Abstract
The conditioning of cocaine's subjective actions with environmental stimuli may be a critical factor in long-lasting relapse risk associated with cocaine addiction. To study the significance of learning factors in persistent addictive behavior as well as the neurobiological basis of this phenomenon, rats were trained to associate discriminative stimuli (SD) with the availability of i.v. cocaine vs. nonrewarding saline solution, and then placed on extinction conditions during which the i.v. solutions and SDs were withheld. The effects of reexposure to the SD on the recovery of responding at the previously cocaine-paired lever and on Fos protein expression then were determined in two groups. One group was tested immediately after extinction, whereas rats in the second group were confined to their home cages for an additional 4 months before testing. In both groups, the cocaine SD, but not the non-reward SD, elicited strong recovery of responding and increased Fos immunoreactivity in the basolateral amygdala and medial prefrontal cortex (areas Cg1/Cg3). The response reinstatement and Fos expression induced by the cocaine SD were both reversed by selective dopamine D1 receptor antagonists. The undiminished efficacy of the cocaine SD to elicit drug-seeking behavior after 4 months of abstinence parallels the long-lasting nature of conditioned cue reactivity and cue-induced cocaine craving in humans, and confirms a significant role of learning factors in the long-lasting addictive potential of cocaine. Moreover, the results implicate D1-dependent neural mechanisms within the medial prefrontal cortex and basolateral amygdala as substrates for cocaine-seeking behavior elicited by cocaine-predictive environmental stimuli.
The conditioning of cocaine's pharmacological actions with discrete environmental stimuli has been implicated as a major factor in the abuse potential of this drug (1). Both retrospective (2) and controlled laboratory studies (3–5) show that such stimuli can evoke drug desire that may lead to the resumption of drug use in abstinent individuals. Drug-related stimuli may also elicit automatic responses that lead to drug-seeking behavior and relapse without the intervention of distinct feelings of craving (6, 7). Learned responses to drug-related stimuli, therefore, represent a possibly critical element contributing to the chronic relapsing nature of cocaine and other drug addiction (8, 9).
Consistent with a role of learning factors in the initiation of drug-seeking behavior, cocaine-related stimuli can elicit strong recovery of responding at a lever previously associated with i.v. cocaine infusions in animal models of relapse (10, 11). However, little information is available about the perseverance of the motivating actions of such stimuli over prolonged periods of abstinence and the neurobiological substrates mediating these effects. In humans, relapse risk is typically greatest during the first 6 months of abstinence but may persist for substantially longer periods of time (1, 8, 12). Better understanding of the environmental conditions contributing to long-lasting vulnerability to relapse and the neurobiological basis of this phenomenon will be of substantial clinical benefit.
In work that has begun to address this issue, the efficacy of a cocaine-predictive discriminative stimulus to elicit responding at a previously active, cocaine-paired lever was found to remain unaltered over 8 days of intermittent testing (11). These findings indicated that the behavioral actions of cocaine-related environmental stimuli are resistant to extinction despite repeated nonreinforced exposure to these cues. Here, we have investigated the significance of drug-related environmental stimuli in enduring vulnerability to relapse by examining whether a drug-predictive stimulus retains its efficacy to induce cocaine-seeking behavior after long-term abstinence. Additionally, to identify sites that may participate in the control of conditioned cocaine-seeking behavior, the rats' brains were examined for regions showing neural activation after exposure to the cocaine cue as measured by increased expression of Fos, the protein product of the immediate-early gene c-fos (13, 14). Lastly, because of evidence that cue-induced cocaine craving in humans is associated with neural activation in dopamine-rich forebrain regions (4, 5, 15) and that cocaine cues increase dopamine release within these regions in rats (11), a third objective was to determine whether the behavioral effects of the cocaine-predictive stimulus and its effects on Fos expression are sensitive to pharmacological antagonism of dopamine neurotransmission.
Materials and Methods
Subjects.
Male Wistar rats (Charles River Breeding Laboratories) weighing 250–300 g at the beginning of the experiment were used. Rats were housed in groups of two or three in a temperature-controlled (22°C) vivarium on a reverse 12-h light/12-h dark cycle with ad libitum access to food and water. All procedures were conducted in strict adherence to the National Institutes of Health guidelines.
Drugs.
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda) was dissolved in sterile physiological saline at a concentration of 0.25 mg/0.1 ml. Drug or vehicle solution was infused at a volume of 0.1 ml over 4 s. SCH 39166 and SCH 23390 (Schering-Plough) were dissolved in ethanol (1 μg/μl) or methanol (1 μg/ml), respectively, diluted with saline to a concentration of 30 μg/ml, and injected s.c. 25–30 min before test sessions.
Behavioral Training and Testing Procedures.
Surgical preparation for i.v. cocaine self-administration was performed as described in ref. 16. Behavioral testing equipment and i.v. self-administration protocols used were also identical to those in ref. 16.
Self-Administration and Discrimination Training.
The self-administration and conditioning procedures have been described in detail (11). Briefly, the purpose of these procedures was to train rats to self-administer i.v. cocaine, while simultaneously establishing discriminative stimuli (SD) associated with cocaine availability vs. nonavailability. Once rats developed stable levels of daily cocaine intake, they were subjected to a discrimination learning regimen as follows. In three daily 1-h sessions, either cocaine or saline was available as the only infusion solution. Each training day included one saline and two cocaine sessions conducted in random sequence. Sessions were initiated by extension of the levers into the chambers and concurrent onset of the respective SD which remained present until termination of the session by retraction of the levers. The SD associated with cocaine availability (S+) consisted of an intermittent tone (7 kHz, 70 dB), whereas the SD, predictive of the saline vehicle solution (that is, S− or nonreward) consisted of continuous illumination of the self-administration chamber's house light. To prevent accidental overdosing, drug infusions were followed by a 20-s time-out period, signaled by illumination of a white cue light during which time the lever remained inactive. Saline infusions produced by lever-presses during S− sessions were similarly followed by a 20-s time-out period, signaled by a white noise (70 dB). In both the S+ and S− conditions, two levers were present. Only the right lever was active and, when depressed, produced an i.v. infusion of the respective solution. The left lever was inactive, but responses at this lever were recorded as a measure of nonspecific activation. At 1 day after reaching a training criterion, defined as stable cocaine-maintained responding (±10% over 3 successive days) and negligible responding during saline availability, each animal was placed on extinction conditions. Extinction sessions began by extension of the levers without presentation of the SD. Responses at the previously active lever activated the syringe pump motor only but had no other programmed consequences. These sessions lasted 1 h and were conducted once daily until a criterion of less than four responses per session over 3 consecutive days was reached.
Cue Exposure Test Procedures.
After completion of extinction training, the rats were tested for the degree of recovery of responding at the previously active lever induced by exposure to either the cocaine S+ or S− alone and the modification of the effects of the cocaine S+ by SCH 39166 (10 μg/kg). These tests were conducted under two conditions as follows. After reaching the extinction criterion, each individual animal was randomly assigned to either an immediate or delayed testing condition. Tests in rats assigned to the immediate condition were conducted on the next day. Rats designated for delayed testing remained in their home cages. Before the delayed test (conducted 4 months after completion of the first extinction phase), these animals were again briefly tested under extinction conditions to reconfirm stable responding at or below the extinction criterion. All tests were conducted by noncontingently presenting rats with either the S+ or S− throughout a single 1 h session without the availability of cocaine or saline. Rats in both the immediate and delayed test conditions were randomly divided into three groups for testing with the S+, the S−, or the S+ preceded by the administration of SCH 39166. Presentation of the respective SD began simultaneously with extension of the levers and terminated with their retraction at the end of the test. As during self-administration training, responses at the active lever were followed by a 20-s signaled time-out period. After completion of the tests, all rats were killed, and their brains were processed for Fos immunohistochemistry as described below.
Test of Dose Dependency of D1 Antagonist Effects on S+-Induced Responding.
To confirm further an involvement of the D1 receptor in the behavioral effects of the cocaine S+ and to establish the dose-dependency of D1 antagonist treatments on cocaine-seeking behavior, an additional group of rats was prepared for testing with three doses of SCH 23390, a D1 antagonist analog of SCH 39166. All experimental procedures were identical to those in the immediate test condition described above, except that a within-subjects design involving cue exposure tests at 3-day intervals was used. All rats were tested with the S− and subsequently with the S+ preceded by vehicle administration before three drug tests involving exposure to the S+ preceded by SCH 23390. To control for order effects, rats received different doses of SCH 23390 (2.5, 5.0, and 10.0 μg/kg) in random sequence across the 3 drug-test days.
Self-Administration Procedures for Tests of Cocaine-Dependent Fos Expression.
To examine whether the distribution of Fos immunoreactivity associated with exposure to the cocaine S+ differs from that produced by cocaine itself, levels and distribution of Fos were determined in rats after 60 min of i.v. self-administration of cocaine. These animals were trained initially to respond for food pellets on a fixed-ratio 1 schedule of reinforcement as described in ref. 11. After acquisition of food-reinforced lever-pressing, the animals were trained to self-administer cocaine on an fixed-ratio 1 schedule and received daily 2-h access to cocaine for a total of 2 weeks (cocaine chronic group). Because repeated cocaine exposure can greatly desensitize cocaine-dependent Fos expression (17, 18), a 1-week abstinence period was imposed before a final 60-min self-administration session to allow for some recovery of cocaine-dependent Fos expression. To provide a measure of acute cocaine effects, Fos immunoreactivity was also measured in a group of previously drug-naive rats after the first cocaine self-administration session (cocaine acute group) after acquisition of food-reinforced responding. A third group was trained and tested identically to the animals in the latter group but received access to i.v. saline only. This group (saline group) served as a control against which cocaine-induced changes in Fos immunoreactivity were compared.
Immunohistochemistry.
Rats remained in the test chambers for 30 min after termination of the cue exposure or cocaine self-administration sessions. The animals then were deeply anesthetized by CO2 inhalation and transcardially perfused with 20 ml of isotonic saline followed by a solution consisting of 250 ml of 4% (vol/vol) paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were removed from the skull, postfixed overnight with 2% (vol/vol) paraformaldehyde in PBS at 4°C, rinsed with PBS, and cryoprotected in 16% (vol/vol) sucrose/PBS solution until sectioning. Brains were sliced coronally into 35-μm sections by using a vibratome (Leica, Deerfield, IL) and stored at −20°C in 30% (vol/vol) ethylene glycol/25% (vol/vol) glycerol/0.05 M phosphate buffer. Sections were rinsed with PBS and incubated with a Fos-specific rabbit polyclonal antibody (Santa Cruz Biotechnology) at a dilution of 1:1,000 for 12 h at 4°C in PBS containing 1.5% (vol/vol) goat serum and 0.3% Triton X-100. Sections then were processed with an ABC kit (Vector Laboratories), and peroxidase activity was revealed by 0.003% H2O2 and 0.05% 3,3-diaminobenzidine-4 HCl and nickel enhanced, resulting in a black-brown precipitate in specifically immunolabeled structures. Sections were mounted on Superfrost+ slides (Fisher Scientific), air dried, coverslipped, and analyzed by using a bright field microscope (Axiophot, Zeiss). Images of the regions of interest were digitally collected and counted by using a computer-assisted image analysis system (mcid, Imaging Research, St. Catherines, ON, Canada). For each of the sections, background was considered to be a portion of the same area not expressing Fos-positive nuclei. For each animal, the mean bilateral counts of Fos-immunoreactive nuclei for six sections per brain region were determined. Brain regions were identified by using the atlas of Paxinos and Watson (19).
Data Analysis.
Differences in the number of lever-press responses among rats assigned to the S+ vs. S− vs. S+ and SCH 39166/SCH 23390 cue exposure conditions as well as differences between the number of cue-induced responses vs. mean extinction responses at criterion (that is, responses averaged across the final three sessions of the extinction phase) were analyzed by mixed-factorial ANOVA. Differences among individual means were verified subsequently by Newman–Keuls post hoc tests and analysis of linear trends. Differences in the number of Fos-positive neurons among groups assigned to the S+, S−, S+ & SCH 39166, and cocaine self-administration conditions were analyzed separately for each brain region by one-way ANOVA, followed by Newman–Keuls post hoc tests.
Results
Conditioning and Extinction Phase.
All rats (n = 34) developed stable cocaine self-administration and ceased responding during saline sessions (Fig. 1a, left column). The mean (±SEM) number of days to training criterion was (16.5 ± 1.17). During subsequent sessions in which i.v. drug or vehicle infusions and the corresponding SD were withheld, the rats reached the extinction criterion (less than four responses per session over 3 consecutive days) within 15.0 ± 2.8 days (Fig. 1 a and b left and center columns).
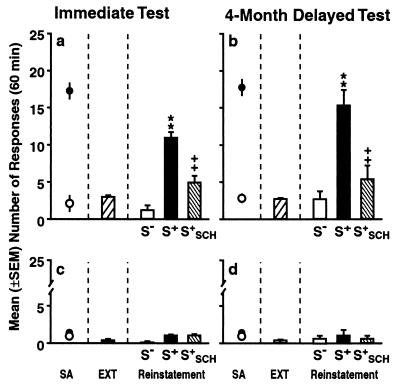
Figure 1.
Lever-press responses during the self-administration training, extinction, and cue exposure test phases at the active (a and b) and inactive (c, d) lever. Self-administration (SA): cocaine-reinforced (●) and saline/nonreinforced (○) responses in the presence of discriminative stimuli respectively associated with cocaine (S+) vs. saline (S−) availability across the final 3 days of the self-administration phase in rats assigned to the immediate (a and c) and 4-month delayed (b and d) cue exposure tests. Extinction (EXT): mean (±SEM) number of responses during the last 3 days of the extinction phase. Reinstatement: responses during exposure to the cocaine S+, the S−, and the S+ preceded by administration of SCH 39166 (S+SCH) during the immediate (a and c) and 4-month delayed (b and d) tests. In both test conditions, presentation of the S+ increased the number of responses over extinction performance, whereas the S− had no effect. SCH 39166 significantly attenuated the effect of the S+, and responding in this condition was not statistically different from extinction levels. **, P < 0.01 (different from the extinction and S− conditions); ++, P < 0.01) (different from the S+ condition).
Behavioral Effects of Reexposure to the Cocaine S+.
In rats assigned to the immediate test condition (n = 18), presentation of the cocaine S+ (n = 6) elicited a significant increase in responding over extinction levels (P < 0.01) at the previously cocaine-paired lever, whereas the S− (n = 6) had no effect (Fig. 1a, right column). The selective D1 antagonist SCH 39166 significantly attenuated the behavioral effect of the S+ (P < 0.01), and the number of responses in this condition (n = 6) was not statistically different from extinction levels or responses in the presence of the S− (post hoc analyses after ANOVA: F2,15 = 29,79; P < 0.001).
An identical pattern of effects was observed in rats (n = 16) tested for the first time after 4 months of abstinence (Fig. 1b). Presentation of the S+ (in n = 6) but not the S− (in n = 6) significantly increased responding over extinction levels (P < 0.01). SCH 39166 inhibited the behavioral effect of the S+ (P < 0.01), and the number of responses in these animals (n = 4) did not differ statistically from either extinction responses or responses in rats presented with the S− (post hoc analyses after ANOVA: F2,13 = 13.59; P < 0.001). In both the immediate and delayed test conditions, responding at an alternative lever that had been present but inactive during the training phase was negligible (Fig. 1 c and d).
Fos Protein Expression After Exposure to the Cocaine S+.
Brains of seven animals were not used or available for Fos immunohistochemistry because of inadequate tissue fixation (n = 5) and loss of brain tissue during freezer storage (n = 2). As a result, final sample sizes in four of the treatment conditions were reduced [immediate test: S+ (n = 4), S− (n = 4), S+ + SCH 39166 (n = 4); delayed test: S+ (n = 5)].
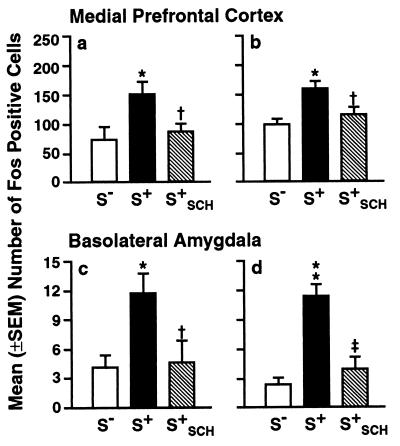
In rats presented with the S+, the number of Fos-immunoreactive nuclei was significantly increased within the basolateral amygdala (immediate test: P < 0.05; delayed test: P < 0.01) and medial prefrontal cortex [prelimbic cortex (Cg3) and area I of the cingulate cortex (Cg1)] (immediate test: P < 0.05; delayed test: P < 0.05) compared with animals of the S− control condition (Figs. 2 and 3). No differences in Fos immunoreactivity between the groups exposed to the cocaine S+ vs. S− were found in any of the other targeted brain regions (Table 1).
Figure 2.
Fos-immunoreactive nuclei in rats after exposure to the cocaine S+, S−, and S+ preceded by administration of SCH 39166 (S+SCH) in the immediate (a and c) and 4-month delayed (b and d) tests. Compared with the S− control condition, the number of Fos-immunoreactive nuclei was significantly increased after exposure to the cocaine S+ in the basolateral amygdala and medial prefrontal cortex (see also caption of Fig. 3), both in the immediate and delayed testing conditions. SCH 39166 reversed the effects of the cocaine S+ on Fos expression. *, P < 0.05; **, P < 0.01 (different from the S− condition). †, P < 0.05, ‡, P < 0.01 (different from the S+ condition).
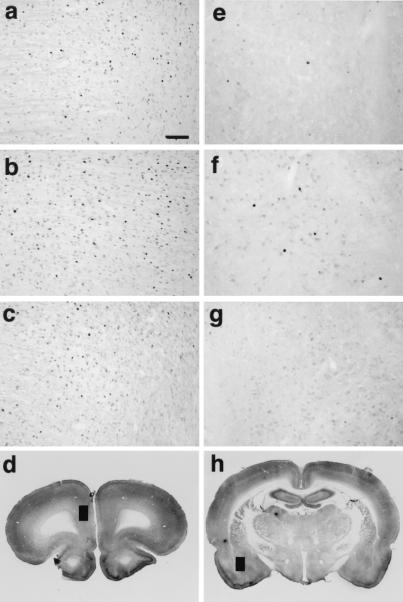
Figure 3.
Representative photomicrographs illustrating the quantitative data shown in Fig. 2. Panels show Fos-immunoreactive nuclei within the medial prefrontal cortex (a–c) and basolateral amygdala (e–g) determined after the delayed cue exposure test. Slices were obtained from rats tested under the S− (a and e), S+ (b and f), and S+SCH (c and g) conditions. (a–c and e–g, bar = 100 μm.) The locations of the fields represented in a–c (medial prefrontal cortex) and e–g (basolateral amygdala) are identified by black rectangles in d and h. The medial prefrontal cortical region sampled includes the prelimbic cortex (Cg3) and area I of the cingulate cortex (Cg1). The anteroposterior (AP) distance of the sections from bregma and approximate dorsal and ventral (DV) borders of the sampled fields are AP 3.2, DV −2.5 to −4.5 (medial prefrontal cortex) and AP −2.12, DV −7.6 to 8.8 (basolateral amygdala; ref. 19).
Table 1.
Fos protein expression induced by exposure to a cocaine-predictive stimulus vs. self-administration of cocaine
| Brain Regions | Cue
exposure: immediate test
|
Cue exposure: 4-month
delay
|
Self-administration
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| S− | S+ | S+ plus SCH39166 | S− | S+ | S+ plus SCH39166 | Saline | Cocaine (acute) | Cocaine (chronic) | |
| Medial prefrontal | |||||||||
| cortex* | 72.7 ± 22.3 | 156.2 ± 24.2‡ | 87.0 ± 13.6§ | 90.6 ± 8.0 | 155.6 ± 15.9‡ | 103.7 ± 12.4§ | 69.5 ± 23.1 | 178.3 ± 24.0† | 131.8 ± 22.9 |
| Frontal cortex | 15.7 ± 4.4 | 9.7 ± 3.5 | 14.7 ± 2.5 | 21.6 ± 6.3 | 30.8 ± 11.2 | 13.5 ± 2.4 | 44.8 ± 25.8 | 121.7 ± 34.0 | 72.2 ± 24.6 |
| Parietal cortex | 56.1 ± 25.7 | 19.0 ± 5.5 | 13.7 ± 4.5 | 35.1 ± 13.0 | 58.6 ± 9.2 | 26.5 ± 11.0 | 42.6 ± 18.2 | 69.7 ± 14.2 | 77.0 ± 18.2 |
| Agranular cortex | 53.2 ± 21.9 | 23.7 ± 2.3 | 14.0 ± 3.3 | 45.0 ± 13.7 | 61.8 ± 17.8 | 49.5 ± 12.1 | 35.0 ± 11.3 | 90.7 ± 8.2 | 122.4 ± 36.0† |
| Claustrum | 65.7 ± 28.1 | 99.5 ± 24.6 | 62.2 ± 12.0 | 51.8 ± 12.4 | 78.8 ± 19.4 | 52.0 ± 14.2 | 150.0 ± 59.3 | 160.7 ± 7.3 | 185.6 ± 31.4 |
| Accumbens shell | 16.0 ± 5.1 | 12.5 ± 4.9 | 15.6 ± 4.8 | 6.6 ± 2.6 | 8.0 ± 2.1 | 4.1 ± 1.0 | 12.0 ± 4.3 | 34.3 ± 5.1‡‡ | 11.8 ± 1.3 |
| Accumbens core | 8.5 ± 3.7 | 7.2 ± 1.8 | 12.2 ± 4.3 | 6.1 ± 3.2 | 7.6 ± 1.9 | 4.7 ± 1.9 | 6.7 ± 2.3 | 18.7 ± 3.6† | 15.1 ± 1.4† |
| Striatum (dorsolateral) | 4.5 ± 2.2 | 2.5 ± 0.8 | 4.0 ± 1.6 | 1.7 ± 0.5 | 1.2 ± 0.5 | 1.1 ± 0.3 | 2.9 ± 1.4 | 58.7 ± 21.5‡‡ | 40.2 ± 1.1‡‡ |
| Striatum (dorsomedial) | 4.2 ± 1.9 | 1.9 ± 1.0 | 2.0 ± 0.9 | 2.1 ± 0.7 | 3.2 ± 2.4 | 0.6 ± 0.1 | 2.3 ± 0.8 | 43.7 ± 18.6‡‡ | 15.4 ± 3.3† |
| Amygdala (basolateral) | 3.3 ± 1.0 | 9.4 ± 1.6‡ | 3.7 ± 1.8§ | 2.3 ± 0.7 | 11.4 ± 1.2†† | 3.9 ± 1.2§§ | 3.4 ± 1.6 | 6.6 ± 01.4 | 5.4 ± 0.9 |
| Amygdala (central) | 4.2 ± 1.2 | 2.4 ± 0.7 | 5.0 ± 1.7 | 1.0 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.5 | 3.7 ± 2.7 | 17.5 ± 2.2‡‡ | 5.8 ± 1.0 |
| Paraventricular thalamic nucleus | 31.2 ± 15.8 | 31.0 ± 12.1 | 40.7 ± 10.7 | 16.3 ± 6.8 | 20.9 ± 10.1 | 16.5 ± 6.5 | 10.0 ± 6.1 | 87.7 ± 23.1‡‡ | 6.8 ± 1.3 |
| Central thalamic nucleus | 15.7 ± 13.1 | 9.7 ± 3.2 | 15.6 ± 4.4 | 6.5 ± 2.7 | 5.9 ± 2.1 | 4.7 ± 2.9 | 3.6 ± 1.7 | 72.3 ± 10.5‡‡ | 6.4 ± 1.2 |
| Ventromedial hypothalamus | 16.1 ± 8.2 | 20.5 ± 9.5 | 14.5 ± 4.9 | 4.6 ± 0.3 | 9.6 ± 2.9 | 13.5 ± 4.5 | 3.0 ± 1.4 | 19.7 ± 5.8‡‡ | 6.5 ± 1.3 |
Shading indicates brain regions showing increased Fos expression after exposure to the cocaine S+.
Prelimbic cortex (Cg3) and cingulate cortex (Cg1).
Newman–Keuls post hoc tests after ANOVA:
, P < 0.05;
, P < 0.01 (different from saline).
, P < 0.05;
, P < 0.01 (different from S−).
, P < 0.05;
, P < 0.01 (different from S+).
In parallel with the reversal of the behavioral effects of the cocaine S+, pretreatment with SCH 39166 reliably reversed the cue-induced increase in Fos immunoreactivity (Figs. 2 and 3) in the basolateral amygdala (immediate test: P < 0.05; delayed test: P < 0.01) as well as in the medial prefrontal cortex (immediate test: P < 0.05; delayed test: P < 0.05), and the number of Fos-positive nuclei in these animals did not differ statistically from that in rats of the S− condition [post hoc tests after ANOVA of counts obtained from the medial prefrontal cortex (immediate test: F2,9 = 4.69; P < 0.05 delayed test: F2,12 = 8.30; P < 0.01) and basolateral amygdala (immediate test: F2,9 = 4.67; P < 0.05; delayed test: F2,12 = 21.58; P < 0.01)].
Dose Dependency of D1 Antagonist Effects on S+-Induced Responding.
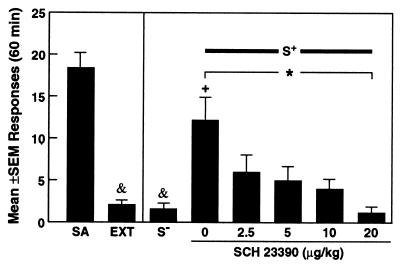
In rats (n = 5) pretreated with the D1 antagonist SCH 23390, the recovery of responding induced by the cocaine S+ was attenuated (Fig. 4). Responses after SCH 23390 treatments differed significantly from those produced by vehicle treatment (P < 0.05) and from cocaine-reinforced responses (P < 0.05) during the self-administration phase (Newman–Keuls post hoc tests after ANOVA: F7,28 = 9.33; P < 0.0001). The dose-dependency of the drug treatment effects was confirmed by analysis of the linear trend across the five means of the dose-response function (0–20 μg/kg). This analysis revealed that the suppression of response by SCH 23390 increased significantly with increasing doses (F1,4 = 17.15; P < 0.05).
Figure 4.
Effects of the D1 antagonist SCH 23390 on response induced by the discriminative stimulus for cocaine (S+). SCH 23390 dose-dependently reversed the effects of the S+. For comparison, the figure also shows the average number of responses during the last 3 days of the self-administration (SA) and extinction (EXT) phases, as well as responses in the presence of the stimulus associated with nonreward (S−). *, P < 0.05, significant linear trend over dose levels; +, P < 0.05, different from the EXT and S− conditions; &, P < 0.01, different from cocaine-reinforced responses (SA).
Cocaine-Dependent Fos Expression in Self-Administering Rats.
The mean (±SEM) number of cocaine infusions during the 1-h self-administration session before rats were killed was 8.83 (±1.17) in rats given access to cocaine for the first time (cocaine acute group; n = 6), and 15.4 (±1.89) in cocaine-experienced rats (cocaine chronic group; n = 5). Rats assigned to the control condition (saline group; n = 6) received an average of 6.5 (±2.89) vehicle infusions. Cocaine self-administration significantly increased the number of Fos-positive nuclei over levels in saline control rats (see Table 1, self-administration). In previously drug-naive animals, Fos immunoreactivity was increased ubiquitously throughout dopamine-rich brain regions including the dorsal striatum, shell and core regions of the nucleus accumbens, the central nucleus of the amygdala and medial prefrontal cortex, as well as within thalamic and hypothalamic nuclei. In rats previously trained to self-administer cocaine, increases in Fos immunoreactivity were confined to the core of the nucleus accumbens, dorsolateral striatum, and agranular cortex.
Discussion
The results confirm that discriminative stimuli associated with the availability and self-administration of cocaine can elicit strong drug-seeking behavior in rats as measured by the recovery of extinguished responding at a previously cocaine-paired lever. Of particular significance is the finding that the cocaine S+ was effective in reinstating drug-seeking behavior not only in rats tested immediately after the initial extinction phase, but that the behavioral effects of this cue were undiminished in rats subjected to an additional 4 months of abstinence. This finding documents that the motivating actions of environmental cues that act as discriminative stimuli for drug availability can remain intact over a substantial length of time and, therefore, that such stimuli may play a significant role in long-lasting relapse risk.
Exposure to the cocaine S+ selectively increased the number of Fos-immunoreactive neurons in the basolateral amygdala and medial prefrontal cortex. Fos immunoreactivity in the striatum and nucleus accumbens, brain regions implicated in the psychomotor stimulant effects of cocaine, was not altered by the cocaine cue. There were also no differences in Fos expression in the motor cortex between the groups exposed to the cocaine S+ vs. S−. Thus, the increased neural activation by the S+ within the basolateral amygdala and medial prefrontal cortex is unlikely to be secondary to motoric activation associated with increased lever-pressing in the animals exposed to the S+. This conclusion is supported also by a related finding showing that Fos protein expression in the basolateral amygdala and cingulate cortex is increased after exposure to a cocaine-paired environment in rats that are not given the opportunity to emit lever-press responses (20).
The undiminished efficacy of the cocaine cue to elicit cocaine-seeking behavior in the delayed test parallels the often long-lasting nature of conditioned cue reactivity and cue-induced cocaine craving in humans (for an example, see ref. 21). An important observation in this regard is that distribution of Fos expression induced by the cocaine S+ also closely parallels data from PET and fMRI brain-imaging studies in humans showing that cue-induced cocaine craving is associated with selective neural activation of the amygdala and cingulate cortex (4, 5, 15). The consistency between neuroanatomical sites showing activation during cocaine craving in brain-imaging studies and the distribution of Fos immunoreactivity associated with exposure to the cocaine S+ in the present study suggests that these procedures may provide an effective research tool for the elucidation of the neural mechanisms underlying cocaine craving and long-lasting relapse risk.
The selective dopamine D1 antagonist SCH 39166 concurrently reversed the increases in Fos immunoreactivity and cocaine-seeking behavior induced by the cocaine S+ in both the immediate and delayed test condition. In addition, the behavioral effects of the cocaine cue were dose-dependently blocked by a second D1 antagonist, SCH 23390. The attenuation of cue-induced responding by SCH 39166 and SCH 23390 cannot readily be attributed to nonspecific impairments in motor performance. Low-dose D1 antagonist treatments decrease the direct reinforcing actions of cocaine without interfering with motoric behavior (22). More importantly, with respect to a role of the D1 receptor in conditioned cocaine-seeking behavior, SCH 23390 dose-dependently reduced responding induced by a light cue conditioned to cocaine but left intact behavior maintained by a conditioned stimulus associated with a food reinforcer (23). Interestingly, SCH 39166 (ecopipam) has been shown to attenuate the euphoric effects of cocaine selectively in humans (that is, without inducing sedation or nonspecific anhedonic effects) and, therefore, may have therapeutic potential as a cocaine antagonist (24). Considering the present results, the D1 receptor may be a promising target not only for treatment approaches aimed at antagonizing the euphoric effects of cocaine, but also for the blunting of cocaine craving and prevention of relapse.
The increase in Fos expression within the basolateral amygdala associated with exposure to the cocaine S+ is consistent with evidence that this brain region is critical for the capacity of stimuli conditioned to both drug and nondrug reinforcers to affect behavior. Lesions of the basolateral amygdala reduce cocaine-seeking behavior elicited (25, 26) or maintained (27) by cocaine-related stimuli, prevent conditioned opiate withdrawal responses (28), and impair conditioned behavior maintained by stimuli associated with sexual reward (29). Recent findings suggest that the conditioned behavioral effects of cocaine-related stimuli may depend on activation of dopamine neurotransmission within the basolateral amygdala (11). The concurrent reversal of S+-induced cocaine-seeking behavior and neural activation by D1 receptor antagonists supports this possibility and, in particular, implicates D1-dependent neural mechanisms within the basolateral amygdala in the control of drug-seeking behavior by external stimuli. The immunohistochemical findings are consistent also with the current understanding of the role of the medial prefrontal cortex in associative learning. Medial prefrontal cortical areas, including the regions sampled here, have been implicated in attentional performance, discrimination learning, and the learning of stimulus attributes predicting reward or no reward (30–33), functions that can be presumed to be essential for the mediation of associations between the subjective effects of cocaine and environmental stimuli. Indeed, contextual stimuli associated with cocaine reward consistently have been found to produce elevations in Fos protein expression within the cingulate cortex in other experimental paradigms (20, 34). The present results further confirm these findings but, in addition, provide direct evidence for a role of medial prefrontal cortical regions in mediating the reinstatement of drug-seeking behavior by drug-associated environmental stimuli. Moreover, as in the case of the basolateral amygdala, the results suggest that these actions may involve D1-dependent neural mechanisms.
A secondary objective of this study was to determine whether the pattern of neural activation associated with drug-seeking behavior induced by the cocaine S+ differs from that produced by the unconditioned effects of cocaine in self-administering rats. In the basolateral amygdala, Fos immunoreactivity was increased selectively after exposure to the cocaine S+ and was not altered either in rats self-administering cocaine acutely or in rats with a history of cocaine self-administration. In contrast, increases in Fos expression in the medial prefrontal cortex were produced by the cocaine S+ as well as by cocaine. Cocaine-induced neural activation in this brain region was, however, seen only in previously drug-naive rats that showed widespread increases in Fos immunoreactivity. No overlap was found between the effects of cocaine and the cocaine S+ in rats with a history of cocaine self-administration. Neural activation in the latter group was restricted to the core of the nucleus accumbens, dorsal striatum, and agranular cortex, suggesting that these brain regions may perhaps recover more rapidly from the desensitization of cocaine-dependent Fos expression associated with repeated cocaine treatments (17, 18) than other sites that showed elevated Fos expression in rats self-administering cocaine acutely. However, the differences between the acute and chronic cocaine groups are not readily accounted for by a differential recovery from desensitization, because factors such as arousal, novelty, and stress associated with i.v. cocaine infusions can be presumed to have contributed to the pattern of neural activation in cocaine-naive rats. Indeed, brain regions activated by cocaine in the chronic rather than the acute group are more likely to represent sites that are more specifically involved in the psychostimulant and rewarding effects of the drug. In agreement with this presumption, it has been shown that cocaine does not increase the expression of Fos-related antigens that are known to be induced selectively by chronic, but not acute, cocaine exposure (18) within the basolateral amygdala and cingulate cortex of rats with a history of chronic self-administration (35). Thus, whereas the cingulate and prelimbic cortex show activation by cocaine in drug-naive rats, these brain regions do not seem to be recruited for the mediation of the primary reinforcing actions of cocaine in animals trained to self-administer the drug. This interpretation is not at variance with a report that Fos protein levels in the cingulate cortex are elevated both after reexposure to a drug-paired environment and by cocaine priming injections in previously cocaine-self-administering rats (20). Priming injections typically signal the response-contingent availability of a drug. Under these conditions, cocaine acts as a discriminative stimulus, and this action is likely to be similar to that of drug-related stimuli or environments but different from the reinforcing effects of the drug in actively self-administering animals. It must, nonetheless, be emphasized that the failure to observe increases in Fos immunoreactivity in rats self-administering cocaine does not necessarily rule out an involvement of the basolateral amygdala and medial prefrontal cortex in the primary reinforcing effects of cocaine. However, the selective neural activation of these brain regions by the cocaine S+ is consistent with evidence that lesions of the basolateral amygdala or medial prefrontal cortex interfere with many of the conditioned behavioral effects of cocaine-associated stimuli without altering behavior reinforced by the drug itself (25–27, 36). When interpreted in the context of this literature, the immunohistochemical data provide further support for a specific role of the basolateral amygdala and medial prefrontal cortex in the mediation of learned responses to cocaine-associated stimuli and strengthen the hypothesis that the conditioned behavioral effects of cocaine-associated contextual stimuli recruit different neuroanatomical substrates than the primary reinforcing actions of cocaine (4, 20, 27).
In conclusion, the results confirm that the efficacy of cocaine-predictive discriminative stimuli to elicit drug-seeking behavior remains intact over prolonged periods of abstinence and, by extension, support the hypothesis that learned responses to drug-related stimuli are an important factor in long-lasting vulnerability to relapse. Moreover, the data implicate the medial prefrontal cortex and basolateral amygdala as candidate sites for the mediation of cue-induced cocaine-seeking behavior and suggest that the D1 receptor may represent an important neuropharmacological substrate for the motivating effects of cocaine-related stimuli.
Acknowledgments
We thank Cindy Simpson for technical assistance with Fos immunohistochemistry, Daniele Vitali for valuable support with image analysis, and Mike Arends for assistance with the preparation of the manuscript. This work was supported by National Institute on Drug Abuse Grants DA 08467 and DA 07348 (to F.W.). This article is publication number 13052-NP from The Scripps Research Institute.
Abbreviations
- SD
discriminative stimulus or stimuli
- S+
SD associated with cocaine availability
- S−
SD associated with lack of cocaine availability
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.O'Brien C P, Childress A R, Ehrman R, Robbins S J. J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 2.Wallace B C. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- 3.Kilgus M D, Pumariega A J. South Med J. 1994;87:1138–1140. doi: 10.1097/00007611-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Childress A R, Mozley P D, McElgin W, Fitzgerald J, Reivich M, O'Brien C P. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maas L C, Lukas S E, Kaufman M J, Weiss R D, Daniels S L, Rogers V W, Kukes T J, Renshaw P F. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 6.Miller N S, Gold M S. Ann Clin Psychiatry. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- 7.Tiffany S T, Carter B L. J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien C P, McLellan A T. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- 9.Leshner A I. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 10.Meil W M, See R E. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- 11.Weiss F, Maldonado-Vlaar C S, Parsons L H, Kerr T M, Smith D L, Ben-Shahar O. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer R E. Lancet. 1996;347:162–166. doi: 10.1016/s0140-6736(96)90345-1. [DOI] [PubMed] [Google Scholar]
- 13.Dragunow M, Faull R. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 14.Herrera D G, Robertson H A. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 15.Garavan H, Pankiewicz J, Bloom A, Cho J K, Sperry L, Ross T J, Salmeron B J, Risinger R, Kelley D, Stein E A. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 16.Caine S B, Lintz R, Koob G F. In: Behavioral Neuroscience: A Practical Approach. Saghal A, editor. Vol. 2. London: Oxford Univ. Press; 1993. pp. 117–143. [Google Scholar]
- 17.Rosen J B, Chuang E, Iadarola M J. Brain Res Mol Brain Res. 1994;25:168–172. doi: 10.1016/0169-328x(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 18.Hope B T, Nye H E, Kelz M B, Self D W, Iadarola M J, Nakabeppu Y, Duman R S, Nestler E J. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. San Diego: Academic; 1998. [Google Scholar]
- 20.Neisewander J L, Baker D A, Fuchs R A, Tran-Nguyen L T, Palmer A, Marshall J F. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Childress A R, Hole A V, Ehrman R N, Robbins S J, McLellan A T, O'Brien C P. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 22.Caine S B, Koob G F. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- 23.Weissenborn R, Deroche V, Koob G F, Weiss F. Psychopharmacology. 1996;126:311–322. doi: 10.1007/BF02247382. [DOI] [PubMed] [Google Scholar]
- 24.Romach M K, Glue P, Kampman K, Kaplan H L, Somer G R, Poole S, Clarke L, Coffin V, Cornish J, O'Brien C P, et al. Arch Gen Psychiatry. 1999;56:1101–1106. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- 25.Grimm J W, See R E. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 26.Meil W M, See R E. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 27.Whitelaw R B, Markou A, Robbins T W, Everitt B J. Psychopharmacology. 1996;127:213–224. [PubMed] [Google Scholar]
- 28.Schulteis G, Ahmed S H, Morse A C, Koob G F, Everitt B J. Nature (London) 2000;405:1013–1014. doi: 10.1038/35016630. [DOI] [PubMed] [Google Scholar]
- 29.Everitt B J, Cador M, Robbins T W. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- 30.Granon S, Passetti F, Thomas K L, Dalley J W, Everitt B J, Robbins T W. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bussey T J, Everitt B J, Robbins T W. Behav Neurosci. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- 32.Bussey T J, Muir J L, Everitt B J, Robbins T W. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 33.Takenouchi K, Nishijo H, Uwano T, Tamura R, Takigawa M, Ono T. Neuroscience. 1999;93:1271–1287. doi: 10.1016/s0306-4522(99)00216-x. [DOI] [PubMed] [Google Scholar]
- 34.Brown E E, Robertson G S, Fibiger H C. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pich E M, Pagliusi S R, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- 36.Weissenborn R, Robbins T W, Everitt B J. Psychopharmacology. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]