Abstract
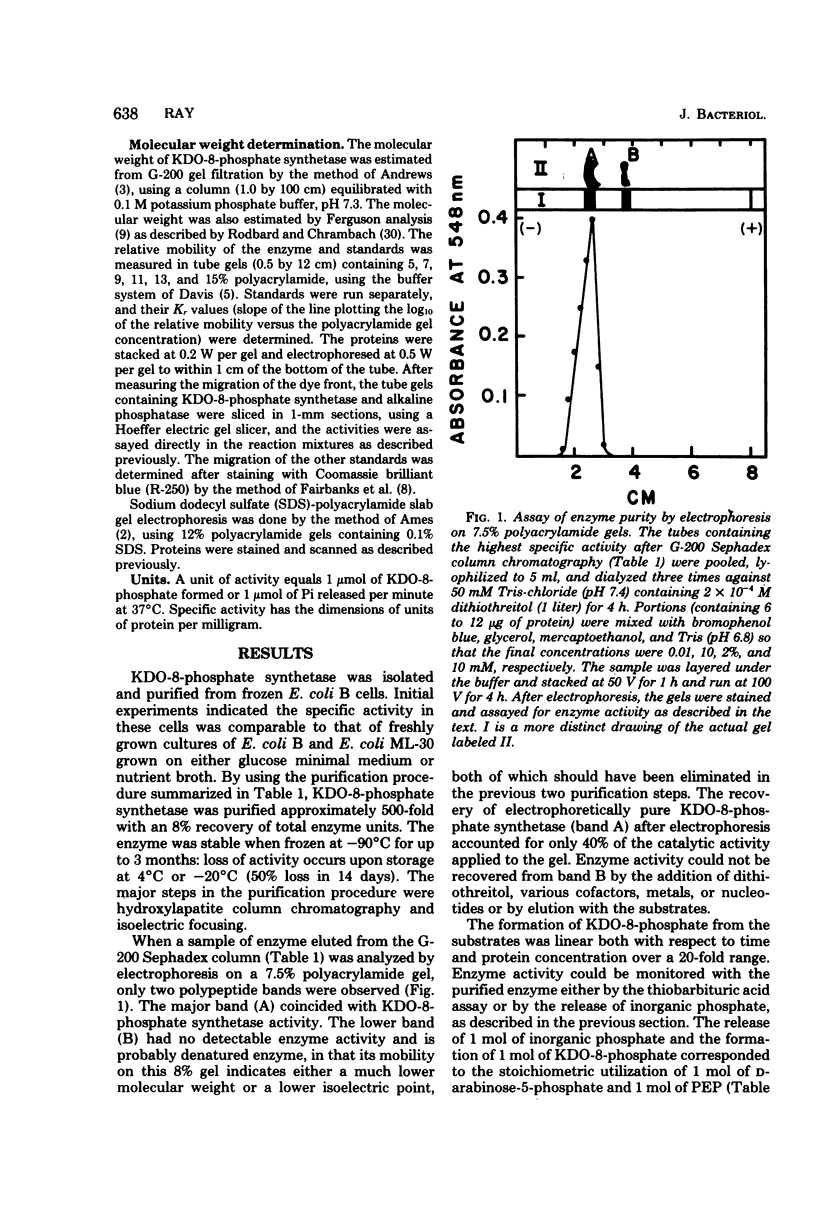
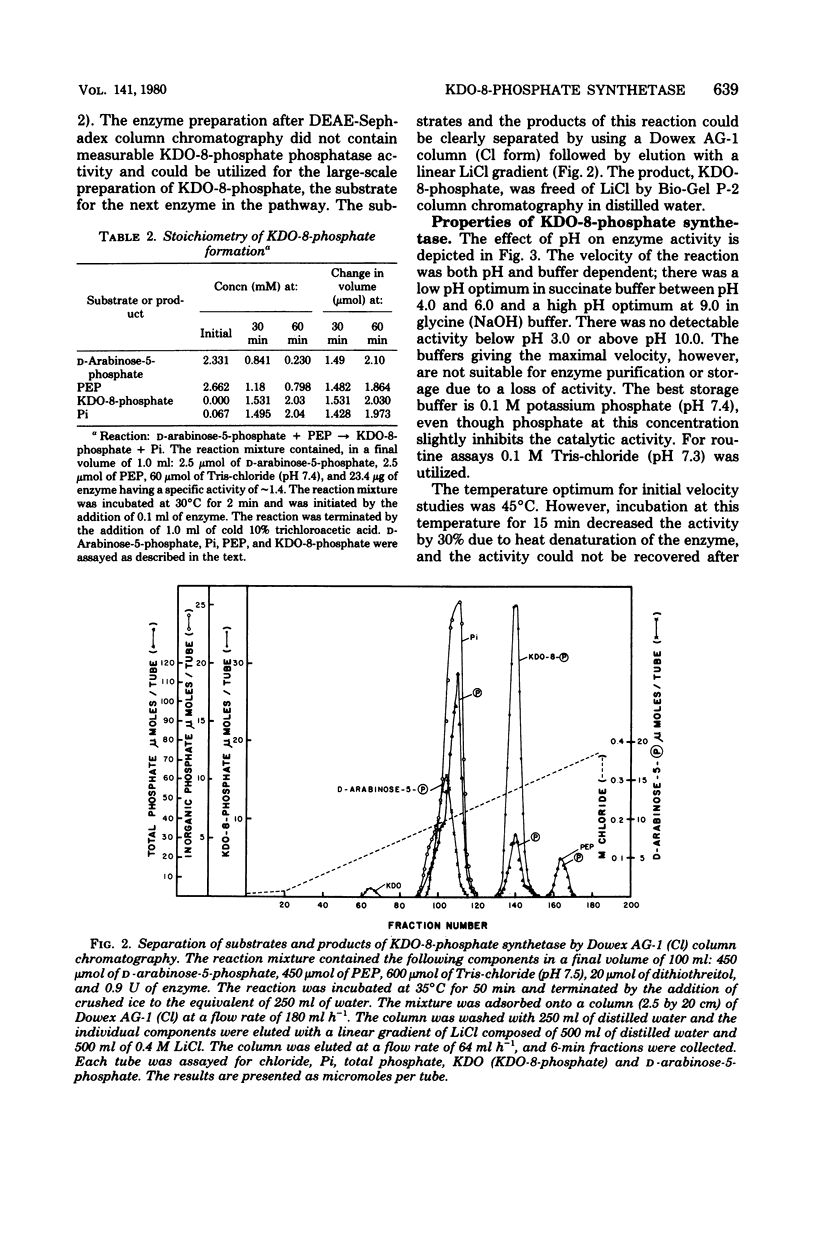
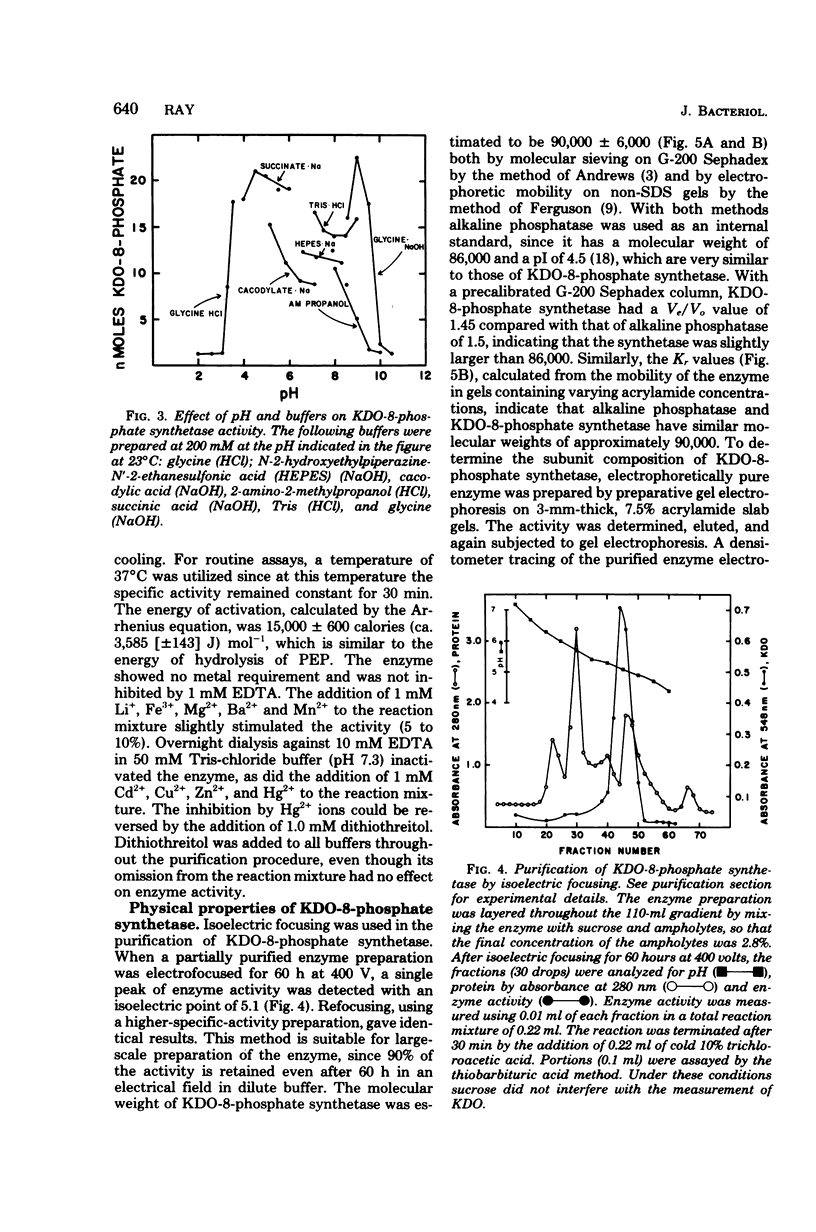
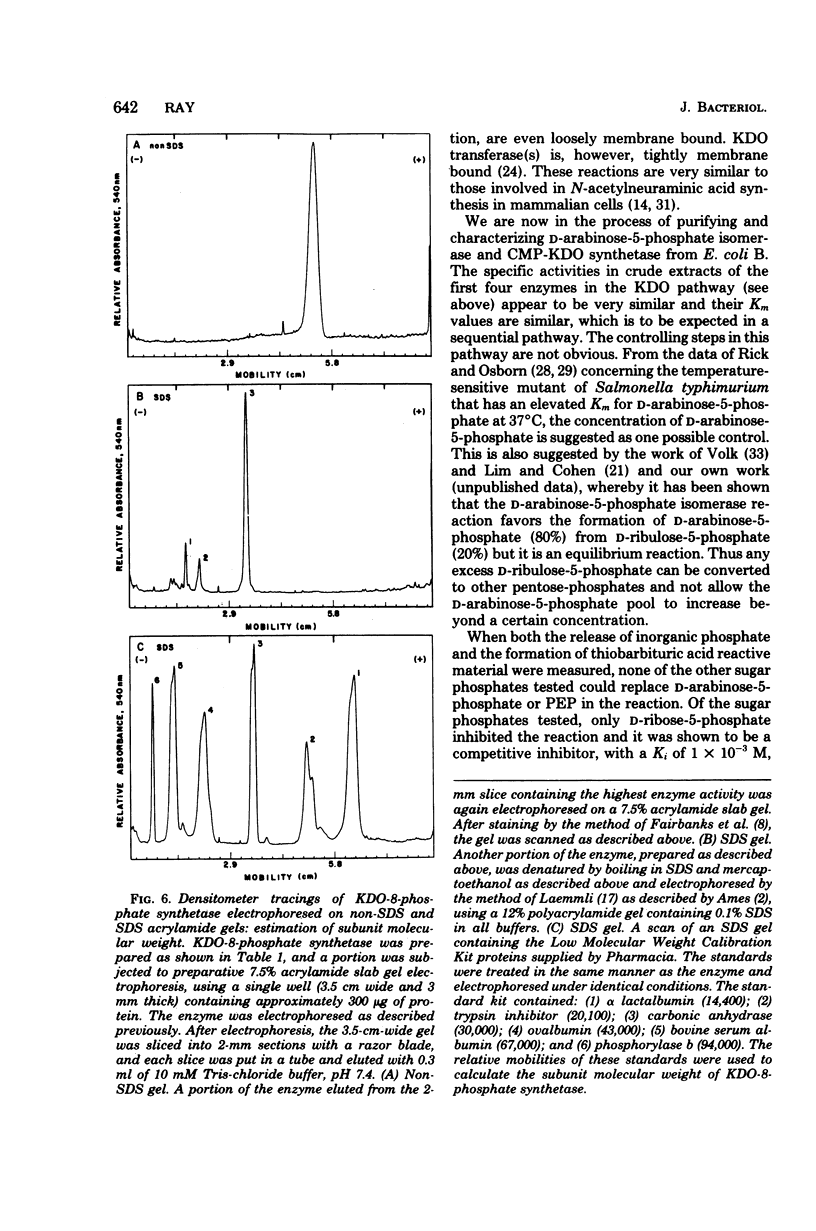
3-Deoxy-D-manno-octulosonate (KDO)-8-phosphate synthetase has been purified 450-fold from frozen Escherichia coli B cells. The purified enzyme catalyzed the stoichiometric formation of KDO-8-phosphate and Pi from phosphoenolpyruvate (PEP) and D-arabinose-5-phosphate. The enzyme showed no metal requirement for activity and was inhibited by 1 mM Cd2+, Cu2+, Zn2+, and Hg2+. The inhibition by Hg2+ could be reversed by dithiothreitol. The optimum temperature for enzyme activity was determined to be 45 degrees C, and the energy of activation calculated by the Arrhenius equation was 15,000 calories (ca. 3,585 J) per mol. The enzyme activity was shown to be pH and buffer dependent, showing two pH optima, one at pH 4.0 to 6.0 in succinate buffer and one at pH 9.0 in glycine buffer. The isoelectric point of the enzyme was 5.1. KDO-8-phosphate synthetase had a molecular weight of 90,000 +/- 6,000 as determined by molecular sieving through G-200 Sephadex and by Ferguson analysis using polyacrylamide gels. Based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the 90,000-molecular-weight native enzyme was composed of three identical subunits, each with an apparent molecular weight of 32,000 +/- 4,000. The enzyme had an apparent Km for D-arabinose-5-phosphate of 2 X 10(-5) M and an apparent Km for PEP of 6 X 10(-6) M. No other sugar or sugar-phosphate could substitute for D-arabinose-5-phosphate. D-Ribose-5-phosphate was a competitive inhibitor of D-arabinose-5-phosphate, with an apparent Ki of 1 X 10(-3) M. The purified enzyme has been utilized to synthesize millimole quantities of pure KDO-8-phosphate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- Ellwood D. C. The distribution of 2-keto-3-deoxy-octonic acid in bacterial walls. J Gen Microbiol. 1970 Mar;60(3):373–380. doi: 10.1099/00221287-60-3-373. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Ghalambor M. A., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. IV. Purification and properties of cytidine monophosphate 3-deoxy-d-manno-octulosonate synthetase. J Biol Chem. 1966 Jul 10;241(13):3216–3221. [PubMed] [Google Scholar]
- Harvey R. J. Metabolic regulation in glucose-limited chemostat cultures of Escherichia coli. J Bacteriol. 1970 Nov;104(2):698–706. doi: 10.1128/jb.104.2.698-706.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOURDIAN G. W., SWANSON A. L., WATSON D., ROSEMAN S. ISOLATION OF SIALIC ACID 9-PHOSPHATASE FROM HUMAN ERYTHROCYTES. J Biol Chem. 1964 Aug;239:PC2714–PC2716. [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- LEVIN D. H., RACKER E. Condensation of arabinose 5-phosphate and phosphorylenol pyruvate by 2-keto-3-deoxy-8-phosphooctonic acid synthetase. J Biol Chem. 1959 Oct;234:2532–2539. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Petitclerc C., Chappelet D., Lazdunski M. On the mechanism of the Zn2+ and Co2+-alkaline phosphatase of E. coli. Number of sites and anticooperativity. Biochem Biophys Res Commun. 1969 Nov 20;37(5):744–749. doi: 10.1016/0006-291x(69)90954-1. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Rupprecht E., Osborn M. J. Isolation of mutants conditionally blocked in the biosynthesis of the 3-deoxy-D-manno-octulosonic-acid--lipid-A part of lipopolysaccharides derived from Salmonella typhimurium. Eur J Biochem. 1977 Jun 1;76(1):41–49. doi: 10.1111/j.1432-1033.1977.tb11568.x. [DOI] [PubMed] [Google Scholar]
- Lim R., Cohen S. S. D-phosphoarabinoisomerase and D-ribulokinase in Escherichia coli. J Biol Chem. 1966 Oct 10;241(19):4304–4315. [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Rasmussen N. S., Osborn M. J. Biosynthesis of lipid A. Enzymatic incorporation of 3-deoxy-D-mannooctulosonate into a precursor of lipid A in Salmonella typhimurium. J Biol Chem. 1978 Mar 10;253(5):1503–1511. [PubMed] [Google Scholar]
- ROSEMAN S. Enzymatic synthesis of cytidine 5'-mono-phospho-sialic acids. Proc Natl Acad Sci U S A. 1962 Mar 15;48:437–441. doi: 10.1073/pnas.48.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Fung L. W., Ho C., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Purification and characterization of a lipid A precursor produced by a mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4904–4912. [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Isolation of a mutant of Salmonella typhimurium dependent on D-arabinose-5-phosphate for growth and synthesis of 3-deoxy-D-mannoctulosonate (ketodeoxyoctonate). Proc Natl Acad Sci U S A. 1972 Dec;69(12):3756–3760. doi: 10.1073/pnas.69.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- VOLK W. A. The enzymatic formation of D-arabinose 5-phosphate from L-arabinose and adenosine triphosphate by Propionibacterium pentosaceum. J Biol Chem. 1959 Aug;234(8):1931–1936. [PubMed] [Google Scholar]
- Vincent W. F., Cameron J. A. Thiobarbiturate-reacting materials in microorganisms. J Bacteriol. 1967 Jan;93(1):156–158. doi: 10.1128/jb.93.1.156-158.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]



