Abstract
Objective
We characterized the state of the vascular endothelium in pediatric obesity by comparing circulating endothelial cell (CEC) number and activation phenotype in severely obese children to normal weight, overweight, and obese children.
Study design
We used immunohistochemical examination of buffy-coat smears to enumerate CEC and immunofluorescence microscopy to quantify activated CEC in 107 children and adolescents. Normal weight (body mass index [BMI] <85th percentile; N=40), overweight (BMI 85th-<95th percentile; N=17), and obese (BMI 95th-<99th percentile; N=23) participants were recruited from a longitudinal study. Severely obese (BMI ≥99th percentile; N=27) participants were recruited from a pediatric obesity clinic. Group means (adiposity; systolic blood pressure [SBP] quartiles) were compared with general linear models, adjusted for sex, age, and race. Pearson correlations characterized relations of CEC with cardiovascular risk factors.
Results
Activated CEC increased across BMI groups (p<0.002) and SBP quartiles (p<0.05). CEC number and activated CEC were highest in the severely obese group. CEC number was significantly associated with SBP, diastolic blood pressure, and triglycerides. Activated CEC were significantly associated with SBP and HDL-cholesterol.
Conclusions
The vascular endothelium was activated in relation to excess adiposity, particularly in the severely obese, and to elevated SBP in children and adolescents.
Keywords: Vascular Endothelium, Obesity, Children, Adolescents
One of the fastest growing obesity categories in children is severe obesity, defined as an age- and sex-specific body mass index (BMI) ≥ 99th percentile. Recent data indicate a 300% increase in severe obesity in the U.S. pediatric population since 1976, with a reported prevalence of 3.8% between 1999-2004.1;2 There is a relative paucity of data in the most extreme forms of obesity. Specifically, few studies have described the cardiovascular risk factor profile, and even fewer have attempted to characterize the vascular status of severely obese children and adolescents.
Perturbation of the vascular endothelium is one of the earliest manifestations of atherosclerosis and is considered a seminal event in its initiation.3 Whole blood circulating endothelial cells (CEC) have detached from the vascular wall and are thought to reflect structural damage and injury to the endothelial layer. Higher numbers may represent more advanced damage to the vascular endothelium.4 In addition to enumeration, CEC phenotype can be characterized by quantifying the surface expression of endothelial biomarkers such as vascular cell adhesion molecule-1 (VCAM-1) to determine whether or not cells are activated (activated CEC).5 Increased numbers of CEC have been demonstrated in various vascular diseases and pathological conditions such as peripheral vascular disease,6 sickle cell anemia,7 acute myocardial infarction and angina pectoris,8 acute coronary syndrome,5 Kawasaki disease,9 systemic inflammation,10 and pulmonary hypertension.11 Importantly, CEC predict future cardiovascular events in individuals with cardiac disease, independent of conventional cardiovascular disease risk factors.12-14 Therefore, we evaluated CEC across a spectrum of adiposity in children and adolescents in order to describe the magnitude of endothelial activation in relation to obesity.
Methods
This cross-sectional study included 107 children and adolescents (age = 13.1 ± 3.8, range 6-22 years; 68 males) who were categorized (following testing) into four adiposity groups based on age- and sex-specific BMI percentiles. Participants in the normal weight BMI < 85th percentile; N = 40), overweight (BMI 85th-<95th percentile; N = 17), and obese (BMI 95th-<99th percentile; N = 23) groups were consecutively enrolled over a period of approximately one year from a longitudinal cohort study investigating the early development of obesity, insulin resistance, and other cardiovascular risk factors. The severely obese (BMI ≥ 99th percentile; N = 27) group was comprised of children and adolescents initially entering the University of Minnesota Pediatric Weight Management Clinic who were consecutively enrolled over the same period of time. No behavioral or drug therapies had yet been initiated in these individuals. All subjects in this study were invited to participate (no exclusion criteria were used). The protocol was approved by the University of Minnesota Institutional Review Board and consent/assent was obtained from parents/participants. Measures were obtained after participants had been fasting ≥10 hours.
Height and weight were obtained using a standard stadiometer and electronic scale, respectively. Waist and hip circumferences were measured to the nearest 0.5 cm. Seated blood pressure was obtained after five minutes of quiet rest, in the right arm using an automatic sphygmomanometer. Fasting lipid profile, glucose, and insulin assays were conducted with standard procedures at the Fairview Diagnostic Laboratories, Fairview-University Medical Center (Minneapolis, MN), a Centers for Disease Control and Prevention–certified laboratory.
Blood samples were collected from patients in Vacutainer tubes (BD Vacutainer Systems, Franklin Lakes, NJ) containing EDTA and were processed immediately for study. CEC analyses were performed in the University of Minnesota Vascular Biology Center as previously described in detail.7;15 For CEC enumeration we used immunohistochemical examination of buffy-coat smears prepared by centrifugation of 1 ml of whole blood placed on Histopague-1077 (Sigma). The antibodies used for staining were specific mouse anti-endothelial P1H12 (anti-CD146), with secondary anti-mouse alkaline phosphatase conjugated antibody (Jackson IRL, Westgrove, PA), and visualized using alkaline phosphatase Fast Red substrate (Vector, Burlingame, CA). Cells positive for P1H12 (CEC) on the smear were counted under a light microscope. The results were expressed as the number of CEC per 1 mL of peripheral whole blood.
The surface phenotyping was achieved by applying immunofluorescent staining to preparations of CEC. In this case, the isolation of CEC was performed by mixing whole blood with immunomagnetic beads (Dynal Oslo, Norway) coated with P1H12 antibody. The beads with CEC attached were spun down using cytospin centrifuge. The panel of antibodies used for double staining included mouse P1H12 (endothelial marker), rabbit anti-VCAM-1 (Santa Cruz, CA), anti-mouse FITC labeled and anti-rabbit TRITC labeled (both from Jackson IRL, Westgrove, PA). Nuclei were counterstained using DAPI.
Slides were viewed under a fluorescent microscope and the results represented a percentage of VCAM-1 positive (activated) CEC among the total population of CEC.
Reproducibility was assessed for CEC enumeration and activation phenotyping in a subset (N = 10) of samples from this study. The coefficients of variation for CEC number and percent activated CEC were 25% and 5%, respectively.
Means across BMI groups (normal weight, overweight, obese, severely obese) were compared with general linear model analysis (GLM procedure, SPSS version 16.0 - SPSS, Inc., Chicago, IL, USA), adjusted for sex, age, and race. The entire sample (N = 107) was grouped by SBP quartile and means across groups were compared with general linear model or Pearson correlation analysis, adjusted for sex, age, race, and BMI. Multivariate linear regression analyses, adjusted for sex, age, and race, were performed to evaluate relations of CEC number and percent of activated cells with cardiovascular risk factors in the entire sample. Statistical significance was considered p < 0.05. Data are presented as mean ± standard deviation.
Results
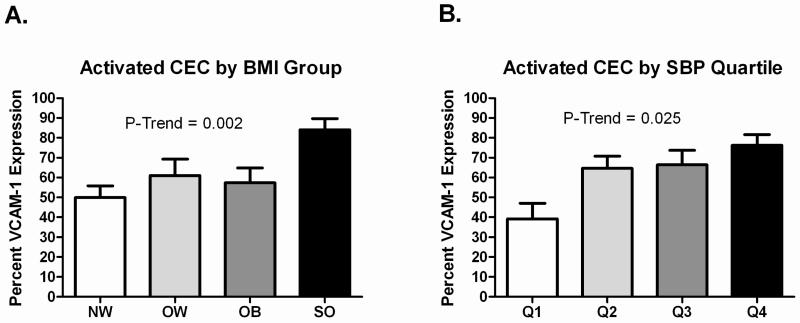
Clinical variables and CEC values by BMI groups are shown in Table I. Age and sex distribution were similar in all four groups. By design, there was a significant graded increase in BMI and waist circumference across groups. Systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, LDL-cholesterol, triglycerides, and insulin significantly increased across groups, and HDL-cholesterol significantly decreased. CEC number did not vary significantly across BMI groups, although it was highest in the severely obese group and was almost double the value of the next highest group (CEC number in the entire sample ranged from 0-4 cells per mL). Activated CEC significantly increased across groups and the highest values were observed in the severely obese group (Figure, A).
Table 1.
Clinical Variables and CEC Values by Adiposity Group
| Variable | NW (N=40) | OW (N=17) | OB (N=23) | SO (N=27) | P-Trend |
|---|---|---|---|---|---|
| Age (years) | 13.7 ± 4.3 | 13.3 ± 4.5 | 12.4 ± 3.0 | 12.7 ± 2.9 | 0.547 |
| Sex (boys, girls) | 25/15 | 12/5 | 16/7 | 15/12 | 0.685 |
| BMI (kg/m2) | 19.1 ± 2.6 | 24.0 ± 3.9 | 26.9 ± 4.4 | 38.4 ± 6.3 | <0.001 |
| Waist Circumference (cm) | 68.4 ± 8.2 | 79.6 ± 12.2 | 88.0 ± 10.0 | 118.6 ± 18.5 | <0.001 |
| SBP (mmHg) | 105.4 ± 10.3 | 106.9 ± 8.9 | 108.9 ± 12.6 | 119.9 ± 13.5 | <0.001 |
| DBP (mmHg) | 57.6 ± 9.4 | 59.7 ± 4.8 | 64.7 ± 7.8 | 65.9 ± 10.9 | 0.001 |
| Total cholesterol (mg/dL) | 144.3 ± 24.9 | 141.6 ± 21.7 | 163.1 ± 32.7 | 163.3 ± 22.3 | 0.006 |
| LDL-cholesterol (mg/dL) | 80.1 ± 20.5 | 80.4 ± 20.8 | 95.2 ± 26.1 | 99.3 ± 17.3 | 0.002 |
| HDL-cholesterol (mg/dL) | 51.0 ± 10.2 | 48.2 ± 10.3 | 47.6 ± 10.8 | 41.3 ± 10.7 | 0.002 |
| Triglycerides (mg/dL) | 65.6 ± 25.0 | 65.7 ± 35.6 | 100.4 ± 72.1 | 113.9 ± 58.1 | <0.001 |
| Glucose (mg/dL) | 81.7 ± 11.1 | 79.8 ± 11.0 | 79.0 ± 10.1 | 82.1 ± 8.5 | 0.591 |
| Insulin (mU/L) | 6.8 ± 3.6 | 8.4 ± 6.8 | 11.8 ± 9.6 | 17.1 ± 11.6 | <0.001 |
| CEC (#/mL blood) | 0.46 ± 0.88 | 0.06 ± 0.24 | 0.39 ± 0.94 | 0.85 ± 1.17 | 0.065 |
| Activated CEC (%) | 49.9 ± 35.9 | 60.9 ± 34.4 | 57.4 ± 36.0 | 84.0 ± 29.3 | 0.002 |
P-values are adjusted for sex, age, and race. Data are presented as mean ± standard deviation.
Figure.
A, Activated CEC across BMI groups in children and adolescents. B, Activated CEC by SBP Quartile in children and adolescents.
Although Table I showed some deviation from a consistent linear relation, CEC number and continuous BMI were significantly correlated (Table II), computed across the entire sample. CEC number was significantly associated with waist circumference, SBP, DBP, and triglycerides. Activated CEC were significantly correlated with continuous BMI, waist circumference, SBP, and inversely with HDL-cholesterol (Table III) across the entire sample.
Table 2.
Correlation of CEC number with Cardiovascular Risk Factors
| Variable | Correlation Coefficient | P-Value |
|---|---|---|
| BMI (kg/m2) | r = 0.21 | 0.030 |
| Waist Circumference (cm) | r = 0.24 | 0.014 |
| SBP (mmHg) | r = 0.28 | 0.005 |
| DBP (mmHg) | r = 0.25 | 0.010 |
| Total Cholesterol (mg/dL) | r = 0.17 | 0.093 |
| LDL-cholesterol (mg/dL) | r = 0.18 | 0.077 |
| HDL-cholesterol (mg/dL) | r = −0.14 | 0.176 |
| Triglycerides (mg/dL) | r = 0.20 | 0.043 |
| Glucose (mg/dL) | r = −0.03 | 0.799 |
| Insulin (mU/L) | r = 0.11 | 0.323 |
P-values are adjusted for sex, age, and race.
Table 3.
Correlation of Activated CEC with Cardiovascular Risk Factors
| Variable | Correlation Coefficient | P-Value |
|---|---|---|
| BMI (kg/m2) | r = 0.36 | <0.001 |
| Waist Circumference (cm) | r = 0.37 | <0.001 |
| SBP (mmHg) | r = 0.38 | <0.001 |
| DBP (mmHg) | r = 0.17 | 0.089 |
| Total Cholesterol (mg/dL) | r = 0.11 | 0.299 |
| LDL-cholesterol (mg/dL) | r = 0.18 | 0.070 |
| HDL-cholesterol (mg/dL) | r = −0.24 | 0.017 |
| Triglycerides (mg/dL) | r = 0.14 | 0.178 |
| Glucose (mg/dL) | r = 0.03 | 0.753 |
| Insulin (mU/L) | r = −0.04 | 0.735 |
P-values are adjusted for sex, age, and race.
Of all the cardiovascular risk factors, SBP was the most closely associated with CEC; therefore, we chose to explore this relation further by separating the entire sample (N = 107) into SBP quartiles. The analysis was adjusted for BMI to address whether associations were independent of level of adiposity. Ranges of SBP in each quartile of SBP were as follows: quartile 1 = 84-99 mmHg (N = 23); quartile 2 = 100-108 mmHg (N = 30); quartile 3 = 109-117 mmHg (N = 27); quartile 4 = 118-150 mmHg (N = 27). There were no significant differences across SBP quartile for CEC number. However, activated CEC increased across groups, independent of BMI (Figure, B).
Discussion
Despite being the fastest growing category of obesity in youth, little is known about the status of the vasculature in the context of severe pediatric obesity. Utilizing ex vivo endothelial vehicles originating from the vascular wall, we show that the endothelium is activated in the context of excess adiposity in children and adolescents, most extremely in the severely obese, and that the magnitude of endothelial activation is associated with the levels of many cardiovascular risk factors (blood pressure, triglycerides, and HDL-cholesterol). In particular, SBP, independent of level of adiposity, was significantly associated with activated CEC.
Since the endothelium is inaccessible in human subjects, non-invasive surrogate measures of endothelial function have been developed and are routinely used in studies of pediatric obesity.16-24 Despite the value of quantifying endothelial function as a metric of vascular risk, more direct measures of endothelial cell biology may offer an opportunity to explore the status of the endothelium at a different level. Since they originate from the vessel wall, CEC may be the closest representation of in situ endothelial biology that can be obtained non-invasively in human subjects. Our data, demonstrating associations of CEC with adiposity and multiple cardiovascular risk factors (particularly SBP) in children and adolescents, provide initial support for their potential use in pediatric research studies as a method of quantifying endothelial activation.
Our finding of substantial endothelial activation in severely obese children are in line with reports suggesting that the number and levels of cardiovascular risk factors are considerably higher in severe pediatric obesity compared with those associated with milder forms of obesity.25-27 Most notably, data from the Bogalusa Heart Study showed that 19% of overweight children (BMI ≥85th-94th percentile) had at least two cardiovascular disease risk factors compared with 39% of children classified as obese (BMI ≥95th percentile).27 Strikingly, this percentage jumped to 59% in children classified as severely obese (BMI ≥99th percentile). Moreover, approximately 84% of the severely obese children in this study had at least one cardiovascular disease risk factor. To our knowledge, the only study to evaluate vascular structure and function in severely obese children showed that FMD was lower, and carotid artery stiffness was higher, in severely obese versus lean controls.18 However, no comparisons were made with overweight and/or obese children to establish the magnitude of vascular risk in the context of severe pediatric obesity compared with milder forms of excess adiposity.
Although mean levels of activated CEC were higher in the overweight and obese groups compared with the normal weight controls, a markedly higher level was observed in the severely obese group. Indeed, the level of activated CEC in this group was similar to levels previously reported in adult patients with sickle cell anemia (84% vs. 85%, respectively), a condition known to be associated with extreme endothelial activation.7 Although our results need to be confirmed in future studies with larger sample sizes, the current data suggest that severe pediatric obesity may be a state of advanced endothelial cell activation, particularly in the presence of elevated SBP. These findings should be considered in light of the fact that most severely obese children become morbidly obese adults.27 At this time it is unclear what mechanisms may be mediating the increased activation of CEC in pediatric obesity. However, the significant correlations of activated CEC with SBP and HDL cholesterol suggest that mechanical stress (SBP) and dyslipidemia (HDL cholesterol) could potentially be mediating factors.
Some limitations of the current study merit discussion. Our study was cross-sectional in design; therefore, we were not able to address the potential role of CEC in risk prediction and possible association with cardiovascular outcomes. We did not perform formal power calculations because, according to our knowledge, CEC have not been adequately evaluated in children. A larger sample size would have provided greater statistical power. We did not perform Tanner stage assessments and therefore cannot confirm that pubertal development was equivalent across groups. No assessments of dietary intake or physical activity patterns were performed. It is likely that behavioral factors differed across groups. Together, the aforementioned issues may explain some of the variability observed in the measures (particularly CEC number).
CEC may be a useful vascular endpoint in pediatric studies. Further work will be required to define pediatric norms for CEC and to evaluate CEC in relation to more widely used vascular methods such as brachial artery FMD, measures of arterial stiffness, and quantification of carotid artery intima-media thickness.
Acknowledgments
We are grateful to Ms. Maria Gomez for her expert analysis of CEC and to the participants who donated their time to this study.
Funding was provided in part by University of Minnesota Vikings Children's Fund (A.K.), Minnesota Medical Foundation (A.K.), National Institutes of Health (P01 HL55552 to R.H.), National Institutes of Health (1RO1DK072124-01A3 to J.S.), and GCRC (M01-RR00400), General Clinical Research Center Program, NCRR/NIH. The authors declare no conflicts of interest.
Abbreviations
- CEC
circulating endothelial cells
- BMI
body mass index
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- FMD
flow-mediated dilation
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr. 2004;80:569–575. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- 2.Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and Trends of Severe Obesity Among US Children and Adolescents. Acad Pediatr. 2009 doi: 10.1016/j.acap.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 4.Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 5.Mutin M, Canavy I, Blann A, Bory M, Sampol J, gnat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93:2951–2958. [PubMed] [Google Scholar]
- 6.Makin AJ, Blann AD, Chung NA, Silverman SH, Lip GY. Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur Heart J. 2004;25:371–376. doi: 10.1016/j.ehj.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- 8.Hladovec J, Prerovsky I, Stanek V, Fabian J. Circulating endothelial cells in acute myocardial infarction and angina pectoris. Klin Wochenschr. 1978;56:1033–1036. doi: 10.1007/BF01476669. [DOI] [PubMed] [Google Scholar]
- 9.Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Tokutomi T, Sekine I. Circulating endothelial cells in Kawasaki disease. Clin Exp Immunol. 2003;131:536–540. doi: 10.1046/j.1365-2249.2003.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George F, Brouqui P, Boffa MC, Mutin M, Drancourt M, Brisson C, et al. Demonstration of Rickettsia conorii-induced endothelial injury in vivo by measuring circulating endothelial cells, thrombomodulin, and von Willebrand factor in patients with Mediterranean spotted fever. Blood. 1993;82:2109–2116. [PubMed] [Google Scholar]
- 11.Bull TM, Golpon H, Hebbel RP, Solovey A, Cool CD, Tuder RM, et al. Circulating endothelial cells in pulmonary hypertension. Thromb Haemost. 2003;90:698–703. doi: 10.1160/TH03-04-0251. [DOI] [PubMed] [Google Scholar]
- 12.Koc M, Richards HB, Bihorac A, Ross EA, Schold JD, Segal MS. Circulating endothelial cells are associated with future vascular events in hemodialysis patients. Kidney Int. 2005;67:1078–1083. doi: 10.1111/j.1523-1755.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee KW, Lip GY, Tayebjee M, Foster W, Blann AD. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood. 2005;105:526–532. doi: 10.1182/blood-2004-03-1106. [DOI] [PubMed] [Google Scholar]
- 14.Quilici J, Banzet N, Paule P, Meynard JB, Mutin M, Bonnet JL, et al. Circulating endothelial cell count as a diagnostic marker for non-ST-elevation acute coronary syndromes. Circulation. 2004;110:1586–1591. doi: 10.1161/01.CIR.0000142295.85740.98. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, Weghuber D, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26:2541–2546. doi: 10.1161/01.ATV.0000245795.08139.70. [DOI] [PubMed] [Google Scholar]
- 17.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 18.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 19.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 20.Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008;29:792–799. doi: 10.1093/eurheartj/ehm633. [DOI] [PubMed] [Google Scholar]
- 21.Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger J, Bank AJ, Dengel DR. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr. 2004;145:731–736. doi: 10.1016/j.jpeds.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr. 2008;152:177–184. doi: 10.1016/j.jpeds.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 23.Rocchini AP, Moorehead C, Katch V, Key J, Finta KM. Forearm resistance vessel abnormalities and insulin resistance in obese adolescents. Hypertension. 1992;19:615–620. doi: 10.1161/01.hyp.19.6.615. [DOI] [PubMed] [Google Scholar]
- 24.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 25.Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia, and respiratory compromise. J Pediatr. 2004;144:766–769. doi: 10.1016/j.jpeds.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 27.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]