Abstract
Signaling through the Tie2 receptor on endothelial cells has been shown to play an important role in normal and pathologic vascular development. We generated K1735 murine melanoma tumor cells that inducibly express soluble Tie2 receptor (Tie2Ex) to study the effects of inhibiting Tie2 signaling on tumor vasculature. Tie2Ex induction rapidly decreased AKT activation but not extracellular signal-regulated kinase (ERK) activation in tumor endothelial cells as detected by immunostaining. This was accompanied by an increase in endothelial cell TUNEL staining but no change in Ki-67 expression. Together with a decrease in the percentage of perfused vessels, this suggested that tumor vessel regression and impaired vascular function rather than angiogenesis inhibition was responsible for the delay in tumor growth following Tie2Ex treatment. However, Tie2Ex failed to inhibit the growth of larger, more established K1735 tumors. These tumors were additionally treated with sorafenib, a multikinase inhibitor that inhibits tumor endothelial cell ERK activation but not AKT activation. Combining Tie2Ex and sorafenib decreased both endothelial cell AKT and ERK activation, decreased endothelial cell survival and proliferation, and significantly inhibited growth of the more established tumors. These studies indicate that activity of specific signaling pathways and prosurvival effects are brought about by Tie2 activation in tumor endothelial cells, and knowledge of the effects of Tie2 inhibition can lead to development of more effective therapeutic regimens for inhibiting tumor neovascularization.
Introduction
Formation of blood vessels by the process of angiogenesis is necessary for sustained tumor growth and requires various factors that trigger intracellular endothelial cell signaling pathways that promote their survival, proliferation, and migration. Antiangiogenic agents commonly target these pathways to inhibit angiogenesis and control tumor growth. Several endothelial cell receptors have been described to play a critical role in vessel formation and survival, including vascular endothelial growth factor receptors and Tie receptors. Studies have shown vascular endothelial growth factor to be critical in endothelial cell survival, proliferation, and migration (reviewed in ref. 1), whereas the Tie receptors are involved in vascular remodeling and vessel stability (reviewed in ref. 2).
Tie1 and Tie2 form the Tie receptor tyrosine kinase family. Tie1 is an orphan receptor tyrosine kinase whose function is poorly understood but appears to play a role in late embryonic vascular development and modulate Tie2 activity (3-9). Tie2, on the other hand, has been studied extensively and has four known binding ligands, angiopoietin (Ang) 1-4 (reviewed in ref. 2). Genetic knockout of the Tie2 receptor and its ligands are embryonic lethal and show a severe vascular defect, suggesting the interplay between receptor and ligand(s) is necessary for vascular development (4, 10, 11). These studies also revealed a defect in recruitment of pericytes to vessels, which suggests the importance of Tie2 on vessel maturation and stability. In vitro, Ang1 stimulation results in Tie2 phosphorylation and has been shown to activate the phosphatidylinositol 3-kinase (PI3K)-AKT (12-15), Raf-MEK-extracellular signal-regulated kinase (ERK; refs. 16-20), and Stat signaling pathways (21) to mediate endothelial cell survival. Ang1 has also been shown to activate Tie1 through a Tie2-dependent mechanism (8, 9). Ang2 was initially identified as an antagonistic ligand of Tie2, inducing changes that destabilize vessels and allow vessel remodeling (22). In certain contexts or at high levels, Ang2 has also been shown to activate Tie2 (23). Studies have indicated that Ang3 and Ang4 are orthologues in mouse and human, respectively (24). However, their functions in vivo are less well characterized. Although the nature and effect of Tie2 signaling have been well studied in vitro, they have not been studied in vivo.
To understand the potential therapeutic utility and application of Tie2 inhibition, we thought it important to understand the effects and consequences of Tie2 inhibition in vivo. Accordingly, we used an inducible decoy receptor of Tie2 (Tie2Ex) in K1735 murine melanoma tumors to study the effects of Tie2 inhibition on tumor endothelial cell signaling and cell fate in vivo. Our studies indicated that inhibition of Tie2 signaling decreases tumor endothelial cell AKT activation and increases vessel apoptosis. This appears to be the mechanism for the delay in K1735 tumor growth following Tie2Ex induction. To determine whether we could improve tumor growth control by inhibiting multiple endothelial cell signaling pathways, we treated well-established K1735 tumors that are refractory to Tie2Ex monotherapy with Tie2Ex and sorafenib, which has been shown to inhibit endothelial cell ERK activation (25). Whereas these larger tumors were refractory to either treatment alone, combined treatment inhibited both endothelial cell AKT and ERK activation and effectively controlled their growth.
Results
Tie2Ex Blocks Recombinant Ang-1-Induced Tie2 Activation and AKT Activation in Endothelial Cells In vitro
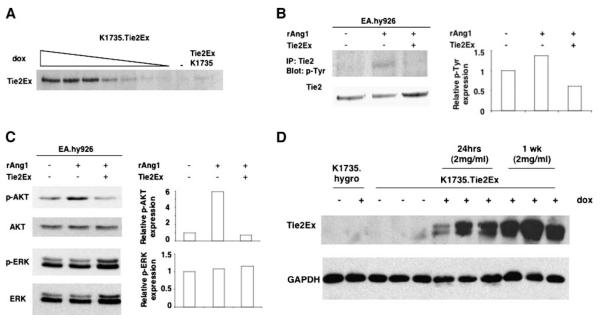
To study the nature and effects of Tie2 signaling in endothelial cells in vivo, we created a system for inducible expression of a Tie2 inhibitor. K1735 murine melanoma cells were engineered using the Tet-on system (26) to express a Tie2 decoy receptor, Tie2Ex (the extracellular domain of murine Tie2), under doxycycline regulation. In addition to these K1735.Tie2Ex cells, K1735.hygro cells were generated to control for effects of doxycycline, which was shown previously to have antiangiogenic effects (27). Regulation of Tie2Ex expression in K1735.Tie2Ex cells was tightly regulated by doxycycline with minimal expression in its absence and substantial expression in its presence (Fig. 1A; Supplementary Fig. S1). To test the ability of Tie2Ex to inhibit Tie2 activation by ligand in vitro, EA.hy926 endothelial cells were stimulated with recombinant Ang1 (rAng1) in the presence or absence of Tie2Ex, and cell lysates were probed with anti-phosphotyrosine antibody following Tie2 immunoprecipitation. The presence of Tie2Ex effectively inhibited Tie2 phosphorylation by rAng1 (Fig. 1B).
FIGURE 1.
Induction of Tie2Ex in K1735.Tie2Ex tumor cells and effect of Tie2Ex on Tie2 activation in EA.hy926 endothelial cells. K1735.Tie2Ex cells were cultured in the presence or absence of doxycycline. A. Western blot of culture supernatant was probed for Tie2Ex using an anti-Tie2 antibody. EA.hy926 endothelial cells were stimulated with rAng1 in the presence or absence of Tie2Ex obtained from conditioned medium. B. Tie2 phosphorylation was detected using an anti-phosphotyrosine antibody following immunoprecipitation of Tie2 from cell lysate. Blot was stripped and reprobed for Tie2 expression (left). Densitometry analysis of p-Tie2 normalized to Tie2 was done and plotted on a histogram (right). C. Western blot of cell lysates was probed for p-AKT or p-ERK and reprobed for AKT or ERK, respectively, after stripping blot. Densitometry analysis of phosphorylated protein expression normalized to unphosphorylated protein was done and plotted on a histogram. D. K1735.Tie2Ex and K1735.hygro tumors were created in C3H mice. Tumor-bearing mice were treated with 2 mg/mL doxycycline for 24 h or 1 wk or without doxycycline in the drinking water. Western blot of tumor lysates was probed for Tie2Ex using an anti-Tie2 antibody and reprobed with an anti-GAPDH antibody.
Activation of Tie2 in endothelial cells has been reported to activate the PI3K-AKT and Raf-MEK-ERK pathways. EA.hy926 cells stimulated with rAng1 showed increased AKT activation (p-AKT) but did not display increased ERK activation (p-ERK; Fig. 1C). In the presence of Tie2Ex, AKT activation by rAng1 was reduced to baseline (Fig. 1C), indicating that Tie2Ex blocked endothelial cell AKT signaling downstream of Tie2. To eliminate the possibility that Tie2 is expressed on K1735 tumor cells and that these cells might be directly affected by Tie2Ex, we looked for Tie2 expression on cultured K1735 wild-type and K1735.Tie2Ex tumor cells by Western blot. Tie2 was not expressed on the tumor cells (data not shown), validating endothelial cells and not tumor cells as the target population for Tie2 inhibition.
Tie2Ex Inhibits Tumor Endothelial Cell AKT Activation but not ERK Activation
To test the effect of Tie2Ex on in vivo tumor endothelial cell signaling, s.c. K1735.Tie2Ex and K1735.hygro tumors were created in syngeneic C3H mice. Mice bearing inducible Tie2Ex tumors treated with 2 mg/mL doxycycline in the drinking water showed Tie2Ex expression at 24 h and maintained expression at 1 week. In comparison, there was little to no Tie2Ex expression in tumors not exposed to doxycycline and no expression in K1735.hygro tumors following doxycycline induction (Fig. 1D). Reverse transcription-PCR done on K1735 tumor lysates revealed the presence of Ang1 and Ang2 transcripts, showing that ligands for Tie2 were likely present in these tumors (Supplementary Fig. S2).
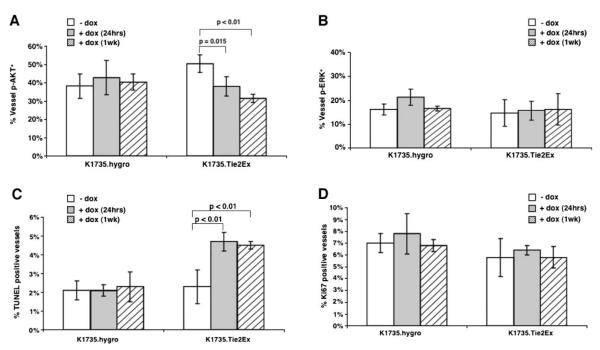
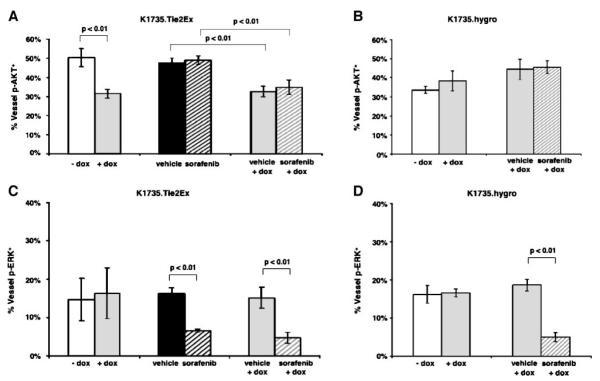
Tumor sections from untreated or doxycycline-treated K1735.Tie2Ex and K1735.hygro tumors were stained for p-AKT or p-ERK along with CD34 to identify endothelial cell signaling changes following Tie2Ex induction. K1735.Tie2Ex tumors treated with doxycycline for 24 h and 1 week showed a significant decrease in the percentage of tumor vessels expressing p-AKT compared with untreated tumors (Fig. 2A; Supplementary Fig. S3; 38.0 ± 5.3% for 24 h and 31.5 ± 2.3% for 1 week versus 50.3 ± 4.8% for control; P ≤ 0.01, t test). In contrast, doxycycline induced no significant change in the percentage of vessels expressing p-ERK (Fig. 2B; 15.7 ± 4.0% for 24 h and 16.3 ± 6.6% for 1 week versus 14.7 ± 5.6% for control; P > 0.1, t test). K1735.hygro tumors showed no significant change in either endothelial cell p-AKT or endothelial cell p-ERK expression following doxycycline treatment (Fig. 2A and B). These studies indicated that both AKT and ERK are activated in tumor endothelial cell and that inhibition of Tie2 signaling decreases tumor endothelial cell AKT activation but not ERK activation.
FIGURE 2.
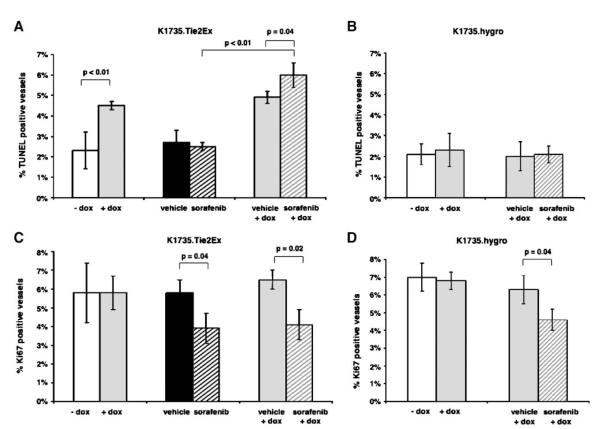
Effect of Tie2Ex on tumor endothelial signaling and fate in vivo. K1735.Tie2Ex and K1735.hygro tumors were created in C3H mice. Tumor-bearing mice were treated with no doxycycline or with doxycycline (2 mg/mL) for 24 h or 1 wk. Paraffin-embedded tumor sections were stained for p-AKT or p-ERK and CD34 to identify endothelial cell p-AKT or p-ERK expression. Percentage of vessels expressing p-AKT (A) or p-ERK (B) was calculated and plotted as a histogram. Tumor sections were stained for TUNEL and CD31 or Ki-67 and CD34 to identify apoptotic or proliferating vessels, respectively. Percentage of apoptotic vessels (C) or proliferating vessels (D) was calculated and plotted as a histogram.
Tie2Ex Decreases Tumor Vessel Integrity and Vessel Perfusion and Delays Tumor Growth
To determine the effect of Tie2Ex on tumor endothelial cell fate following the signaling changes, tumor sections were stained with TUNEL and anti-CD31 antibody to identify apoptotic vessels and with anti-Ki-67 and anti-CD34 antibodies to identify proliferating vessels. K1735.Tie2Ex tumors treated with doxycycline for 24 h or 1 week had increased apoptotic vessels compared with untreated tumors (Fig. 2C; 4.7 ± 0.5% for 24 h and 4.5 ± 0.2% for 1 week versus 2.3 ± 0.9% for control; P < 0.01, t test). These changes are a consequence of Tie2Ex expression, as vessel apoptosis was not induced in K1735.hygro tumors treated with doxycycline (Fig. 2C). Staining of K1735.Tie2Ex tumors for Ki-67 revealed no significant change in vessel proliferation with doxycycline treatment (Fig. 2D; 6.4 ± 0.4% for 24 h and 5.8 ± 0.9% for 1 week versus 5.8 ± 1.6% for control; P > 0.1, t test). These results show that Tie2Ex inhibition of Tie2 signaling alters tumor endothelial cell survival but not proliferation. An examination of earlier time points showed a trend toward a decrease in endothelial cell p-AKT and an increase in endothelial cell TUNEL as early as 6 to 12 h after doxycycline administration (given by gavage to be certain of time of onset of doxycycline action; Supplementary Fig. S4). Notably, this was before the beginning of the increase in TUNEL staining in K1735 tumor cells (Supplementary Fig. S4).
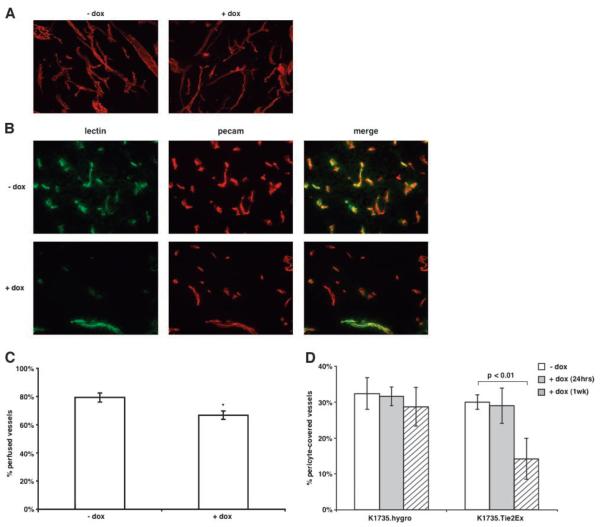
To examine the effect of endothelial cell signaling and fate changes on the tumor vascular morphology, we stained thick tumor sections with an anti-CD31 (PECAM) antibody and imaged the sections using confocal microscopy. The caliber and branching of tumor vessels was similar in the presence or absence of doxycycline (Fig. 3A). To determine the effect of Tie2Ex of vascular perfusion, we injected fluorescently labeled tomato lectin i.v. into tumor-bearing mice to highlight perfused vessels and stained sections of their tumors with anti-CD31 antibody to identify anatomic vessels. Tie2Ex-treated tumors had fewer anatomic vessels stained with lectin compared with control tumors (Fig. 3B and C; 66.8 ± 3.0% for doxycycline-treated tumors versus 79.3 ± 3.2% for control tumors; P < 0.01, t test). These data suggest endothelial cell signaling and fate changes following Tie2 inhibition significantly impair tumor vessel function.
FIGURE 3.
Effect of Tie2Ex on tumor vessel morphology, perfusion, and maturity. A. Thick (60 μm) sections of tumors from mice untreated or treated with doxycycline (18 h) were stained with an anti-CD31 (PECAM) antibody followed by Texas red-conjugated secondary antibody. Tumor sections were viewed using a confocal microscope and the projected images are shown (total magnification, ×100). B. Tumor-bearing mice receiving no doxycycline or doxycycline (18 h) were injected with FITC-conjugated tomato lectin via tail vein to label vessels before euthanasia. Thin (10 μm) sections were stained with an anti-CD31 (PECAM) antibody followed by Texas red-conjugated secondary antibody. Representative images of tumor sections from doxycycline-treated or untreated mice (total magnification, ×200). C. All vessels from the thin tumor sections were counted and the percent of PECAM0positive vessels (total vessels) that are FITC positive (perfused vessels) was calculated. Values were plotted on a histogram plot as the percentage of perfused vessels. *, P < 0.01, t test. D. Frozen tumor sections from K1735.hygro and K1735.Tie2Ex tumors untreated or treated with doxycycline for 24 h and 1 wk were stained with anti-smooth muscle actin antibody and anti-CD31 antibody to identity pericyte-covered vessels. All vessels were counted and plotted as a histogram as percentage of vessels pericyte-covered. Histogram plot represents values obtained from counting vessels in two sections of at least three different tumors for each group. Student’s t test statistical analysis was done. P < 0.01 was considered highly significant.
Angiopoietin-Tie2 interaction has also been shown to be important in vessel maturation and pericyte recruitment to vessels (reviewed in ref. 28). To determine whether inhibition of Tie2 signaling by Tie2Ex affects pericyte coverage of vessels in K1735 tumors, tumor sections were stained for pericytes using anti-smooth muscle actin antibody and CD31 for vessel stain. Tie2Ex induction resulted in a decrease in pericyte coverage only after 1 week of induction (Fig. 3D).
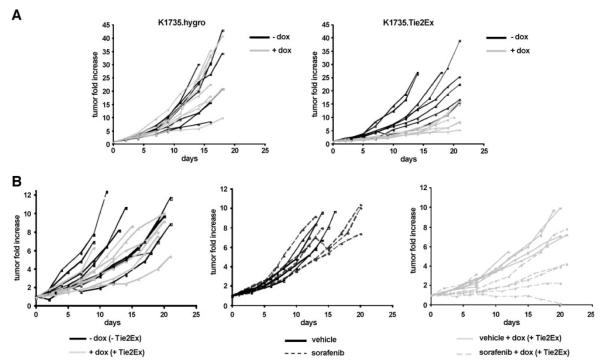
To study the effects of Tie2Ex on tumor growth, doxycycline treatment was initiated when K1735.Tie2Ex and K1735.hygro tumors reached ~3 mm in diameter, and tumor growth was monitored. Growth was delayed in doxycycline-treated K1735.Tie2Ex tumors compared with untreated tumors, whereas growth of K1735.hygro tumors was not delayed following doxycycline treatment (Fig. 4A; Supplementary Fig. S5). H&E staining of tumor sections from K1735.Tie2Ex tumors revealed very small regions of necrosis after 24 h induction. Necrosis was more prominent in tumors after 1 week of induction (Supplementary Fig. S6; 17.6 ± 4.7% necrosis for 24 h doxycycline and 43.1 ± 17.3% necrosis for 1 week versus 1 ± 1% necrosis for no doxycycline; P < 0.01, t test). Together with the rapid induction of endothelial cell death and a decrease in vessel perfusion, these observations suggest that vessel regression and impaired vascular function leading to tumor necrosis was responsible for Tie2Ex control of tumor growth.
FIGURE 4.
Growth of K1735.hygro and K1735.Tie2Ex tumors. K1735.hygro and K1735.Tie2Ex tumors were created in C3H mice. A. Tumor-bearing mice were treated with or without doxycycline starting at 3 to 4 mm in tumor diameter for up to 3 wk. Growth of individual K1735.hygro and K1735.Tie2Ex tumors untreated (black line) or treated with doxycycline (gray line). B. Tumor-bearing mice were treated starting at 5 to 6 mm in tumor diameter for up to 3 wk. Growth of individual K1735.Tie2Ex tumors untreated (black lines) or treated with doxycycline (gray lines), treated with vehicle (black lines) or sorafenib (dotted lines), and treated with vehicle and doxycycline (solid gray lines) or sorafenib and doxycycline (dotted gray lines).
Tie2Ex and Sorafenib Treatment Controls Growth of Larger Tumors
To provide a more clinically relevant model of therapy, doxycycline was initiated when K1735.Tie2Ex tumors were more established (~6 mm in diameter). In contrast to tumors treated at the smaller size, these tumors were not delayed in their growth (Fig. 4B; Supplementary Fig. S7), indicating that inhibition of Tie2 signaling was insufficient to produce a therapeutic effect in the larger tumors. To determine whether inhibiting a different endothelial cell signaling pathway not activated by Tie2, the Raf-MEK-ERK pathway, might improve control of growth of tumors at the larger size, we treated K1735.Tie2Ex tumors with sorafenib, a multikinase inhibitor that inhibits ERK activation in K1735 tumor endothelial cells (25). Large K1735.Tie2Ex tumors treated with sorafenib alone were not inhibited in their growth (Fig. 4B; Supplementary Fig. S7). However, combining sorafenib treatment with doxycycline induction of Tie2Ex produced a clear delay in tumor growth (Fig. 4B; Supplementary Fig. S7).
Effect of Combined Tie2Ex and Sorafenib Treatment on Endothelial Cell Signaling, Apoptosis, and Proliferation
The larger K1735.Tie2Ex tumors treated or untreated with doxycycline, sorafenib, or a combination of the two for 1 week were analyzed for changes in endothelial cell AKT and ERK activation. Doxycycline-treated K1735.Tie2Ex tumors showed a significant decrease in activated AKT expression in endothelial cells compared with untreated tumors, and the addition of sorafenib did not further inhibit endothelial cell p-AKT expression (Fig. 5A and B). Sorafenib treatment significantly decreased endothelial cell p-ERK expression, and the addition of Tie2Ex did not enhance endothelial cell p-ERK inhibition (Fig. 5C and D).
FIGURE 5.
Endothelial cell signaling of K1735.Tie2Ex tumors treated with Tie2Ex and sorafenib. Paraffin-embedded tumor sections from K1735.Tie2Ex and K1735.hygro tumors treated starting at a larger size (~6 mm diameter) were stained for p-AKT or p-ERK and CD34 to identify endothelial cell p-AKT (A and B) or p-ERK (C and D) expression. Percentage of vessels expressing p-AKT in K1735.Tie2Ex tumors (A) and K1735.hygro tumors (B) was calculated and plotted as a histogram. Percentage of vessels expressing p-ERK in K1735.Tie2Ex tumors (C) and K1735.hygro tumors (D) was calculated and plotted as a histogram. Histogram plot represents values obtained from counting vessels in two sections of at least three different tumors for each group. Student’s t test statistical analysis was done. P < 0.01 was considered highly significant.
Endothelial cell apoptosis and proliferation were examined in these tumors to examine the effect of Tie2Ex and sorafenib treatment on endothelial cell fate. Whereas Tie2Ex induced endothelial cell apoptosis in large K1735.Tie2Ex tumors, sorafenib treatment alone did not. Treatment with sorafenib plus Tie2Ex further increased endothelial cell death compared with Tie2Ex treatment alone (Fig. 6A). The fact that endothelial cell apoptosis was not induced in K1735.hygro tumors treated with doxycycline (Fig. 6B) showed that apoptosis was due to Tie2Ex induction and was not an effect of doxycycline itself. Sorafenib treatment significantly decreased endothelial cell proliferation, whereas Tie2Ex produced no significant effect on proliferation either alone or in combination with sorafenib (Fig. 6C and D).
FIGURE 6.
Endothelial cell fate of K1735.Tie2Ex tumors treated with Tie2Ex and sorafenib. Tumor sections were stained for TUNEL and CD31 or Ki-67 and CD34 to identify apoptotic vessels (A and B) or proliferating vessels (C and D). Percentage of apoptotic vessels in K1735.Tie2Ex tumors (A) and K1735.hygro tumors (B) was calculated and plotted as a histogram. Percentage of proliferating vessels in K1735.Tie2Ex tumors (C) and K1735.hygro tumors (D) was calculated and plotted as a histogram. Histogram plot represents values obtained from counting vessels in two sections of at least three different tumors for each group. Student’s t test statistical analysis was done. P < 0.05 and P < 0.01 were considered highly significant.
Tie2Ex as an Antitumor Therapeutic Agent
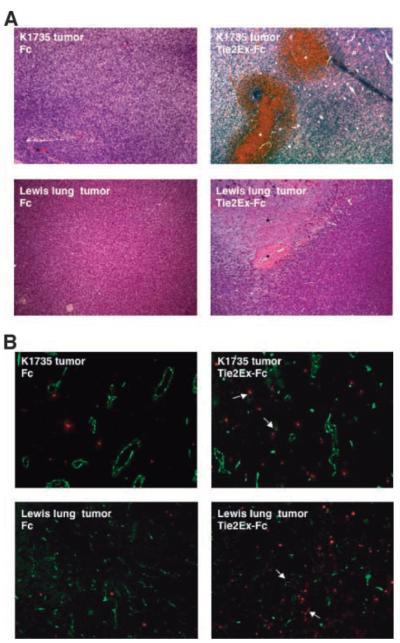
To show that the effect of Tie2Ex on K1735.Tie2Ex tumors was not restricted to that tumor cell clone, Tie2Ex fused to a Fc was expressed in 293T cells, purified from culture supernatant, and injected intratumorally into wild-type K1735 and Lewis lung tumors. Purified Fc was injected as a control agent. Tumors injected with Tie2Ex-Fc had regions of thrombosis and hemorrhage compared with little to no hemorrhage in Fc-injected tumors (Fig. 7A). Tumors injected with Tie2Ex-Fc also had increased cellular apoptosis (Fig. 7B). These results indicate that Tie2Ex has the potential to be a tumor therapeutic agent that disrupts tumor vessel integrity.
FIGURE 7.
Intratumoral injection of Tie2Ex in K1735 wild-type and Lewis lung tumors. Purified Tie2Ex-Fc or Fc in PBS was injected intratumorally into K1735 wild-type tumors or Lewis lung tumors. Tumors were excised 16 to 18 h after injection. A. H&E staining of tumor sections from K1735 wild-type tumors (top) or Lewis lung tumors (bottom) with Fc (left) or Tie2Ex-Fc (right). Total magnification, ×100. B. Frozen tumor sections from K1735 wild-type or Lewis lung tumors injected with Fc or Tie2Ex-Fc were stained with TUNEL (red) and CD31 (green) to identify apoptotic cells and vessels. Total magnification, ×100. Asterisks, regions of hemorrhage or cell death; arrows, apoptotic endothelial cells/vessels.
Discussion
In vitro studies have identified that multiple signaling pathways are activated downstream of Ang1-activated Tie2 signaling in endothelial cells, including the Raf-MEK-ERK pathways and PI3K-AKT pathways (reviewed in ref. 2). Many of these studies agree in finding that the PI3K-AKT pathway is the major pathway activated by Tie2 signaling (12-15). This may be explained by the presence of binding sites for the p85 regulatory subunit of PI3K on Tie2 receptor tyrosine residues following receptor activation (15). Our studies using an inducible tumor model expressing Tie2Ex to inhibit Tie2 signaling support the thesis that Tie2 signaling in vivo, at least in tumor endothelial cells, is through the AKT pathway and that ongoing Tie2 signaling is needed for maintenance of full AKT activation in tumor endothelial cells. Published in vitro studies have reported conflicting results on ERK activation following Ang1 stimulation of Tie2 (15-20). The studies showing activation of ERK have not reported sustained ERK activation. Our data do not address whether Raf-MEK-ERK signaling is induced transiently by Tie2 activation in vivo. However, our results showing that inhibition of Tie2 signaling does not affect tumor endothelial cell ERK activation indicate that ongoing Tie2 signaling is not needed for maintenance of ERK activation in tumor endothelial cells and that activation of the ERK pathway in tumor endothelial cells is most likely attributed to signaling from other receptors or integrins (30-34).
Following Tie2Ex induction, a fraction of vessels expressing p-AKT continue to express p-AKT. One explanation may be that Tie2Ex does not completely inhibit Tie2 activation. This could be due to limited access of the decoy receptor to endothelial cell-pericyte junctions, where paracrine signaling has been shown to occur (35). Another possibility may be that other endothelial cell receptors, such as vascular endothelial growth factor receptors, contribute to endothelial cell AKT activation. Preliminary studies in the laboratory have shown that treatment with a vascular endothelial growth factor inhibitor decreases tumor endothelial cell p-AKT expression by about a third. Combined with the current results, this suggests that signaling originating from Tie2 and vascular endothelial growth factor receptors may be responsible for most or all of AKT activation in tumor endothelial cells. These results also indicate that activation of tumor endothelial cell AKT signaling occurs through a combination of factors and that inhibiting the activity of a single factor may not completely suppress signaling through AKT.
The fact that Tie2 inhibition decreased endothelial cell AKT activation and increased endothelial cell apoptosis but had no effect on endothelial cell proliferation suggests that AKT activation downstream of Tie2 plays a critical role in tumor endothelial cell survival but is not a determinant of endothelial cell proliferation. In vitro studies have shown that endothelial cell survival downstream of Ang1-induced Tie2 activation is mediated through the forkhead transcription factor FOXO1 and survivin (13, 14, 36). Ang2 can also function as an antiapoptotic factor through an autocrine mechanism by regulating FOXO1 expression (37). Inhibition of Tie2 signaling in vitro was also shown to decrease endothelial cell AKT activation and up-regulate thrombospondin-1, an endogenous antiangiogenic molecule that induces endothelial cell apoptosis (38). These are candidate factors for regulating tumor endothelial cell survival downstream of Tie2 via AKT activation. Our data also showed a decrease in vascular perfusion following Tie2Ex induction, suggesting that Tie2 signaling, by promoting endothelial cell survival and possibly additional mechanisms, helps maintain tumor vascular function.
Our studies show that the therapeutic effect of Tie2Ex is a result of decreased tumor endothelial cell signaling and survival following Tie2 inhibition. Previous studies designed to inhibit Tie2 signaling by using soluble Tie2 or specifically blocking Ang2 interaction with Tie2 have shown inhibition of tumor growth and tumor metastasis (39-41), but these reports provided little or no information on mechanism. The influence of Tie2 inhibition on vessel perfusion and endothelial cell survival but not endothelial cell proliferation suggests that tumor growth delay is not due to angiogenesis inhibition but rather a result of decreased tumor vascular perfusion and vessel regression. This is consistent with the regions of necrosis that develops following Tie2Ex induction in K1735 tumors, which contrasts with the absence of necrotic regions in K1735 tumors treated with agents that tend to have a strong angiogenesis inhibitory component, such as recombinant interleukin-12 (42, 43). More recently, studies have focused on understanding the role of Tie1 in endothelial cells. These studies showed the potential of Tie1/Tie2 interaction to affect their downstream signaling (5, 7-9). Although the contribution of Tie1 signaling to tumor endothelial cell survival is currently unknown, our use of Tie2Ex to modulate Tie2 signaling may affect signaling through Tie1, and some of the effects we observe may be attributable to inhibition of Tie1 actions.
Angiopoietin-Tie2 interaction has also been shown to be important in vessel maturation and pericyte recruitment to vessels (reviewed in ref. 28). Tie2Ex induction resulted in a decrease in pericyte coverage only after 1 week of induction. The delayed kinetics in decreased pericyte coverage compared with a more rapid change in endothelial cell signaling suggests that cellular changes resulting from Tie2 inhibition may be secondary to changes in endothelial cell signaling. Our results do not indicate whether Tie2 inhibition directly or indirectly affects pericyte engagement. Recent studies investigating the molecular mechanisms of angiopoietin-mediated smooth muscle cell recruitment to endothelial cells identified a paracrine interplay between endothelial cells and smooth muscle cells. Ang1 was shown to stimulate endothelial cell production of hepatocyte growth factor and heparin-binding epidermal growth factor-like growth factor, which resulted in the recruitment of smooth muscle cells to endothelial cells (44, 45). Combined with our results, this would suggest that blocking Tie2 signaling in tumor vessels may inhibit endothelial cell production of paracrine factors that recruit pericytes, resulting in destabilized vessels.
Tie2 inhibition by Tie2Ex induced K1735 tumor growth delay in recently established, smaller tumors but was not noticeably effective in controlling growth of larger, more established tumors. Interestingly, the combination of Tie2Ex and sorafenib, a multikinase inhibitor that inhibits ERK activation in K1735 tumor endothelial cells (25), resulted in a dramatic improvement in growth control of the more established tumors. This appears to be the result of increased endothelial cell apoptosis and decreased endothelial cell proliferation with combination therapy compared with treatment with Tie2Ex alone, which only increased endothelial cell apoptosis, or treatment with sorafenib alone, which only decreased endothelial cell proliferation. From a signaling standpoint, a decrease in activated endothelial cell AKT expression was seen only with Tie2Ex treatment and a decrease in activated endothelial cell ERK expression was seen only with sorafenib therapy. The correlation of changes in endothelial cell fate with changes in endothelial cell signaling following different treatments reinforces the idea that endothelial cell Tie2 activates PI3K-AKT signaling and specifies survival while having little or no effect on Raf-MEK-ERK pathway activation and endothelial cell proliferation. These insights into the association of tumor endothelial cell signaling with endothelial cell fate in vivo suggest a more rational way to apply targeted agents for effective antiangiogenic therapies. Previous studies in the laboratory showed that sorafenib treatment of small K1735 tumors increased endothelial cell apoptosis and decreased endothelial cell proliferation (25). In the current studies, sorafenib treatment of the more established tumors contributed to endothelial cell apoptosis only when combined with Tie2Ex. This suggests that Tie2 inhibition may sensitize tumor vessels to damage by other agents through its ability to impair tumor endothelial cell survival. Tumors in patients tend to be well established by the time of diagnosis and therapy. Whatever therapeutic effect Tie2 inhibitors may produce on their own, they may be able to improve the efficacy of other antiangiogenic or antivascular agents in treating these established tumors. Recent studies showed that tumors develop resistance to monotherapy with antiangiogenic agents targeting individual angiogenic factors and that treatment with multiple agents targeting multiple factors can overcome resistance (refs. 46, 47; reviewed in refs. 48, 49). Our studies also support therapy using multiple agents but invoke a different rationale, inhibition of complementary endothelial cell signaling pathways, to select targets and drug combinations to control tumor growth.
Materials and Methods
Mice and Tumors
Inducible Tie2Ex K1735 murine melanoma tumor cells were generated using the Tet-on system (BD Biosciences). Briefly, cells were stably transfected with the rtTA component and single-cell clones were isolated by G418 selection and screened for highest expression of transiently transfected reporter construct in the presence of doxycycline. The extracellular domain of Tie2 (gift from D. Dumont) was inserted into the TRE-hygro plasmid, which was then transfected into the K1735 tumor cells stably expressing rtTA. Single-cell clones were again isolated and screened for Tie2Ex expression regulated by doxycycline.
Female C3H/HeN mice (6-8 weeks old) were purchased from the National Cancer Institute and maintained in micro-isolate cages. Tumors were generated s.c. by injecting 2 × 106 cells into the hind limb of mice. Tumor growth was measured at regular intervals using calipers and tumor volume was calculated using the formula for approximating the volume of a spheroid [0.5 × width × width × length]. Mice were treated with doxycycline at 2 mg/mL in 5% sucrose water and changed every other day. Treatment with sorafenib at 30 mg/kg by daily gavage was done as described previously (25).
Biochemistry
EA.hy926 cells (50) were grown to confluence in 100 mm plate. Cells were serum starved overnight and then pretreated with 1 mmol/L sodium vanadate for 10 min before incubation with rAng1 (R&D Systems) at 50 ng/mL. Tie2Ex conditioned medium was produced by transiently transfecting HEK 293T cells with a pEF2-TekFc plasmid (Dr. Newman Yeilding) followed by collection and filtration of the supernatant with a 0.22 μm syringe filter (Millipore). Cells were stimulated with rAng1 or rAng1 + Tie2Ex for 10 min and then lysed in lysis buffer [50 mmol/L Tris (pH 7.4), 0.25% Triton X-100, 5 mmol/L EDTA, 0.1% NP-40, 250 mmol/L NaVO4, 20 mmol/L β-glycerophosphate, 10 mmol/L NaF, and complete protease inhibitor cocktail (Sigma)].
Protein concentration was determined using the MicroBCA kit (Pierce). For Tie2 phosphorylation studies, an anti-Tie2 antibody (sc-324; Santa Cruz Biotechnology) was added to cell lysate and mixed gently overnight at 4°C with protein A agarose beads (Invitrogen). Beads were washed five times with 1 × PBS/1% NP-40. Proteins were eluted with loading buffer, resolved on a 10% SDS-polyacrylamide gel by electrophoresis, and transferred to nitrocellulose membrane (Protran; Schleicher and Schuell). Membrane was blotted with an anti-phosphotyrosine antibody (clone 4G10; Upstate), stripped, and reprobed with an anti-Tie2 antibody (Ab33; Upstate). ERK and AKT activation was detected by loading 50 μg cell lysate onto a 10% SDS-polyacrylamide gel, transferred to nitrocellulose membrane, and probed with anti-p-ERK (Cell Signaling) or anti-p-AKT (Cell Signaling), respectively. Blots were stripped and reprobed with an anti-ERK (Cell Signaling) or anti-AKT antibody (Cell Signaling), respectively. Relative p-Tie2, p-ERK, and p-AKT expression was quantitated by calculating absorbance of phosphorylated proteins/absorbance of unphosphorylated proteins and normalized to control levels (Image J; NIH). Tie2Ex in tumor lysate was detected by using an anti-Tie2 antibody (Ab33; Upstate). The blot was stripped and reprobed with an anti-GAPDH antibody as a loading control (Cell Signaling).
Tumor Cell RNA Isolation and Reverse Transcription-PCR Analysis
Total RNA was isolated from frozen tumor fragments homogenized in Trizol solution according to the manufacturer’s protocol (Invitrogen). cDNAs were generated from total RNA using the SuperScript First-Strand kit (Invitrogen). Samples receiving no reverse transcriptase enzyme were used as negative controls. Ang1 transcripts (1,496 bp) were detected using PCR with primer pairs: 5′-ATGACAGTTTTCCTTTCCTTT-3′ and 5′-TCAAAAGTCCAAGGGCCGGAT-3′. Ang2 transcripts (1,488 bp) were detected using PCR with primer pairs: 5′-ATGTGGCAGATCATTTTCCTA-3′ and 5′-TTAGAAATCTGCTGGCCGGAT-3′. Reaction conditions were 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min 30 s.
Tie2Ex-Fc Protein Purification
HEK 293T cells were adapted to serum-free SFM4HEK293 medium conditions according to the manufacturer’s protocol (Hyclone). Adapted cells were transferred into DMEM containing 10% serum 1 day before transfection with pEF2-Tie2Ex-Fc plasmid (from Dr. Newman Yeilding). Transfection was done using SuperFect Transfection Reagent according to the manufacturer’s protocol (Qiagen) and transferred into SFM4HEK293 medium overnight. Culture supernatant was tested for Tie2Ex expression using Western blot analysis by probing with an anti-Tie2 antibody. Viable cells were transferred into CELLine device (BD Biosciences) at 2 × 106/mL and allowed to grow for 7 days. After 7 days, cells were spun down and the supernatant was collected. Supernatant medium was changed into binding buffer [20 mmol/L sodium phosphate (pH 7.0)] using Amicon Ultra centrifugal filter device (Millipore). Tie2Ex-Fc was purified using HiTrap protein A affinity columns (GE Healthcare) according to the manufacturer’s protocol. Protein was eluted with 0.1 mol/L citric acid (pH 3.0) and buffer was exchanged into PBS (pH 7.0) using Amicon Ultra centrifugal filter device (Millipore).
Immunohistologic Staining and Analysis
Staining for endothelial cell p-AKT, p-ERK, TUNEL, and Ki-67 expression were done as described previously (25, 51). Paraffin-embedded tumor sections were stained with an anti-p-AKT (Ser473) antibody (736E11; Cell Signaling), anti-p-p44/42 mitogen-activated protein kinase (Thr202/Tyr204) antibody (20G11; Cell Signaling), or anti-Ki-67 antibody (Vector Laboratories) and an anti-CD34 antibody (Abcam) to identify endothelial cell p-AKT, p-ERK, or Ki-67, respectively. Vessels were considered positive for the antigen if at least one endothelial cell (identified by CD34) in the vessel was positive for the antigen. Frozen tumor sections (10 μm) were stained with an anti-CD31 antibody (Pharmingen) and an anti-smooth muscle actin antibody (Sigma) or a TUNEL labeling kit (Millipore) to identify pericyte-covered vessels or apoptotic vessels. For vessel perfusion analysis, tumor-bearing mice were injected i.v. with FITC tomato lectin (Vector Laboratories) and frozen tumor sections (10 μm) were stained with an anti-CD31 antibody followed by a Texas red-conjugated fluorescent secondary. All vessels were counted in the viable regions of the tumor. Confocal microscopy of vessel morphology and branching was done as described previously (42, 51). Percentage of tumor necrosis was determined by quantitating necrotic areas, as identified by H&E staining of tumor sections, using Image J software (NIH).
Statistical Analysis
Assessment of statistical significance for survival analysis was done by log-rank test (GraphPad Prism software). All other statistical analysis was done by Student’s t test (Microsoft Excel).
Supplementary Material
Acknowledgments
We thank Dr. Newman Yeilding for the Tie2Ex construct, Steve Huang for advice on protein purification, Danielle Murphy for advice on immunohistology, Wiem Lassoued and Sosina Makonnen for technical assistance, Bayer for providing sorafenib, Center for Molecular Studies of Liver and Digestive Diseases Morphology Core (center grant P30 DK50306) for histology imaging, and Biomedical Imaging Core for confocal images.
Grant support: Cancer Research Institute training grant and Department of Defense predoctoral training grant W81XWH-04-1-0338 (J.H. Tsai) and DAMD17-03-1-036, CA77851, and CA99519 (W.M.F. Lee).
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 2.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–41. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Rodewald HR, Sato TN. Tie1, a receptor tyrosine kinase essential for vascular endothelial cell integrity, is not critical for the development of hematopoietic cells. Oncogene. 1996;12:397–404. [PubMed] [Google Scholar]
- 4.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 5.Marron MB, Singh H, Tahir TA, et al. Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J Biol Chem. 2007;282:30509–17. doi: 10.1074/jbc.M702535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–23. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–37. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 8.Saharinen P, Kerkela K, Ekman N, et al. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J Cell Biol. 2005;169:239–43. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan HT, Venkatesha S, Chan B, et al. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 2007;21:3171–83. doi: 10.1096/fj.07-8487com. [DOI] [PubMed] [Google Scholar]
- 10.Dumont DJ, Gradwohl G, Fong GH, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 11.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–91. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp Cell Res. 2004;298:167–77. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–9. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–5. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 15.Kontos CD, Stauffer TP, Yang WP, et al. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18:4131–40. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujikawa K, de Aos Scherpenseel I, Jain SK, Presman E, Christensen RA, Varticovski L. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp Cell Res. 1999;253:663–72. doi: 10.1006/excr.1999.4693. [DOI] [PubMed] [Google Scholar]
- 17.Harfouche R, Gratton JP, Yancopoulos GD, Noseda M, Karsan A, Hussain SN. Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. 2003;17:1523–5. doi: 10.1096/fj.02-0698fje. [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Ryu YS, Kwak HJ, et al. EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin 1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. FASEB J. 2002;16:1126–8. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- 19.Yoon MJ, Cho CH, Lee CS, Jang IH, Ryu SH, Koh GY. Localization of Tie2 and phospholipase D in endothelial caveolae is involved in angiopoietin-1-induced MEK/ERK phosphorylation and migration in endothelial cells. Biochem Biophys Res Commun. 2003;308:101–5. doi: 10.1016/s0006-291x(03)01341-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu WH, MacIntyre A, Nicosia RF. Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am J Pathol. 2002;161:823–30. doi: 10.1016/S0002-9440(10)64242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korpelainen EI, Karkkainen M, Gunji Y, Vikkula M, Alitalo K. Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant Tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene. 1999;18:1–8. doi: 10.1038/sj.onc.1202288. [DOI] [PubMed] [Google Scholar]
- 22.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 23.Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–52. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 24.Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96:1904–9. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy DA, Makonnen S, Lassoued W, Feldman MD, Carter C, Lee WM. Inhibition of tumor endothelial ERK activation, angiogenesis, and tumor growth by sorafenib (BAY43-9006) Am J Pathol. 2006;169:1875–85. doi: 10.2353/ajpath.2006.050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson M, Wong D, Lacroix S, Stanisz J, Singh G. Inhibition by doxycycline of angiogenesis in the chicken chorioallantoic membrane (CAM) Cancer Chemother Pharmacol. 2005;56:1–9. doi: 10.1007/s00280-004-0955-2. [DOI] [PubMed] [Google Scholar]
- 28.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 29.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65:6820–7. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- 30.Lee MJ, Thangada S, Claffey KP, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–12. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 31.Eliceiri BP, Cheresh DA. The role of αv integrins during angiogenesis. Mol Med. 1998;4:741–50. [PMC free article] [PubMed] [Google Scholar]
- 32.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–7. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 33.Pedram A, Razandi M, Levin ER. Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 1998;273:26722–8. doi: 10.1074/jbc.273.41.26722. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Sato JD. MAP kinases, phosphatidylinositol 3-kinase, and p70 S6 kinase mediate the mitogenic response of human endothelial cells to vascular endothelial growth factor. J Cell Physiol. 1999;178:235–46. doi: 10.1002/(SICI)1097-4652(199902)178:2<235::AID-JCP13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-β activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–37. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 36.Daly C, Wong V, Burova E, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–71. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly C, Pasnikowski E, Burova E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15491–6. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niu Q, Perruzzi C, Voskas D, Lawler J, Dumont DJ, Benjamin LE. Inhibition of Tie-2 signaling induces endothelial cell apoptosis, decreases Akt signaling, and induces endothelial cell expression of the endogenous anti-angiogenic molecule, thrombospondin-1. Cancer Biol Ther. 2004;3:402–5. doi: 10.4161/cbt.3.4.735. [DOI] [PubMed] [Google Scholar]
- 39.Lin P, Buxton JA, Acheson A, et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc Natl Acad Sci U S A. 1998;95:8829–34. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–8. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol. 2003;162:183–93. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gee MS, Koch CJ, Evans SM, et al. Hypoxia-mediated apoptosis from angiogenesis inhibition underlies tumor control by recombinant interleukin 12. Cancer Res. 1999;59:4882–9. [PubMed] [Google Scholar]
- 44.Iivanainen E, Nelimarkka L, Elenius V, et al. Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J. 2003;17:1609–21. doi: 10.1096/fj.02-0939com. [DOI] [PubMed] [Google Scholar]
- 45.Taher TE, Derksen PW, de Boer OJ, et al. Hepatocyte growth factor triggers signaling cascades mediating vascular smooth muscle cell migration. Biochem Biophys Res Commun. 2002;298:80–6. doi: 10.1016/s0006-291x(02)02397-5. [DOI] [PubMed] [Google Scholar]
- 46.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1α-deficient colon cancer cells. Nat Med. 2005;11:992–7. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 48.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 50.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80:3734–7. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai JH, Makonnen S, Feldman M, Sehgal CM, Maity A, Lee WM. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther. 2005;4:1395–400. doi: 10.4161/cbt.4.12.2331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.