Abstract
Purpose of Review
Describe ion and water homeostatic mechanisms in the inner ear, how they are compromised in hearing disorders, and what treatments are employed to restore auditory function.
Recent Findings
The ion and water transport functions in the inner ear help maintain the proper endolymph K+ concentration required for hair cell function. Gene defects and idiopathic alterations in these transport functions cause hearing loss, but often the underlying cause is unknown. Current therapies largely involve glucocorticoid treatment, although the mechanisms of restoration are often undeterminable. Recent studies of these ion homeostatic functions in the ear are characterizing their cellular and molecular control. It is anticipated that future management of these hearing disorders will be more targeted to the cellular processes involved and improve the likelihood of hearing recovery.
Summary
A better understanding of the ion homeostatic processes in the ear will permit more effective management of their associated hearing disorders. Sufficient insight into many homeostatic hearing disorders has now been attained to usher in a new era of better therapies and improved clinical outcomes.
Keywords: Ion homeostasis, Meniere's disease, hearing loss, inflammation, middle ear, inner ear, endolymph
I. Introduction
Many recent studies are providing a clearer picture of ion and water transport mechanisms in the ear required to maintain the critical high K+ levels of endolymph. Numerous hearing maladies result from disruption of these homeostatic processes. Many cases are spontaneously or therapeutically reversible, suggesting management is possible. Unfortunately many are due to genetic defects and irresolvable. The focus of this review will be to evaluate the latest research into the ion homeostatic processes of the ear, their associated hearing disorders, and what therapeutic approaches have been successful in their management.
II. Ion Homeostasis in Ear
The inner ear is an ion transport organ. The hair cell depolarizes following transduction of some motion, releases neurotransmitter, and elicits nerve conduction signals to the brain. All of these processes require a unique environment of endolymph (high K+) surrounding the hair cell stereocilia and perilymph (high Na+) around the hair cell body. K+ enters the hair cell as part of the transduction process, is recycled back to the lateral wall via a series of gap junctions and other transport mechanisms, and is secreted back into the endolymph by the stria vascularis (Fig. 1). Thus, the ion homeostasis mechanisms are simply the life support system for the hair cell. An extensive description of these channels and transporters is beyond the scope of this paper and one is referred to recent reviews of hair cell function [1–2], inner ear ion channels and transporters [3–6], and the genetic disorders that impact them [7–8].
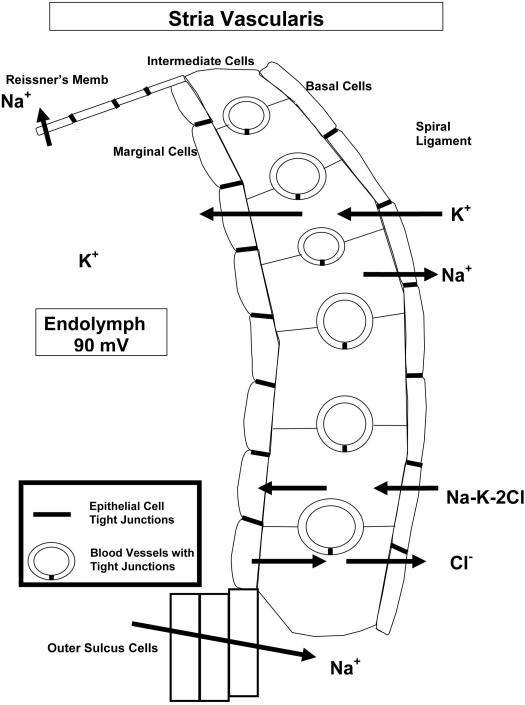
Fig. 1.
Movement of K+ ions in the endolymph. Hair cell transduction causes movement of K+ ions through the hair cell, after which they are transported along the supporting cells and spiral ligament (SL) to the stria vascularis (SV) for secretion back into the endolymph.
The various cell layers of the stria vascularis have unique ion channels for moving K+ into the endolymph (Fig. 2). The basal cells and marginal cells are sealed by tight junctions to control movement of ions and other substances. The capillary endothelial cells, also sealed by tight junctions, make up the blood labyrinth barrier. The spiral ligament also has an extensive vascular network and is occupied largely by fibrocytes. The fibrocytes in the spiral ligament, the basal and intermediate cells of the stria, and the endothelial cells of the strial vessels are all joined by gap junctions that mediate K+ transport. This extensive syncytium reflects the significant control of ion homeostasis in the ear, as well as the potential for transport dysfunction that can occur.
Fig. 2.
Schematic of the stria vascularis showing the general directional flow of ions through the stria vascularis. K+ is moved into the endolymph through a series of channels in the various cell layers. Tight junctions occur between basal cells, marginal cells, and vascular endothelial cells, the latter making up the blood labyrinth barrier.
III. Disorders of Homeostasis
There are numerous hearing disorders that are the direct result of disrupted ion homeostasis. While the initial cause may be something else (inflammation, ototoxicity, noise, etc.), the ultimate impact on the ear is the interference of some ion or water transport mechanism. Thus, impaired ion homeostasis is essentially the final common pathway for many inner ear diseases.
A. Ear vasculature/homeostasis processes
The endothelial cell monitors its environment and responds accordingly when stimulated by bacteria, virus, trauma, circulating antibodies, and immune complexes [9–11].
Mechanisms
The endothelial cell is not a victim of inflammation, but rather a major player in the local inflammatory response. It produces cytokines and other pro-inflammatory factors, such as adhesion molecules that bind leukocytes, monocytes, and macrophages, to move them across intercellular junctions into the tissue for host defense [12–14]. These inflammatory response processes in the brain involve microglia, astrocytes, and endothelial cells, leading to disruption of the blood brain barrier [15–17]. Debate still is ongoing as to whether such inflammation is protective, destructive, or both, but the ultimate goal is to protect the host from the pathogen or other inflammatory attack [16, 18]. Inner ear studies show a similar role played by the cochlear vasculature. The lateral wall vasculature is a complex of endothelial cells, pericytes, and resident perivascular macrophages [19–21], all the components needed for the vascular inflammatory response to occur in the inner ear.
Maladies
Inner ear homeostasis is susceptible to numerous insults that compromise the vasculature of the lateral wall (stria vascularis, spiral ligament) and modiolar artery. Unfortunately, the programmed endothelial cell response to open intercellular junctions to permit immune cell entry into the perivascular space will open the blood labyrinth barrier in the ear. Because integrity of the strial endothelial cell tight junction is critical for maintenance of the endolymph, such disruption will equilibrate ion potentials between the serum and endolymph to cancel the endocochlear potential. Thus, such an inflammatory response, while generally protective of the host, is not compatible with hearing because of disrupted K+ levels in the endolymph (Fig. 2).
Numerous insults to the inner ear have been shown to cause vascular inflammatory responses and potentially hearing loss. This may be a factor in sudden hearing loss, rapidly progressing hearing loss, immune-mediated hearing loss, autoimmune disease hearing loss, labyrinthitis, etc. Several recent studies have shown pro-inflammatory insults impact the vasculature and ion homeostatic processes of the lateral wall or endolymphatic sac:
- Circulating pathogens induce production of cytokines by endothelial cells [22].
- Circulating immune complexes and autoantibodies impact the ear [23–25].
- Noise trauma induces lateral wall inflammatory processes [19, 26–30].
- Ototoxic drugs induce infiltrating lymphocytes [31].
- Hyperlipidemia occurs in Apo E null mice [32].
- Dysfunction of endolymphatic sac by inflammatory cytokine IFN-γ [33].
Management
The traditional glucocorticoid therapy (prednisone, dexamethasone, prednisolone) would be beneficial in suppressing vascular inflammatory processes and protecting/restoring hearing. However, one must be careful in interpreting whether the treatment affected the underlying inflammatory process or the disrupted inner ear homeostatic process. If inflammation disrupts stria vascularis ion homeostatic mechanisms, then therapies may treat the inflammation, the ion transport problem, or both. For example, autoimmune disease mice have significant hearing loss that develops due to elevated serum immune complexes that compromise stria vascularis ion transport [25]. The mineralocorticoid aldosterone upregulates the epithelial sodium channel (ENaC) and Na+,K+-ATPase, and is as effective as glucocorticoids in managing or preventing this hearing loss in mice. However, glucocorticoids also bind to the mineralocorticoid receptor, essentially upregulating genes related to both receptors' functions. Furthermore, recent studies have shown glucocorticoids in the ear may directly drive ENaC function via the glucocorticoid receptor also [34–35]. Thus, the line between glucocorticoid and mineralocorticoid driven processes is blurring. The recent characterization of serum- and glucocorticoid kinase -1 (SGK-1), which is produced following binding by either steroid group, directly controls ENaC activation [36–37]. It also upregulates expression of numerous other ion transport channels, many of which are found in the inner ear [38]. How much SGK-1 impacts inner ear steroid treatment outcomes is currently uninvestigated.
Because one seldom can determine the exact cause of the hearing loss, it is often not clear whether the steroid given is suitable for the underlying homeostatic problem. This may be why recent meta-analyses debate whether steroids or vasodilators are beneficial [39–41]. Further compounding the confusion over outcomes is the fact the lateral wall will regenerate, causing recovery in 50–60% of sudden hearing loss cases. Unfortunately most reports have small sample sizes and power analyses suggest one would need 1,000 patients to effectively demonstrate a statistically significant improvement over this spontaneous recovery rate [41]. Until more effective diagnostic tools are available to determine the actual underlying ion homeostatic process involved in a patient's hearing loss, glucocorticoid therapy will continue to be the major therapy. Mineralocorticoid treatment (aldosterone, fludrocortisone) has shown some promise for treating human hearing loss, presumably by directly regulating ion transport functions via the mineralocorticoid receptor.
B. Middle ear processes
Although most research of otitis media has focused on the inflammatory cytokines, recent studies have begun characterizing the role of ion and water transport channels in the middle ear mucosa.
Mechanisms
Many of the ion transporters and aquaporins found in the inner ear also occur in the middle ear. These include several K+ channels, ENaC, Na+,K+-ATPases, gap junctions, tight junction claudins, the sodium potassium chloride cotransporter (NKCC), and chloride channel (Clcnka) (42). These presumably are involved in the clearance of fluid to keep the middle ear clear [43–46].
Maladies
Inflammation in the middle ear suppresses gene expression of many of these ion channels and aquaporins [42–46], which possibly is responsible for failure of effusions to clear. If inflammation is to protect the host from pathogens, effusion formation may help kill bacteria by providing a concentrated pool of cytokines and inflammatory cells. Furthermore, inflammatory cytokines in the middle ear cause similar cytokine expression and pathology in the inner ear [47–52], leading to downregulation of these same ion and water transport channels (unpublished data). The sensorineural hearing loss accompanying otitis media may include inflammatory depression of these cochlear ion channels to decrease K+ transport into the endolymph (Fig. 2).
Management
Because antibiotics are the only treatment for otitis media, little is known about the potential management of the ion homeostatic disruption in the middle ear. Recent efforts to manage these ion homeostasis channels in middle ear disease has focused on the glucocorticoids to suppress inflammation and mineralocorticoids to enhance K+ and Na+ exchange via ENaC and Na+,K+-ATPase to clear middle ear fluid [53–54]. However, it appears the glucocorticoid dexamethasone is also influential in driving middle ear ENaC function [55]. Future studies will undoubtedly clarify this role of ion and water transport in middle ear effusions and its control with therapeutics. Effusion control will reduce pain and discomfort in children, lower the risk to the inner ear, and potentially eliminate the need for many prescriptions of antibiotics that have limited effectiveness anyway.
C. Mineralocorticoid - Glucocorticoid processes
Several ion channels and transporters in the lateral wall are driven by natural and therapeutic steroid hormones.
Mechanisms
Several ion transport channels in the stria vascularis and spiral ligament are responsible for moving K+ into the endolymph and Na+ out (Fig. 2). These include the K+ channels (KCNJ10, KCNE1, KCNQ1), ENaC, Na+,K+-ATPase, NKCC, gap junctions, aquaporins, TRPV4, purinergic receptors, and tight junction claudins. Mineralocorticoids and glucocorticoids are the hormone drivers for some of these [4–5, 25, 34–35, 56], as well as arginine vasopressin, atrial natriuretic peptide, insulin, and endothelin [57]. However, the drivers of many of these channels are thus far undetermined.
Maladies
Gene defects affect many of these channels [7–8], the most common of which are the gap junction connexins [3–4, 8, 58]. These act primarily in the supporting cells and the interconnected fibrocytes in the spiral ligament. Although fibrocyte gap junctions move K+, recent studies show they move other molecules relevant to cochlear homeostasis [58–60]. It is not clear if some forms of sudden and rapidly progressing hearing loss are the result of disruption of these pathways. Meniere's disease does not appear to involve these channels [61], instead involving those in the aquaporins-vasopressin complex (below). The vascular inflammatory diseases above also may directly impact the function of these channels. Aging and noise damage can have a considerable effect on ion homeostasis. Noise exposure reduces purinergic signaling, potentially leading to reduction in K+ secretion [62], while sound-induced expression of osmotic stress protein 94 in the lateral wall may help protect ion homeostasis [28]. Gene polymorphisms in these K+ channels and heat shock proteins also appear to predispose individuals to noise-induced hearing loss [63–64]. Aging leads to degenerative changes in the lateral wall and decreased ion and water transport [65–66]. Loss of tight junction claudins leads to unregulated K+ transport and hearing loss [67–68]. Loss of the pendrin function of anion exchange in the lateral wall impairs cochlear pH regulation, leading to hearing loss and enlarged vestibular aqueduct [69–70].
Management
The various therapeutic steroids have an impact on these channels, particularly ENaC, Na+,K+-ATPase, and some of the K+ channels. Both glucocorticoids and mineralocorticoids upregulate KCNJ10, aquaporin 1, and gap junction connexin 26, with greater effect seen following intratympanic delivery (unpublished data). This supports the improved results seen with intratympanic steroid delivery clinically [71–74], although this is still debated [75]. Speculation regarding antioxidant therapy to prevent age-related degeneration of vascular and lateral wall functions raises an interesting possibility for the future [76].
D. Aquaporin-Vasopressin processes
Recent studies are beginning to clarify the role of certain aquaporins and vasopressin in the control of endolymph volume.
Mechanisms
Aquaporin 2 channels and vasopressin (anti-diuretic hormone) are responsible for moving K+ and water into the endolymph. These channels are active in the stria vascularis [77–78], and endolymphatic sac [79]. Vasopressin supplementation will cause expression of numerous ion transport genes in the ear [80] and lead to hydrops [81–82]. The endolymphatic sac also contains purinergic receptors [83] and TRPV4 channels [84] that are proposed to control fluid balances, but whether they act in tandem with aquaporins and vasopressin is unknown.
Maladies
Meniere's disease is often associated with dysfunction of vasopressin and aquaporin 2 channels. Meniere's patients often have elevated levels of vasopressin during active disease [85]. It has been challenging to develop the causative relationship between elevated serum vasopressin and Meniere's disease since the symptoms are usually unilateral. Recently it was determined that mRNA of the vasopressin receptor is expressed at much higher levels in the endolymphatic sac of Meniere's patients [86–87]. This lead to the conclusion that it is the unilateral cochlear elevation of receptor expression, coupled with slightly higher serum levels, that causes unilateral Meniere's. Recent studies also have identified potential aquaporin gene polymorphisms in Meniere's patients that may impact water movement and hydrops formation [88]. Decreased aquaporin 4 expression has been suggested as a mechanism of aging related hearing loss as well [65].
Hydrops results from excess K+ being moved into the endolymph, but it is not clear if this is always due to aquaporins 2 – vasopressin functions. For example, endogenous ouabain suppresses Na+,K+-ATPase, which is responsible for moving K+ into the endolymph. Meniere's patients have low levels of endogenous ouabain [89], which may increase K+ transport and cause hydrops. Also, a gene polymorphism in adducin, which increases Na+,K+-ATPase function, is increased in Meniere's patients [90]. ENac is also suppressed in the endolymphatic sac by inflammation [91], suggesting reduced movement of Na+ out of the endolymph may increase hydrops. Salicylates downregulate cochlear aquaporin 6, which suggests a link between the hearing loss and tinnitus seen with nonsteroidal anti-inflammatories [92].
Management
Steroids have been shown to be effective in many cases of Meniere's disease, but it is not clear why. They may be effective because of underlying inflammatory causes of vasopressin and receptor abnormalities, or those responding to steroids may not have aquaporins 2 – vasopressin issues at all. Dexamethasone does impact aquaporin 3 expression [79], but glucocorticoids also would help clear fluid buildup by restoring normal ion homeostasis. For example, we have shown that glucocorticoids upregulate cochlear gene expression of the K+ channel KCNJ10, aquaporin 1, and gap junction connexin 26 (unpublished data). Diuretic treatments have been suggested for Meniere's disease, but it is not clear if they would operate on the vasopressin receptor to reduce water transport, act predominantly through the NKCC channel to suppress K+ movement into the endolymph, or both.
Conclusion
Recent research on ion homeostatic mechanisms of the ear demonstrates the relevance of many channels to clinical hearing disorders. Basic research is gradually building the molecular profile of these channels and transporters that someday will make targeted therapies possible. Medical management of such disorders may eventually include genetic hearing loss by upregulating parallel transport systems to restore partial cochlear function. The molecular armamentarium available to researchers, coupled with the translational research mind set of our clinician scientists, set the stage for an exciting future.
Acknowledgments
Research Supported by NIH-NIDCD grants R01 DC05593 and R01 DC009455
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fettiplace R. Defining features of the hair cell mechanoelectrical transducer channel. Pflugers Arch. 2009;458:1115–23. doi: 10.1007/s00424-009-0683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Review of hair cell transduction channel.
- 2.Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Reviews latest findings on hair cell mechanotransduction.
- 3.Couloigner V, Sterkers O, Ferrary E. What's new in ion transports in the cochlea? Pflugers Arch. 2006;453:11–22. doi: 10.1007/s00424-006-0103-4. [DOI] [PubMed] [Google Scholar]
- 4.Lang F, Vallon V, Knipper M, Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol. 2007;293:C1187–208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]
- 5.Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology (Bethesda) 2009;24:307–16. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Review of ion transport mechanisms in the ear as basis for gene mutations.
- 6.Hibino H, Kurachi Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 2006;21:336–45. doi: 10.1152/physiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- 7.Hilgert N, Smith RJ, Van Camp G. Function and expression pattern of nonsyndromic deafness genes. Curr Mol Med. 2009;9:546–64. doi: 10.2174/156652409788488775. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Reviews current understanding of gene mutations in the ear and how some relate to ion transport dysfunction.
- 8.Wang WH, Yang JJ, Lin YC, Yang JT, Chan CH, Li SY. Identification of novel variants in the C×29 gene of nonsyndromic hearing loss patients using buccal cells and restriction fragment length polymorphism method. Audiol Neurootol. 2010;15:81–7. doi: 10.1159/000231633. [DOI] [PubMed] [Google Scholar]; * Demonstrates gene variants in connexin 29 as a potential cause of hearing loss.
- 9.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–22. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 10.Lemichez E, Lecuit M, Nassif X, Bourdoulous S. Breaking the wall: targeting of the endothelium by pathogenic bacteria. Nat Rev Microbiol. 2010;8:93–104. doi: 10.1038/nrmicro2269. [DOI] [PubMed] [Google Scholar]; ** Summarizes the impact of bacterial infection on endothelial cell response.
- 11.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 12.Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol. 2010;184:5242–52. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Shows the branching points of vessels as preferred sites for leucocyte transmigration.
- 13.van Zonneveld AJ, de Boer HC, van der Veer EP, Rabelink TJ. Inflammation, vascular injury and repair in rheumatoid arthritis. Ann Rheum Dis. 2010;69(Suppl 1):i57–60. doi: 10.1136/ard.2009.119495. [DOI] [PubMed] [Google Scholar]; * Discusses the pro-inflammatory cytokine production by endothelial cells.
- 14.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–30. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Discusses the mechanisms of endothelial cells control over leukocyte transmigration.
- 15.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 16.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–93. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 17.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]; * Describes the role of microglia in brain inflammatory control.
- 18.Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, Weaver LC. Debate: “is increasing neuroinflammation beneficial for neural repair?”. J Neuroimmune Pharmacol. 2006;1:195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]
- 19.Dai M, Nuttall A, Yang Y, Shi X. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear Res. 2009;254:100–7. doi: 10.1016/j.heares.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Provides evidence for spiral ligament blood flow control by perivascular pericytes.
- 20.Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL. The cochlear pericytes. Microcirculation. 2008;15:515–29. doi: 10.1080/10739680802047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. doi: 10.1007/s00441-010-1040-2. In Press. [DOI] [PubMed] [Google Scholar]; ** Stria vascularis perivascular macrophages are derived from bone marrow and may play a role in strial homeostasis, as well as vascular repair following inflammation.
- 22.Gloddek B, Lamm K, Arnold W. Pharmacological influence on inner ear endothelial cells in relation to the pathogenesis of sensorineural hearing loss. Adv Otorhinolaryngol. 2002;59:75–83. doi: 10.1159/000059254. [DOI] [PubMed] [Google Scholar]
- 23.Kanzaki J, Kanzaki S, Ogawa K. Long-term prognosis of steroid-dependent sensorineural hearing loss. Audiol Neurootol. 2009;14:26–34. doi: 10.1159/000151587. [DOI] [PubMed] [Google Scholar]; * Discussses the potential for vascular inflammation to underlie hearing loss.
- 24.George DL, Pradhan S. Idiopathic sensorineural hearing disorders in adults--a pragmatic approach. Nat Rev Rheumatol. 2009;5:505–12. doi: 10.1038/nrrheum.2009.150. [DOI] [PubMed] [Google Scholar]; ** Relationship of vascular inflammation with human hearing loss.
- 25.Trune DR, Kempton JB. Blocking the glucocorticoid receptor with RU-486 does not prevent glucocorticoid control of autoimmune mouse hearing loss. Audiol Neurootol. 2009;14:423–31. doi: 10.1159/000241899. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Impact of autoimmune disease on ion transport mechanisms in the ear.
- 26.Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575–83. doi: 10.1002/jnr.20764. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto H, Omelchenko I, Shi X, Nuttall AL. The influence of NF-kB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J Neurosci Res. 2009;87:1832–40. doi: 10.1002/jnr.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Shows inflammatory processes in lateral wall induced by noise.
- 28.Yamamoto H, Shi X, Nuttall AL. The influence of loud sound stress on expression of osmotic stress protein 94 in the murine inner ear. Neuroscience. 2009;158:1691–8. doi: 10.1016/j.neuroscience.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Shows osmotic stress protein 94 may protect the ear during loud sound.
- 29.Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am J Pathol. 2009;174:1692–704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Provides evidence for cochlear pericyte role in inflammatory response to noise.
- 30.Dai M, Yang Y, Omelchenko I, Nuttall AL, Kachelmeier A, Xiu R, Shi X. Bone marrow cell recruitment for repair of acoustically damaged blood-labyrinth-barrier mediated by a local iNOS/SDF-1α pathway. Amer J Pathol. doi: 10.2353/ajpath.2010.100340. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** iNOS controls recruitment of bone marrow cells to the stria vascularis to repair its vessels following noise trauma.
- 31.Sato E, Shick HE, Ransohoff RM, Hirose K. Expression of fractalkine receptor CX3CR1 on cochlear macrophages influences survival of hair cells following ototoxic injury. J Assoc Res Otolaryngol. 2010;11:223–34. doi: 10.1007/s10162-009-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Shows the role of invading macrophages on inner ear damage during kanamycin ototoxicity.
- 32.Zhu S, Du X, Cai Q, Guo Y, Liu L, Cheng W, Tao Z, Yoo T. Impaired stria vascularis in the inner ear of apolipoprotein E gene knockout mice. ORL J Otorhinolaryngol Relat Spec. 2008;70:373–80. doi: 10.1159/000163033. [DOI] [PubMed] [Google Scholar]
- 33.Son EJ, Moon IS, Kim SH, Kim SJ, Choi JY. Interferon-gamma suppresses Na+ -H+ exchanger in cultured human endolymphatic sac epithelial cells. J Cell Biochem. 2009;107:965–72. doi: 10.1002/jcb.22201. [DOI] [PubMed] [Google Scholar]; * Shows susceptibility of the N+-H+ exchanger to inflammation in endolymphatic sac.
- 34.Kim SH, Kim KX, Raveendran NN, Wu T, Pondugula SR, Marcus DC. Regulation of ENaC-mediated sodium transport by glucocorticoids in Reissner's membrane epithelium. Am J Physiol Cell Physiol. 2009;296:C544–57. doi: 10.1152/ajpcell.00338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Provides evidence ENaC function driven by glucocorticoid receptor.
- 35.Kim SH, Marcus DC. Endolymphatic sodium homeostasis by extramacular epithelium of the saccule. J Neurosci. 2009;29:15851–8. doi: 10.1523/JNEUROSCI.3044-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Shows evidence for Na+ transport by saccular epithelium.
- 36.Musch MW, Lucioni A, Chang EB. Aldosterone regulation of intestinal Na absorption involves SGK-mediated changes in NHE3 and Na+ pump activity. Am J Physiol Gastrointest Liver Physiol. 2008;295:G909–19. doi: 10.1152/ajpgi.90312.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuetz F, Kumar S, Poronnik P, Adams DJ. Regulation of the voltage-gated K(+) channels KCNQ2/3 and KCNQ3/5 by serum- and glucocorticoid-regulated kinase-1. Am J Physiol Cell Physiol. 2008;295:C73–80. doi: 10.1152/ajpcell.00146.2008. [DOI] [PubMed] [Google Scholar]
- 38.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 39.Agarwal L, Pothier DD. Vasodilators and vasoactive substances for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD003422.pub4. CD003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: II. A Meta-analysis. Arch Otolaryngol Head Neck Surg. 2007;133:582–6. doi: 10.1001/archotol.133.6.582. [DOI] [PubMed] [Google Scholar]
- 41.Wei BP, Mubiru S, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003998.pub2. CD003998. [DOI] [PubMed] [Google Scholar]
- 42.MacArthur CJ, Hausman F, Kempton JB, Trune DR. Murine middle ear inflammation and ion homeostasis gene expression. Otol Neurotol. doi: 10.1097/MAO.0b013e31820e6de4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Multiple ion and water transport channels occur in the middle ear and are downregulated during inflammation, potentially compromising effusion clearance.
- 43.Song JJ, Kown SK, Kim EJ, Lee YS, Kim BY, Chae SW. Mucosal expression of ENaC and AQP in experimental otitis media induced by Eustachian tube obstruction. Int J Pediatr Otorhinolaryngol. 2009;73:1589–93. doi: 10.1016/j.ijporl.2009.08.011. [DOI] [PubMed] [Google Scholar]; * Describes altered aquaporin and ENaC with otitis media.
- 44.Zhang Q, Liu C, Gao X, Hu Y, Guo W, Sun J, Li X. Expression pattern of aquaporin 1 in the middle ear of the guinea pig with secretory otitis media. ORL J Otorhinolaryngol Relat Spec. 2009;71:70–7. doi: 10.1159/000182419. [DOI] [PubMed] [Google Scholar]; ** Decribes altered aquaporin function in the middle ear with inflammation.
- 45.Zhang Q, Liu C, Wang J, Sun J, Hu Y, Chen G, Li X. Expression pattern of aquaporin 4 and 5 in the middle ear of guinea pigs with secretory otitis media. Acta Otolaryngol. 2009:1–7. doi: 10.3109/00016480902974183. [DOI] [PubMed] [Google Scholar]; ** Describes increased gene expression of aquaporins 4 and 5 with secretory otitis media.
- 46.Kang SH, Chang KH, Ohcho S, Lee HY, Cha K, Moon SK, Andalibi A, Lim DJ. Expression of water channel proteins (aquaporins) in the rat Eustachian tube and middle ear mucosa. Acta Otolaryngol. 2007;127:687–92. doi: 10.1080/00016480500452574. [DOI] [PubMed] [Google Scholar]
- 47.Tsuprun V, Cureoglu S, Schachern PA, Ferrieri P, Briles DE, Paparella MM, Juhn SK. Role of pneumococcal proteins in sensorineural hearing loss due to otitis media. Otol Neurotol. 2008;29:1056–60. doi: 10.1097/MAO.0b013e31818af3ad. [DOI] [PubMed] [Google Scholar]
- 48.Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine acute otitis media. Laryngoscope. 2007;117:22–9. doi: 10.1097/01.mlg.0000240170.48584.73. [DOI] [PubMed] [Google Scholar]
- 49.Moon SK, Woo JI, Lee HY, Park R, Shimada J, Pan H, Gellibolian R, Lim DJ. Toll-like receptor 2-dependent NF-kappaB activation is involved in nontypeable Haemophilus influenzae-induced monocyte chemotactic protein 1 up-regulation in the spiral ligament fibrocytes of the inner ear. Infect Immun. 2007;75:3361–72. doi: 10.1128/IAI.01886-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juhn SK, Jung MK, Hoffman MD, Drew BR, Preciado DA, Sausen NJ, Jung TT, Kim BH, Park SY, Lin J, Ondrey FG, Mains DR, Huang T. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117–38. doi: 10.3342/ceo.2008.1.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joglekar S, Morita N, Cureoglu S, Schachern PA, Deroee AF, Tsuprun V, Paparella MM, Juhn SK. Cochlear pathology in human temporal bones with otitis media. Acta Otolaryngol. 2010;130:472–6. doi: 10.3109/00016480903311252. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Shows quantitative loss of stria vascularis and spiral ligament with long term otitis media.
- 52.Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol Head Neck Surg. 2007;137:332–7. doi: 10.1016/j.otohns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 53.MacArthur CJ, Kempton JB, DeGagne J, Trune DR. Control of chronic otitis media and sensorineural hearing loss in C3H/HeJ mice: glucocorticoids vs mineralocorticoids. Otolaryngol Head Neck Surg. 2008;139:646–53. doi: 10.1016/j.otohns.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacArthur CJ, DeGagne JM, Kempton JB, Trune DR. Steroid control of acute middle ear inflammation in a mouse model. Arch Otolaryngol Head Neck Surg. 2009;135:453–7. doi: 10.1001/archoto.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Showed mineralocorticoids, as well as glucocorticoids, were effective in clearing middle ear inflammation.
- 55.Son EJ, Kim SH, Park HY, Kim SJ, Yoon JH, Chung HP, Choi JY. Activation of epithelial sodium channel in human middle ear epithelial cells by dexamethasone. Eur J Pharmacol. 2009;602:383–7. doi: 10.1016/j.ejphar.2008.11.012. [DOI] [PubMed] [Google Scholar]; ** Describes efficacy of dexamethasone in activating ENaC in middle ear.
- 56.Kim CH, Kim HY, S OC, Oh SH, Lee JE, Lee JH. Developmental change of Na(+)-absorptive function in Reissner's membrane epithelia. Neuroreport. 2009;20:1275–8. doi: 10.1097/WNR.0b013e32833017eb. [DOI] [PubMed] [Google Scholar]; * Shows amiloride sensitive Na+ transport in developing Reissner's membrane.
- 57.Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–54. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 58.Hoang Dinh E, Ahmad S, Chang Q, Tang W, Stong B, Lin X. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009;1277:52–69. doi: 10.1016/j.brainres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Excellent review of latest research on connexins and gap junctions mutations in the ear.
- 59.Adams JC. Immunocytochemical traits of type IV fibrocytes and their possible relations to cochlear function and pathology. J Assoc Res Otolaryngol. 2009 doi: 10.1007/s10162-009-0165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Describes role for spiral ligament fibrocytes besides K+ transport.
- 60.Chang Q, Tang W, Ahmad S, Stong B, Leu G, Lin X. Functional studies reveal new mechanisms for deafness caused by connexin mutations. Otol Neurotol. 2009;30:237–40. doi: 10.1097/MAO.0b013e318194f774. [DOI] [PubMed] [Google Scholar]; * Presents evidence for gap junctions transporting biochemical factors and potential impact of gene defects that prevent this function.
- 61.Campbell CA, Della Santina CC, Meyer NC, Smith NB, Myrie OA, Stone EM, Fukushima K, Califano J, Carey JP, Hansen MR, Gantz BJ, Minor LB, Smith RJ. Polymorphisms in KCNE1 or KCNE3 are not associated with Meniere disease in the Caucasian population. Am J Med Genet A. 2010;152A:67–74. doi: 10.1002/ajmg.a.33114. [DOI] [PubMed] [Google Scholar]; ** Evidence does not support K+ channel gene defects in Meniere's disease.
- 62.Lee JH, Marcus DC. Purinergic signaling in the inner ear. Hear Res. 2008;235:1–7. doi: 10.1016/j.heares.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pawelczyk M, Van Laer L, Fransen E, Rajkowska E, Konings A, Carlsson PI, Borg E, Van Camp G, Sliwinska-Kowalska M. Analysis of gene polymorphisms associated with K ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann Hum Genet. 2009;73:411–21. doi: 10.1111/j.1469-1809.2009.00521.x. [DOI] [PubMed] [Google Scholar]; ** Describes gene polymorphisms in K+ channels that may relate to susceptibility to noise-induced hearing loss.
- 64.Konings A, Van Laer L, Michel S, Pawelczyk M, Carlsson PI, Bondeson ML, Rajkowska E, Dudarewicz A, Vandevelde A, Fransen E, Huyghe J, Borg E, Sliwinska-Kowalska M, Van Camp G. Variations in HSP70 genes associated with noise-induced hearing loss in two independent populations. Eur J Hum Genet. 2009;17:329–35. doi: 10.1038/ejhg.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Establishes a potential link between heat shock protein gene polymorphisms and susceptibility to noise induced hearing loss.
- 65.Christensen N, D'Souza M, Zhu X, Frisina RD. Age-related hearing loss: aquaporin 4 gene expression changes in the mouse cochlea and auditory midbrain. Brain Res. 2009;1253:27–34. doi: 10.1016/j.brainres.2008.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Showed altered aquaporin gene expression in aging cochlea and brainstem.
- 66.Ohlemiller KK, Rice ME, Lett JM, Gagnon PM. Absence of strial melanin coincides with age-associated marginal cell loss and endocochlear potential decline. Hear Res. 2009;249:1–14. doi: 10.1016/j.heares.2008.12.005. [DOI] [PubMed] [Google Scholar]; ** Relates aging hearing loss with stria vascularis degeneration.
- 67.Nakano Y, Kim SH, Kim HM, Sanneman JD, Zhang Y, Smith RJ, Marcus DC, Wangemann P, Nessler RA, Banfi B. A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet. 2009;5:e1000610. doi: 10.1371/journal.pgen.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Shows role of tight junction claudins in protecting base of hair cell from high K+.
- 68.Elkouby-Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res. 2008;333:427–38. doi: 10.1007/s00441-008-0621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dai P, Stewart AK, Chebib F, Hsu A, Rozenfeld J, Huang D, Kang D, Lip V, Fang H, Shao H, Liu X, Yu F, Yuan H, Kenna M, Miller DT, Shen Y, Yang W, Zelikovic I, Platt OS, Han D, Alper SL, Wu BL. Distinct and novel SLC26A4/Pendrin mutations in Chinese and U.S. patients with nonsyndromic hearing loss. Physiol Genomics. 2009;38:281–90. doi: 10.1152/physiolgenomics.00047.2009. [DOI] [PubMed] [Google Scholar]; * Shows pendrin mutations correlate with hearing loss and enlarged vestibular acqueduct.
- 70.Wangemann P, Kim HM, Billings S, Nakaya K, Li X, Singh R, Sharlin DS, Forrest D, Marcus DC, Fong P. Developmental delays consistent with cochlear hypothyroidism contribute to failure to develop hearing in mice lacking Slc26a4/pendrin expression. Am J Physiol Renal Physiol. 2009;297:F1435–47. doi: 10.1152/ajprenal.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Establishes relationship between pendrin mutation and developmental defects due to hypothyroidism in the ear.
- 71.Salt AN, Plontke SK. Principles of local drug delivery to the inner ear. Audiol Neurootol. 2009;14:350–60. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Discusses perilymph sampling in assessing intracochlear drug distribution to compare effectivness of middle ear delivery methods.
- 72.She W, Dai Y, Du X, Yu C, Chen F, Wang J, Qin X. Hearing evaluation of intratympanic methylprednisolone perfusion for refractory sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2010;142:266–71. doi: 10.1016/j.otohns.2009.10.046. [DOI] [PubMed] [Google Scholar]; * Better hearing outcomes with intratympanic steroid delivery.
- 73.Hong SM, Park CH, Lee JH. Hearing outcomes of daily intratympanic dexamethasone alone as a primary treatment modality for ISSHL. Otolaryngol Head Neck Surg. 2009;141:579–83. doi: 10.1016/j.otohns.2009.08.009. [DOI] [PubMed] [Google Scholar]; * Potential frequency recovery differences between oral and intratympanic steroid delivery methods.
- 74.Mikulec AA, Plontke SK, Hartsock JJ, Salt AN. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otol Neurotol. 2009;30:131–8. doi: 10.1097/mao.0b013e318191bff8. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Provides evidence of middle ear delivered drugs going through the bone of the otic capsule in addition to the round window.
- 75.Hu A, Parnes LS. Intratympanic steroids for inner ear disorders: a review. Audiol Neurootol. 2009;14:373–82. doi: 10.1159/000241894. [DOI] [PubMed] [Google Scholar]; ** Good review of literature on efficacy of intratympanic steroid delivery.
- 76.Bielefeld EC, Tanaka C, Chen GD, Henderson D. Age-related hearing loss: is it a preventable condition? Hear Res. 2010;264:98–107. doi: 10.1016/j.heares.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Provides evidence for antioxidant treatments to prevent age-related hearing loss.
- 77.Nishioka R, Takeda T, Kakigi A, Okada T, Takebayashi S, Taguchi D, Nishimura M, Hyodo M. Expression of aquaporins and vasopressin type 2 receptor in the stria vascularis of the cochlea. Hear Res. 2010;260:11–9. doi: 10.1016/j.heares.2009.09.011. [DOI] [PubMed] [Google Scholar]; ** Good description of aquaporins and vasopressin locations in the ear.
- 78.Lopez IA, Ishiyama G, Lee M, Baloh RW, Ishiyama A. Immunohistochemical localization of aquaporins in the human inner ear. Cell Tissue Res. 2007;328:453–60. doi: 10.1007/s00441-007-0380-z. [DOI] [PubMed] [Google Scholar]
- 79.Kitahara T, Fukushima M, Uno Y, Mishiro Y, Kubo T. Up-regulation of cochlear aquaporin-3 mRNA expression after intra-endolymphatic sac application of dexamethasone. Neurol Res. 2003;25:865–70. doi: 10.1179/016164103771953989. [DOI] [PubMed] [Google Scholar]
- 80.Gu FM, Han HL, Zhang LS. Effects of vasopressin on gene expression in rat inner ear. Hear Res. 2006;222:70–8. doi: 10.1016/j.heares.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 81.Marshall AF, Jewells VL, Kranz P, Lee YZ, Lin W, Zdanski CJ. Magnetic resonance imaging of guinea pig cochlea after vasopressin-induced or surgically induced endolymphatic hydrops. Otolaryngology - Head & Neck Surgery. 2010;142:260–5. doi: 10.1016/j.otohns.2009.10.006. [DOI] [PubMed] [Google Scholar]; * Induction of endolymphatic hydrops with vasopressin treatment.
- 82.Nishimura M, Kakigi A, Takeda T, Okada T, Doi K. Time course changes of vasopressin-induced enlargement of the rat intrastrial space and the effects of a vasopressin type 2 antagonist. Acta Otolaryngol. 2009;129:709–15. doi: 10.1080/00016480802419115. [DOI] [PubMed] [Google Scholar]; * Showed vasopressin causes hydrops as a potential mechanisms for Meniere's disease.
- 83.Mori T, Miyashita T, Akiyama K, Inamoto R, Mori N. The expression of P2Y1, 2, 4, and 6 receptors in rat endolymphatic sac epithelia. Neuroreport. 2009;20:419–23. doi: 10.1097/WNR.0b013e328325a926. [DOI] [PubMed] [Google Scholar]; * Results suggest purinergic receptors may control sodium transport in endolymphatic sac.
- 84.Kumagami H, Terakado M, Sainoo Y, Baba A, Fujiyama D, Fukuda T, Takasaki K, Takahashi H. Expression of the osmotically responsive cationic channel TRPV4 in the endolymphatic sac. Audiol Neurootol. 2009;14:190–7. doi: 10.1159/000180290. [DOI] [PubMed] [Google Scholar]
- 85.Takeda T, Takeda S, Kakigi A, Okada T, Nishioka R, Taguchi D, Nishimura M, Nakatani H. Hormonal aspects of Meniere's disease on the basis of clinical and experimental studies. ORL J Otorhinolaryngol Relat Spec. 2010;71(Suppl 1):1–19. doi: 10.1159/000265113. [DOI] [PubMed] [Google Scholar]; ** Showed relationship between elevated vasopressin and active Meniere's.
- 86.Kitahara T, Doi K, Maekawa C, Kizawa K, Horii A, Kubo T, Kiyama H. Meniere's attacks occur in the inner ear with excessive vasopressin type-2 receptors. J Neuroendocrinol. 2008;20:1295–300. doi: 10.1111/j.1365-2826.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 87.Kitahara T, Maekawa C, Kizawa K, Horii A, Doi K. Plasma vasopressin and V2 receptor in the endolymphatic sac in patients with delayed endolymphatic hydrops. Otol Neurotol. 2009;30:812–9. doi: 10.1097/MAO.0b013e3181b11db5. [DOI] [PubMed] [Google Scholar]; ** Correlates vasopressin receptor in sac as major factor in vasopressin induced Meniere's disease.
- 88.Mallur PS, Weisstuch A, Pfister M, Mhatre AN, Lalwani AK. Aquaporin-2 and -4: single nucleotide polymorphisms in Meniere's disease patients. Audiol Med. 2010;8:18–23. [Google Scholar]; ** Provided evidence that aquaporin gene polymorphisms may contribute to Meniere's disease.
- 89.Teggi R, Zagato L, Delli Carpini S, Messaggio E, Casamassima N, Lanzani C, Manunta P, Bussi M. Endogenous ouabain in Meniere's disease. Otol Neurotol. 2010;31:153–6. doi: 10.1097/MAO.0b013e3181c0eaba. [DOI] [PubMed] [Google Scholar]; * Showed plasma levels of ouabain drop in Meniere's disease patients.
- 90.Teggi R, Lanzani C, Zagato L, Delli Carpini S, Manunta P, Bianchi G, Bussi M. Gly460Trp alpha-adducin mutation as a possible mechanism leading to endolymphatic hydrops in Meniere's syndrome. Otol Neurotol. 2008;29:824–8. doi: 10.1097/MAO.0b013e318180a4b1. [DOI] [PubMed] [Google Scholar]
- 91.Kim SH, Park HY, Choi HS, Chung HP, Choi JY. Functional and molecular expression of epithelial sodium channels in cultured human endolymphatic sac epithelial cells. Otol Neurotol. 2009;30:529–34. doi: 10.1097/MAO.0b013e31819a8e0e. [DOI] [PubMed] [Google Scholar]; * Demonstrated inflammation induced upregulation of ENaC in endolymphatic sac.
- 92.Perin P, Tritto S, Botta L, Fontana JM, Gastaldi G, Masetto S, Tosco M, Laforenza U. Aquaporin-6 expression in the cochlear sensory epithelium is downregulated by salicylates. J Biomed Biotechnol. 2010;2010:264704. doi: 10.1155/2010/264704. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Establishes a connection between aquaporin 6 expression and nonsteroidal anti-inflammatories.