Abstract
Background and Purpose
Functional Magnetic Resonance Imaging (fMRI) is a powerful tool to investigate recovery of brain function in stroke patients. An inherent assumption in fMRI data analysis is that the Blood Oxygenation Level Dependent (BOLD) signal is stable over the course of the exam. In this study, we evaluated the validity of such assumption in chronic stroke patients.
Methods
Fifteen patients performed a simple motor task with repeated epochs using the paretic and the unaffected hand in separate runs. The corresponding BOLD signal time courses were extracted from the primary (M1) and supplementary motor areas (SMA) of both hemispheres. Statistical maps were obtained by the conventional General Linear Model (GLM) and by a parametric-GLM (p-GLM).
Results
Stable BOLD amplitude was observed when the task was executed with the unaffected hand. Conversely, the BOLD signal amplitude in both M1 and SMA was progressively attenuated in every patient when the task was executed with the paretic hand. The conventional GLM analysis failed to detect brain activation during movement of the paretic hand. However, the proposed p-GLM corrected the misdetection problem and showed robust activation in both M1 and SMA.
Conclusions
The use of data analysis tools that are built upon the premise of a stable BOLD signal may lead to misdetection of functional regions and underestimation of brain activity in stroke patients. The present data urges the use of caution when relying upon the BOLD response as a marker of brain reorganization in stroke patients.
Keywords: stroke patients, BOLD signal instability, primary motor cortex, supplementary motor cortex, cortical plasticity
INTRODUCTION
In recent years, functional neuroimaging has made significant contributions to the understanding of neurophysiological and neuropathological processes that occur after an ischemic brain insult1, 2. Among all neuroimaging techniques, functional Magnetic Resonance Imaging (fMRI) has clearly proven advantageous due to its non-invasiveness and good spatiotemporal resolution. Furthermore, the vast literature of fMRI studies in the healthy brain can provide the normal standard against which studies of neuropathological cases can be compared, such as stroke. Most of such studies are based on Blood Oxygenation Level Dependent (BOLD) mechanism, which relies on net changes in the oxidative state of hemoglobin during neural activity. As regional cerebral blood flow increases more than the increase in the regional cerebral metabolic rate of oxygen (rCMRO2), a net reduction of the local concentration of deoxyhemoglobin results, leading to local MRI signal increases of T2*-weighted images.
To properly perform fMRI studies using standard block design paradigms, one needs to assume that the BOLD signal is stable throughout data acquisition3, 4. Among other factors, this stability is highly dependent on a normal coupling between neural activity and vascular response. However, it is well known that neurovascular coupling is disrupted in pathological diseases of the brain5, 6. Therefore, the assumption of BOLD stability may not be valid in fMRI studies of stroke patients, leading to misinterpretation of results obtained with conventional statistical analysis, such as the General Linear Model (GLM).
The purpose of the present study was three-fold. First, to evaluate the dynamic stability of the BOLD signal amplitude in the primary motor cortex (M1) and supplementary motor area (SMA) in response to a motor task performed by chronic stroke patients in a single fMRI run. Second, to compare the BOLD response to movement of the paretic versus the unaffected hand in these patients. Third, to evaluate the performance of the conventional GLM, and to propose an alternative strategy to analyze fMRI of stroke patients.
MATERIALS AND METHODS
Subjects
Fifteen patients with chronic ischemic stroke on unilateral Middle Cerebral Artery (MCA) territory (7 women; mean age: 57y) were included in the study. Two were excluded from analyses due to uncorrectable amounts of head movement during the MRI acquisition, leaving 13 participants (5 women) in the final dataset. The study was approved by the local Research Ethics Committee. All patients gave their written, informed consent.
For each patient, the affected territory of the MCA stroke was evaluated by computer tomography and MRI. The motor function was evaluated by the Fugl-Meyer motor assessment, using a validated scale that varies from zero (most impairment) to 100 (no motor impairment)7. Demographic and clinical details of relevance are presented in Table 1. None of the patients presented carotid or intracranial stenosis.
Table 1.
Demographic and clinical details of the fifteen chronic stroke survivors evaluated in this study.
| Case | Gender | Age | Time from stroke (years) | Anatomic Location | Paretic side | Motor impairment (Fugl-Meyer score) |
|---|---|---|---|---|---|---|
| 1 | M | 61 | 10 | Corona Radiata | Left | 69 |
| 2 | F | 57 | 1,5 | Corona Radiata | Left | 84 |
| 3 | M | 64 | 1 | Inf. Frontal Gyrus | Right | 59 |
| 4 | F | 38 | 1,5 | Internal Capsule | Left | 89 |
| 5 | M | 67 | 1,3 | Post Central Gyrus | Right | 74 |
| 6 | M | 71 | 1 | Corona Radiata | Left | 42 |
| 7 | M | 69 | 9 | Sup. Temporal Gyrus | Right | 62 |
| 8 | M | 58 | 2 | Angular Gyrus | Left | 68 |
| 9 | F | 57 | 1,5 | Corona Radiata | Left | 82 |
| 10 | M | 55 | 4,5 | Corona Radiata | Right | 73 |
| 11 | F | 48 | 2 | Internal Capsule | Left | 75 |
| 12 | M | 55 | 5 | Corona Radiata | Right | 69 |
| 13 | F | 45 | 2 | Supramarginal Gyrus | Right | 57 |
| 14 | F | 64 | 1,5 | Inf. Parietal Lobule | Left | 63 |
| 15 | F | 43 | 3 | Internal Capsule | Left | 51 |
MRI acquisition
MR images were acquired in a 1.5T scanner (Siemens, Magneton Vision). The functional data set was acquired with an EPI sequence (TR/TE=4500/66.0ms, FA = 90°, voxel size = 1.64mm × 1.64mm × 5.00mm). Also a high-resolution T1-weighted image (1mm3) was acquired using a 3D fast spoiled gradient echo (TR/TE=9.7/4.0ms, FA=12°).
A block paradigm was designed with six blocks of rest (27 seconds), intercalated by five blocks of activity (27 seconds), when the patient was cued to open and close her hand. It consisted of two runs, one with the paretic and the other with the unaffected hand. Prior to being placed in the MRI scanner, each patient performed a “dry run” to ensure task compliance, and to guarantee a stable frequency of movement throughout the exam.
Image Processing and Analysis
Regions of interest (ROI) were chosen over M1 and SMA of each hemisphere of all patients. The average amplitude of the BOLD response was computed in every ROI for each hand (unaffected and paretic). Absolute BOLD amplitude differences between the two hemispheres were evaluated using a Student-t test. Statistical differences along task performance were evaluated using a parametric one-way analysis of variance (ANOVA) test with Bonferroni’s post-hoc correction for multiple comparisons (five time points). Unless otherwise specified, statistical significance was taken at p < 0.05.
fMRI was analyzed in BrainVoyager QX 1.9 (Brain Innovation, Maastricht, The Netherlands). Pre-processing included three dimensional motion correction, linear trend removal, temporal high-pass filtering at 0.01Hz and slice-scan-time correction with a sinc interpolation. To improve anatomical localization, statistical maps were co-registered onto high-resolution T1 images.
fMRI statistical maps were computed by two methods: a conventional General Linear Model (GLM) and a parametric modulation method (p-GLM), capable of detecting data trending. Specifically, p-GLM includes two predictors to explain the variance of the data. The first one is based on the conventional hemodynamic response function, with no weighting. The second presents a hemodynamic model weighted according to a specific modulation (Fig. 1). Both maps were corrected for multiple comparisons using a false discovery rate (FDR), with a threshold set to q(FDR < 0.05).
Figure 1.
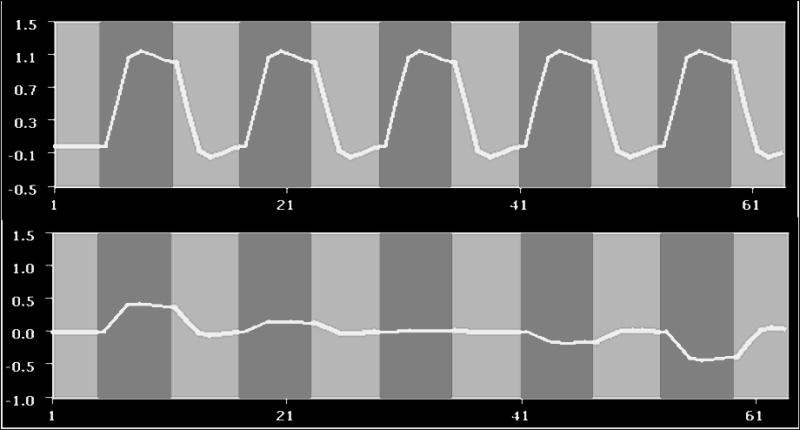
Two predictors created to extract the modulated fMRI response. Light gray columns correspond to periods when the motor task was being performed and the dark gray columns correspond to rest periods. (A) The top row predictor is commonly used in block paradigm fMRI statistical analysis and is designed to be stable throughout task performance; (B) the predictor at the bottom row is weighted to take into account progressive attenuation of the fMRI response in each epoch.
RESULTS
All patients presented stroke of the region supplied by the MCA, of either the right or left hemisphere, and the time of stroke varied from 1 to 10 years (mean: 3.25y). Fugl-Meyer evaluation showed a mean score of 69.5, varying from 42 to 89, which is considered a severe motor impairment7 (Table 1).
To investigate for asymmetries in signal amplitude, the average BOLD response (% change) was calculated for both M1 and SMA of each hemisphere (affected and unaffected). Table 2 shows the mean BOLD signal values, obtained from the average of all patients in each ROI. To allow comparison across patients, signal amplitude was normalized to the first activation block. Signal asymmetry was statistically significant when all five blocks of task were taken into account (p < 0.024 for M1 and p < 0.042 for SMA), and it increases when the average was computed with respect only to the last block (p < 0.002 for M1 and p < 0.027 for SMA).
Table 2.
The average BOLD signal (% change) in response to the motor task performed with the paretic or unaffected hand. Mean values were obtained from the average of all patients, for a given hemisphere, over all five blocks and only the last block. A paired Student t-test was applied to inspect for statistical difference across hemispheres.
| Paretic Hand | Unaffected Hand | ||||
|---|---|---|---|---|---|
| Mean of Five Blocks BOLD (% signal change) | Mean | SD | Mean | SD | p value |
| M1 | 2.24 | 0.81 | 3.08 | 0.70 | 0.024 |
| SMA | 1.85 | 0.86 | 2.71 | 0.89 | 0.042 |
| Mean of the Last Block BOLD (% signal change) | |||||
| M1 | 1.58 | 0.76 | 2.94 | 0.91 | 0.002 |
| SMA | 1.43 | 0.63 | 2.68 | 0.80 | 0.027 |
In order to further investigate this finding, and to demonstrate that this is representative, the amplitude and standard deviation of the normalized BOLD response was calculated for each of the 5 task epochs, averaged across all patients (Fig. 2), for each hemisphere and every ROI. Figure 2 shows the results obtained in SMA and M1 for the unaffected (Fig. 2A) and paretic hand (Fig. 2B). There was no significant difference in the response of the unaffected hand, showing that the BOLD amplitude is preserved across repeated epochs, in either in SMA (Fig. 2A, left) or M1 (Fig. 2A, right). Conversely, the BOLD response of the paretic hand progressively attenuates in SMA (Fig. 2B, left) as well as in M1 (Fig. 2B, left). The responses in M1 in the last two epochs are significantly smaller than the response in either the first or second epochs (p<0.001). The same trend was found in SMA, where the amplitudes to the fourth and fifth epochs are significantly smaller than the initial one (p<0.05).
Figure 2.
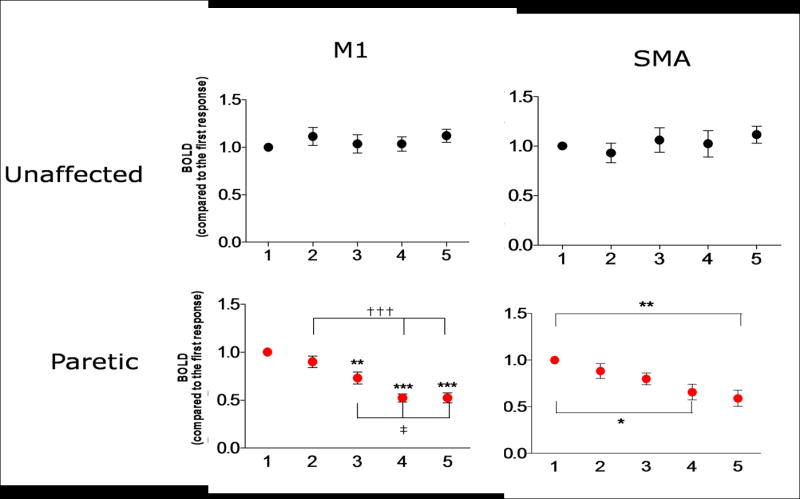
Amplitude and standard deviation of the mean BOLD signal amplitude in SMA (left) and M1 (right) throughout a single run of five epochs (x axis), normalized to the first epoch and averaged across patients, obtained in response to movement of the unaffected hand (A) and the paretic hand (B). *p<0.05, **p<0.01, ***p<0.0001 compared to the first response. †††p<0.001 compared to the second response. ‡p<0.05 compared to the third response.
In order to evaluate the impact of such attenuation over the computation of fMRI statistical maps, the conventional GLM and p-GLM methods were applied to the data of every patient. Results from a representative patient are shown in Figures 3 and 4.
Figure 3.
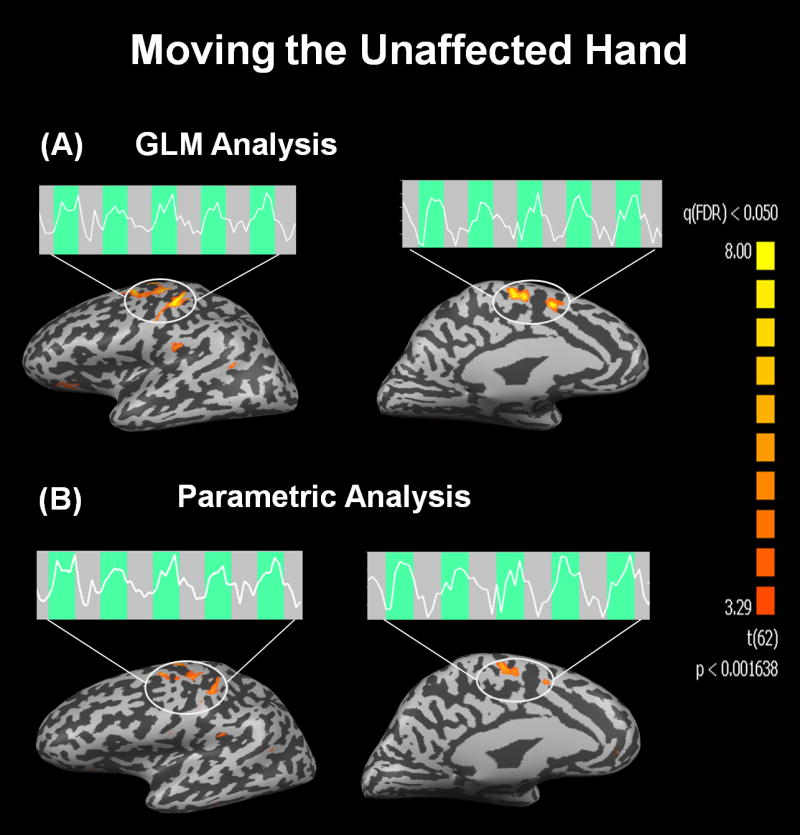
Functional activation maps obtained from a representative patient when moving the unaffected hand. Statistically significant results [q(FDR)<0.05] were found in M1 and SMA by both analysis methods: the conventional GLM (A) and the parametric modulated GLM (B). The BOLD signal time courses show a stable response throughout task execution both in M1 as well as in SMA.
Figure 4.
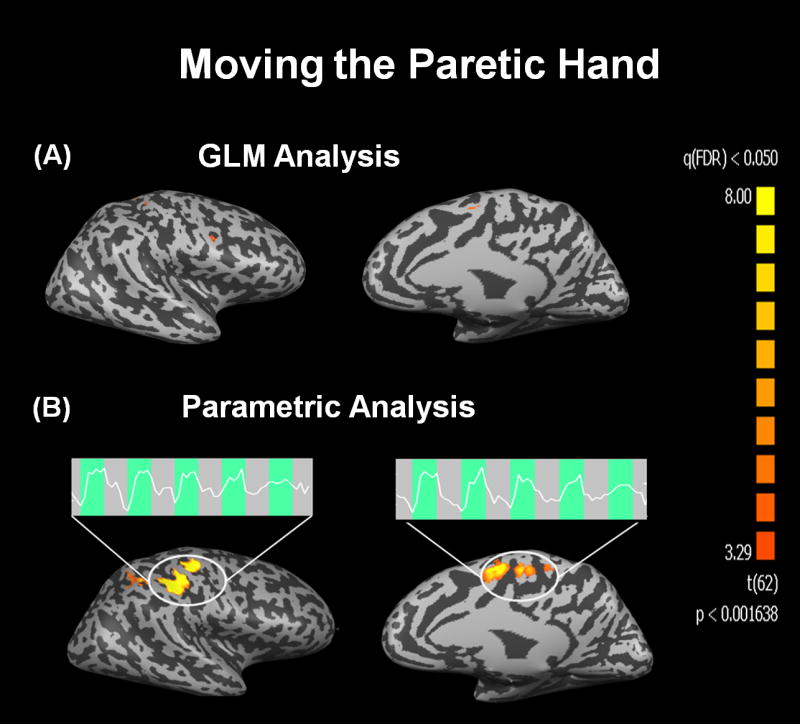
Functional activation maps obtained from a representative patient when moving the paretic hand. Application of the conventional GLM analysis failed to identify regions of activation in all but an extremely limited area close to M1 and SMA (A). On the other hand the parametric modulated GLM successfully detected activation in both M1 and SMA (B). In both regions, the BOLD signal time courses show a remarkable and progressive attenuation during the execution of the motor task.
Figure 3 presents statistical maps obtained by GLM (Fig. 3A) and p-GLM (Fig. 3B) when the patient was moving the unaffected hand, and the extracted BOLD time-courses from M1 and SMA. The functional maps are quite similar, demonstrating that both analysis methods provide robust detection of activation in M1 and SMA in response to the task. Moreover, it is clear that the BOLD response is stable throughout the 5 epochs comprising movement of the unaffected hand.
Figure 4 shows the functional maps and corresponding BOLD time series obtained when the same patient moved the paretic hand. The conventional GLM failed to detect activation in M1 and SMA (Fig. 4A). Conversely, when p-GLM was applied, robust activation in both M1 and SMA were observed (Fig. 4B). The fMRI time courses in both regions show a progressive and significant attenuation of the BOLD response to movement of the paretic hand.
DISCUSSION
In the present study, the stability of the BOLD response was quantitatively analyzed across repeated epochs when chronic stroke patients performed a motor task. The key finding of this work is the remarkable progressive attenuation of the BOLD response when stroke survivors were moving their paretic hand8. Such short-term BOLD attenuation was consistently observed in every single patient in the present study, and led to a siginificant underestimation of the region of activation when the conventional GLM analysis was applied to the data. Besides being an unforeseen phenomenon, this result puts into question the simple use of presence or absence of activation in ipsilateral and contralateral M1 and SMA, as detected with standard fMRI analysis techniques, such as the GLM, as a marker of functional status in stroke patients.
Although a few previous studies have already reported a decrease in BOLD signal in chronic stroke patients, such amplitude variations were observed in different fMRI sessions6, 9-12. Herein, a remarkable and consistent rapid decrease of the BOLD signal amplitude was detected during a single fMRI session in all studied patients. A similar finding has been reported by our group in a single stroke patient study8.
As noted here, individual characteristics of the BOLD response may confound interpretation of brain activation. Besides signal analysis strategies, such as the one implemented in this study, many attempts have been made to calibrate the BOLD signal and so to have a more reliable evaluation of fMRI signal. Most of them are based on hypercapnia challenges13, 14, which attempts to model inherent individual brain perfusion characteristics in order to better estimate the observed BOLD signal15.
The present results can most probably be explained according to three hypothesis: muscular fatigue or weakness; habituation associated with brain plasticity; or altered neurovascular coupling.
Muscular fatigue hypothesis needs to be considered in light of the simplicity of the motor task executed, although none of the patients reported difficulty in executing the task. It is well known that damage of brain tissue following stroke affects corticospinal and other supraspinal motor pathways. The reduction in neural activity along these pathways promotes a disruption of primary force control mechanisms, affecting the fast recruitment of spinal motoneurons and resulting in slower movements and a decreased muscle strength16. Previously conducted studies of motor unit activity in hemiplegic patients have suggested that motor unit firing rates tend overall to be decreased relative to ipsilesional limbs, which affects the capacity to produce fused contraction17. Moreover, previous fMRI studies have demonstrated that, while the response in M1 is dependent on the duration and motor strength of the task, the response in SMA is invariably constant18. In our study, we found that the BOLD response in SMA was also progressively attenuated. Therefore, taken together, the simplicity of the motor task and the decreased response in SMA it is unlikely that muscular fatigue alone can explain the observed phenomena.
A second explanation relates to a possible plasticity-induced habituation. It is well known that the brain shows a remarkable capacity of reorganization as a consequence of acquiring new skills and behaviors, or learning new tasks19. In our case, the repetitive nature of the task could have induced habituation, leading to a progressive attenuation of the BOLD response. Previously, Karni and colleagues conducted longitudinal fMRI studies to evaluate the effect of task performance on M1 BOLD response20, 21. They observed that the performance of a repeated motor task by healthy subjects in different sessions leads to a smaller area of activation than that obtained the first time subjects executed the task. Furthermore, Carel and colleagues studied two groups of healthy subjects, one with and the other without daily passive training22. Two fMRI sessions were accomplished, separated by one month. The untrained group showed a decrease activity of M1 and SMA, consistent with a habituation effect22. Another study from the same group failed to observe any habituation effects within a single fMRI section, and only comparisons of the fMRI response to different sessions separated by 5 hours or by 49 days showed significant changes in the extent of the area of activation23. We believe pure habituation is not the most probable explanation for the observed attenuation, mainly for two reasons. First, only the regions responding to movement of the paretic hand, but not the ones responding to movement of the unaffected hand, exhibited habituation. Second, should habituation be the main cause for the signal decrease, one should expect the regions associated with the unaffected hand to yield the strongest signal attenuation due to a greater ability of the patients in executing the task with this hand.
A third explanation for the present results focuses on an altered neurovascular coupling. Previous studies have documented impairment of the cerebrovascular reactivity (CVR) in stroke2, 11, 24. For instance, analysis of the BOLD response to finger tapping showed that stroke survivors present a slower rate of rise and maximum signal intensity than healthy controls11. Interestingly, such changes were found in both hemispheres (affected and unaffected), suggesting that the differences may be due to a diffuse functional neurovascular pathology11. Moreover, stroke survivors showed decreased BOLD amplitudes both in M1 and in SMA in the ipsilesional hemisphere, regardless of whether a unilateral or a bilateral motor task was performed2,much like in the present study (Table 2). Furthermore, a recent arterial spin labeling study demonstrated a general hypoperfusion, and an increased transit time, in sensorimotor and supplementary motor cortices of stroke survivors25. Therefore, we believe that the present data is consistent with this hypothesis: as hypoperfusion is temporally preserved, the maintained local increase of metabolic demand by neuronal activity would result in a progressive local attenuation of the BOLD signal.
Conclusion
In conclusion, the present study reports on the existence of a progressive short-term attenuation of the BOLD signal in stroke. Regardless of the cause of the observed phenomena, the present data urges the use of caution when relying upon the magnitude and spatial extent of the BOLD response in M1 and SMA of stroke survivors as a marker of brain plasticity and reorganization. Therefore, the use of new strategies should be considered, as, for instance, the analysis method proposed in this study.
Acknowledgments
The authors would like to thank the Brazilian Financial Agencies FAPESP (05/03225-7), CNPq, CAPES and FINEP for financial support. This research was supported in part by the Intramural Research Program of the NIH, NINDS (Alan P. Koretsky, Scientific Director).
Footnotes
Conflict of interest The authors state no conflict of interest.
References
- 1.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults - a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 2.Krainik A, Hund-Georgiadis M, Zysset S, von Cramon DY. Regional impairment of cerebrovascular reactivity and bold signal in adults after stroke. Stroke. 2005;36:1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7. [DOI] [PubMed] [Google Scholar]
- 3.de Araujo DB, Tedeschi W, Santos AC, Elias J, Neves UPC, Baffa O. Shannon entropy applied to the analysis of event-related fmri time series. Neuroimage. 2003;20:311–317. doi: 10.1016/s1053-8119(03)00306-9. [DOI] [PubMed] [Google Scholar]
- 4.Sturzbecher MJ, Tedeschi W, Cabella BCT, Baffa O, Neves UPC, De Araujo DB. Non-extensive entropy and the extraction of bold spatial information in event-related functional mri. Physics in Medicine and Biology. 2009;54:161–174. doi: 10.1088/0031-9155/54/1/011. [DOI] [PubMed] [Google Scholar]
- 5.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and alzheimer disease. Journal of Applied Physiology. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 6.Rossini PM, Altamura C, Ferretti A, Vernieri F, Zappasodi F, Caulo M, Pizzella V, Del Gratta C, Romani GL, Tecchio F. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain. 2004;127:99–110. doi: 10.1093/brain/awh012. [DOI] [PubMed] [Google Scholar]
- 7.Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the fugl-meyer assessment for testing motor-performance in patients following stroke. Physical Therapy. 1993;73:447–454. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho SMF, Pontes-Neto OM, Fabio SRC, Leite JP, Santos AC, de Araujo DB. Rapid bold fmri signal loss in the primary motor cortex of a stroke patient. Arquivos De Neuro-Psiquiatria. 2008;66:885–887. doi: 10.1590/s0004-282x2008000600022. [DOI] [PubMed] [Google Scholar]
- 9.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the bold fmri signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 10.Hamzei F, Knab R, Weiller C, Rother J. The influence of extra- and intracranial artery disease on the bold signal in fmri. Neuroimage. 2003;20:1393–1399. doi: 10.1016/S1053-8119(03)00384-7. [DOI] [PubMed] [Google Scholar]
- 11.Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Altered hemodynamic responses in patients after subcortical stroke measured by functional mri. Stroke. 2002;33:103–109. doi: 10.1161/hs0102.100482. [DOI] [PubMed] [Google Scholar]
- 12.Rother J, Knab R, Hamzei F, Fiehler J, Reichenbach JR, Buchel C, Weiller C. Negative dip in bold fmri is caused by blood flow - oxygen consumption uncoupling in humans. Neuroimage. 2002;15:98–102. doi: 10.1006/nimg.2001.0965. [DOI] [PubMed] [Google Scholar]
- 13.Andrade KC, Pontes-Neto OM, Leite JP, Santos AC, Baffa O, de Araujo DB. Quantitative aspects of brain perfusion dynamic induced by bold fmri. Arquivos De Neuro-Psiquiatria. 2006;64:895–898. doi: 10.1590/s0004-282x2006000600001. [DOI] [PubMed] [Google Scholar]
- 14.Leoni RF, Mazzeto-Betti KC, Andrade KC, de Araujo DB. Quantitative evaluation of hemodynamic response after hypercapnia among different brain territories by fmri. Neuroimage. 2008;41:1192–1198. doi: 10.1016/j.neuroimage.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Thomason ME, Foland LC, Glover GH. Calibration of bold fmri using breath holding reduces group variance during a cognitive task. Human Brain Mapping. 2007;28:59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: Effects on individuated finger movements. Journal of Neuroscience. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle & Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 18.Peck KK, Sunderland A, Peters AM, Butterworth S, Clark P, Gowland PA. Cerebral activation during a simple force production task: Changes in the time course of the haemodynamic response. Neuroreport. 2001;12:2813–2816. doi: 10.1097/00001756-200109170-00012. [DOI] [PubMed] [Google Scholar]
- 19.Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional mri evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 21.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carel C, Loubinoux I, Boulanouar K, Manelfe C, Rascol O, Celsis P, Chollet F. Neural substrate for the effects of passive training on sensorimotor cortical representation: A study with functional magnetic resonance imaging in healthy subjects. Journal of Cerebral Blood Flow and Metabolism. 2000;20:478–484. doi: 10.1097/00004647-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F. Within-session and between-session reproducibility of cerebral sensorimotor activation: A test-retest effect evidenced with functional magnetic resonance imaging. Journal of Cerebral Blood Flow and Metabolism. 2001;21:592–607. doi: 10.1097/00004647-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Murata Y, Sakatani K, Hoshino T, Fujiwara N, Kano T, Nakamura S, Katayama Y. Effects of cerebral ischemia on evoked cerebral blood oxygenation responses and bold contrast functional mri in stroke patients. Stroke. 2006;37:2514–2520. doi: 10.1161/01.STR.0000239698.50656.3b. [DOI] [PubMed] [Google Scholar]
- 25.Brumm KP, Perthen JE, Liu TT, Haist F, Ayalon L, Love T. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. Neuroimage. 51:995–1005. doi: 10.1016/j.neuroimage.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


