Abstract
Cocaine abuse is an ongoing and serious problem which has lead to the growth of a brutal criminal enterprise, particularly in the Americas and Europe. At present, there are no effective pharmacological agents available to treat the addiction by blocking cocaine or reversing its effects. In order to help motivated addicts conquer their addiction, vaccines against cocaine are being developed, and one has progressed to clinical trials. This review will discuss the concept of anti-drug vaccines in general, the successes and limitations of the various anti-cocaine vaccine approaches, the results of the clinical trials with an anti-cocaine vaccine, and some new vaccine-mediated approaches to combat cocaine addiction.
Keywords: Substance Abuse, Vaccination, Cocaine, Immunotherapy
History
Cocaine is one of the oldest known drugs. The leaves of the coca plant have been chewed by the indigenous peoples of South America for thousands of years to increase stamina for high altitude work and to afford feelings of well-being. In 1859, the active ingredient in coca was isolated and named cocaine, and it was considered a rather benign drug until the beginning of the 20th century, when the negative aspects of its use became widely recognized (1). The sale and distribution of opium, coca leaves, and their derivatives was restricted in the United States under the 1914 Harrison Narcotics Tax Act to the practice of medicine, dentistry, and veterinary medicine. However, the law was not strictly enforced for cocaine, and there was open use of the drug for a number of years, although this was generally limited to the minority and low income urban populations, artists, and musicians (2). The addictive properties of cocaine were again gradually recognized, but in spite of this and increased efforts to enforce drug laws, the use of cocaine, particularly the smoked free base form known as crack, exploded during the 1970's and 80's. There is some light on the horizon, however, as data gathered by SAMSHA, the Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services shows that admissions to substance abuse treatment for cocaine decreased from 1995 to 2005, and the age of those admitted went up substantially (3). Even though there was a decrease in admissions among African-Americans, there was an increase among whites, while use prevalence among the former remained substantially higher than among the latter. There is an urgent need for new treatments for cocaine addiction, especially since there are no effective pharmacological interventions (4), as there are for morphine/heroin addiction (methadone) (5).
Anti-drug Vaccines
It has been known and studied for at least 50 years that small molecules (haptens) will not usually elicit an immune response unless they are covalently attached to an immunogenic protein (6, 7). This results in the body's tolerance for most medications and chemical exposures without significant harmful immune reactions. Allergic reactions to medicines like penicillin occur when the immune system recognizes the chemical or its metabolic products after they have been bound to native proteins, allowing for antigen presentation and initiation of the immune response (8). Therefore, to produce an anti-drug vaccine, the hapten is covalently attached to a foreign carrier protein, permitting both cross-linking of the antibody molecules expressed on B cells to activate them, as well as induction of the T cell help that is required to elicit high level antibody responses (9).
The first effort at an anti-drug vaccine, in this case against morphine/heroin, was published in the early 1970's. A useful review by Spector in 1973 discusses the effectiveness of three vaccines prepared from different derivatives of morphine, and whether the antibodies so produced bound to other morphine-like molecules (10). Work on these vaccines withered away subsequently, probably due to the availability of methadone for heroin addiction, as that intervention seemed to help addicts stop using the abused drug. Even though methadone is itself addictive, it calms the craving for heroin, has few side effects and has been successfully used for more than thirty years in Western countries (11). Interest in anti-drug vaccines reawakened starting in 1992 when an anti-cocaine vaccine was reported (12). In 2001, Janda prepared a hapten for use in making anti-nicotine vaccines (13). Several other types of nicotine vaccines quickly followed (14), and clinical trials have been undertaken using some of them (15) with modest results. Methamphetamine vaccines have also been produced (16, 17), and their use in rodents has given positive results, suggesting that clinical trials for anti-methamphetamine vaccines may not be far off.
Anti-cocaine Vaccines Leading to Catalytic Antibodies
The methyl ester of cocaine can spontaneously hydrolyze in serum to give benzoyl ecgonine (BE) (18). There are also esterases in tissue that will accomplish this hydrolysis (19) and the phenyl ester can be hydrolyzed by human butyryl cholinesterase (HBC) to give ecgonine methyl ester and benzoic acid (20). These metabolites have little if any pharmacological activity. There was considerable interest in the 1990's in the creation of catalytic antibodies which would act along the lines of HBC, and papers were published by Chandrakumar (21), Landry (22), Basmadjian (23) and Berkman (24). Clever chemistry was used to create haptens known as transition state analogs in order to mimic the intermediate, usually unstable chemical structures that lead to the hydrolysis reaction, so that the antibodies produced would act as enzymes to catalyze the release of the benzoyl group from cocaine. Basmadjian reported that polyclonal antibodies in the sera of vaccinated mice had this effect, and Landry generated monoclonal antibodies that did so as well. Berkman, by a laborious chemical synthesis, prepared a hapten that was claimed to be stable and long-lived and conjugated it to keyhole limpet hemocyanin (KLH). Later on, the vaccine was given to mice, and the monoclonal antibodies which were generated were identified by a high-throughput method (25). About the same time, Janda reported that the position of the linker in the cocaine transition state hapten was important, such that a linker attached in the methyl ester position rather than the tropane nitrogen gave better results, and having an amide group in the carbon chain of the linker was even more effective (26). The catalytic antibody idea is attractive in that a relatively small number of antibodies produced by a vaccine might be able to destroy cocaine molecules and then be ready to take on more. However, some questions about the quantity, affinity and efficacy of these antibodies still need to be answered. Furthermore, in none of these studies were the vaccinated mice tested for the in vivo behavioral outcomes of cocaine dosing, in spite of high titers of the catalytic antibodies being reported. Anti-cocaine catalytic antibodies continue to be studied (27), but no clinical trials have been initiated as yet.
Anti-cocaine Vaccines
Anti-cocaine vaccines began to be made in 1992. The first report, by Bagasra (12), used questionable chemistry to create the vaccine (28), but the door was opened. In 1995, Janda prepared the first credible anti-cocaine vaccine (29). A hapten (named GNC) with a six-carbon linker terminating in a carboxylic acid group was synthesized starting with BE and conjugated KLH by standard methods. When vaccinated rats were dosed with cocaine, there was suppression of locomotor activity as compared with controls and lower levels of cocaine were found in the brain. Another study using the same vaccine showed prevention of cocaine reinstatement in rats (30). Janda also synthesized a hapten (dubbed GND) similar to GNC only with two amide groups in place of the two ester groups of GNC. This change was said to lead to a more stable and effective vaccine (31), and indeed vaccinated rats displayed suppression of the psychomotor effects of cocaine (32). However, these results were only compared with those occurring from injection of a monoclonal antibody generated from a GNC-KLH vaccination (GNC92H2).
In 1996, Fox, Kantak et al. published the creation of a vaccine consisting of succinyl norcocaine (SNC) conjugated to bovine serum albumin (BSA) using very accessible chemistry (33), and demonstrated that less cocaine was found in the brains of vaccinated mice after a cocaine dose. Soon thereafter, Ettinger prepared a vaccine by a photoactivation method (34) whereby a linker attached to a protein is inserted into a carbon-carbon bond of cocaine, but this method does not allow one to tell which or how many of the carbon-carbon bonds of the cocaine has been accessed. Nevertheless, hot-plate and place preference conditioning assays as well as attenuation of the discriminative properties of cocaine (35) confirmed that anti-cocaine antibodies had been formed in vaccinated rats. In 2000, an interesting idea was reported on by Schabacker (36), whereby a monoclonal anti-cocaine antibody was used as an anti-idiotype vaccine whose configuration mimics that of cocaine, presenting an internal image of the cocaine molecule to the immune system. The vaccinated mice showed reduced levels of cocaine in the brain following a challenge as well as similar levels of anti-cocaine antibodies in the serum as compared with mice vaccinated with a cocaine-KLH conjugate vaccine.
Meanwhile, Fox and Kantak continued work on their vaccine, now made from succinyl norcocaine conjugated to cholera toxin B (dubbed TA-CD) and, significantly, prepared by a commercial company, Cantab Pharmaceuticals, UK. Studies in 2000 and 2001 showed that the vaccine was effective in eliciting levels of antibodies sufficient to antagonize self-administration of cocaine in rats (37, 38). At about the same time, Landry treated 3 rhesus monkeys with a succinyl norcocaine-BSA vaccine and reported that the level of antibodies in the blood corresponded to suppression of operant responding to a food reward, and that there were no side effects resulting from the vaccination (39). In 2005, Hrafnkelsdottir vaccinated mice with a succinyl norcocaine –KLH vaccine both by an intranasal route using a glyceride adjuvant (RhinoVax) and by a subcutaneous route (40). After a challenge with cocaine, the levels of cocaine in the serum, brain and olfactory bulb were measured, and the amount of cocaine found in the brain was less for all cocaine immunized groups as compared to controls. The amount of cocaine in the brains of intranasally vaccinated mice was only 2 times higher than in the brains of the subcutaneously vaccinated mice, in spite of the fact that the serum level of antibodies was fivefold higher in the latter group. This suggested that mucosal antibodies could be playing a role in sequestering the cocaine, and intranasal vaccination could be useful for cocaine taken in by smoking or snorting.
Clinical Trials of an Anti-Cocaine Vaccine (TA-CD)
In 2002, using the TA-CD vaccine, Kosten reported on the first clinical trial of an anti-cocaine vaccine (41), using subjects recruited from a residential treatment program. The vaccine was well tolerated, and three different doses were given three times at monthly intervals. Twenty-four subjects completed the trial. Anti-cocaine antibodies appeared after the second injection, peaked at three months and fell to baseline by 1 year. The level of antibodies correlated with the vaccine dose. In 2005, another trial was undertaken, this time in cocaine-dependent subjects recruited from an outpatient treatment center, with 16 patients completing the study. (42). The effects of two different doses of the vaccine were studied, and the subjects who received the higher dose had higher levels of antibodies in their serum and fewer positive urines indicating cocaine use. They also reported less of a euphoric effect if they did take cocaine. Antibodies did not appear in the serum before 2 weeks and had waned by 6 months, but booster vaccinations in a few subjects increased the antibody titers. However, in both trials, 25-30% of subjects produced relatively low levels of antibodies.
A larger trial involving 115 subjects from an outpatient methadone maintenance program was carried out in 2009 (43). Antibody levels were measured monthly after the first 2 weeks, and urine samples were tested three times a week for the entire study. As in the previous trials, the level of antibodies reached varied substantially among individuals. Based on animal studies showing the concentration of high affinity antibodies required to bind cocaine in the serum, and the concentration of cocaine needed in humans to give a euphoric effect, it was estimated that a concentration of 43 μg/mL of antibody would be needed to bind and capture sufficient cocaine to significantly reduce its access to the brain. Only 38% of the subjects in this trial attained this target concentration of antibodies, but this high responder group had significantly more cocaine-free urine samples than either the low antibody group or the placebo group. A trial to determine how the subjective effects of smoked cocaine varied with peak plasma antibody levels was reported in 2010 by Haney (44). The subjects were 10 non-treatment-seeking cocaine dependent men. As before, the peak antibody levels were highly variable, but the subjects with the higher levels offered their impression that the cocaine they used had less “good drug effect” and was of “lower quality”.
Alternate Approaches for Anti-Cocaine Vaccines
David Jackson at the University of Melbourne in Australia has developed a totally synthetic self-adjuvanting vaccine which has shown promise as an anti-cocaine vaccine in animal studies in our laboratories. In effect, a component of lipopolysaccharide is attached to a short peptide, to which a T cell peptide epitope (required for T cell helper function) and the hapten of interest are also attached (45). This vaccine construction thus delivers both the antigen and the co-stimulatory signaling molecular structure to the same microenvironment, enhancing the likelihood of an effective immune activation. In addition, this vaccine preparation approach has the advantage of ease of modification and ease of production.
Steven Brimijoin at the Mayo Clinic (46) and Chan-Guo Zhan of the University of Kentucky (47, 48) have developed an improved versions of HBC which can be administered as a biological treatment or delivered by a viral vector (49) to block the activity of cocaine by rapidly hydrolyzing cocaine in circulation. If this enzyme treatment were used together with anti-cocaine antibodies elicited by vaccination, a synergistic effect would be achieved, as the cocaine would be bound rapidly by the antibody on initial exposure, and then hydrolyzed as it is slowly released from an antibody, further reducing its potential access to the brain (50).
Expert Commentary
As the investigators in the clinical trials reported, there are several factors that need improving before anti-cocaine vaccines can achieve their full potential. It is not understood why there is so much variability in antibody responses to cocaine among the human subjects, having no clear relationship with the antibody responses to the carrier protein. In the early clinical studies, antibody responses to cholera toxin B were generally high and the pattern of responses did not appear to correlate quantitatively with the anti-cocaine responses. About a third of patients in the large outpatient study responded with adequately high levels for blocking cocaine effects, and studies should be undertaken to find correlates to this observation, such as the health of the subjects, their genetic makeup, their patterns of prior drug use, or their immunological response to other hapten stimuli. To increase the overall level of antibodies, a better vaccine and/or adjuvant should be sought, with perhaps an improved vaccine booster schedule. The amount and density of hapten conjugated to the immunogenic carrier protein can influence the antibody response both positively and negatively (51), and this variable has not been investigated in the cocaine studies, and may have an effect on the efficacy of the vaccine. The potential desirability or liability of very high-affinity antibody production should be more thoroughly probed. Finally, cocaine is a molecule which allows attachment of linkers in several places. Head-to-head studies rating the different attachment locales and the chemical structure of the linkers is needed.
Five Year View
Additional studies for the current cocaine vaccine are planned to confirm and extend the discussed outpatient studies, and there are ongoing developmental studies of alternative adjuvants and vaccine constructs which will likely improve the quantity and quality of antibodies produced, as well as the proportion of high response subjects. Such results would lead to clinical application of these vaccines for in the treatment of cocaine abusers. Better vaccines or newer methods will not be the end of the game for treating substance abuse, however. The motivated cocaine addict will need other interventions such as therapy and rehabilitation programs in order to overcome this seductive addiction. Anti-drug programs in schools should be strengthened, as cocaine addiction often starts before age 20. The criminal justice system should reconsider the wholesale incarceration of cocaine users, and offer help rather than punishment. Let us hope that in the years ahead anti-cocaine vaccination will be one of numerous arrows in our therapeutic quiver to combat drug addiction.
Key Issues
Anti-cocaine vaccines have shown promise in clinical trials, validating the potential of such an approach to help treat substance abuse.
Better vaccines and/or better vaccination methods will be needed to ensure that antibody levels in most patients will be adequate to sequester commonly used doses of cocaine,
Readily available rehabilitation and therapy programs will need to be instituted along with vaccination in order to ensure the best results for addiction eradication.
Figure 1.
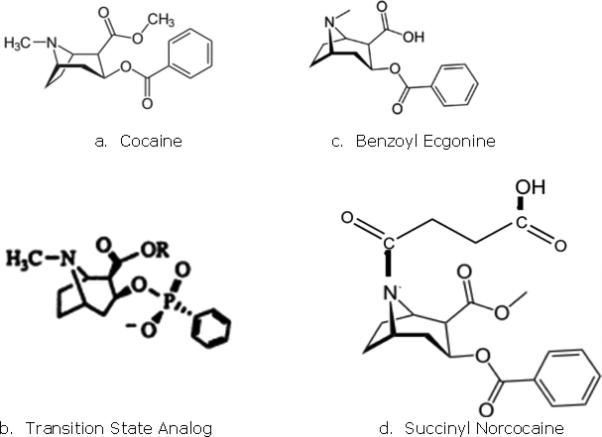
Structure of cocaine and cocaine derivatives
Figure 2.
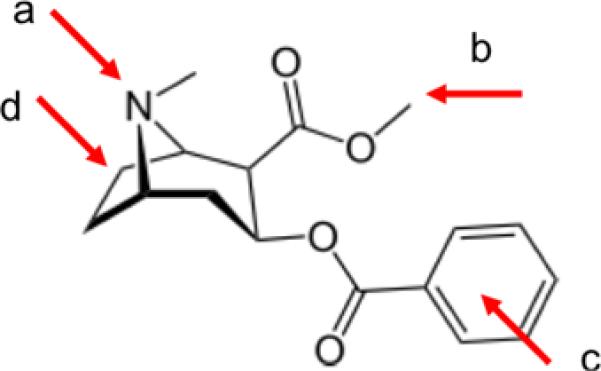
Sites for attachment of linkers to cocaine for conjugation to proteins
Acknowledgements
Supported by the Department of Veterans Affairs (VA) Merit Review Program and VISN 16 Mental Illness Research, Education and Clinical Center (MIRECC), the VA National Substance Use Disorders Quality Enhancement Research Initiative (QUERI), and the National Institute on Drug Abuse grants 5R01 DA 26859, K05 DA 0454 (TRK), P50-DA18197.
References
- 1.Ruetsch YA, Boni T, Borgeat A. From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem. 2001;1:175–182. doi: 10.2174/1568026013395335. [DOI] [PubMed] [Google Scholar]
- 2.Wilson R. Drug abuse prevention: A school and community partnership. 3 ed. Jones & Bartlett; Sudbury, MA: 2010. [Google Scholar]
- 3.The DASIS Report: Cocaine Route of Administration Trends: 1995-2005. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Rockville, MD: Sep 13, 2007. [online]. Available at: http://www.oas.samhsa.gov/2k7/crackTX/crackTX.htm. [Google Scholar]
- 4.Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 5.Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- 6.Berzofsky JA, Berkower IJ, editors. Immunogenicity and Antigen Structure. Second ed. Raven Press; New York: 1989. [Google Scholar]
- 7.Eisen HN, Siskind GW. Variations in Affinities of Antibodies During the Immune Response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 8.deShazo RD, Kemp SF. Allergic reactions to drugs and biologic agents. Jama. 1997;278:1895–1906. [PubMed] [Google Scholar]
- 9.Falugi F, Petracca R, Mariani M, et al. Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: a model for new conjugate vaccines. Eur J Immunol. 2001;31:3816–3824. doi: 10.1002/1521-4141(200112)31:12<3816::AID-IMMU3816>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Spector S, Berkowitz B, Flynn EJ, Peskar B. Antibodies to morphine, barbiturates, and serotonin. Pharmacol Rev. 1973;25:281–291. [PubMed] [Google Scholar]
- 11.Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis. 29:200–216. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagasra O, Forman LJ, Howeedy A, Whittle P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology. 1992;23:173–179. doi: 10.1016/0162-3109(92)90023-6. [DOI] [PubMed] [Google Scholar]
- 13.Isomura S, Wirsching P, Janda KD. An immunotherapeutic program for the treatment of nicotine addiction: hapten design and synthesis. J Org Chem. 2001;66:4115–4121. doi: 10.1021/jo001442w. [DOI] [PubMed] [Google Scholar]
- 14.de Villiers SH, Lindblom N, Kalayanov G, et al. Nicotine hapten structure, antibody selectivity and effect relationships: results from a nicotine vaccine screening procedure. Vaccine. 2010;28:2161–2168. doi: 10.1016/j.vaccine.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 15.Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. quiz 322-283, 325. [DOI] [PubMed] [Google Scholar]
- 16.Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine. 2009;27:2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]
- 17.Gentry WB, Ruedi-Bettschen D, Owens SM. Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin. 2009:5. doi: 10.4161/hv.5.4.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Zhao K, Deng S, Landry DW. Nonenzymatic Hydrolysis of Cocaine via Intramolecular Acid Catalysis. Helvetica Chimica Acta. 1999;82:85–89. [Google Scholar]
- 19.Warner A, Norman AB. Mechanisms of cocaine hydrolysis and metabolism in vitro and in vivo: a clarification. Ther Drug Monit. 2000;22:266–270. doi: 10.1097/00007691-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Lynch TJ, Mattes CE, Singh A, Bradley RM, Brady RO, Dretchen KL. Cocaine detoxification by human plasma butyrylcholinesterase. Toxicol Appl Pharmacol. 1997;145:363–371. doi: 10.1006/taap.1997.8187. [DOI] [PubMed] [Google Scholar]
- 21.Chandrakumar NS, Carron CP, Meyer DM, Beardsley PM, Nash SA, Tam LL, Rafferty M. Phenylphosphonate monoester analogs of cocaine. Potential haptens for the generation of catalytic antibodies. Bioorg Med Chem Lett. 1993;3:309–312. [Google Scholar]
- 22.Landry DW, Zhao K, Yang GX, Glickman M, Georgiadis TM. Antibody-catalyzed degradation of cocaine. Science. 1993;259:1899–1901. doi: 10.1126/science.8456315. [DOI] [PubMed] [Google Scholar]
- 23.Basmadjian GP, Singh S, Sastrodjojo B, et al. Generation of polyclonal catalytic antibodies against cocaine using transition state analogs of cocaine conjugated to diphtheria toxoid. Chem Pharm Bull (Tokyo) 1995;43:1902–1911. doi: 10.1248/cpb.43.1902. [DOI] [PubMed] [Google Scholar]
- 24.Berkman CE, Underiner GE, Cashman JR. Synthesis of an immunogenic template for the generation of catalytic antibodies for (–)-cocaine hydrolysis. J Org Chem. 1996;61:5686–5689. [Google Scholar]
- 25.Cashman JR, Berkman CE, Underiner GE. Catalytic antibodies that hydrolyze (-)-cocaine obtained by a high-throughput procedure. J Pharmacol Exp Ther. 2000;293:952–961. [PubMed] [Google Scholar]
- 26.Matsushita M, Hoffman TZ, Ashley JA, Zhou B, Wirsching P, Janda KD. Cocaine catalytic antibodies: the primary importance of linker effects. Bioorg Med Chem Lett. 2001;11:87–90. doi: 10.1016/s0960-894x(00)00659-4. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie KM, Mee JM, Rogers CJ, Hixon MS, Kaufmann GF, Janda KD. Identification and characterization of single chain anti-cocaine catalytic antibodies. J Mol Biol. 2007;365:722–731. doi: 10.1016/j.jmb.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallacher G. A potential vaccine for cocaine abuse prophylaxis? Immunopharmacology. 1994;27:79–84. doi: 10.1016/0162-3109(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 29.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 30.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci U S A. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakurai M, Wirsching P, Janda KD. Design and synthesis of a cocaine-diamide hapten for vaccine development. Tetrahedron Lett. 1996;37:5479–5482. [Google Scholar]
- 32.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci U S A. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox BS, Kantak KM, Edwards MA, et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 34.Ettinger RH, Ettinger WF, Harless WE. Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacol Biochem Behav. 1997;58:215–220. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 35.Johnson MW, Ettinger RH. Active cocaine immunization attenuates the discriminative properties of cocaine. Exp Clin Psychopharmacol. 2000;8:163–167. doi: 10.1037//1064-1297.8.2.163. [DOI] [PubMed] [Google Scholar]
- 36.Schabacker DS, Kirschbaum KS, Segre M. Exploring the feasibility of an anti-idiotypic cocaine vaccine: analysis of the specificity of anticocaine antibodies (Ab1) capable of inducing Ab2beta anti-idiotypic antibodies. Immunology. 2000;100:48–56. doi: 10.1046/j.1365-2567.2000.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantak KM, Collins SL, Bond J, Fox BS. Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology (Berl) 2001;153:334–340. doi: 10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- 38.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 39.Koetzner L, Deng S, Sumpter TL, Weisslitz M, Abner RT, Landry DW, Woods JH. Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. J Pharmacol Exp Ther. 2001;296:789–796. [PubMed] [Google Scholar]
- 40.Hrafnkelsdottir K, Valgeirsson J, Gizurarson S. Induction of protective and specific antibodies against cocaine by intranasal immunisation using a glyceride adjuvant. Biol Pharm Bull. 2005;28:1038–1042. doi: 10.1248/bpb.28.1038. [DOI] [PubMed] [Google Scholar]
- 41.Kosten TR, Biegel D. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2002;1:363–371. doi: 10.1586/14760584.1.3.365. [DOI] [PubMed] [Google Scholar]
- 42.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 43.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine Vaccine for the Treatment of Cocaine Dependence: A Randomized Double-Blind Placebo-Controlled Efficacy Trial. Arch Gen Psych. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deliyannis G, Kedzierska K, Lau YF, Zeng W, Turner SJ, Jackson DC, Brown LE. Intranasal lipopeptide primes lung-resident memory CD8+ T cells for long-term pulmonary protection against influenza. Eur J Immunol. 2006;36:770–778. doi: 10.1002/eji.200535217. [DOI] [PubMed] [Google Scholar]
- 46.Brimijoin S, Gao Y, Anker JJ, et al. A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology. 2008;33:2715–2725. doi: 10.1038/sj.npp.1301666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng F, Zhan CG. Rational design of an enzyme mutant for anti-cocaine therapeutics. J Comput Aided Mol Des. 2008;22:661–671. doi: 10.1007/s10822-007-9144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narasimhan D, Nance MR, Gao D, et al. Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng Des Sel. 2010 doi: 10.1093/protein/gzq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y, Brimijoin S. Lasting reduction of cocaine action in neostriatum--a hydrolase gene therapy approach. J Pharmacol Exp Ther. 2009;330:449–457. doi: 10.1124/jpet.109.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Y, Orson FM, Kinsey BM, Kosten TR, Brimijoin S. The concept of pharmacologic cocaine interception as a treatment for drug abuse. Chem Biol Interact. 2010 doi: 10.1016/j.cbi.2010.02.036. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malaitsev VV, Azhipa O. The effect of epitope density on the immunogenic properties of hapten-protein conjugates. Biull Eksp Biol Med. 1993;115:645–646. [PubMed] [Google Scholar]


