Abstract
The Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene has been associated with aspects of schizophrenia that are possibly related to the disorder’s pathogenesis. The present study investigated the Val158Met polymorphism in relation to anhedonia—a construct central to negative schizotypy. Anhedonia and other schizotypal characteristics were assessed in relatives of schizophrenia patients, relatives of bipolar patients, and nonpsychiatric controls using the Chapman schizotypy scales and the Schizotypal Personality Questionnaire. Compared to controls, relatives of individuals with schizophrenia had elevated scores on Chapman scales for Social Anhedonia and Physical Anhedonia, while relatives of bipolar disorder patients exhibited only increased scores on the Social Anhedonia scale. As a group, relatives of schizophrenia patients who were homozygous for the val allele of the COMT polymorphism showed the highest elevations in self-reported social and physical anhedonia. Associations with the COMT polymorphism were absent in relatives of bipolar patients and control participants. Findings suggest that anhedonia is manifest in individuals who carry genetic liability for schizophrenia and is associated with the Val158Met polymorphism of the COMT gene.
Keywords: Catechol-O-methyltransferase (COMT), Schizophrenia, Anhedonia, Schizotypy, Phenotype
Introduction
Determining how schizotypy phenotypes are related to specific genes is important for developing a working model of psychosis proneness tied to the genetics of schizophrenia (Meehl, 2001; Horan et al., 2004; Lenzenweger et al., 2006). The Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene has been associated with various phenotypes in schizophrenia patients (Bilder et al., 2004) but has yet to be thoroughly tested for associations with schizotypy phenotypes in individuals who carry genetic liability for schizophrenia (e.g., first-degree biological relatives of schizophrenia patients).
The COMT gene encodes an enzyme involved in the inactivation of catecholamines (dopamine, adrenaline, and noradrenaline) and incorporates codon 158 of the chromosomal region 22q11 (Dunham et al., 1992). The Val158Met polymorphism influences variation in enzyme availability—with activity three to four times lower for the met allele than for the val allele (Lotta et al., 1995; Lachman et al., 1996) and appears associated with D1 receptor availability in the human cortex (Slifstein et al., 2008). Evidence suggests an association between the val allele (i.e., less catecholamine activity) and the diagnosis of schizophrenia (Wonodi et al., 2003), decreased performance on cognitive tasks (Bilder et al., 2002; MacDonald et al., 2007), and decreased efficiency of prefrontal cortex (Egan et al., 2001). Nonetheless, in studies of schizophrenia the met allele has also been related to impaired shifting of a response rule based on feedback (Nolan et al., 2004), reduced ability to sustain smooth pursuit eye movements in the brief absence of a target (Thaker et al., 2004), greater vulnerability to visual backward masking effects (Goghari & Sponheim, 2008), and increased low frequency electroencephalography activity over frontal brain regions during resting state (Venables et al., in press). It is also important to note that the region of the genome where COMT resides has been associated with both schizophrenia and bipolar disorder (Badner & Gershon, 2002) with some studies pointing to an association between bipolar disorder and the met allele (Li et al., 1997; Mynett-Johnson et al., 1998). The met allele at codon 158 has also been associated with anxiety in women, panic disorder, and sensitivity to pain in adults (Enoch et al., 2003; Woo et al., 2004; Zubieta et al., 2003). Thus, the role of Val158Met polymorphism of the COMT gene in psychopathology is unclear. It appears the polymorphism may exert varying effects on different forms of psychopathology depending on the allele and the mental disorder.
Reviews of the literature show that when investigators have used a diagnosis of schizophrenia as a phenotype, associations with the COMT polymorphism are variable. Although Fan et al. (2002) found family-based association studies to provide evidence for the val allele to be related to schizophrenia, a recent meta-analysis failed to show such an association (Glatt et al., 2003). Glatt and colleagues suggested that the association of the COMT polymorphism with schizophrenia may be most evident in individuals of European ancestry; however, a recent analysis of large samples of subjects with European ancestry failed to yield association of schizophrenia with the COMT Val158Met polymorphism (Sanders et al., 2008). On the other hand, a study of Irish families with a high density of schizophrenia provided evidence that the val allele of the polymorphism contributes to genetic risk of schizophrenia (Chen et al., 2004).
Several studies have investigated schizotypal characteristics as phenotypes for the COMT Val158Met polymorphism. Avramopoulos et al (2002) reported that the val allele was associated with high schizotypy scores in a male normative sample. Stefanis et al., (2004) replicated these results and found that the val allele was specifically related to negative and disorganization dimensions of schizotypy. More recently, these investigators showed that in the general population the relationship between negative schizotypy and cognitive deficit was moderated by the val allele (Smyrnis et al., 2007). Schurhoff et al. (2007) studied relatives of schizophrenia and bipolar patients and found the presence of the val allele to be associated with independent dimensions of negative and positive schizotypy. Across these variable findings, only an association of the val allele of the COMT polymorphism with negative schizotypy has been replicated. Thus, aspects of negative schizotypy such as anhedonia may be tied to the Val158Met polymorphism. Investigators have yet to specifically examine individuals who carry genetic liability for schizophrenia for associations between the elements of schizotypy and the COMT polymorphism, or employ measures that comprehensively characterize the phenomenology of schizotypy associated with liability for schizophrenia.
Social and physical anhedonia are constructs central to “negative schizotypy” (Blanchard et al., 2000, Gooding & Braun, 2004). It has been shown that high scores on the Social Anhedonia Scale (SAS) predict vulnerability to psychosis in college and community populations (Chapman, Chapman, Kwapil, Eckblad, & Zinser, 1994; Kwapil, 1998). Social anhedonia is also separable from elements of “positive schizotypy” such as magical ideation (Horan et al., 2007). The SAS and Physical Anhedonia Scale (PAS) extensively assess anhedonia and indifference to social and physical experiences, and may serve as sensitive measures for detecting genetic effects related to schizophrenic phenomenology. Kendler et al. (1996) found that the SAS differentiated relatives of individuals with schizophrenia from controls in a large community adult sample. Earlier, Katsanis et al. (1990) successfully differentiated relatives of schizophrenia patients and controls with the PAS. Moreover, physical anhedonia scores tend to be higher in family members of schizophrenia patients with severe anhedonic symptoms (Berenbaum & McGrew, 1993; Fanous et al., 2001). In bipolar disorder, physical anhedonia has failed to be associated with increased familial risk for the condition (Etain et al., 2007).
The Schizotypal Personality Questionnaire (SPQ: Raine, 1991) is a brief measure that assesses all nine criteria of DSM-III-R Schizotypal Personality Disorder and functions as a useful screen for the disorder (Raine, 1991). Thus far it has served as the primary phenotypic measure in studies associating schizotypy with the Val158Met COMT polymorphism. Higher scores on the Interpersonal factor of the SPQ have been associated with poorer sustained attention (Chen, 1997) and a greater spatial working memory deficit (Park & McTigue, 1997) perhaps suggesting an association with cognitive functions involving frontal cortex. Although it may be considered an aspect of the Interpersonal factor, anhedonia is not expressly measured by the SPQ. Thus, additional instruments that specifically assess anhedonia may prove to be useful in examining the role of the COMT gene in risk for schizophrenia.
Given evidence for elevated anhedonia scores in relatives of schizophrenia patients and recent findings of negative schizotypal characteristics being associated with variability in the COMT gene, it is possible that anhedonia is influenced by the COMT polymorphism in people with increased genetic liability for schizophrenia. The hypotheses of the present study were 1) that anhedonia reflects liability for schizophrenia but not for bipolar disorder, 2) that anhedonia is increased in relatives possessing the val allele of the COMT polymorphism, and 3) that associations of anhedonia with the val allele are specific to schizophrenia as compared to bipolar disorder.
Materials and Methods
Participants
Subjects were 94 first-degree relatives of schizophrenia (N = 79) or schizoaffective disorder (N = 15) patients, 45 first-degree relatives of bipolar patients, and 85 healthy nonpsychiatric control participants. Data were gathered within the context of a family study in which patients with schizophrenia, schizoaffective disorder, or bipolar disorder were recruited from the Minneapolis VA Medical Center, community outpatient programs, and a county mental health clinic. First-degree biological relatives were identified through interviews with patients and invited by letter and telephone to participate. We identified potential nonpsychiatric control participants through posting announcements at community libraries, fitness centers, the Minneapolis VA Medical Center, and in newsletters for veterans and fraternal organizations. Staff excluded potential control subjects with personal or family histories of psychotic symptoms or affective disorder as defined by the DSM-IV. Patients, relatives, and control subjects were excluded for histories of substance dependence but were not excluded for past alcohol dependence, as long as they had not abused alcohol in the past month.
Relatives and control subjects completed the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 1996), the Structured Interview for Schizotypy (SIS; Kendler et al., 1989), and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II; First et al., 1997) when indicated by item endorsements on the SCID-II Personality Questionnaire (Ekselius et al., 1994). Lifetime Axis I and II diagnoses for subjects were determined by doctoral-level psychologists and trained advanced graduate students through a consensus process consistent with published guidelines (Leckman et al., 1982) which involved review of SCID-I, SCID-II, SIS, medical history, and family informant material. See Sponheim et al. (2004) for complete description of exclusion criteria, recruitment, and assessment procedures. All participants completed an informed consent process, and the Minneapolis VA Medical Center and University of Minnesota Institutional Review Boards approved the study protocol. For the 53 relatives who were unable to travel for in-person assessments, questionnaires were mailed to them after they had completed informed consent procedures by telephone and a mailed consent form had returned with their signature. These individuals completed SCID-I assessment via telephone and collected buccal swab specimens from themselves after being instructed to follow printed guidelines. Participants returned buccal swabs and completed questionnaires by U.S. Postal Service.
Questionnaires
Relatives and control subjects completed the SPQ (Raine, 1991), a 74-item true-false questionnaire that measures interpersonal, cognitive-perceptual, and disorganized schizotypal characteristics with nine subscales: constricted affect, suspiciousness, no close friends, social anxiety, odd beliefs, odd behavior, odd speech, unusual perceptual experiences, and ideas of reference. While the many scales of the SPQ may provide a detailed characterization of schizotypy, and the SPQ total score has been shown to possess good internal reliability with a coefficient alpha of .91 (Raine, 1991), reliabilities for individual SPQ subscales have been reported below .70 (Calkins, Curtis, Grove, & Iacono, 2004; Kerns, 2007). The SPQ has shown good criterion and discriminant validity for the detection of schizotypal personality. Fifty-five percent of individuals in a normative sample who scored in the top 10% of SPQ total scores had a DSM-III-R diagnosis of schizotypal personality disorder as assessed by the SCID-I. In addition, the SPQ shows low correlations with scales that assess aspects of psychosis-proneness not included in schizotypal personality disorder criteria (e.g., .18 to .19 with Anhedonia) (Raine, 1991).
All participants completed the “Survey of Attitudes and Experiences” which included a pseudo-random mixture of the true-false questions from the Revised Social Anhedonia Scale (SAS; Eckblad et al., 1982), the Revised Physical Anhedonia Scale (PAS; Eckblad and Chapman, 1983), the Magical Ideation Scale (Chapman et al., 1976), the Perceptual Aberration Scale (Chapman et al., 1978), the Chapman Infrequency Scale (Chapman & Chapman, 1983) as well as the L and K Scales from the Minnesota Multiphasic Personality Inventory-2 (MMPI-2; Butcher et al., 2001). The Infrequency Scale was used to exclude subjects for careless responding, and to examine group differences in careless responding. The L and K scales were used to examine differences in defensive responding between groups. The Chapman scales demonstrate good reliability in schizophrenia and control groups, having yielded Cronbach α’s of greater than .7 (ie. recently Kerns, 2006, Horan et al., 2008). Subjects were excluded from analyses if answers to items on a schizotypy scale were missing. No more than seven subjects were excluded from analyses of any given measure.
COMT Genotyping
We determined the COMT Val158Met genotype for each individual by a restriction fragment length polymorphism technique. Whole blood specimens were collected on FTA Matrix specimen collection cards.i Punches from the FTA blood cards were then prepared for PCR analysis according to Whatman FTA protocol. The washed punch was used directly for PCR amplification. Amplification was carried out as described by Bergman-Jungestrom and Wingren (2001). PCR reactions were initially denatured at 94° Celsius for 3 minutes followed by 39 cycles of denaturation at 93° C for 45 seconds, annealing at 55° C for 1 minute and extension at 72° C for 1 minute with a final 4 minute extension at 72° C. The PCR products were digested with NlaIII (New England Biolabs) for 3 hours at 37° C followed by incubation at 60° C for 20 minutes to denature the enzyme. The digestion was then separated by polyacrylamide gel electrophoresis and the digestion products visualized by staining with ethidium bromide. The COMT val allele has a G at position 1947 yielding a 114 base pair fragment after digestion with NlaIII, whereas the COMT met allele has an A at this position, which allows digestion of the 114 base pair fragment into two products of 96 and 18 base pairs. Genotypes were called by two independent and trained technicians. If it was difficult to determine genotype from gels the specimen was again amplified and submitted to electrophoresis. Discordance was resolved by first running an additional gel and if further disagreement or uncertainty was noted, it was resolved by a doctoral-level molecular biologist. Later we completed genotyping of specimens using a Sequenom MassArray platform that yielded complete agreement with genotypes determined through electrophoresis.
Sample Characteristics
Not all relatives and control subjects were genotyped. Thirteen relatives of schizophrenia patients and seven relatives of bipolar patients failed to provide usable specimens. Specimens were not initially gathered from control participants, hence only 35 recently individuals provided useable specimens. Table I reports demographic information regarding genotyped and non-genotyped participants from the three subject groups. Table II presents demographic characteristics and rates of lifetime psychopathology for each participant group by COMT genotype. All participant groups were in Hardy-Weinburg Equilibrium (relatives of schizophrenia patients, X2 (2, N = 81) =.02, p = .99; relatives of bipolars, X2 (2, N = 38) =.24, p = .89; controls, X2 (2, N = 30) = .53, p = .77). Analyses of the total sample revealed differences in gender across groups (X2 = 6.54, p <.05) with a higher ratio of female to male relatives for schizophrenia patients as compared with controls. Relative and control groups differed in age. Within each of the relative groups age was not correlated with any of the measures found to be associated with group differences (e.g., For PAS and constricted affect in relatives of schizophrenia patients, r = .04, p = .71, and r = .15, p = .15, respectively. For SAS in relatives of bipolar patients, r = -.15, p = .33). Age was positively correlated with constricted affect within the control group (r = .28, p = .01).ii
Table I.
Demographic Characteristics of Participant Groups: Genotyped, Non-Genotyped, and Total Sample
| Group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Relatives of SC Patients |
Relatives of BPD Patients |
Nonpsychiatric Controls |
|||||||
| G | NG | Total | G | NG | Total | G | NG | Total | |
| N | 81 | 13 | 94 | 38 | 7 | 45 | 30 | 55 | 85 |
| % female | 68 | 62 | 67 | 53 | 71 | 56 | 50 | 47 | 48 |
| Mean age (SD) | 50(12)b | 63(12) | 52(13)a | 50(17) | 50(12) | 50(16)c | 39(12) | 44(17) | 42(15) |
Note: SC = Schizophrenia (79 relatives) or schizoaffective disorder (15 relatives); BPD = Bipolar disorder; G = Genotyped subjects; NG = Non-genotyped subjects (subjects without genotype information)
p < .001; comparing relatives of SC and control group
p < .005; comparing genotyped and non-genotyped relatives within group
p < .01; comparing relatives of BPD and control group
Table II.
Demographic Characteristics of Genotyped Individuals by Group and Genotype
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Relatives of SC Patients | Relatives of BPD Patients | Nonpsychiatric Controls | |||||||
| Val/Val | Val/Met | Met/Met | Val/Val | Val/Met | Met/Met | Val/Val | Val/Met | Met/Met | |
| N | 22 | 41 | 18 | 6 | 20 | 12 | 9 | 13 | 8 |
| Mean Age (SD) | 53(9)a | 48(10) | 49(12)b | 51(10)b | 51(20) | 45(15) | 36(13) | 44(9) | 34(13) |
| % Female | 71 | 60 | 64 | 83 | 47 | 36 | 44 | 69 | 25 |
| % Siblings | 64 | 73 | 83 | 50 | 35 | 50 | n/a | n/a | n/a |
| % Parents | 36 | 24 | 11 | 50 | 40 | 33 | n/a | n/a | n/a |
| % Offspring | 0 | 2 | 6 | 0 | 25 | 17 | n/a | n/a | n/a |
| %Lifetime DSM Axis I Dis. | 32 | 37 | 33 | 67 | 40 | 50 | 0 | 0 | 0 |
| % Major Depression | 23 | 29 | 33 | 67 | 20 | 50 | 0 | 0 | 0 |
| % Alcohol Dependence | 4 | 5 | 11 | 0 | 0 | 0 | 0 | 0 | 0 |
| % Bipolar Disorder | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| % Anxiety Disorder1 | 4 | 7 | 0 | 17 | 15 | 17 | 0 | 0 | 0 |
| % Lifetime DSM Axis II Dis2. | 24 | 0 | 0 | 0 | 0 | 22 | 0 | 0 | 0 |
| % Lifetime SCZ Spect. Dis. | 24 | 3 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
Note SC = Schizophrenia or schizoaffective disorder; BPD = Bipolar disorder; DSM = Diagnostic and Statistical Manual; Dis = Disorder ; SCZ Spect. Dis. = DSM-IV Psychotic Disorders and Cluster A Personality Disorders; n/a = not applicable
p < .005; compared with controls of same genotype.
p < .05; compared with controls of same genotype.
Two individuals in the nonpsychiatric control group were given a diagnosis of a lifetime anxiety disorder (one had Panic Disorder, and one had a history of PTSD) but neither of these controls were genotyped.
Axis II disorders were not assessed for 22 relatives of schizophrenia patients and 13 relatives of bipolar disorder patients because they did not travel to the study site. The percentages reflect the proportion of individuals with genotype data affected for whom Axis II diagnoses were available.
Of 94 relatives of schizophrenia and schizoaffective patients, 21 were determined to have a history of either Major Depressive Disorder or Depressive Disorder Not Otherwise Specified. Nine of the 45 relatives of bipolar patients had a history of such a disorder. In order to rule out the influence of vulnerability to depression on schizotypy indices we carried out several one-way analysis of variances (ANOVAs) for each relative group specifying lifetime mood disorder as the fixed factor (present, absent) and schizotypy variables (e.g. social anhedonia) as the dependent variables. No analysis for either relative group revealed an effect of lifetime mood disorder on schizotypy indices with the exception of a trend toward relatives of bipolar disorder participants with a history of mood disorder having increased Interpersonal factor scores compared to those without such a history, F (1,39) = 3.72, p = .06.
Statistical Analysis
Because of non-normal distribution within groups, a logarithmic transformation of the schizotypy variables was performed. To determine whether the groups of relatives showed elevation on schizotypy indices we carried out a repeated-measures ANOVA for the three SPQ factors, specifying subject group (schizophrenia relative, bipolar relative, control) and gender (male, female) as between subjects factors and scale as a within subjects factor. Similar analyses were carried out for the Chapman Social and Physical Anhedonia scales and for the Chapman Perceptual Aberration and Magical Ideation scales, specifying group and gender as between subjects factors and scale as a within subjects factor. We also carried out a repeated measures ANOVA on the validity scales (L, K, and Chapman Infrequency Scale) to determine whether groups or genders were discrepant in their overall tendency to endorse questionnaire items. After determining effects of group, gender, and group by gender interaction for the entire sample, we conducted repeated measures ANOVAs for individuals with available genotype data to test for effects of the COMT Val158Met polymorphism on selected sets of measures that had yielded elevated schizotypy scores for relatives. For these analyses we specified genotype (val/val, val/met, met/met), group (relative, control), and gender as between-subject factors. When ANOVA’s yielded significant effects, pairwise comparisons were computed to detail group differences.
Results
Schizotypal Phenotypes in Biological Relatives of Schizophrenia and Bipolar Disorder Patients
Table III presents means and standard deviations of the SPQ factors and Chapman scale indices for the two groups of relatives and the control group, as well as statistics for group comparisons. Analysis of the SPQ factors yielded a nearly significant effect of group across all factors, F(2,191) = 2.84, p = .06. There were no interactions of scale and group, F(3.8,213.8) = 1.11, p = .35, and scale and gender, F(1.9, 213.8) = .251, p = .77, indicating that regardless of gender the relatives and controls did not vary in their differences across SPQ factors assessing schizotypy. Because differences have been found previously between relatives and controls on the Interpersonal factor (Grove et al., 1991; Calkins et al., 2004), exploratory univariate tests of each factor were conducted. Only the Interpersonal factor demonstrated an effect of group (see Table III) with relatives of schizophrenia patients having higher scores than controls. Effect sizes indicated that the relatives of schizophrenia patients were the most elevated on the factor although relatives of bipolar patients tended toward high scores. There were no group differences on the Cognitive-Perceptual and Disorganization factors of the SPQ. Univariate tests also failed to show group differences in SPQ total score.
Table III.
Means, Standard Deviations, and Univariate Test Statistics for Chapman Scales and SPQ Factors using Total Sample
| Rels of SC | 1d | Rels of BPD | 2d | Controls | (df) F | p | |
|---|---|---|---|---|---|---|---|
| SPQ | |||||||
| Total Score | 14.4 (11.2) | .35 | 14.8 (14.2) | .17 | 10.2 (7.1) | (2,196) 2.24 | .11 |
| Interpersonal Factor | 7.6 (6.2)b | .38 | 7.0 (7.7) | .11 | 4.9 (4.7) | (2,191) 4.40 | .01 |
| Cognitive-Perceptual Factor | 4.3 (4.9) | .08 | 4.9 (6.2) | .16 | 3.2 (2.6) | (2,191) .60 | .55 |
| Disorganization Factor | 3.9 (3.3) | .17 | 4.2 (4.1) | .10 | 3.0 (2.4) | (2,191)1.62 | .20 |
| Chapman Scales | |||||||
| Social Anhedonia Scale (SAS) | 8.6 (5.9)a | .44 | 7.9 (6.2)b | .31 | 6.6 (5.7) | (2,178) 6.88 | .001 |
| Physical Anhedonia Scale (PAS) | 13.4 (6.1)b | .33 | 13.1 (7.5) | .15 | 11.7 (6.1) | (2,178) 3.33 | .04 |
| Magical Ideation Scale | 2.6 (2.7) | .03 | 2.9 (3.9) | -.10 | 2.3 (2.0) | (2,178) .04 | .97 |
| Perceptual Aberration Scale | 1.7 (2.5) | .16 | 1.9 (3.1) | .11 | 1.2 (1.3) | (2,178) .15 | .87 |
| Validity Scales | |||||||
| Chapman Infrequency Scale | 2.3 (.6) | .00 | 2.4 (.7) | .15 | 2.3 (.6) | (2,195) .36 | .70 |
| MMPI-2 L Scale | 4.3(2.1) | .29 | 3.6 (1.9) | -.05 | 3.7 (2.1) | (2,195) .81 | .45 |
| MMPI-2 K Scale | 17.4 (4.6)b | -.42 | 16.9 (4.9)a | -.51 | 19.2 (4.0) | (2,195) 4.54 | .01 |
Note. Effect sizes and test statistics are based on logarithmically transformed scores for each schizotypy measure in order to correct for non-normal distributions. Validity scales were not log transformed. Rels of SC = Relatives of schizophrenia patients; Rels of BPD = relatives of bipolar disorder patients; Controls = Nonpsychiatric control subjects.
effect size (Cohen, 1977) for differences between relatives of schizophrenia patients and controls.
effect size (Cohen, 1977) for differences between relatives of bipolar patients and controls.
different from controls p < .01
different from controls p < .05
To detail how the three groups compared on specific elements of the Interpersonal factor we carried out separate ANOVAs for each factor subscale. Analyses of specific subscales revealed that groups differed only in constricted affect, F(2,191) = 4.77, p = .01, with relatives of schizophrenia patients (M = 1.55, SD = 1.67) having higher scores than controls, (M = 1.06, SD = 1.28), p = .002, and relatives of bipolar patients (M =1.48, SD = 1.95) failing to differ from controls, p = .16.
Univariate tests with gender as the fixed factor showed an effect of gender on the Interpersonal factor only within the relatives of schizophrenia patients, F(1, 84) = 4.80, p = .03. Due to the difference between genders in this group, each gender was compared with same-gendered control participants in scores on the constricted affect subscale of the Interpersonal factor. Male relatives of schizophrenia patients had significantly higher constricted affect scores (M = 2.32, SD = 1.70) than male controls (M = 1.30, SD = 1.37), p = .02, while female relatives of schizophrenia patients (M = 1.14, SD = 1.52) did not differ from female controls (M = .80, SD = 1.13), p = .24. When age was entered into the ANOVA group difference in constricted affect remained, p = .05.
To examine distinct negative and positive elements of schizotypy, we carried out separate analyses for anhedonia scales and perceptual aberration/magical ideation scales. Analysis of the two Chapman anhedonia scales yielded group, F(2,183) = 7.852, p = .001, and gender main effects, F(1,183) = 15.24, p <.001, a trend toward an interaction of group and scale, F(2,183) = 2.16, p = .12), but not interaction of group and gender, F(2,183) = 1.05, p = .35. ANOVAs for each Chapman scale yielded group effects for the SAS and PAS (see Table III). Both relatives of schizophrenia patients and relatives of bipolar patients had elevated scores on the SAS compared to controls. Only relatives of schizophrenia patients had higher PAS scores than controls. Inspection of effect sizes in Table III revealed that relatives of schizophrenia patients most strongly deviated from control subjects on measures of anhedonia although both groups of relatives tended to have elevated mean scores.
Follow-up comparisons were carried out to detail gender differences on the Chapman scales to test for gender differences on measures of psychosis proneness noted in other studies (i.e, Miller & Burns, 1995). Both male and female relatives of schizophrenia patients had higher scores on the SAS (M = 10.59, SD = 7.02; M = 7.45, SD = 4.95, respectively) than same-gendered controls (M = 7.54, SD = 6.54; M = 5.54, SD = 4.49, respectively, p = .006 and .03, respectively), but male relatives of schizophrenia patients also had elevated SAS scores compared to female relatives of schizophrenia patients (t [80] = 2.23, p = .03). Male relatives of schizophrenia patients had elevated PAS scores (M = 15.89, SD = 7.89) compared to same-gendered controls (M = 12.03, SD = 6.07; t [65] = 2.16, p = .03) and female relatives of schizophrenia patients (M = 12.13, SD = 4.47; t [79] = 2.11, p = .04). Only male relatives of bipolar patients showed elevated scores on the SAS compared to same-gendered controls (p = .04 for males; p = .43 for females). Male relatives of bipolar patients had significantly higher scores on the SAS compared to female relatives of bipolar patients (M = 10.00, SD = 6.65; M = 6.17, SD = 5.41, respectively; t[40] = 3.04, p = .004). Male relatives of bipolar patients also had significantly higher scores on the PAS compared to same-gendered controls (M = 15.94, SD = 7.04; M = 12.03, SD = 6.07, respectively; t[55] = 2.02, p < .05) and female relatives (M = 10.82, SD = 7.27; t[38] = 2.60, p = .01).
Analysis of the Magical Ideation and Perceptual Aberration scales yielded no main or interaction effects. Neither the relatives of schizophrenia patients nor the relatives of bipolar disorder patients were elevated on the Magical Ideation Scale or the Perceptual Aberration Scale, and genders did not differ on either scale. The two genders in the control group did not differ on any of the Chapman scales (range of p values = .13 to .98).
Analyses of L, K, and Chapman Infrequency validity scales yielded an interaction of group and scale, F(2.7, 259) = 4.94, p = .004, but no interaction of group, scale, and gender, F(2.7, 5.3) = .51, ns, indicating that groups differed in response bias but that this was not influenced by gender. Follow-up ANOVAs for each scale revealed that compared to controls, K scale scores were lower in relatives of schizophrenia patients (p = .02) and relatives of bipolar patients (p = .008). Lower K scores in the relatives of schizophrenia patients are consistent with the two groups admitting to more psychopathology than controls and an absence of defensive responding as has been suggested in other family studies (Katsanis et al., 1990). There were no effects involving group for the Infrequency and L scales.
Schizotypal Phenotypes Evident in COMT Val Homozygote Relatives of Schizophrenia Patients
Because constricted affect, social anhedonia, and physical anhedonia most strongly characterized relatives of schizophrenia patients, we sought to determine whether these indices served as phenotypes for the Val158Met polymorphism of the COMT gene in the first-degree biological relatives of schizophrenia patients. The subset of subjects with genotype data was submitted to analyses of genotype effectsiii iv.
Table IV presents means and standard deviations of relatives of schizophrenia patients and controls by COMT genotype on the Chapman scales and subscales of the SPQ Interpersonal factor. A repeated measures ANOVA of the four subscales of the SPQ Interpersonal factor failed to yield any effects involving COMT genotype indicating that elevated scores of relatives of schizophrenia patients on SPQ subscales were not associated with the Val158Met polymorphism. Analysis of the four Chapman scales yielded an interaction of group, scale, and genotype, F(5.23, 206.6) = 3.82, p = .002, and a trend toward an interaction of group, scale, and gender, F(2.61, 206.6) = 2.43, p = .07. There were no other effects involving genotype or gender, indicating that gender distribution did not account for the observed interaction involving group and genotype.
Table IV.
Means and Standard Deviations for Relatives of Schizophrenia Patients and Controls Subjects by COMT Val158Met Genotype
| Relatives of SC Patients | Nonpsychiatric Controls | |||||
|---|---|---|---|---|---|---|
| Val/Val | Val/Met | Met/Met | Val/Val | Val/Met | Met/Met | |
| Chapman Scales | ||||||
| Social Anhedonia | 9.1 (6.9)a | 8.5 (6.4) | 7.5 (4.3) | 4.5 (4.2) | 5.6 (2.7) | 5.5 (3.0) |
| Physical Anhedonia | 15.2 (5.3)a,b | 12.5 (5.4) | 11.9 (6.0) | 10.6 (6.0) | 9.1 (4.3) | 14.1 (6.6) |
| Magical Ideation Scale | 1.8 (2.1) | 3.2 (3.2) | 2.2 (1.3) | 2.2 (1.8) | 1.5 (1.4) | 1.5 (1.5) |
| Perc. Aberration Scale | 1.0(1.3) | 2.1 (2.9) | 2.2 (3.2) | 1.1 (1.1) | 1.0 (1.0) | .4 (.7) |
| SPQ Interpersonal Factor Subscales | ||||||
| No Close Friends | 2.1 (2.8) | 1.7 (2.3) | 1.6 (1.8) | .3 (.7) | 1.2 (2.0) | .3 (.5) |
| Constricted Affect | 1.6 (1.8) | 1.4 (1.7) | 1.9 (1.7) | .6 (.9) | .7 (1.3) | .8 (.7) |
| Excessive Social Anxiety | 2.4 (2.1) | 3.3 (2.8) | 3.3 (2.2) | 2.0 (1.8) | 1.7 (1.2) | 1.1 (1.4) |
| Suspiciousness | 1.0 (1.3) | 1.0 (1.4) | 1.2 (1.4) | .4 (.7) | .3 (.5) | .3 (.5) |
= significantly different controls of same genotype, p <.05
= significantly different from met/met relatives of schizophrenia patients, p <.05
SC = First-degree relatives of schizophrenia patients.
Perc. = Perceptual
To determine how group differences on each scale contributed to the interaction of group, scale and genotype, we examined the relatives of schizophrenia patients and controls by carrying out an ANOVA for each Chapman scale with group and genotype as the between subjects factors. There was an interaction of group and genotype for the PAS, F(2, 90) = 3.43, p = .04. The val-homozygote relatives of schizophrenia patients had elevated PAS scores compared to met homozygote relatives of schizophrenia patients (t[33] = 2.07, p = .05; Cohen’s d = .70) and a trend toward higher PAS scores than val/met relatives of schizophrenia patients (t[55] = 1.9, p = .06, Cohen’s d = .55). The three genotypes for the control subjects failed to differ from one another in PAS scores. When relatives of schizophrenia patients with different COMT genotypes were contrasted with the entire group of controls to determine which allele was associated with abnormal elevations in scores of anhedonia, the val homozygote relatives of schizophrenia patients had higher PAS scores (t[94] = 3.19, p = .003, Cohen’s d = .73), while other genotypes failed to differ from control subjects (val/met relatives and controls, t[113] = 1.02, p = .30, Cohen’s d = .21; met/met relatives and controls, t[91] = .27, p = .79, Cohen’s d = .08). Although the follow-up ANOVA for the SAS failed to yield a genotype effect, F(2, 90) = .58, p = .56, tests of which genotype deviated from normative levels of social anhedonia revealed that the val homozygote relatives of schizophrenia patients did differ from the mean score of the control subjects on the SAS (t[99] = 1.97, p = .05, Cohen’s d = .51). The val/met genotypes showed a trend, and the met/met genotypes failed to differ from controls (val/met relatives and controls, t[116] = 1.87, p = .06, Cohen’s d = .38; met/met relatives and controls, t[93] = 1.29, p = .20, Cohen’s d = .41). The Figure depicts social and physical anhedonia scores for relatives of schizophrenia patients in contrast with the control group mean. The val allele was associated with greater scores in the relatives.
Diagnostic Specificity of the Anhedonia Phenotype for COMT
Availability of the relatives of bipolar patients allowed for examination of whether the association between anhedonia and the val allele of the COMT polymorphism in relatives of schizophrenia patients was evident for another heritable disorder shown to be associated with the locus. Because relatives of bipolar disorder patients had elevated SAS scores compared to controls we carried out a repeated measures ANOVA of the Chapman scalesv. Although the analysis failed to yield any effects involving genotype we carried out an ANOVA for each Chapman scale, with group and genotype as the between subjects factors, to fully test the specificity of the anhedonia phenotype to the val allele in relatives of schizophrenia patients. There was no group-by-genotype interaction for any of the four Chapman scales. Follow-up ANOVA of PAS scores failed to yield a genotype effect, F(2, 32) = .52, p = .60, and differences between the genotypes were small or failed to indicate a clear association with the COMT polymorphism in the relatives of bipolar patients (val/val compared to val/met, Cohen’s d = .49; val/val compared to relatives, Cohen’s d = .22; val/met compared to met/met, Cohen’s d = -.24). Differences between the genotypes and the control group on the PAS were also small or not consistent with a direct association with the gene (val homozygotes, Cohen’s d = .59; val/met, Cohen’s d = .15, met/met, Cohen’s d = .35). Thus, PAS scores of relatives of bipolar patients were largely unrelated to COMT genotype. A similar analysis for SAS scores failed to yield a genotype effect, F(2, 34) = .83, p = .45. Effect sizes for differences between genotypes were all modest or inconsistent with an association with SAS scores in the relatives of bipolar patients (val/val compared to val/met, Cohen’s d = - .54; val/val compared to met/met, Cohen’s d = -.36; val/met compared to met/met, Cohen’s d = .24). Differences between each genotype in the relatives of bipolar patients and the control group were also inconsistent with genotype being associated elevations on the SAS (val/val to controls, Cohen’s d = .02; val/met to controls, Cohen’s d = .56; met/met to controls, Cohen’s d = .38). No genotypes showed significant elevations in scores on the Magical Ideation and Perceptual Aberration scales relative to other genotypes within the relatives of bipolar patients or in contrast with the controls.vi
Finally, inspection of Table II revealed that schizophrenia spectrum pathology was only evident in relatives of schizophrenia patients with the val allele. An aggregation of schizophrenia spectrum conditions in val homozygote relatives of schizophrenia patients is evidence that the val allele of the Val158Met polymorphism may be associated with increased pathology. Schizophrenia spectrum disorders in relatives of bipolar patients were rare and failed to be associated with the val allele.
Discussion
Of several schizotypy phenotypes we found anhedonia was most evident in first-degree biological relatives of schizophrenia patients and that physical anhedonia was associated with the Val158Met polymorphism of the COMT gene. The relatives of schizophrenia patients as a group had increased anhedonia scores, but the val homozygote relatives showed the greatest levels of social and physical anhedonia as assessed by the Chapman scales. Relatives of schizophrenia patients also had elevated scores on the Interpersonal Factor of the SPQ suggestive of social difficulties and concerns, but the factor failed to be associated with the Val158Met polymorphism.
Although first-degree biological relatives of bipolar disorder patients had elevated social anhedonia scores, in this group the COMT polymorphism failed to be associated with the schizotypy phenotype. Anhedonia may not be specific to first-degree relatives of schizophrenia patients; however, the association of anhedonia with the COMT gene may be unique to schizophrenia amongst severe mental disorders. High scores of physical anhedonia in biological relatives of schizophrenia patients is consistent with the notion that anhedonia is an indicator of predisposition to psychosis. An association between anhedonia and COMT polymorphism in relatives of schizophrenia patients also suggests the presence of trait-based anhedonic characteristics in this group. Because relatives of schizophrenia patients and relatives of bipolar patients failed to differ in physical anhedonia the diagnostic specificity of the scale elevation is not necessary indicated; however, increased physical anhedonia scores in bipolar relatives may reflect affective lability and episodic depression evident in people with liability for mood disorders. This interpretation is consistent with work showing that more trait-like anhedonic characteristics are evident in schizophrenia as compared to depression (Blanchard, Horan, & Brown, 2001).
Because of its possible effects on regulation of prefrontal and striatal catecholamines, the Val158Met polymorphism has been proposed to be causally related to frontal cortical dysfunction, deficits in executive function, and impaired working memory in schizophrenia (Tunbridge et al., 2006). Studies have documented an association of the COMT polymorphism with indices of prefrontal dysfunction in first-degree biological relatives of schizophrenia, (Egan et al., 2001; Callicot et al., 2003) but a clear tie to schizophrenia was absent in other investigations (Goldberg et al., 2003; Rosa et al., 2004). The present finding of the COMT polymorphism as related to a schizotypy phenotype provides evidence that the biological effects of the COMT gene may be relevant to the pathophysiology of schizophrenia. The balance of dopamine in prefrontal and striatal regions has been considered in etiologic formulations of schizophrenia (Weinberger et al., 1988; Meyer-Lindenberg et al., 2002; McIntosh, 2006) and may relate to schizotypal characteristics evident in individuals who carry genetic liability for the condition. For example, Siessmeier et al (2006) found that a tendency to differentiate positive from neutral visual stimuli was positively correlated with the size of ventral striatum in healthy controls. Also, administration of COMT antagonist tolcapone in mice inhibits stress-induced anhedonia-related behavior (Moreau et al., 1994) suggesting that greater availability of dopamine reduces anhedonic responses.
Meehl proposed that primary hypohedonia is a heritable element of pleasure impairment that reflects genetic predisposition for schizophrenia (for a discussion see Meehl, 2001). Recent work has provided evidence that aspects of anhedonia assessed by the SAS and PAS function as latent taxa (Blanchard et al., 2000; Horan et al., 2004) and are abnormally prevalent in individuals who carry genetic liability for schizophrenia (Glatt et al., 2007; Kendler et al., 1996; Clementz et al., 1991; Katsanis et al., 1990). The present study provides the first evidence that anhedonia is associated with a candidate gene for schizophrenia in biological first-degree relatives of schizophrenia patients. Similarly, we observed schizophrenia spectrum disorders to only be present in relatives of schizophrenia patients who carried the val allele. Perhaps COMT can be considered a schizogene akin to the descriptions of Meehl (ie. Archives of General Psychiatry, 1989).
Previous investigations that show varied associations of schizotypy phenotypes with the COMT polymorphism have included subjects from the general population (Avramopoulos et al., 2002; Stefanis et al., 2004; Ma et al., 2007), compared genotypes within relatives of schizophrenia and bipolar disorder as one group, without distinguishing between schizophrenia and bipolar disorder (Schurhoff et al., 2007), or used a schizotypy measure not expressly measuring psychosis-proneness (Ma et al., 2007; Schurhoff et al., 2007; Avramopoulos et al., 2002) thus limiting the conclusions one can draw about characteristics of schizotypy and genetic liability for schizophrenia. The present study yielded evidence that the SAS and PAS sensitively measure anomalies in biological relatives of schizophrenia patients. Other studies have found that of schizotypy factors derived from the SPQ, only the Interpersonal factor reveals anomalies in biological relatives (Grove et al., 1991; Calkins et al., 2004). But it appears that anhedonia, and not the broader construct of interpersonal functioning, is associated with the influence of the val allele of the COMT polymorphism in families affected by schizophrenia. The present results also suggest that anhedonia may be evident in relatives of bipolar patients but that it is largely independent of the Val158Met polymorphism. Thus, although anhedonia is observed in relatives of schizophrenia and bipolar patients, and the COMT gene has been associated with both disorders, the relationship between anhedonia and the COMT polymorphism appears to be only evident in relatives of schizophrenia patients. The finding of an association between anhedonia and the COMT gene as specific to schizophrenia may relate to differences in emotional responding identified in schizophrenia and mood disorders (Berenbaum & Oltmanns, 1992).
Given the limited sample size of the relatives of bipolar patients the present findings regarding diagnostic specificity need to be replicated in a larger sample of relatives. Future studies of COMT gene would also benefit from inclusion of multiple SNPs relevant to dopamine transmission to further explore the role of dopamine genes in predisposing an individual to schizotypy and schizophrenia. Ideally, these studies would compose their samples with similar proportions of the two genders. Nevertheless, the present study provides support for a link between the val allele of the COMT gene and anhedonic characteristics in individuals who carry genetic liability for schizophrenia.
Figure 1.
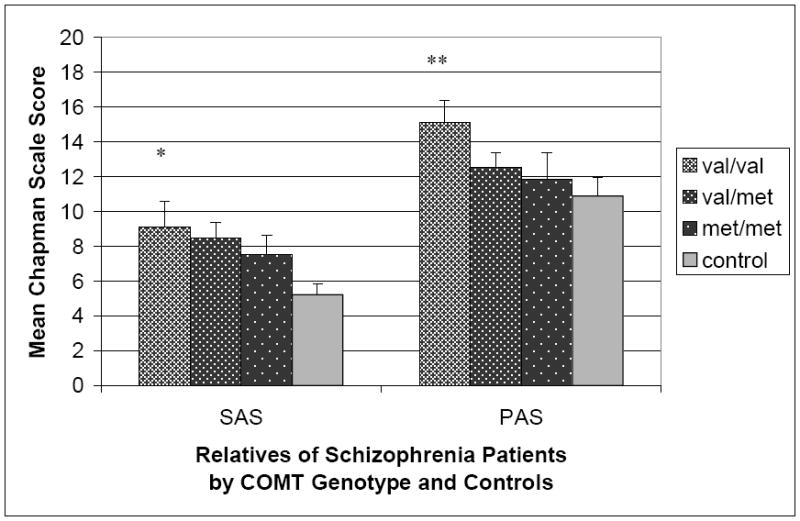
Social Anhedonia Scale (SAS) and Physical Anhedonia Scale (PAS) scores in nonpsychiatric control participants and first-degree biological relatives of schizophrenia patients by COMT Val158Met genotype. Error bars represent standard errors of the mean.
** p < .005 for difference with control subjects.
* p ≤ .05 for difference with control subjects.
Acknowledgments
The authors would like to thank Bridget Hegeman, Ph.D., Tricia Bender, Amy Silberschmidt, Jessica Barker, Laurie Shekels Ph.D., Tasha Nienow Ph.D., and Althea Noukki, Ph.D. for their assistance with this project.
Footnotes
This work was supported by grants from the Department of Veterans Affairs Medical Research Service, the National Institute of Mental Health (5R24MH069675) and the Minnesota Medical Foundation (SMF-2075-99) to Dr. Sponheim, and the Mental Health Patient Service Line at the Veterans Affairs Medical Center, Minneapolis Minnesota.
As described under Participants, for those relatives who were unable to travel buccal swab specimens were collected by participants via printed instruction and sent back to the study with the questionnaires. DNA was isolated from buccal swabs and then subjected to the same procedures as DNA isolated from whole blood in order to characterize the Val158Met polymorphism.
For scales failing to differentiate groups, age was correlated with the disorganized factor in controls (r = -.23, p = .04), odd speech and unusual perceptual experiences in relatives of schizophrenia patients (r = .25, p = .02; r = .24, p = .02, respectively), and magical ideation (r = -.38, p = .01), odd behavior (r = -.36, p = .02), odd speech (r = -.34, p = .02), the cognitive perceptual factor (r = -.40, p = .01), and the disorganized factor (r = -.38, p = .01) in relatives of bipolar patients.
Note that for analyses involving genotype, a maximum of three subjects were excluded due to missing data on one or more of the schizotypy measures. At least seven subjects were included in genotypes for relatives of schizophrenia and controls.
T-tests were performed to examine differences within groups between genotyped and non-genotyped participants on each measure. Differences between genotyped and non-genotyped participants were found only within the control group, with genotyped participants showing significantly lower ‘no close friends’, ‘odd behavior’, and ‘suspiciousness’ subscale scores than non-genotyped individuals (t1,81 = -2.55, p = .013; t1,81 = -2.18, p = .032; t1,82 = -2.82, p = .002, respectively). No significant differences were found within groups for any of the Chapman measures.
Because a small number of relatives of bipolar patients of specific genders and genotypes were available we did not include gender as a factor in this analysis.
Examination of individual PAS scores for the val homozygote relatives of bipolar patients revealed that one relative had a score of 41. When this person was excluded the mean PAS score for the val homozygote relatives of bipolar patients fell to 11.2 (1.9) and the mean score was no longer significantly elevated (p =.39).
Contributor Information
Anna R. Docherty, Veterans Affairs Medical Center, Minneapolis, Minnesota Department of Psychological Sciences University of Missouri-Columbia, Columbia, Missouri.
Scott R. Sponheim, Veterans Affairs Medical Center, Minneapolis, Minnesota Departments of Psychiatry and Psychology, University of Minnesota, Minneapolis, Minnesota.
References
- Avramopoulos D, Stefanis NC, Hantoumi I, Smyrnis N, Evdokimidis I, Stefanis CN. Higher scores of self reported schizotypy in healthy young males carrying the COMT high activity allele. Molecular Psychiatry. 2002;7(7):706–711. doi: 10.1038/sj.mp.4001070. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Molecular Psychiatry. 2002;7(4):405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, McGrew J. Familial resemblance of schizotypic traits. Psychological Medicine. 1993;23(2):327–33. doi: 10.1017/s0033291700028427. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101(1):37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman-Jungestrom M, Wingren S. Catechol-O-Methyltransferase (COMT) gene polymorphism and breast cancer risk in young women. British Journal of Cancer. 2001;85(6):859–862. doi: 10.1054/bjoc.2001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biological Psychiatry. 2002;52(7):701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–61. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Gangestad SW, Brown SA, Horan WP. Hedonic capacity and schizotypy revisited: a taxometric analysis of social anhedonia. Journal of Abnormal Psychology. 2000;109(1):87–95. doi: 10.1037//0021-843x.109.1.87. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. Journal of Abnormal Psychology. 2001;110(3):363–71. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Curtis CE, Grove WM, Iacono WG. Multiple dimensions of schizotypy in first degree biological relatives of schizophrenia patients. Schizophrenia Bulletin. 2004;30(2):317–25. doi: 10.1093/oxfordjournals.schbul.a007081. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. American Journal of Psychiatry. 2003;160(12):2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Infrequency Scale. 1983 Unpublished test. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. Journal of Abnormal Psychology. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103(2):171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang X, O’Neill AF, Walsh D, Kendler KS. Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Molecular Psychiatry. 2004;9(10):962–7. doi: 10.1038/sj.mp.4001519. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioural sciences, Revised Edition. New York: Academic Press; 1977. [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, et al. Catechol O-methyltransferase val(158)met genotype and neural mechanisms related to affective arousal and regulation. Archives of General Psychiatry. 2006;63(12):1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Dunham I, Collins J, Wadey R, Scambler P. Possible role for COMT in psychosis associated with velo-cardio-facial syndrome. Lancet. 1992;340(8831):1361–1362. doi: 10.1016/0140-6736(92)92553-r. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. TheRevised Social Anhedonia Scale. 1982 Unpublished test. [Google Scholar]
- Ekselius L, Lindstrom E, von Knorring L, Bodlund O, Kullgren G. SCID II interviews and the SCID Screen questionnaire as diagnostic tools for personality disorders in DSM-III-R. Acta Psychiatrica Scandinavica. 1994;90(2):120–3. doi: 10.1111/j.1600-0447.1994.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biological Psychiatry. 2001;50(2):98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences USA. 2001;98(12):6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatric Genetics. 2003;13(1):33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Etain B, Roy I, Henry C, Rousseva A, Schurhoff F, Leboyer M, et al. No evidence for physical anhedonia as a candidate symptom or an endophenotype in bipolar affective disorder. Bipolar Disorders. 2007;9(7):706–12. doi: 10.1111/j.1399-5618.2007.00413.x. [DOI] [PubMed] [Google Scholar]
- Fan JB, Chen WY, Tang JX, Li S, Gu NF, Feng GY, et al. Family-based association studies of COMT gene polymorphisms and schizophrenia in the Chinese population. Molecular Psychiatry. 2002;7(5):446–7. doi: 10.1038/sj.mp.4001001. [DOI] [PubMed] [Google Scholar]
- Fanous A, Gardner C, Walsh D, Kendler KS. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Archives of General Psychiatry. 2007;58(7):669–73. doi: 10.1001/archpsyc.58.7.669. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. American Journal of Psychiatry. 2003;160(3):469–76. doi: 10.1176/appi.ajp.160.3.469. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: from phenotype to genotype. Biological Psychiatry. 2007;62(4):282–93. doi: 10.1016/j.biopsych.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Braun JG. Visuoconstructive performance, implicit hemispatial inattention, and schizotypy. Schizophrenia Research. 2004;68(2-3):261–9. doi: 10.1016/S0920-9964(03)00157-9. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry. 2003;60(9):889–96. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Grove WM, Lebow BS, Clementz BA, Cerri A, Medus C, Iacono WG. Familial prevalence and coaggregation of schizotypy indicators: a multitrait family study. Journal of Abnormal Psychology. 1991;100(2):115–121. doi: 10.1037//0021-843x.100.2.115. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH. The factorial structure of schizotypy: Part I. Affinities with syndromes of schizophrenia. Schizophrenia Bulletin. 1996;22(4):611–620. doi: 10.1093/schbul/22.4.611. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. BDNF variant linked to anxiety-related behaviors. Bioessays. 2007;29(2):116–119. doi: 10.1002/bies.20534. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Gangestad SW, Kwapil TR. The psychometric detection of schizotypy: do putative schizotypy indicators identify the same latent class? Journal of Abnormal Psychology. 2004;113(3):339–57. doi: 10.1037/0021-843X.113.3.339. [DOI] [PubMed] [Google Scholar]
- Horan WP, Brown SA, Blanchard JJ. Social anhedonia and schizotypy: the contribution of individual differences in affective traits, stress, and coping. Psychiatry Research. 2007;149(1-3):147–56. doi: 10.1016/j.psychres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Horan WP, Reise SP, Subotnik KL, Ventura J, Nuechterlein KH. The validity of psychosis proneness scales as vulnerability indicators in recent-onset schizophrenia patients. Schizophrenia Research. 2008;100(1-3):224–36. doi: 10.1016/j.schres.2007.12.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, Beiser M. Anhedonia and perceptual aberration in first-episode psychotic patients and their relatives. Journal of Abnormal Psychology. 1990;99(2):202–6. doi: 10.1037//0021-843x.99.2.202. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophrenia Bulletin. 1989;15(4):559–71. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- Kendler KS. “A gene for…”: the nature of gene action in psychiatric disorders. American Journal of Psychiatry. 2005;162(7):1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Psychiatric genetics: a methodologic critique. American Journal of Psychiatry. 2005;162(1):3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thacker L, Walsh D. Self-report measures of schizotypy as indices of familial vulnerability to schizophrenia. Schizophrenia Bulletin. 1996;22(3):511–520. doi: 10.1093/schbul/22.3.511. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Schizotypy facets, cognitive control, and emotion. Journal of Abnormal Psychology. 2006;115(3):418–27. doi: 10.1037/0021-843X.115.3.418. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. Journal of Abnormal Psychology. 1998;107(4):558–565. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- Lachman H, Morrow B, Shprintzen R, Veit S, Parsia S, Faedda G, et al. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. American Journal of Medical Genetics. 1996;67(5):468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Archives of General Psychiatry. 1982;39(8):879–83. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF. Schizotaxia, schizotypy, and schizophrenia: Paul E. Meehl’s blueprint for the experimental psychopathology and genetics of schizophrenia. Journal of Abnormal Psychology. 2006;115(2):195–200. doi: 10.1037/0021-843X.115.2.195. [DOI] [PubMed] [Google Scholar]
- Li T, Vallada H, Curtis D, Arranz M, Xu K, Cai G, et al. Catechol-O-methyltransferase Val158Met polymorphism: frequency analysis in Han Chinese subjects and allelic association of the low activity allele with bipolar affective disorder. Pharmacogenetics. 1997;7(5):349–53. doi: 10.1097/00008571-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Ma X, Sun J, Yao J, Wang Q, Hu X, Deng W, et al. A quantitative association study between schizotypal traits and COMT, PRODH and BDNF genes in a healthy Chinese population. Psychiatry Research. 2007;153(1):7–15. doi: 10.1016/j.psychres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Carter C, Flory JD, Ferrell RE, Manuck SB. COMT Val158Met and executive control: a test of the benefit of specific deficits to translational research. Journal of Abnormal Psychology. 2007;116(2):306–12. doi: 10.1037/0021-843X.116.2.306. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Baig BJ, Hall J, Job D, Whalley HC, Lymer GK, et al. Relationship of Catechol-O-Methyltransferase Variants to Brain Structure and Function in a Population at High Risk of Psychosis. Biological Psychiatry. 2007;61(10):1127–34. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Meehl PE. Schizotaxia revisited. Archives of General Psychiatry. 1989;46(10):935–44. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Primary and secondary hypohedonia. Journal of Abnormal Psychology. 2001;110(1):188–93. doi: 10.1037//0021-843x.110.1.188. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature Neuroscience. 2002;5(3):267–71. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Miller LS, Burns SA. Gender differences in schizotypic features in a large sample of young adults. Journal of Nervous and Mental Disease. 1995;183(10):657–61. doi: 10.1097/00005053-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Mishlove M, Chapman LJ. Social anhedonia in the prediction of psychosis proneness. Journal of Abnormal Psychology. 1985;94(3):384–396. doi: 10.1037//0021-843x.94.3.384. [DOI] [PubMed] [Google Scholar]
- Mynett-Johnson LA, Murphy VE, Claffey E, Shields DC, McKeon P. Preliminary evidence of an association between bipolar disorder in females and the catechol-O-methyltransferase gene. Psychiatric Genetics. 1998;8(4):221–225. doi: 10.1097/00041444-199808040-00004. [DOI] [PubMed] [Google Scholar]
- Park S, McTigue K. Working memory and the syndromes of schizotypal personality. Schizophrenia Research. 1997;26(2-3):213–220. doi: 10.1016/s0920-9964(97)00051-0. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophrenia Bulletin. 1991;17(4):555–64. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. American Journal of Psychiatry. 2008;165(4):420–23. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- Schurhoff F, Szoke A, Chevalier F, Roy I, Meary A, Bellivier F, et al. Schizotypal dimensions: An intermediate phenotype associated with the COMT high activity allele. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(1):64–68. doi: 10.1002/ajmg.b.30395. [DOI] [PubMed] [Google Scholar]
- Shadish WR. Revisiting field experimentation: field notes for the future. Psychological Methods. 2002;7(1):3–18. doi: 10.1037/1082-989x.7.1.3. [DOI] [PubMed] [Google Scholar]
- Siessmeier T, Kienast T, Wrase J, Larsen JL, Braus DF, Smolka MN, et al. Net influx of plasma 6-[18F]fluoro-L-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. European Journal of Neuroscience. 2006;24(1):305–13. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- Smyrnis N, Avramopoulos D, Evdokimidis I, Stefanis CN, Tsekou H, Stefanis NC. Effect of schizotypy on cognitive performance and its tuning by COMT val158 met genotype variations in a large population of young men. Biological Psychiatry. 2007;61(7):845–53. doi: 10.1016/j.biopsych.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Iacono WG, Thuras PD, Beiser M. Using biological indices to classify schizophrenia and other psychotic patients. Schizophrenia Research. 2001;50(3):139–150. doi: 10.1016/s0920-9964(00)00160-2. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Steele VR, McGuire KA. Verbal memory processes in schizophrenia patients and biological relatives of schizophrenia patients: intact implicit memory, impaired explicit recollection. Schizophrenia Research. 2004;71(2-3):339–348. doi: 10.1016/j.schres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, et al. Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biological Psychiatry. 2004;56(7):510–515. doi: 10.1016/j.biopsych.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Otowa T, Hibino H, Kato C, Otani T, Umekage T, et al. Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neuroscience Research. 2006;54(3):180–185. doi: 10.1016/j.neures.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tunbridge E, Harrison P, Weinberger D. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological Psychiatry. 2006;60(2):141–51. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Weinberger D, Berman K, Chase T. Mesocortical dopaminergic function and human cognition. Annual N Y Academy of Sciences. 1988;537:330–8. doi: 10.1111/j.1749-6632.1988.tb42117.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Biological phenotypes and genetic research on schizophrenia. World Psychiatry. 2002;1(1):2–6. [PMC free article] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Mitchell BD, Buchanan RW, Thaker GK. Association between Val108/158 Met polymorphism of the COMT gene and schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2003;120(1):47–50. doi: 10.1002/ajmg.b.20037. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH. The association between panic disorder and the L/L genotype of catechol-O-methyltransferase. Journal of Psychiatric Research. 2004;38(4):365–370. doi: 10.1016/j.jpsychires.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wuthrich VM, Bates TC. Confirmatory factor analysis of the three-factor structure of the schizotypal personality questionnaire and chapman schizotypy scales. Journal of Personality Assessment. 2006;87(3):292–304. doi: 10.1207/s15327752jpa8703_10. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Heitzeg M, Smith Y, Bueller J, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]


