater percent body fat compared with males, regardless of age.
This study was limited with respect to the size of the cohort of non-transfused subjects. Additionally, due to the present study design, we are unable to further explore the relationship between low hemoglobin and poor growth and bone mineralization. We hypothesize that these effects may be due to increased metabolic rate due to increased red cell turnover, particularly in non-transfused patients who have ineffective erythropoiesis and high marrow turnover, problems which are ameliorated by chronic transfusion. Correcting severe anemia through transfusion therapy will diminish ineffective erythropoiesis and studies from decades ago documented improved growth with transfusion in Thal [30,31].
These preliminary findings support the need for routine growth monitoring in the patients with Thal, which may factor into whether or not to initiate transfusion therapy in a child with ß-Thal intermedia. Though the majority of adult patients with Thal had healthy body composition with rare obesity, young, non-transfused patients appear at risk for being underweight. Optimizing physical activity and appropriate use of transfusion therapy may improve growth and bone health in these patients.
Acknowledgments
The authors would like to thank the patients who volunteered their time to participate in this study, as well as Ashutosh Lal, MD and Melanie Kirby-Allen, MD, who assisted in the preparation of the manuscript.
Supported by a cooperative agreement with the National Heart, Lung, and Blood Institute, National Institutes of Health (U01-HLE65232 to Children’s Hospital of Philadelphia, U01-HLE 65233 to University Health Network Toronto General Hospital, U01-HLE65239 to Children’s Hospital & Research Center at Oakland, U01-HLE65244 to Weill Medical College of Cornell University, U01-HLE65260 to Children’s Hospital Boston, and U01-HLE65238 to New England Research Institutes), and at the Children’s Hospital & Research Center at Oakland by NIH-M01E RR01271 and the Children's Hospital Boston by NIH-NCRR grant M01-RR02172 and NIH grant K24HL004184 (E.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42:864–81. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Rocha VZ, Libby P. Obesity, inflammation and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 3.Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance: a mini review. Gerontology. 2009;55:379–86. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- 4.Heilman K, Zilmer M, Zilmer K, Tillmann V. Lower bone mineral density in children with type 1 diabetes is associated with poor glycemic control and higher serum ICAM-1 and urinary isoprostane levels. J Bone Miner Metab. 2009;27:598–604. doi: 10.1007/s00774-009-0076-4. [DOI] [PubMed] [Google Scholar]
- 5.Cashman KD. Altered bone metabolism in inflammatory disease: role for nutrition. Proc Nutr Soc. 2008;67:196–205. doi: 10.1017/S0029665108007039. [DOI] [PubMed] [Google Scholar]
- 6.Zemel BS, Kawchak DA, Fung EB, Ohene-Frempong K, Stallings VA. Effect of Zinc Supplementation on Growth and Body Composition in Children with Sickle Cell Disease. Am J Clin Nutr. 2002;75:300–07. doi: 10.1093/ajcn/75.2.300. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson RD, Conaway M, Chumlea WC, Rosenbaum P, Fung EB, Henderson RC, et al. Growth and health in children with moderate to severe cerebral palsy. Pediatrics. 2006;118:1010–18. doi: 10.1542/peds.2006-0298. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez HF, Perez NB, Malpeli A, Martinez MI, DelBuono B, Viteri FE. Nutrition and immunological status in log-term follow up of children with short bowel syndrome. J Parenteral Enteral Nutr. 2005;29:186–91. doi: 10.1177/0148607105029003186. [DOI] [PubMed] [Google Scholar]
- 9.Filosa A, Di Maio S, Esposito G, De Martinis F, De Terlizzi F. Persistence of delayed adrenarche in boys with thalassemia. J Pediatr Endocrinol. 2001;14:407–14. doi: 10.1515/jpem.2001.14.4.407. [DOI] [PubMed] [Google Scholar]
- 10.Tienboon P. Effect of nutrition support on immunity in paediatric patients with beta-thalassaemia major. Asia Pacific Journal of Clinical Nutrition. 2003;12:61–5. [PubMed] [Google Scholar]
- 11.Fuchs GJ, Tienboon P, Linpisarn S, Nimsakul S, Leelapat P, Tovanabutra S, et al. Nutritional factors and thalassemia major. Arch Disease Childhood. 1996;74:224–7. doi: 10.1136/adc.74.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soliman AT, El-Matary W, Fattah MM, Nasr IS, El Alaily RK, Thabet MA. The effect of high-calorie diet on nutritional parameters of children with beta-thalassaemia major. Clinical Nutrition. 2004;23:1153–8. doi: 10.1016/j.clnu.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Kalef-Ezra J, Zibis A, Chaliassos N, Hatzikonstantinou I, Karantanas A. Body composition in homozygous B-Thalassemia. Annals NY Acad Sci. 2000;904:621–624. doi: 10.1111/j.1749-6632.2000.tb06527.x. [DOI] [PubMed] [Google Scholar]
- 14.Vogiatzi M, Macklin EA, Fung EB, Cheung A, Lane J, Schneider R, et al. Bone disease in thalassemia: a frequent and still unresolved problem. Journal Bone Mineral Research. 2009;24:543–57. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC Growth Charts: United States. Advance Data. 2000:314-1–28. [PubMed] [Google Scholar]
- 16.Prentice AM, Jebb SA. Beyond Body Mass Index. Obesity Reviews. 2001 August;2:141–7. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height and body mass index, United States 1960-2002. Advance Data. 2004;(347) USDHHS. [PubMed] [Google Scholar]
- 18.Kelly TL, Wilson KE, Heymsfield SB. Dual Energy X-ray Absorptiometry Body Composition Reference Values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs GJ, Tienboon P, Khaled MA, Nimsakul S, Linpisarn S, Faruque ASG, Yutrabootr Y, Dewler M, Suskind RM. Nutritional support and growth in thalassemia major. Arch Dis Child. 1997;76:509–12. doi: 10.1136/adc.76.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr DA, Papalia S, Morton A, Dick I, Dhaliwal S, Prince RL. Bone mass in young women is dependent on lean body mass. J Clin Dens. 2007;10:319–326. doi: 10.1016/j.jocd.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang MC, Bachrach LK, VanLoan M, Hudes M, Flegal KM, Crawford PB. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Rosen CJ, Klibanski A. Bone, Fat and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–41. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley GA, Kelley KS. Effects of aerobic exercise on non-high density lipoprotein cholesterol in children and adolescents: a meta-analysis of randomized controlled trials. Prog Cardiovasc Nurs. 2008;23:128–32. doi: 10.1111/j.1751-7117.2008.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24:158–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zemel MB. The role of dairy foods in weight management. J Amer Coll Nutrition. 2005;24(6 Suppl):537S–46S. doi: 10.1080/07315724.2005.10719502. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RW, Jones IE, Williams SM, Goulding A. Body fat percentages measured by dual energy x-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3-18 y. Am J Clin Nutrition. 2002;76:1416–21. doi: 10.1093/ajcn/76.6.1416. [DOI] [PubMed] [Google Scholar]
- 30.De Sanctis V, Wonke B. Growth and Endocrine complications in thalassemia. Mediprint; Rome, Italy: 1998. [Google Scholar]
- 31.Viprakasit V, Tanphaichitr VS, Mahasandana C, Assteerawatt A, Suwantol L, Veerakul G, et al. Linear growth in homozygous beta thalassemia and beta-thalassemia/hemoglobin E patients under different regimens. J Med Assoc Thai. 2001;84:929–41. [PubMed] [Google Scholar]