Abstract
Access to liver transplantation is reportedly inequitable for racial/ethnic minorities, but inadequate adjustment for geography and disease progression precludes any meaningful conclusions. We aimed to evaluate the association between candidate race/ethnicity and liver transplant rates after thorough adjustment for these factors, and to determine how uniform racial/ethnic disparities were across MELD scores.
Methods
Chronic end-stage liver disease candidates initially waitlisted between 02/28/2002 and 02/27/2007 were identified from Scientific Registry for Transplant Recipients data. The primary outcome was deceased donor liver transplantation (DDLT); the primary exposure covariate was race/ethnicity (White; African-American; Hispanic; Asian; Other). Cox regression was used to estimate the covariate-adjusted DDLT rates by race/ethnicity, stratified by donation service area (DSA) and Model of End-stage Liver Disease (MELD) score.
Results
Averaging across all MELD scores, African-Americans, Asians, and Others had similar adjusted DDLT compared to Whites. However, Hispanics had an 8% lower DDLT rate compared to Whites (HR=0.92, p=0.011). The disparity among Hispanics was concentrated among patients with MELD scores < 20, with HR=0.84 (p=0.021) for MELD 6–14, and HR=0.85 (p=0.009) for MELD 15–19. Asians with MELD scores <15 had a 24% higher DDLT rate relative to Whites (HR=1.24, p=0.024). However, Asians with MELD scores 30–40 had 46% lower DDLT rates (HR=0.54, p=0.004).
Conclusions
While African-Americans did not have significantly different DDLT rates compared to similar White candidates, race/ethnicity-based disparities were prominent among subgroups of Hispanic and Asian candidates. This inequity may lead to excess mortality for minority candidates by precluding a survival benefit with liver transplantation.
Keywords: access to care, liver transplantation, health disparities, organ allocation
Introduction
The U.S. Census Bureau projects that the United States population will undergo rapid racial and ethnic diversification over the next fifty years. Minority populations are expected to grow by 2% per year over the next three decades in the U.S. The African-American population is expected to grow to encompass 15% of the U.S. population, while Asian and Pacific Islanders will make up approximately 9%. While Hispanics are currently the single largest ethnic minority group, making up 15% of the U.S. population, they are projected to account for nearly a quarter of the population by 2050 and total 130 million (1). Recent data also suggest that the incidence of chronic liver disease and hepatocellular carcinoma is growing fastest in minority populations, particularly among Hispanics (2–8). The rapid diversification of the U.S. population coupled with the growing burden of liver disease in minority communities suggests that the liver transplant waitlist of the future will have greater racial, ethnic, and cultural pluralism than it does currently.
The need for racial/ethnic equity in liver transplantation among patients with the same medical urgency is necessary, but previous studies in this area are narrow in their conception of race, potentially biased, or derived from candidate cohorts who were waitlisted under obsolete allocation rules. African-Americans reportedly had significantly lower transplant rates in the era prior to adoption of the Model for End Stage Liver Disease (MELD)-based liver allocation system (9, 10), with improvement in the disparity reportedly occurring in the MELD era (3, 10). These studies fail to provide a complete evaluation of other minority groups, such as Hispanics and Asians, who account for a growing proportion of the waitlist (11). Further, previous disparities-related studies do not correctly adjust for geographic factors that may impact the receipt of a liver transplant. The variation in the likelihood of receiving a liver transplant in different parts of the country has been well documented (12–14), and is tied to the local availability of organs from deceased donors, performance of organ procurement organizations, and transplant program practices (15, 16). In order to better understand and address racial/ethnic disparities in liver transplantation in a policy framework, studies on racial/ethnic equity in liver transplantation must provide a more precise estimate of the scope of the problem by accounting for potential confounding factors.
Given the growing racial/ethnic diversity of the liver waitlist, the intent of our study was to comprehensively characterize racial/ethnic disparities in access to liver transplantation in the context of the liver allocation system. Using data from the Scientific Registry of Transplant Recipients, we aimed to evaluate any potential racial/ethnic inequity in waitlist event rates by accounting for patient differences, changes in disease over time, and geographic factors, which all affect transplant rates (17). We also aimed to determine to what extent different levels of geographic adjustment (the local DSA level, the Organ Procurement and Transplant Network (OPTN) regional level, and groups of OPTN regions) affect measured differences in adjusted liver transplant rates between racial/ethnic groups. The liver allocation system is driven by the principle of medical urgency, and theoretically is able to achieve equity in access to transplantation by remaining true to objective characterizations of liver disease severity (18). Using a carefully designed statistical approach that modeled access to liver transplant based on the rules of liver allocation, we intended to identify any racial/ethnic disparities that were byproducts of the design of the allocation system.
Methods
Data were obtained from the Scientific Registry of Transplant Recipients (SRTR) and based on patient-level data submitted by transplant centers to the Organ Procurement and Transplantation Network (OPTN). After excluding patients with acute liver failure (Status 1), those under the age of 18 years, those with previous liver transplants, the study population (n=39,114) consisted of adult chronic end-stage liver disease candidates initially added to the waitlist between February 28, 2002 and February 27, 2007. The start date corresponded to the initiation date of MELD-based liver allocation by the OPTN, while the end date was chosen such that up to 5 years of follow-up were available.
In our analysis, race and ethnicity were considered jointly. Both race and ethnicity represent a social context that frames personal and cultural identity, attitudes toward health, health practices, and behavioral risks that may precipitate disease. These constructions may also shape patient interactions with the health care system by affecting the expectations and perceptions of both the patients and providers in health care encounters (19). We used race and ethnicity definitions provided by transplant centers using the OPTN data collection infrastructure. Race and ethnicity were defined as non-Hispanic White (White), African-American, Hispanic, Asian, or Other; with the latter including Native Americans, Native Alaskans, Native Hawaiians, and those of undefined or mixed race/ethnicity (e.g., Black Hispanics). Our principal aim was to determine whether deceased donor liver transplant rates differed by candidate race/ethnicity.
Cox regression was used to compare race/ethnicity-specific rates of deceased donor liver transplantation among active patients (20). Patients began follow-up on the date they were initially added to the waitlist. They were followed until the earliest of liver transplantation, death, granting of a MELD exception score, or the end of the observation period (02/27/2007). In order to assess the workings of the allocation system, we modeled the rate of DDLT among active patients, since donor livers cannot be allocated to those who are inactivated, removed, or who have died on the waitlist (21). Correspondingly, follow-up of patients while removed or inactive from the waitlist for any period of time (e.g., for temporarily illnesses or other issues that precluded liver transplantation) was excluded. A patient’s observation was subsequently reinitiated if and when the patient was reactivated. Patients were censored from the analysis at the time at which they received a living donor liver transplant or a MELD exception score (e.g., for hepatocellular carcinoma), if applicable. Differences in access to liver transplantation were represented by hazard ratios (HR) and expressed as percent changes in relative rates.
The Cox models were stratified by integer MELD score, and included several covariates from the SRTR candidate file, some of which were: age, gender, diagnosis, diabetes, body mass index, hospitalization status at listing, receipt of dialysis, albumin, and prior malignancy. Note that MELD score, albumin and the use of dialysis were time-dependent covariates, meaning that all of a candidate’s changes with respect to such factors recorded in data submitted to the OPTN were incorporated into the analysis. A total of 98 patients had at least one missing data element in the baseline adjustment covariates and these patients were excluded.
In order to study the effect of geography on measured differences in transplant rates between minority groups and whites, we created three separate geographic adjustment models. A previous article identified African-Americans as having significantly decreased transplant rates relative to Whites, using a model adjusted for geography using consolidated OPTN regions (10). We created two additional models, one adjusted for each individual OPTN region, and the final one adjusted for donation service area (DSA), the primary level of organ distribution. By comparing these three models, we could identify how transplant rates differed between minority groups and whites registered in the same geographic unit. Subsequent comparisons of racial/ethnic differences in adjusted deceased donor liver transplant rates were obtained using models stratified by the donation service area (DSA) in addition to MELD score.
Further analysis was directed at evaluating whether the association between race/ethnicity and liver transplant rates was modified by medical urgency or geography (i.e., interactions). First, to determine if the impact of race/ethnicity differed by medical urgency, we fitted models which estimated separate MELD category-specific race/ethnicity effects. This involved fitting models with product terms defined by indicators for the race/ethnicity group and MELD category. MELD categories were based on a modification of the ranges based on liver transplant survival benefit (22). These categories were MELD 6–14, 15–19, 20–29, 30–40, and were derived by grouping candidates with adjacent MELD scores together to ensure an adequate number of transplant events in each group. Second, we fitted models which enabled direct comparisons of relative transplant rates between racial/ethnic minorities and Whites registered in the same DSAs within each OPTN region. We also fitted additional models to determine whether race/ethnicity affected the respective transitions from being an active waitlist candidate to inactivation, removal, and death, stratified by integer MELD and DSA. These models were censored at transplantation and MELD exception. Models to determine the effect of race/ethnicity-MELD interactions on these other outcomes were also fitted.
This study was approved by the U.S. Health Resources and Services Administration (HRSA) SRTR project officer. HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)(5) and HRSA Circular 03. All statistical analysis was performed using SAS v9.2 (SAS Institute; Cary, NC). Statistical significance was defined by p < 0.05.
Results
Clinical characteristics of the 39,114 patients in the study population are displayed in Table 1. Whites made up 74.1% of waitlisted patients, Hispanics 13.9%, African-Americans 7.3%, Asians 3.7%, and Others less than 1%. Racial/ethnic subgroups were significantly different with respect to the following factors: age at waitlisting, sex, diagnosis, MELD score at listing, body mass index and percent of patients on dialysis; with p<0.0001 in each case. The mean age at waitlist registration ranged from 50 (African-Americans) to 54 years (Asians). The proportion of male candidates was lowest in the Other group (58% male) and highest in the Asian population (68% male). Regarding diagnosis, African-Americans also had the highest proportion of hepatitis C candidates. African-Americans also had the highest proportion of patients with cholestatic liver disease, and Asian and Other candidates had the highest proportions of non-cholestatic liver disease. Hepatitis B was not the sole cause of liver failure for any individual racial/ethnic group, congruent with previously reported national data (23). The median MELD at listing was at least two points higher for African-Americans compared to Whites, Hispanics or Asians. At transplant, African-Americans and Hispanics had the highest median MELD scores. Hispanics had a significantly higher proportion of blood type O candidates. Whites had the highest proportion of blood type A candidates. Asians had the highest respective proportions of blood type B and AB candidates. With regards to co-morbidities, African-Americans had the highest proportion of dialysis dependent candidates. 8–10% of candidates had diabetes, and Hispanics had significantly more diabetics versus the other groups. Mean body mass index was lowest among Asian candidates and highest among Hispanic candidates. African-Americans had the highest proportion of candidates on dialysis at registration of all groups.
Table 1.
Clinical Characteristics of Liver Transplant Waitlist Candidates in the Study Cohort by Race/Ethnicity in the MELD Era (n=39,114)*
| Variable | White (n=28989) | African-American (n=2865) | Hispanic (n=5452) | Asian (n=1450) | Other (n=358) | p-value |
|---|---|---|---|---|---|---|
| Age at waitlist registration (yrs) (SD) | 52.6 (9.3) | 49.5 (10.5) | 52.0 (9.5) | 53.6 (10.5) | 50.5 (9.5) | <.0001 |
| Sex | <.0001 | |||||
| Male (%) | 19203 (66.2) | 1702 (59.4) | 3341 (61.3) | 989 (68.2) | 207 (57.8) | |
| Female (%) | 9786 (33.8) | 1163 (40.6) | 2111 (38.7) | 461 (31.8) | 151 (42.2) | |
| Diagnosis | <.0001 | |||||
| Cholestatic (n) (%) | 2850 (9.8) | 314 (11.0) | 299 (5.5) | 62 (4.3) | 29 (8.1) | |
| Non-Cholestatic (n) (%) | 10311 (35.6) | 674 (23.5) | 1944 (35.7) | 638 (44.0) | 145 (45.5) | |
| Hepatitis C (n) (%) | 11511 (39.7) | 1405 (49.0) | 2566 (47.1) | 378 (26.1) | 122 (34.1) | |
| Acute Hepatic Necrosis (n) (%) | 429 (1.5) | 74 (2.6) | 98 (1.8) | 83 (5.7) | 13 (3.6) | |
| Malignant Neoplasm (Non- HCC) (n) (%) | 650 (2.2) | 65 (2.3) | 141 (2.6) | 99 (6.8) | 18 (5.0) | |
| Metabolic Disease (n) (%) | 630 (2.2) | 18 (0.6) | 41 (0.8) | 13 (0.9) | 5 (1.4) | |
| Other (n) (%) | 2608 (9.0) | 315 (11.0) | 363 (6.7) | 177 (12.2) | 26 (7.3) | |
| Blood Type | <.0001 | |||||
| A (n) (%) | 11857 (40.9) | 786 (27.4) | 1673 (30.7) | 362 (25.0) | 122 (34.0) | |
| B (n) (%) | 3130 (10.8) | 589 (20.6) | 551 (10.1) | 383 (26.4) | 37 (10.3) | |
| O (n) (%) | 12901 (44.5) | 1371 (47.9) | 3102 (56.9) | 582 (40.1) | 189 (52.7) | |
| AB (n) (%) | 1098 (3.8) | 119 (4.2) | 126 (2.3) | 123 (8.5) | 11 (3.1) | |
| Mean Body Mass Index (SD) | 28.6 (5.8) | 28.2 (6.0) | 28.9 (5.7) | 24.6 (4.3) | 29.4 (6.1) | <.0001 |
| Renal Failure (% dialysis) | 817 (2.8) | 173 (6.0) | 225 (4.1) | 56 (3.9) | 16 (4.5) | <.0001 |
| Diabetes (n) (%) | 2551 (8.8) | 264 (9.2) | 561 (10.3) | 117 (8.1) | 37 (10.3) | .0050 |
| Coronary Artery Disease (n) (%) | 460 (1.6) | 36 (1.3) | 60 (1.1) | 10 (0.7) | 5 (1.4) | .0007 |
| Chronic Obstructive Pulmonary Disease (n) (%) | 492 (1.7) | 30 (1.1) | 47 (0.9) | 8 (0.6) | 6 (1.7) | <.0001 |
| MELD Score at Listing, (median) (25th,75th %ile) | 14 (11, 19) | 17 (12, 23) | 15 (11, 20) | 13 (9, 19) | 16 (12, 22) | |
| MELD Score at Transplant (median)(25th, 75th %ile) | 18 (13,24) | 20 (13,26) | 20 (12,27) | 14 (7, 21) | 19 (14, 24) |
Percentages are column percents.
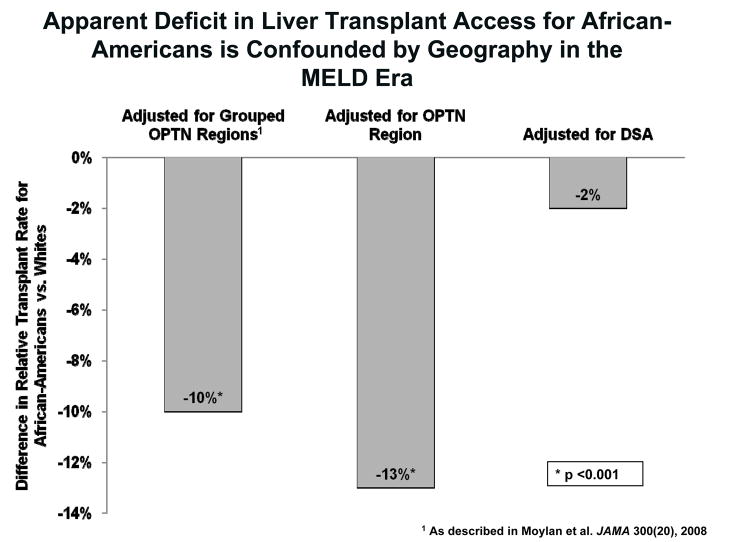
The geographic variation in measured disparities in liver transplant rates between minorities and Whites is represented in Figure 1. Each bar represents the difference in adjusted liver transplant rates between African-Americans and Whites for a defined unit of geographic comparison. When comparing relative transplant rates between African-Americans and Whites registered in the same quadrant of the country (grouped contiguous OPTN regions: Northeast, Southeast, Northwest, Southwest), consistent with the geography adjustment in (10), African-Americans had a significant 10% lower adjusted transplant rate than Whites, with a covariate-adjusted hazard ratio (HR) of 0.90 with p=0.0001. Similarly, when comparing African-Americans and Whites registered in the same OPTN region, the relative transplant rate was also significantly lower for African-Americans (HR=0.87, p=0.0001). However, when comparing African-Americans and Whites registered in the same DSA, the disparity between African-Americans and Whites was minimal and not statistically significant (HR=0.98, p=0.50).
Figure 1.
Apparent Deficit in Liver Transplant Access for African-Americans is Confounded by Geography in the MELD Era. This figure demonstrates the differences in relative transplant rates between African-Americans and Whites when compared using three different methods of geographic adjustment. When adjusting for geography by grouping adjacent OPTN regions together, or even by individual OPTN regions, African-Americans appeared to have a significantly lower liver transplant rate compared to Whites in those same geographic areas. The DSA is the primary geographic unit of liver allocation in the United States. When comparing African-Americans and Whites registered in the same DSA, no differences in access to liver transplantation from the waiting list are noted.
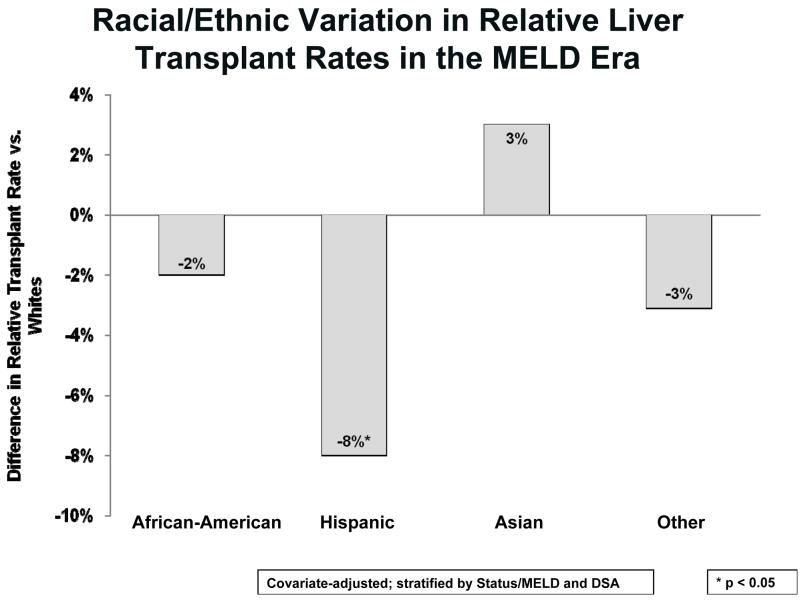
Figure 2 displays the risk-adjusted differences in relative liver transplant rates for Hispanics, Asians, and those of Other race/ethnicity, each versus Whites. The non-significant 2% lower rate for African-Americans is as described in Figure 1 adjusted for DSA. In contrast to African-Americans, Hispanics had a significantly lower liver transplant rate than Whites by 8% (HR=0.92, p=0.011). Candidates who were Asian or of Other race/ethnicity had no significant differences in liver transplant rates compared to Whites. Interactions tests between race/ethnicity and sex and diagnosis were not significant.
Figure 2.
Racial/Ethnic Variation in Relative Liver Transplant Rates in the MELD Era. Based on DSA-level and other risk-adjustment, the relative transplant rates for Hispanics are significantly lower than Whites. African-Americans had relatively similar transplant rates compared to Whites, as in Figure 1. Despite a small trend toward a 3% higher transplant rate for Asians compared to Whites, this was not significant. For Other or mixed race candidates, there was a non-significant trend toward a 3% lower liver transplant rate compared to Whites. The models incorporated all changes in MELD score reported to the SRTR.
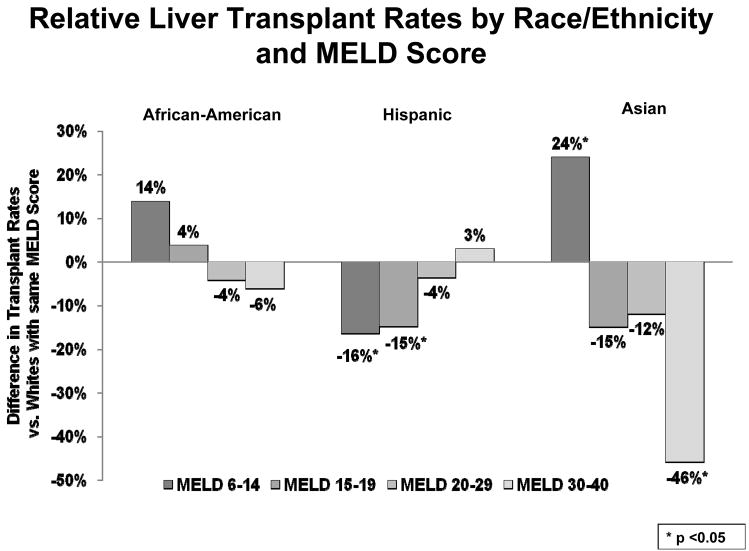
In order to sharpen our understanding of which subsets of minority candidates affected by the disparity, we determined differences in transplant rates within each MELD strata (Figure 3). African-Americans and Whites had similar liver transplant rates at all MELD scores, but displayed slightly lower, but non-significant, transplant rates in the higher MELD spectrum. Asian candidates with lower MELD scores had a 24% higher transplant rate relative to Whites at the same scores (HR=1.24, p=0.024), but the sickest Asian patients had nearly half the transplant rate of their White counterparts (HR=0.54, p=0.004) When registered in the same DSA, Hispanic candidates displayed a 15–16% lower transplant rates compared to Whites when they had MELD scores less than 20, with HR=0.84 (p=0.021) for MELD 6–14 and HR=0.85 (p=0.009) for MELD 15–19. At MELD scores above 20, Hispanics and Whites did not have different transplant rates within DSA.
Figure 3.
Relative Liver Transplant Rates by Race/Ethnicity and MELD Score. After further stratifying each racial group by MELD score, several findings were prominent. African-Americans did not have significantly different transplant rates compared to Whites at any MELD score. Hispanic candidates had significantly lower transplant rates compared to Whites with similar disease severity at MELD scores less than 20, by 15–16%. Asian candidates demonstrated 24% higher liver transplant rates at the lowest MELD scores, but 46% lower transplant rates at the highest MELD scores. Of note, the analysis incorporated all changes in MELD score reported to the SRTR, and these transplant rate differences reflect the outcomes while candidates remained at the MELD score within that range.
Racial/ethnic differences were noted in other waitlist events as well. Compared to Whites, African-Americans had significantly lower relative wait-list removal rates for reasons other than transplant (HR=0.93, p=0.004). Similarly, Asians also had significantly lower non-transplant removal rates (HR=0.89, p=0.009). Hispanics trended toward a lower removal rate compared to Whites, but this was not statistically significant (HR=0.96, p=0.073). Regarding racial/ethnic differences in waitlist mortality, African-Americans had a 37% lower mortality rate while active on the waitlist compared to Whites (HR=0.63, p<0.0001). Asians maintained a 27% lower mortality rate versus Whites (HR=0.73, p=0.007). Hispanics did not have a significantly different death rate compared to Whites. There were no significant differences in waitlist inactivation rates by race/ethnicity.
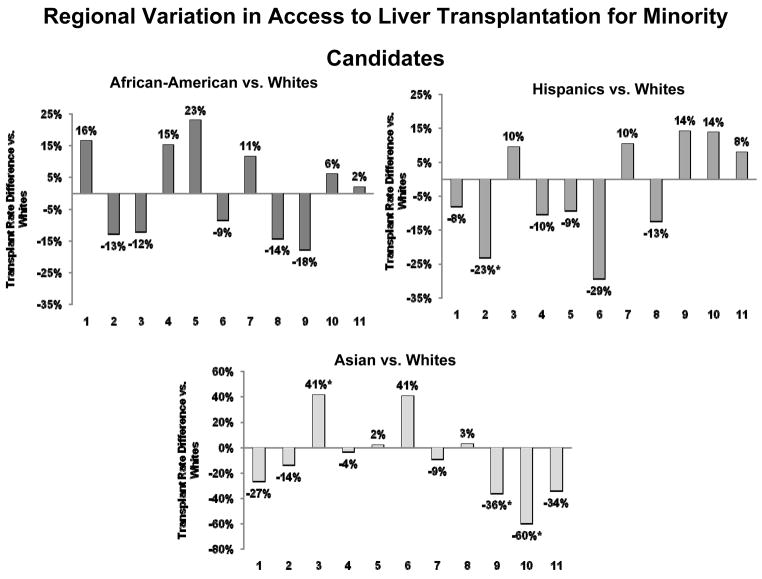
DSA-adjusted transplant rates for African-Americans, Hispanics, and Asians each varied extensively across OPTN regions (Figure 4). However, after covariate adjustment, 7 of 11 OPTN regions displayed no differences in transplant rates when the respective minority groups were compared to Whites. No single region demonstrated significant disparities for all minority groups. African-Americans did not have any statistically significant differences in transplant rates compared to Whites in any OPTN region. Region 2, which includes Pennsylvania, Maryland, New Jersey, Delaware, West Virginia, Washington DC and northern Virginia, demonstrated significantly lower transplant rates for Hispanics compared to Whites (HR=0.77, p=0.021). Asian candidate access to liver transplantation varied widely. In Region 3, which includes most of the southeastern United States, Asians demonstrated a 41% higher transplant rate than Whites (HR=1.41, p=0.051). Regions 9 and 10, which contain the DSAs that serve New York state, western Vermont, Ohio, Indiana, and Michigan, demonstrated 37–60% lower transplant rates for Asian candidates, with HR=0.63 (p=0.009) for Region 9 and HR=0.40 (p=0.037) for Region 10.
Figure 4.
Regional Variation in Access to Liver Transplantation for Minority Candidates. Across all minority groups, the 11 OPTN regions that cover the United States demonstrated an impressive degree of variation in relative transplant rates compared to White candidates. Four regions demonstrated significantly different transplant rates for a minority group compared to Whites. Five regions trended toward lower transplant rates for African-Americans compared to Whites in those respective regions. Five regions demonstrated trends toward lower liver transplant rates for Hispanics, and Region 2 reached statistical significance in this regard. For Asian candidates, seven regions trended toward lower access to liver transplantation compared to Whites. Regions 9 and 10 had much lower transplant rates for these candidates compared to their respective White counterparts, but Region 3 had significantly higher transplant rates for Asians. The analysis incorporated all changes in MELD score reported to the SRTR,
Discussion
Racial/ethnic disparities in transplantation have been framed as the culmination of a series of successive barriers in access to care that marginalize minorities with organ failure (2, 17, 24–26). Our findings demonstrate racial/ethnic variation in access to liver transplantation at a critical step – the transition from transplant candidate to transplant recipient. Racial/ethnic disparities at this step in the transplant process are important, because liver transplant candidates are a highly selected population that have overcome clinical and non-clinical barriers to get on the waitlist (17). The clinical outcome of these candidates is based on individual disease progression, the continued ability to access specialized hepatological care, and on efficacy of the liver allocation system. We observed significantly lower liver transplant rates for subgroups of minority candidates compared to their White counterparts, particularly among Hispanic candidates and Asians with high MELD scores. Importantly, these lower relative transplant rates were not accompanied by higher rates of alternate waitlist outcomes including death, removal for non-transplant reasons, and inactivation. We also demonstrated a “difference in differences” in liver transplant rates, by showing geographic heterogeneity in racial/ethnic differences in access to transplant. To our knowledge, our study is the first to broadly address disparities in access to liver transplantation across all racial/ethnic groups, and to identify the importance of properly accounting for geographic variation.
Geographic variation is being increasingly recognized as a threat to optimizing the use of donated organs (12, 27), and in our study had a clear effect on the measured differences in transplant rates between minorities and Whites. African-Americans did not have different adjusted transplant rates compared to Whites when compared at the DSA level. Moylan and colleagues previously studied access to liver transplant for African-American and White candidates and also found no differences in transplant rates (10). Their study imprecisely adjusted for geography by grouping OPTN regions together, suggesting, for example, that candidates in Florida have similar access to transplant as those in Texas, even though organ availability is highly variable across DSAs. Further, they did not evaluate ethnic minorities other than African- Americans, and did not account for changes in the severity of liver disease over time while candidates were waitlisted. It was therefore appropriate to evaluate racial/ethnic disparity in access to transplantation at the DSA level to improve precision in our estimates. Our findings suggest that the locale where candidates seek care for their liver disease modifies the effect of race/ethnicity on access to liver transplantation.
Volk and colleagues assessed disparities in access to liver transplantation and suggested that differences in median waiting time between DSAs, a proxy for organ availability, mediated racial/ethnic inequity (16). Our analyses differ from their work in several important ways. In contrast to Volk et al., which was focused on African-Americans and Hispanics, our approach evaluated disparities across all racial/ethnic groups, including Whites, African-Americans, Hispanics, Asians, and those of mixed or other ethnic heritage. We specifically focused on identification of residual disparities within MELD subgroups and geographic areas. Methodologically, this focus was manifested in our measurement of racial/ethnic differences in transplant rates during active waitlist time and by our exhaustive treatment of the intersection of race/ethnicity, MELD score, and geography. In addition, we gave due consideration to competing risks including inactivation, removal for reasons other than transplant, and death. Volk et al. considered any difference in transplant rates after registration to be a disparity. However, in view of the dynamic nature of the liver transplant waitlist, their failure to identify a disparity in access among Hispanics was predicated, in the absence of analysis, on there being no racial/ethnic differences in rates of inactivation, re-activation, removal for reasons other than transplant, and granting of MELD exception scores. Our approach addressed these realities for African-American, Hispanic, and Asian candidates in the context of allocation rules that are intended to apply to all candidates, and also accounted for the extent to which geography modified the effect of race and ethnicity.
An important finding in this study was the lack of an overall disparity in transplant rates between African-Americans and Whites. This was also true across MELD scores and OPTN regions. This contrasts with Reid et al., a pre-MELD era study, which reported significantly disparities in access to transplant for African-Americans (9). African-Americans did present with more advanced disease at registration compared to Whites in our study, but the equitable transplant rate that was observed after accounting for this advanced disease is the intended result of a medical urgency-based organ allocation system. However, registration on the waitlist with more advanced disease signifies an important and persistent issue in the continuum of care of patients with liver disease, as suggested by Moylan et al. (10). The delay in waitlist registration likely symbolizes impaired access to quality pre-transplant care, and may indicate that African-Americans suffer from greater disease-related morbidity before registration (2, 17, 28, 29). Further study on the timeliness and appropriateness of physician referral of African-Americans with chronic liver disease to transplant centers is clearly a necessity.
The disparities noted in this analysis must be considered in the context of whether patients receive a survival benefit with a liver transplant. Once a patient reaches a MELD of 12 or higher, the survival benefit of receiving liver transplant is realized (30). Hispanics with MELD scores of 6–14 had a 16% lower transplant rate and those with MELD scores 15–20 had a 15% lower transplant rate than Whites with the same respective MELD scores. Asians with MELD scores of 6–14 had a 24% higher transplant rate, while those with highest MELD scores had a 46% lower transplant rate compared to Whites with the same respective MELD scores. Minority candidates who have lower relative transplant rates at the lowest MELD scores arguably do not suffer from a “disparity”, as these patients with well-compensated disease are unintentionally spared the risk of a liver transplant. Conversely, Asians were potentially harmed by a relatively higher transplant rate at low MELD scores (22). The lower transplant rate at high MELD scores also disadvantaged Asians because liver transplantation provides, at this end of the MELD spectrum, the most survival benefit. In aggregate, our data indicate which subgroups of minority candidates truly have impaired access to liver transplantation.
Another contribution of this analysis is the demonstration of variation in relative transplant rates for members of individual racial/ethnic groups by geography. For Hispanic candidates, Region 2 had the lowest transplant rates, with notable trends toward lower access in five other OPTN regions. Transplant rates for Asian candidates varied tremendously across the country, with three regions showing significantly different access for those candidates compared to Whites. Region 3 demonstrated more than a 40% higher relative rate of transplant for Asians, and Regions 9 and 10 had significantly lower relative transplant rates for Asians versus Whites. Additionally, five other regions trended toward lower transplant rates for Asians. Although African-Americans trended toward lower transplant rates in five regions, there was no statistically significant difference in adjusted transplant rates between African-Americans and Whites in any region. The geographic analysis has some limitations with regard to statistical power, but identification of the geographic areas that have marginal access for a particular racial/ethnic group in a given area may help providers gain insight into practice patterns that may be responsible for these observations. This variation may be tied to differences in clinical decision-making by transplant surgeons that may be driven by the candidate’s clinical condition, but also the local and regional realities of organ availability. Further, providers may manage their waitlist differently depending on their program’s specific candidates, the proximity of other liver transplant programs, organ acceptance patterns, and many other factors. Geographic variation may modify racial/ethnic effects via providers themselves. Some areas may have more diversity among transplant providers, and or may provide patients the opportunity for more racially/ethnically concordant patient-physician interactions that may ultimately decrease undesirable waitlist outcomes for minority candidates.
Our study has some limitations. While our study cohort represents the largest to date to describe racial/ethnic disparities in liver transplant rates, our statistical models are limited by the covariates in the SRTR data. We have previously recommended the collection of more granular data specifically with regard to measuring disparities in transplantation (17). Patients were also censored at the time they received a MELD exception for hepatocellular carcinoma, which is growing in incidence among minorities and accounts for 15 to 20% of the U.S. liver transplant volume (11, 31). Our focus was to identify disparities by using a clinically homogenous population, and MELD exceptions artificially boost access to liver transplant. Differential access to these exceptions depending on variation in pathophysiology, geographic differences in the granting of MELD exceptions, and the use of adjuvant therapies might have biased our understanding of disparities if we had continued observation of patients beyond the granting of MELD exceptions. Finally, these data represent summative population-based estimates, and are not immediately usable by individual patients trying to determine what their chances of receiving a liver transplant compared to others, or deciding where to pursue liver transplant candidacy. The effect of race or ethnicity on transplant rates cannot be ascribed to individual patients in that group.
Previous authors have noted that while not specifically prescribed by the Final Rule, allocation based on medical urgency should have the beneficent effect of racial/ethnic equity (32). Most transplant physicians, surgeons, and policy makers would agree that the current liver allocation system is fairer than a decade ago, but as we have demonstrated, it is not yet equitable. Our work demonstrates that members of rapidly growing segments of the population that develop liver disease have impaired access to a life-saving treatment. Balancing values in the allocation of scarce medical resources is unique to transplantation, and will continue to challenge the transplant community until organ supply meets demand. While providing members of certain racial/ethnic groups with an advantage in allocation in order to improve equity seems unethical, the public and the transplant community have a responsibility to thoroughly evaluate allocation policy changes for the potential to precipitate or exacerbate unintended disparities. By remaining vigilant and cognizant of these important issues in the development of transplant policy development and in research on disparities, equity and sensible liver transplant allocation policy need not be mutually exclusive.
Footnotes
Presented in part at the Plenary Session of the American Transplant Congress 2009, Boston, Massachusetts, June 3, 2009.
References
- 1.Day JC U.S. Bureau of the Census CPR. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. Washington, DC: U.S. Government Printing Office; 1996. [Google Scholar]
- 2.Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: Biological, cultural, or socioeconomic factors. Hepatology. 2008;47(3):1058–1066. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]
- 3.Kemmer N, Zacharias V, Kaiser TE, Neff GW. Access to Liver Transplantation in the MELD Era: Role of Ethnicity and Insurance. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 4.Kemmer N, Neff GW. Ethnic variations in chronic liver diseases. Dig Dis Sci. 2008;53(5):1339–1344. doi: 10.1007/s10620-007-9992-0. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167(18):1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Siegel AB, Davila JA, Shaib YH, Cayton-Woody M, McBride R, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol. 2006;44(1):158–166. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 9.Reid AE, Resnick M, Chang Y, Buerstatte N, Weissman JS. Disparity in use of orthotopic liver transplantation among blacks and whites. Liver Transpl. 2004;10(7):834–841. doi: 10.1002/lt.20174. [DOI] [PubMed] [Google Scholar]
- 10.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300(20):2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scientific Registry of Transplant Recipients. 2009 Available from: http://www.ustransplant.org.
- 12.Roberts JP, Dykstra DM, Goodrich NP, Rush SH, Merion RM, Port FK. Geographic differences in event rates by model for end-stage liver disease score. Am J Transplant. 2006;6(10):2470–2475. doi: 10.1111/j.1600-6143.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 13.Trotter JF, Osgood MJ. MELD scores of liver transplant recipients according to size of waiting list: impact of organ allocation and patient outcomes. JAMA. 2004;291(15):1871–1874. doi: 10.1001/jama.291.15.1871. [DOI] [PubMed] [Google Scholar]
- 14.Ubel PA, Caplan AL. Geographic favoritism in liver transplantation--unfortunate or unfair? N Engl J Med. 1998;339(18):1322–1325. doi: 10.1056/NEJM199810293391811. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad J, Bryce CL, Cacciarelli T, Roberts MS. Differences in access to liver transplantation: disease severity, waiting time, and transplantation center volume. Ann Intern Med. 2007;146(10):707–713. doi: 10.7326/0003-4819-146-10-200705150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Volk ML, Choi H, Warren GJ, Sonnenday CJ, Marrero JA, Heisler M. Geographic variation in organ availability is responsible for disparities in liver transplantation between Hispanics and Caucasians. Am J Transplant. 2009;9(9):2113–2118. doi: 10.1111/j.1600-6143.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 17.Mathur AK, Sonnenday CJ, Merion RM. Race and Ethnicity in Access to and Outcomes of Liver Transplantation: A Critical Literature Review. American Journal of Transplantation. 2009;9(12):2662–2668. doi: 10.1111/j.1600-6143.2009.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4 (Suppl 9):114–131. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington DC: National Academic Press; 2002. [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2. Hoboken, N.J.: Wiley; 2002. [Google Scholar]
- 21.Organ Procurement and Transplantation Network. Health Resources and Services Administration, HHS. Final rule Fed Regist. 1999;64(202):56650–56661. [PubMed] [Google Scholar]
- 22.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg CL, Steffick DE, Edwards EB, Heimbach JK, Magee JC, Washburn WK, et al. Liver and intestine transplantation in the United States 1998–2007. Am J Transplant. 2009;9(4 Pt 2):907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280(13):1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 25.Alexander GC, Sehgal AR. Why hemodialysis patients fail to complete the transplantation process. Am J Kidney Dis. 2001;37(2):321–328. doi: 10.1053/ajkd.2001.21297. [DOI] [PubMed] [Google Scholar]
- 26.Ladin K, Rodrigue JR, Hanto DW. Framing disparities along the continuum of care from chronic kidney disease to transplantation: barriers and interventions. Am J Transplant. 2009;9(4):669–674. doi: 10.1111/j.1600-6143.2009.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemmer N, Safdar K, Kaiser T, Zacharias V, Neff GW. Impact of geographic location on access to liver transplantation among ethnic minorities. Transplantation. 2008;85(2):166–170. doi: 10.1097/TP.0b013e31816223f8. [DOI] [PubMed] [Google Scholar]
- 28.Kemmer N, Neff GW. Liver Transplantation in the Ethnic Minority Population: Challenges and Prospects. Dig Dis Sci. 2009 doi: 10.1007/s10620-009-0803-7. [DOI] [PubMed] [Google Scholar]
- 29.Kemmer N, Zacharias V, Kaiser TE, Neff GW. Access to liver transplantation in the MELD era: role of ethnicity and insurance. Dig Dis Sci. 2009;54(8):1794–1797. doi: 10.1007/s10620-008-0567-5. [DOI] [PubMed] [Google Scholar]
- 30.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemmer N, Neff G, Secic M, Zacharias V, Kaiser T, Buell J. Ethnic differences in hepatocellular carcinoma: implications for liver transplantation. Dig Dis Sci. 2008;53(2):551–555. doi: 10.1007/s10620-007-9872-7. [DOI] [PubMed] [Google Scholar]
- 32.Axelrod DA, Pomfret EA. Race and sex disparities in liver transplantation: progress toward achieving equal access? JAMA. 2008;300(20):2425–2426. doi: 10.1001/jama.2008.732. [DOI] [PubMed] [Google Scholar]