Abstract
Background
Almost half of women treated with aromatase inhibitors (AI) develop AI-associated musculoskeletal symptoms (AIMSS) such as arthralgias, but the etiology is unclear. The upper extremities are frequently affected, especially wrists, hands, and fingers. AI use may also increase the risk of developing carpal tunnel syndrome. Tendon sheath fluid and tenosynovial changes have been demonstrated by imaging symptomatic AI-treated patients. We hypothesized that these abnormalities correlate with AIMSS.
Methods
Thirty consecutive patients initiating adjuvant therapy with letrozole or exemestane on a prospective clinical trial enrolled in a pilot study evaluating tendon and joint abnormalities at baseline and after 3 months of AI therapy. Patients underwent high resolution ultrasonography of the wrists bilaterally and completed the Health Assessment Questionnaire (HAQ) and pain Visual Analog Scale (VAS). AIMSS were defined as increase in HAQ or VAS score during AI therapy that exceeded a predefined cutoff.
Results
Twenty-five subjects completed both baseline and 3 month assessments. During the first 12 months of AI therapy, 15 subjects developed AIMSS, and 13 discontinued therapy because of musculoskeletal symptoms. There was a trend toward an association between presence of tendon sheath abnormalities on wrist ultrasound at baseline and development of AIMSS (p=0.06).
Conclusions
Clinically relevant musculoskeletal symptoms develop in AI-treated women, leading to treatment discontinuation in a substantial percentage. However, patient-reported symptoms were not associated with changes visible on wrist ultrasonography in this pilot study.
Keywords: breast cancer, aromatase inhibitor, arthralgia, ultrasonography, musculoskeletal
Introduction
Aromatase inhibitors (AI) substantially decrease circulating estrogen concentrations in postmenopausal women via blockade of aromatase, the enzyme responsible for the conversion of androgens to estrogens. This class of drugs is increasingly used for the adjuvant treatment of postmenopausal women with hormone receptor-positive invasive breast cancer, in part because of data to support their superiority compared to tamoxifen, a selective estrogen receptor modulator.1–9 Although AIs were reported to be relatively well tolerated in the large prospective randomized clinical trials,4, 8, 10, 11 more recent studies have demonstrated that up to half of treated patients develop AI-associated musculoskeletal symptoms (AIMSS) such as arthralgias, joint stiffness, and carpal tunnel syndrome (CTS), which can lead to treatment discontinuation.12–15 A previous study found that symptoms of AIMSS appear, on average, within 3 months of initiation of AI therapy.14
The pathophysiology of AIMSS remains unclear. Previously reported studies using magnetic resonance imaging (MRI) have demonstrated tenosynovitis in a substantial proportion of patients with severe wrist symptoms.16 A prospective study of 12 AI treated women demonstrated a high incidence of MRI-detected wrist tenosynovitis after 6 months of AI therapy, and new abnormalities on imaging studies were associated with a decrease in grip strength.17 However, in these studies, associations between the objective findings and the development of patient-reported musculoskeletal symptoms were not evaluated.
High resolution ultrasound is an effective and readily accessible tool that identifies abnormalities of the tendon sheath, can quantify changes in the median nerve in CTS,18 and may be applicable for the evaluation of musculoskeletal changes that occur with AI therapy. The objective of this pilot study was to prospectively evaluate the anatomy of the wrist using serial high resolution ultrasonography in women initiating treatment with AIs as part of their adjuvant breast cancer treatment plan. We hypothesized that the presence of ultrasound-detected abnormalities of the wrist prior to treatment initiation, or their appearance during AI therapy, may correlate with development of AI-associated musculoskeletal symptoms.
Methods
Subjects
Women who enrolled in the multicenter prospective randomized Exemestane and Letrozole Pharmacogenomics (ELPh) clinical trial (ClinicalTrials.gov #NCT00228956) between October 2007 and September 2008 at the University of Michigan Comprehensive Cancer Center were offered concomitant enrollment in this ultrasound substudy. As per the parent study, patients were randomized to exemestane (Aromasin, Pfizer, New York, NY) 25 mg orally daily or letrozole (Femara, Novartis, East Hanover, NJ) 2.5 mg orally daily. Key eligibility criteria included female gender, postmenopausal status, age ≥ 18 years, early stage hormone receptor positive breast cancer, and intent to initiate adjuvant therapy with an AI. No prior AI therapy was permitted. All indicated surgery, radiation therapy, and chemotherapy for breast cancer was completed prior to study enrollment. This substudy excluded patients with a history of prior significant wrist injury, wrist surgery, or carpal tunnel syndrome. The protocol was approved by the Institutional Review Board, and all enrolled patients provided written informed consent.
Study Design and Interventions
This prospective study of high resolution wrist ultrasonography was conducted as a substudy of the ELPh trial. The study design of the ELPh trial has previously been reported.14 Briefly, patients were evaluated at baseline, and after 1, 3, 6, and 12 months of AI therapy to assess changes in medical history and concomitant medications, and to complete symptom questionnaires and the modified Health Assessment Questionnaire (HAQ),19 which includes a pain Visual Analog Scale (VAS).
AIMSS was previously reported to develop in the majority of AI-treated patients within 3 months of initiation of therapy.14 Therefore, all patients in this substudy also completed the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire20 and underwent evaluation of the bilateral wrists with high resolution ultrasonography using 12 – 17 Mhz linear transducers (Model iU22, Philips, Bothell, WA) at baseline and after 3 months of AI therapy.
Definition of AIMSS
In the ELPh trial, AIMSS was defined as meeting one or more of the following criteria at any point during study participation: 1) HAQ score increased by more than 0.4 over baseline score, 2) Pain VAS value ≥ 5 cm (of 10 cm VAS) for patients with no pain (VAS = 0) at baseline, and 3) Pain VAS value increased and pain rated much worse or very much worse pain on self-rated clinical global impression scale (graded as 6 or 7)21 for patients reporting pain at baseline (VAS > 0).14
Wrist ultrasonography
Patients underwent baseline and 3 month bilateral wrist high resolution ultrasound imaging. The ultrasound examination included static images and video clips, as well as a written report of imaging findings. Imaging data included: 1) presence or absence of fluid (compressible anechoic or hypoechoic distention), hyperemia on color or power Doppler imaging, or synovitis (non-compressible, hypoechoic distention) in the volar or dorsal joint spaces (distal radioulnar, radiocarpal, and midcarpal), 2) presence or absence of fluid, hyperemia, or synovitis in the flexor or extensor tendon sheaths, 3) presence or absence of tendinosis (hypoechoic and enlarged), full thickness tear (complete discontinuity), or partial thickness tear (partial anechoic cleft) in the flexor or extensor tendons, 4) median nerve area (mm2), measured at the wrist crease (using circumferential trace) at the level of the pisiform, and 5) presence of subcutaneous edema (hypoechoic, linear, and branching distended lymphatics). Images were analyzed in a blinded manner by an academic musculoskeletal radiologist after all patients had undergone both evaluations.
Statistical analyses
The primary objective of this study was to estimate the 1) incidence of tendon sheath, joint space, and tendon abnormalities and 2) increase in median nerve cross-sectional area prior to and 3 months following initiation of AI therapy, and to quantify those changes based on high-resolution ultrasonography of the wrist. The secondary objective was to evaluate associations between objective, ultrasound-detected changes in the median nerve and tendon sheaths and treatment-emergent musculoskeletal symptoms.
For analysis, the ultrasound abnormalities were grouped into those affecting the tendon, the tendon sheath, and the joint space. Change in average median nerve area prior to and 3 months following initiation of AI therapy was tested using paired t-test. Univariate associations between baseline findings and development of AIMSS or early treatment discontinuation were tested using chi-square tests. Logistic regression was used to model the probability of developing AIMSS or early treatment discontinuation (two separate models) as a function of baseline characteristics and change in VAS, HAQ, and DASH scores between baseline and 3 months. A p value of <0.05 was considered statistically significant.
Results
Patient characteristics
Characteristics of the 29 eligible subjects are listed in Table 1. One patient was found to be ineligible for the parent study because she was not postmenopausal, and was excluded from all analyses. Of the 29 eligible patients, 25 underwent repeat evaluation at 3 months. Two patients discontinued AI therapy prior to the 3 month study visit because of musculoskeletal symptoms. One developed intolerable aches and pains, and the other had fatigue, headache, and myalgias. Of the other two subjects who did not undergo ultrasonography at the 3 month timepoint, one had been non-compliant with study procedures and was taken off study, and one developed fatigue, headaches, and inability to concentrate and discontinued AI therapy.
Table 1.
Patient characteristics. Values are given for patient-reported outcomes at baseline and after 3 months of AI therapy
| Characteristic | Baseline N = 29 |
3 Months N = 25 |
P value | |
|---|---|---|---|---|
| Median age (range) | 61 (47–83) | 59 (47–83) | ||
| Race/ethnicity | Non-Hispanic White | 25 (86%) | 22 (88%) | |
| Hispanic White | 2 (7%) | 1 (4%) | ||
| Black | 1 (3%) | 1 (4%) | ||
| Asian | 1 (3%) | 1 (4%) | ||
| BMI | <25 kg/m2 | 9 (31%) | 8 (32%) | |
| 25–30 kg/m2 | 4 (14%) | 4 (16%) | ||
| >30 kg/m2 | 16 (55%) | 13 (52%) | ||
| Prior chemotherapy | Any | 15 (52%) | 13 (52%) | |
| Taxane | 12 (41%) | 11 (44%) | ||
| Prior tamoxifen | 10 (34%) | 9 (36%) | ||
| Median HAQ score (range) |
0 (0–0.625) | 0 (0–0.750) | 0.97 | |
| Median VAS (range) | 2.8 (0–8.1) | 2.6 (0–5.7) | 0.49 | |
| Median DASH (range) | 5.8 (0–44.8) | 6.9 (0–55) | 0.95 | |
| Patient-reported joint pain |
None | 11 (38%) | 7 (28%) | |
| Mild | 8 (28%) | 7 (28%) | 0.41 | |
| Moderate/Severe | 10 (34%) | 11 (44%) | ||
| Patient-reported neuropathy |
None | 13 (45%) | 16 (64%) | |
| Mild | 11 (38%) | 4 (16%) | 0.44 | |
| Moderate/Severe | 5 (17%) | 5 (20%) | ||
Wrist Ultrasonography Findings
Abnormalities on the baseline high resolution wrist ultrasound were common, especially in the joint recess (Table 2; representative finding shown in Figure 1A). Almost half of subjects developed new findings on ultrasound after 3 months of AI therapy, also primarily located in the joint recess (Table 3; representative finding shown in Figure 1B). Abnormalities of the tendon itself were noted rarely. Change in mean median nerve area with 3 months of AI therapy was not statistically different (10.4 ± 2.2 mm at baseline versus 10.8 ± 2.5 mm at 3 months).
Table 2.
Proportion of subjects (n=29) with abnormalities on wrist ultrasonography prior to initiation of AI therapy. Numbers do not necessarily add up to totals because some subjects had more than one abnormality present (e.g., both fluid and synovitis of the extensor tendon sheath).
| Site | # with abnormalities unilaterally (%) |
# with abnormalities bilaterally (%) |
Total # with abnormalities (%) |
Total # with abnormalities (%) |
|---|---|---|---|---|
| Tendon sheath (flexors) | 3 (10.3%) | 1 (3.4%) | 4 (13.8%) | 14 (48.3%) |
| - Fluid | 3 (10.3%) | 1 (3.4%) | 4 (13.8%) | |
| - Synovitis | 0 | 0 | 0 | |
| - Hyperemia | 0 | 0 | 0 | |
| Tendon sheath (extensors) | 6 (20.7%) | 5 (17.2%) | 11 (37.9%) | |
| - Fluid | 6 (20.7%) | 5 (17.2%) | 11 (37.9%) | |
| - Synovitis | 1 (3.4%) | 0 | 1 (3.4%) | |
| - Hyperemia | 1 (3.4%) | 0 | 1 (3.4%) | |
| Tendon (flexor + extensor) | 1 (3.4%) | 1 (3.4%) | 2 (6.9%) | 2 (6.9%) |
| - Tendinosis | 1 (3.4%) | 1 (3.4%) | 2 (6.9%) | |
| - Partial thickness tear | 0 | 0 | 0 | |
| - Full thickness tear | 0 | 0 | 0 | |
| Joint recess (volar) | 6 (20.7%) | 7 (24.1%) | 12 (41.4%) | 24 (82.8%) |
| - Fluid | 4 (13.8%) | 6 (20.7%) | 10 (34.5%) | |
| - Synovitis | 2 (6.9%) | 3 (10.3%) | 5 (17.2%) | |
| - Hyperemia | 0 | 0 | 0 | |
| Joint recess (dorsal) | 12 (41.4%) | 18 (62.3%) | 24 (82.8%) | |
| - Fluid | 5 (17.2%) | 16 (55.2%) | 21 (72.4%) | |
| - Synovitis | 4 (13.8%) | 9 (31.0%) | 13 (44.8%) | |
| - Hyperemia | 5 (17.2%) | 2 (6.9%) | 7 (24.1%) | |
Figure 1.
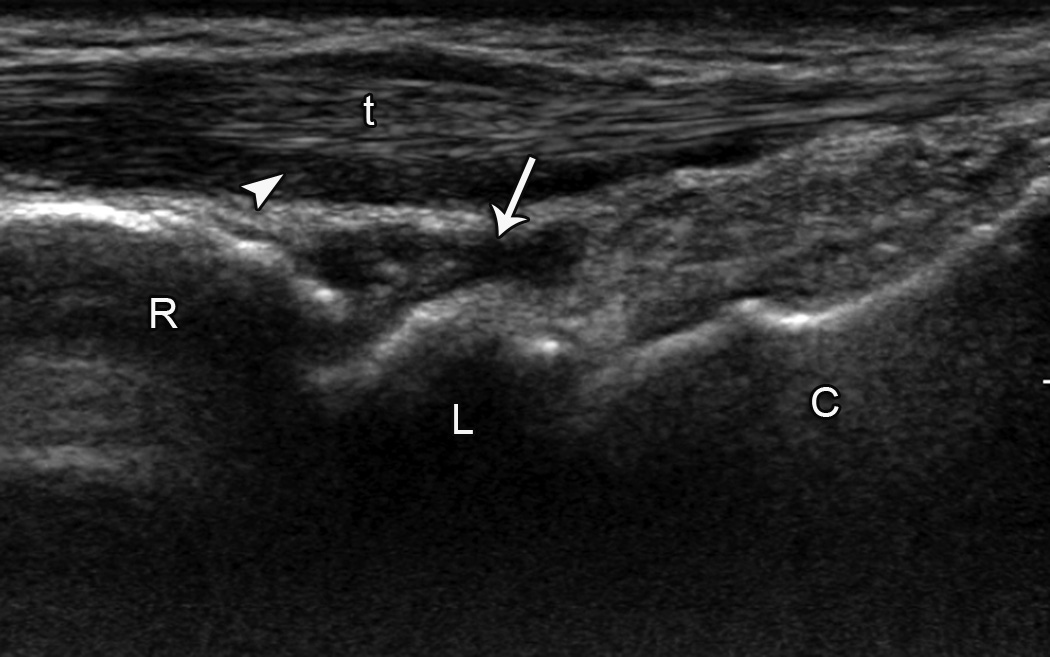
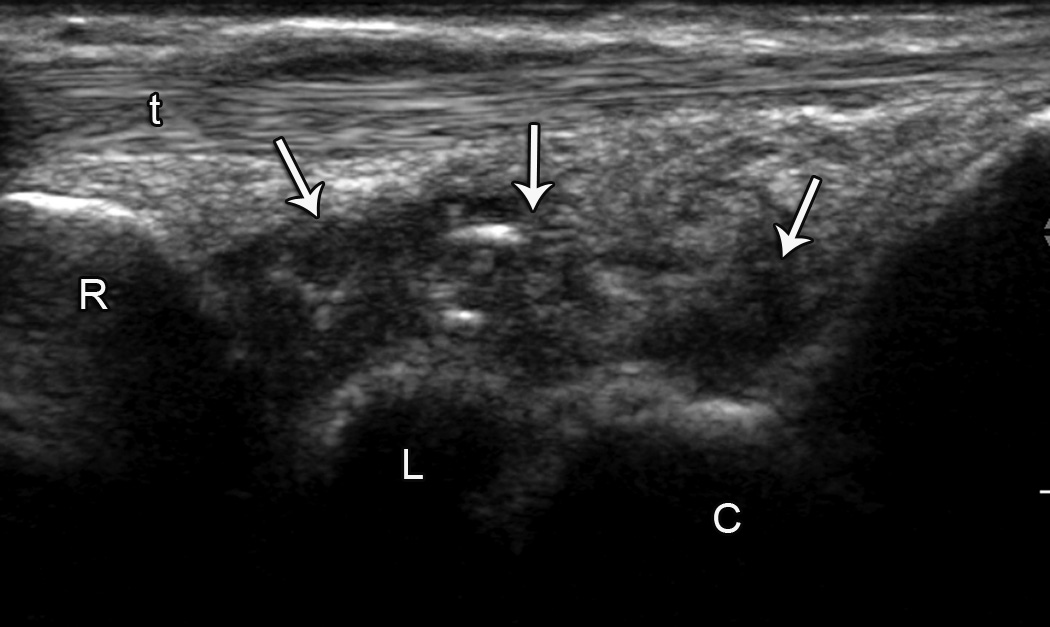
Joint recess and extensor tendon abnormalities. A. Baseline sagittal ultrasound image over dorsal wrist shows hypoechoic fluid distending the (arrow) dorsal recess of the radiocarpal joint and (arrowhead) surrounding the extensor tendon. B. Three month sagittal ultrasound image over dorsal wrist shows heterogeneous hypoechoic fluid and synovitis distending the (arrows) dorsal recesses of the radiocarpal and midcarpal joints. (C = capitate, L = lunate, R = radius, t = extensor tendon, right side of image is distal).
Table 3.
Proportion of subjects (n=25) with new abnormalities on ultrasonography after 3 months of AI therapy
| Site | # with abnormalities unilaterally (%) |
# with abnormalities bilaterally (%) |
Total # with abnormalities (%) |
Total # with abnormalities (%) |
|---|---|---|---|---|
| Tendon sheath (flexors) | 1 (4%) | 0 | 1 (4%) | 5 (20%) |
| - Fluid | 1 (4%) | 0 | 1 (4%) | |
| - Synovitis | 0 | 0 | 0 | |
| - Hyperemia | 0 | 0 | 0 | |
| Tendon sheath (extensors) | 4 (16%) | 0 | 4 (16%) | |
| - Fluid | 4 (16%) | 0 | 4 (16%) | |
| - Synovitis | 1 (4%) | 0 | 1 (4%) | |
| - Hyperemia | 0 | 0 | 0 | |
| Tendon (flexor + extensor) | 0 | 0 | 0 | 0 |
| - Tendinosis | 0 | 0 | 0 | |
| - Partial thickness tear | 0 | 0 | 0 | |
| - Full thickness tear | 0 | 0 | 0 | |
| Joint recess (volar) | 3 (12%) | 3 (12%) | 5 (20%) | 12 (48%) |
| - Fluid | 3 (12%) | 2 (8%) | 5 (20%) | |
| - Synovitis | 1 (4%) | 2 (8%) | 3 (12%) | |
| - Hyperemia | 0 | 0 | 0 | |
| Joint recess (dorsal) | 7 (28%) | 4 (16%) | 11 (44%) | |
| - Fluid | 1 (4%) | 4 (16%) | 5 (20%) | |
| - Synovitis | 5 (20%) | 2 (8%) | 7 (28%) | |
| - Hyperemia | 2 (8%) | 0 | 2 (8%) | |
Changes in Patient-Reported Symptoms
After three months of AI therapy, 72% of subjects reported joint pain, with 44% of the total study population reporting moderate to severe pain (Table 1). Median scores on the HAQ and VAS for all subjects were unchanged between baseline and 3 months. Of note, half of the study patients reported pre-existing neuropathy, primarily of mild severity, and this symptom decreased during AI therapy. Since subjects with carpal tunnel syndrome were excluded from participation, baseline neuropathy symptoms were likely due to alternate etiologies.
Subjects were followed on AI therapy until treatment discontinuation or for at least 1 year. Fifteen subjects (51.7% of eligible cohort) developed AIMSS after a median of 6.2 months of AI therapy (range 1.2–18.6 months). Thirteen subjects (44.8% of eligible cohort) discontinued AI therapy because of musculoskeletal symptoms after a median duration of therapy of 10.7 months (range 0.9–21.2 months). The majority of subjects subsequently switched to a different AI therapy. One additional patient discontinued AI therapy after the 3 month timepoint because of intractable diarrhea.
Association between Baseline Findings and AIMSS or Early Treatment Discontinuation
On univariate analysis, higher body mass index was associated with decreased risk of early treatment discontinuation (Odds Ratio (OR) across BMI groups 0.16 (95% confidence interval (C.I.) 0.03–0.84), p=0.03; Table 4). There was a trend toward prior tamoxifen usage and lower risk of early treatment discontinuation (OR 0.05 (95% C.I. 0.002–1.0), p=0.05). No other significant associations were noted between baseline patient characteristics or questionnaire scores and either development of AIMSS or early treatment discontinuation because of musculoskeletal symptoms (Table 4). There was a trend toward an association between presence of tendon sheath abnormalities on ultrasound at baseline and subsequent development of AIMSS (Figure 2A). Ten of the 15 subjects (67%) who developed AIMSS during 12 months of AI therapy had tendon sheath abnormalities at baseline, compared to 4 of 14 (29%) who did not develop AIMSS (p=0.06). No statistically significant associations between baseline ultrasound findings and either development of AIMSS (Figure 2A) or early treatment discontinuation (Figure 2B) were noted.
Table 4.
Associations between subject characteristics and patient-reported outcomes and a) development of AIMSS and b) early treatment discontinuation due to musculoskeletal symptoms.
| Development of AIMSS |
Early Treatment Discontinuation |
|||
|---|---|---|---|---|
| Baseline Characteristic | Odds ratio (95% CI) |
P value |
Odds ratio (95% CI) |
P value |
| Age (<55 vs ≥55) | 0.18 (0.01–3.1) | 0.24 | 0.23 (0.01–4.2) | 0.32 |
| BMI (<25, 25–30, >30) | 0.43 (0.11–1.7) | 0.22 | 0.16 (0.03–0.84) | 0.03 |
| Prior tamoxifen (no, yes) | 0.21 (0.02–3.1) | 0.26 | 0.05 (0.002–1.0) | 0.05 |
| Prior chemotherapy (no, yes) | 0.26 (0.04–1.9) | 0.19 | 0.19 (0.02–1.74) | 0.14 |
| HAQ score | 0.59 (0.31–1.1) | 0.11 | 0.73 (0.38–1.43) | 0.36 |
| VAS score | 1.26 (0.77–2.1) | 0.36 | 0.90 (0.52–1.55) | 0.70 |
| DASH score | 1.04 (0.92–1.2) | 0.52 | 1.09 (0.96–1.25) | 0.17 |
| Change in Measurement (baseline and 3 mo) |
||||
| HAQ | 1.15 (0.57–2.3) | 0.70 | 1.39 (0.68–2.88) | 0.37 |
| VAS | 1.56 (0.42–5.8) | 0.51 | 1.43 (0.46–4.48) | 0.54 |
| DASH | 1.05 (0.91–1.2) | 0.52 | 1.09 (0.93–1.29) | 0.28 |
| Change in Measurement (baseline and 12 mo) |
||||
| HAQ | 2.76 (0.3–26.6) | 0.38 | 6.02 (0.4–83.8) | 0.18 |
| VAS | 2.11 (1.1–4.2) | 0.03 | 2.12 (1.1–4.1) | 0.02 |
Figure 2.
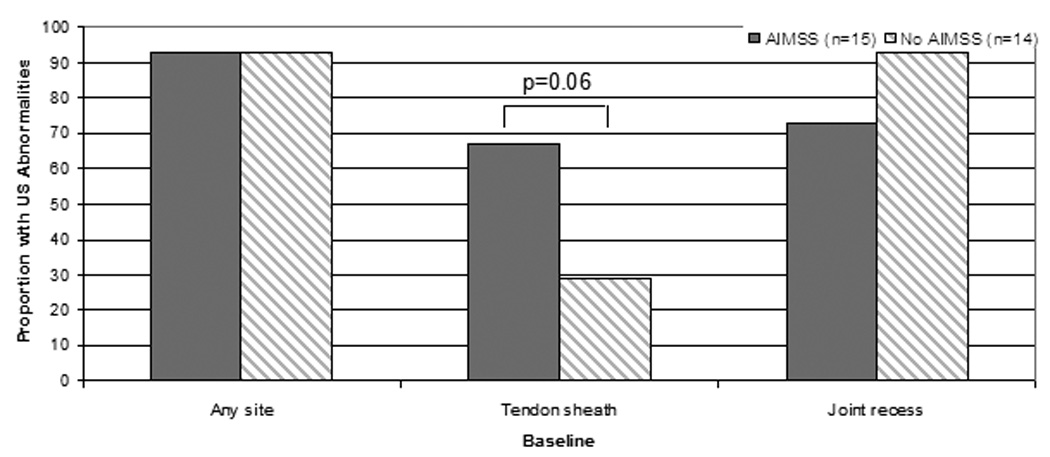
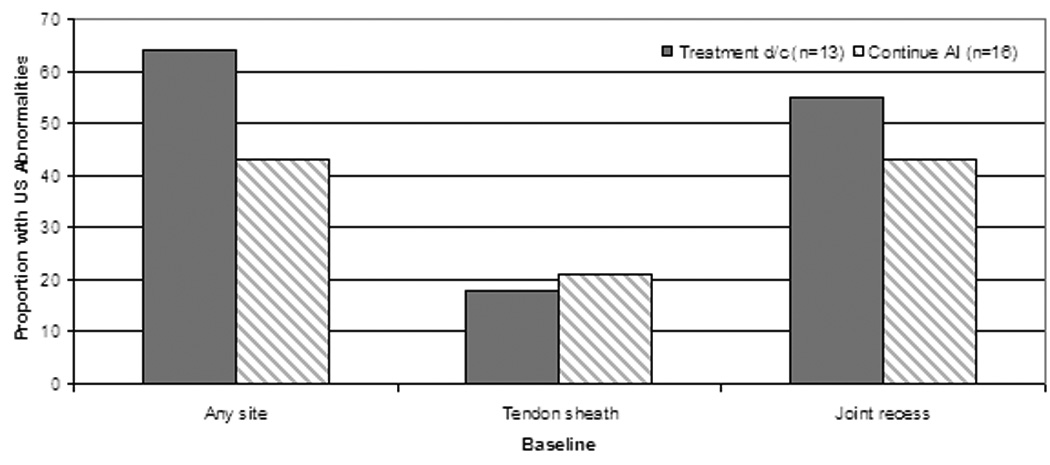
Association between presence of ultrasonographic (US) abnormalities at baseline and treatment-emergent AI-musculoskeletal symptoms (AIMSS) (A) and early treatment discontinuation due to musculoskeletal symptoms (B). D/c = discontinue, n = number. All comparisons are non-statistically significant.
Association between Change in Findings after 3 Months of AI Therapy and AIMSS or Early Treatment Discontinuation
No associations were noted between change from baseline to 3 months of AI therapy in HAQ, VAS, or DASH scores and development of AIMSS or early treatment discontinuation (Table 4). Similar to the findings at baseline, we did not observe statistically significant associations between new findings on ultrasound and either development of AIMSS or early treatment discontinuation (Figure 3).
Figure 3.
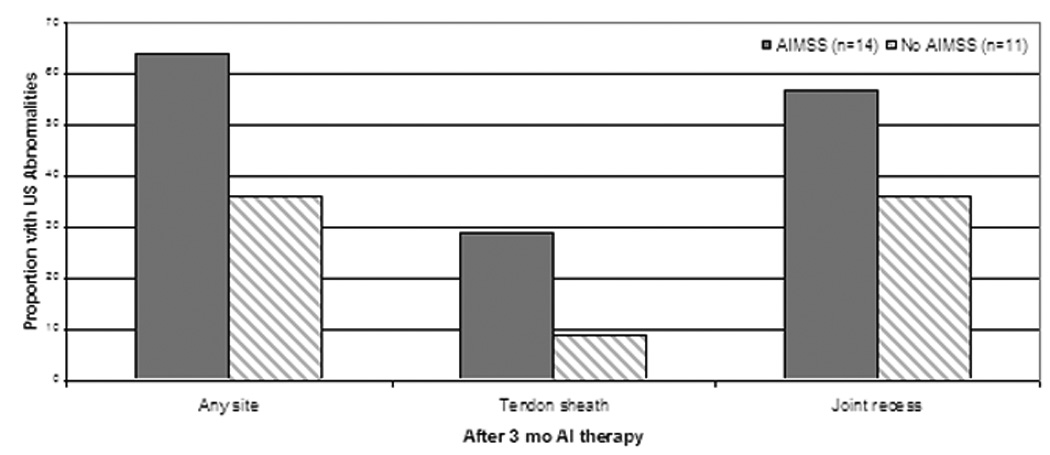
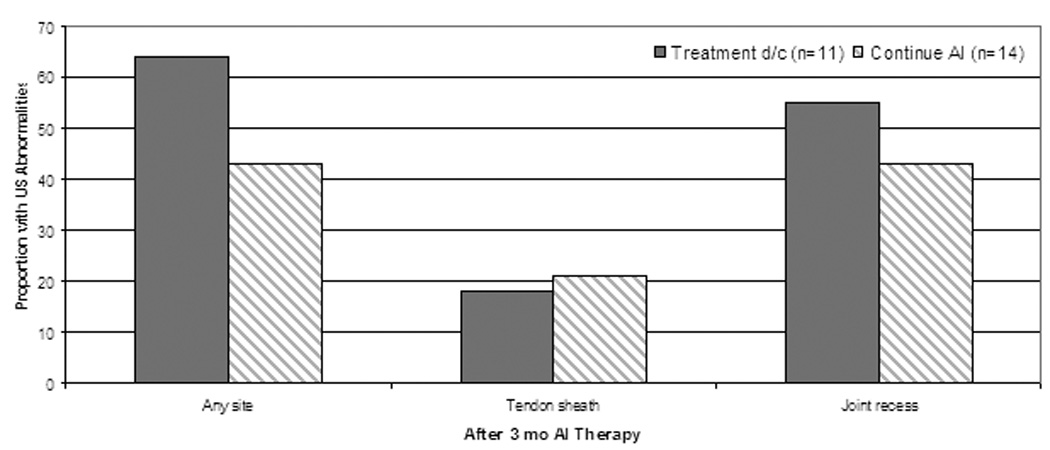
Association between percentage of new ultrasonographic (US) abnormalities after 3 months of AI therapy and treatment-emergent AI-musculoskeletal symptoms (AIMSS) (A) and early treatment discontinuation due to musculoskeletal symptoms (B). D/c = discontinue, n = number. All comparisons are non-statistically significant.
Although a previous analysis that suggested that the majority of AI-treated subjects develop symptoms within 3 months of treatment initiation, the median time to development of AIMSS in the cohort enrolled in this substudy was 6.2 months. Subjects enrolled in this substudy completed the HAQ and VAS questionnaires at multiple additional timepoints as part of the parent study. Therefore, we performed an unplanned analysis of the correlation between development of AIMSS and early treatment discontinuation and change in HAQ and VAS from baseline to either 12 months or the time of early treatment discontinuation, whichever came first. We found a significant association between change in VAS and development of AIMSS (OR 2.11, 95% CI 1.1–4.2; p=0.03) and early treatment discontinuation (OR 2.12, 95% CI 1.1–4.1; p=0.02). A similar finding was not noted with change in HAQ (data not shown).
Discussion
In this study, high resolution ultrasound-detected abnormalities of the tendon sheath and joint space at the bilateral wrist were very common in women prior to initiation of AI therapy. We observed a trend toward an association between tendon sheath abnormalities on ultrasonography prior to treatment initiation and development of AIMSS. Identification of factors predictive of increased likelihood of development of AIMSS would be useful for counseling and managing patients initiating treatment with adjuvant endocrine therapy. Although this study does not provide new insight into the mechanism of AIMSS, our results suggest that pre-existing abnormalities within the tendon sheath may predict which women are likely to experience AI-associated musculoskeletal pain.
Our findings are similar to those previously reported by others, the majority of which have been cross-sectional studies.16, 17, 22 A strength of our study is the prospective, serial assessment of subjects using both high-resolution ultrasound and patient self-report questionnaires.
A higher than anticipated number of subjects discontinued therapy because of musculoskeletal symptoms, possibly due to chance in this small patient cohort, or possibly because they were enrolled in a clinical trial with a specific focus on musculoskeletal symptoms, which may have led to greater patient- and physician-awareness of toxicity. Similar to other reports, subjects in this cohort who were previously treated with tamoxifen were less likely to discontinue AI therapy.13 Contrary to other reports,12, 13 however, there was an association between obesity and decreased risk of treatment discontinuation due to musculoskeletal symptoms.
A limitation of this study is the lack of multiple, long term ultrasound assessments during AI therapy. Subjects were assessed after 3 months of therapy, but the median time to reported AI-associated musculoskeletal symptoms in this cohort was approximately 6 months. Therefore, it is possible that we were unable to detect a statistically significant association between ultrasound-detected abnormalities and patient-reported symptoms because of insufficient timepoint assessments. This restriction to analysis of the data from the 3 month timepoint may also have led to the lack of noted association between change in HAQ and VAS score and development of AIMSS or early treatment discontinuation, which is also supported by our finding of a statistically significant association between change in VAS between baseline and 12 months and both development of AIMSS and early treatment discontinuation. A second limitation of this study is the relatively small subject cohort, which was further decreased by early treatment discontinuation by 5 subjects, which reduced statistical power.
Another possible limitation is the use of ultrasonography as opposed to MRI. One potential criticism of ultrasound is operator-dependent variability. In this study we attempted to decrease this variability as much as possible through the collection and storage of both static images and cine clips for each subject at each timepoint, for later analysis by a single blinded reviewer. Advantages of high-resolution ultrasound include lower cost, shorter evaluation time, and lack of factors which can preclude assessment with MRI, such as claustrophobia and presence of metal in the body.
In summary, the findings in this pilot study suggest that presence of abnormalities of the wrist tendon sheath prior to AI initiation may predict for subsequent development of AI-associated musculoskeletal symptoms both at the wrist and at other anatomic sites. Future studies are warranted to confirm the trend noted in this pilot study, and to further elucidate the predictive role for imaging when making treatment decisions about adjuvant endocrine therapy.
Acknowledgments
Sources of support:
Pharmacogenetics Research Network Grant # U-01 GM61373 from the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, MD (IU); Grant Number M01-RR000042 to UM from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Grants from Pfizer (DFH), Novartis Pharma AG (DFH), Barbara Padnos Breast Cancer Research Fund, University of Michigan Comprehensive Cancer Center (NLH) and Expedition Inspiration Fund for Breast Cancer Research (NLH)
Footnotes
ClinicalTrials.gov number: NCT00228956
Financial disclosures:
Research funding: Pfizer (DFH, VS, AMS), Novartis (DFH, VS, AMS), AstraZeneca (NLH, DFH)
Honoraria: AstraZeneca (VS)
Other support (equipment): Philips (JJ), SonoSite (JJ), Terason (JJ)
Consultant: BioClinica (JJ)
Royalties (book): Elsevier (JJ)
References
- 1.Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 2.Boccardo F, Rubagotti A, Puntoni M, Guglielmini P, Amoroso D, Fini A, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole trial. J Clin Oncol. 2005;23:5138–5147. doi: 10.1200/JCO.2005.04.120. [DOI] [PubMed] [Google Scholar]
- 3.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA. 17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 5.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 6.Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–1971. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 7.Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 8.Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 9.Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Austrian Breast and Colorectal Cancer Study Group Trial 6a. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized 6a. J Natl Cancer Inst. 2007;99:1845–1853. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]
- 10.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 11.Coombes RC, Paridaens R, Jassem J, Van de Velde CJ, Delozier T, Jones SE, et al. First mature analysis of the Intergroup Exemestane Study. J Clin Oncol. 2006;24 LBA527. [Google Scholar]
- 12.Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 13.Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 14.Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales L, Pans S, Paridaens R, Westhovens R, Timmerman D, Verhaeghe J, et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat. 2006;104:87–91. doi: 10.1007/s10549-006-9394-6. [DOI] [PubMed] [Google Scholar]
- 17.Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, et al. Prospective Study to Assess Short-Term Intra-Articular and Tenosynovial Changes in the Aromatase Inhibitor-Associated Arthralgia Syndrome. J Clin Oncol. 2008;26:3147–3152. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- 18.Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31:726–732. doi: 10.1016/j.jhsa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30:167–178. [PubMed] [Google Scholar]
- 20.Hudak PL, Amadio PC, Bombardier C The Upper Extremity Collaborative Group (UECG) Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected] Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. Clinical global impressions. [Google Scholar]
- 22.Dizdar O, Ozcakar L, Malas FU, Harputluoglu H, Bulut N, Aksoy S, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor-related arthralgia. J Clin Oncol. 2009;27:4955–4960. doi: 10.1200/JCO.2008.20.5435. [DOI] [PubMed] [Google Scholar]