Abstract
Objective
In addition to the increasingly recognized role of physical exercise in maintaining cognition, exercise may influence Alzheimer's disease (AD) pathology as transgenic mouse studies show lowered levels of AD pathology in exercise groups. The objective of this study was to elucidate the association between exercise and AD pathology in humans using Pittsburgh Compound B (PIB), amyloid-β (Aβ)42, tau, and phosphorylated tau (ptau)181 biomarkers.
Methods
Sixty-nine older adults (17 males, 52 females) aged 55–88 were recruited and confirmed to be cognitively normal. A questionnaire on physical exercise levels over the last decade was administered to all. Cerebrospinal fluid (CSF) samples were collected from 56 participants, and amyloid imaging with PIB was performed on 54 participants.
Results
Participants were classified based on biomarker levels. Those with elevated PIB (p=.030), tau (p=.040) and ptau181 ((p=.044) had significantly lower exercise with a non-significant trend for lower Aβ42 (p=.135) to be associated with less exercise. Results were similar for PIB after controlling for covariates; tau (p=.115) and ptau181 (p=.123) differences were reduced to non-significant trends. Additional analyses also demonstrated that active individuals who met the exercise guidelines set by the American Heart Association (AHA) had significantly lower PIB binding and higher Aβ42 levels with and without controlling for covariates (PIB: p=.006 and p=.001; Aβ42: p=.042 and p=.046). Lastly, the associations between exercise engagement and PIB levels were more prominent in APOE epsilon 4 non-carriers.
Interpretation
Collectively, these results are supportive of an association between exercise engagement and AD biomarkers in cognitively normal older adults.
Introduction
With the disproportionate growth of the elderly population, Alzheimer's disease (AD) -the most common form of dementia among older adults - is poised to become a public health crisis. Although research on novel therapeutic strategies is gathering momentum with several new drugs entering clinical trials, it is still unclear what will be successful.1 Coupled with the lack of current treatments that can halt this disease, there is great impetus toward efforts concerning early detection and prevention of AD. Lifestyle practices that could potentially deter or slow disease progression are especially important considering that neurodegeneration in AD begins up to a decade or more before the appearance of clinical symptoms.2, 3 Physical exercise may be an effective tool in this regard with its increasingly recognized role in preserving not only cardiovascular but also brain and cognitive health in older adults.4 Recommended by the Alzheimer's Association5 to clinicians as a way to maintain cognitive functioning in AD and enhance a patient's quality of life (e.g., by lowering depression), studies now suggest that exercise may also reduce risk of cognitive decline and dementia.6, 7
There has been recent attention on how exercise affects AD pathology as quantified by imaging and neurochemical measures. The accumulation of amyloid plaques and neurofibrillary tangles involved in the neurodegenerative process of AD are correlated with several useful biomarkers. First, fibrillar amyloid-β deposition can be detected through positron emission tomography (PET) studies with Pittsburgh Compound B (PIB) as radiotracers to image the amyloid-β (Aβ) aggregates, with plaque formation indicated by higher uptake of PIB.8 In addition, amyloid deposition can be assayed using lumbar puncture to measure the cerebrospinal fluid (CSF) level of Aβ42 (a primary constituent of plaques) with reductions in CSF levels of Aβ42 indicative of plaque formation. Third, neurofibrillary tangles are composed of hyperphosphorylated tau proteins (ptau), and an increased CSF level of ptau181 is observed in AD.9 Last, AD axonal death and neuronal degeneration is reflected in atrophy of brain regions such as the hippocampus and increased CSF levels of normally intracellular tau protein.10, 11 These biomarkers - PIB, Aβ42, tau, ptau181, and MRI brain volumetry - chart AD pathology and may serve as early diagnostic indicators.12, 13
Using these biomarkers researchers have investigated effects of physical exercise on structural brain integrity and AD pathology. Structural imaging studies indicate beneficial effects of exercise and aerobic fitness on brain structure in healthy older adults14, 15 and individuals with AD16, 17. Investigations of the effects of environmental enrichment, including wheel running, in transgenic AD mouse models have demonstrated improvements in cognitive performance but varying results in terms of amyloid deposition, with reports of reduced18-20, stable21 and increased22 levels. Examination of exercise specifically in transgenic AD mice has also produced discrepant results, with lower levels of amyloid deposition and lower tau observed in some investigations23-25, but no effect of exercise on amyloid reported by others26, 27. Divergent results in these studies may be attributable to use of different genetic mouse models, whether exercise onset is before or at the time of symptom onset, and/or type and duration of exercise.28 The association between exercise and human AD pathology, however, as measured by PET-PIB and CSF neurochemical biomarkers such as Aβ42, tau, and ptau181 remains unexplored. The primary goal of the current study was to examine associations between exercise engagement and biomarker levels in older adults without clinical symptoms of AD.
Materials and Methods
Participants
Older adults, age 55–88 years, were recruited from the Washington University Alzheimer Disease Research Center and screened for neurologic illnesses or injury (e.g., cerebrovascular disease, Parkinson's disease). Based on the Washington University Clinical Dementia Rating (CDR), a validated and reliable interview-based measurement sensitive in detecting the earliest stages of dementia29, 30, all participants were classified as nondemented (CDR=0). An exercise engagement questionnaire (see below for details) was administered to all 69 participants, cerebrospinal fluid (CSF) samples were collected from 56 participants, and amyloid imaging with PIB was performed on 54 participants. Power was .80 to detect an effect size (Cohen's d) of .77 in the CSF sample and .79 in the PIB sample.
Individuals were CDR=0 at all clinical assessments. Clinical assessment was within ±6.0 months (SD=4.5) of the PET scan and ±4.0 months (SD=2.2) of the CSF assessment. Exercise assessment was within ±2.8 years (SD=2.1) of the PET scan and ±1.7 years (SD=0.7) of CSF assessment. The interval between clinical and exercise assessments was ±5.8 months (SD=5.4) for the PIB sample and ±6.2 months (SD=4.9) for the CSF sample. Exercise assessment was subsequent to the PET scan for 45/54 participants and subsequent to CSF assessment for 54/56 participants. All participants consented to participation in accordance with guidelines of the Washington University Human Research Protection Office. Exercise and structural MRI data on a subset of these individuals (n=52) was reported previously.14
Measurement of physical exercise engagement
Validity
A validated questionnaire assessing history of walking, running and jogging (WRJ) activity for the past 10 years was used to estimate exercise engagement.31 The measure was significantly correlated with cardiorespiratory fitness measured via treadmill test in a sample of 5063 individuals aged 18-80 years. Stable correlations were observed between retrospective self-report of activity for a particular year and aerobic fitness for that year across the 10 1-year assessment periods, suggesting participants across the examined age range were capable of relatively accurate self-report over this extended time span.
Procedure
The questionnaire was administered by telephone, and participants reported number of months/year, number of workouts/week, average number of miles/workout, and average time/mile for each year in which they engaged in WRJ activity for the preceding 10 years. A physical exercise engagement score for each participant was derived by estimating metabolic equivalent (MET) values using the compendium of physical activities32, as described previously.31 The index of exercise engagement was average MET hours/week over the past 10 years. As a reference, an individual who followed the American Heart Association's (AHA) physical exercise recommendation for older adults (30 minutes of moderate exercise 5 days/week) would score 7.5 MET-hours/week.31, 33
Cerebrospinal fluid collection, processing, and biomarker measurement
CSF (20 – 30ml) free from any blood contamination was collected by lumbar puncture in polypropylene tubes at 8:00 AM after overnight fasting as described previously.8 Samples were gently inverted to avoid gradient effects, briefly centrifuged at low speed to pellet any cellular elements, and aliquoted (500μl) into polypropylene tubes before freezing at −84°C. Analyses for total tau, ptau181, and Aβ42 were completed using commercial enzyme-linked immunosorbant assay (Innotest; Innogenetics, Ghent, Belgium). For all biomarker measures, samples were continuously kept on ice with only a single thaw after initial freezing before assays.
PET-PIB imaging
In vivo amyloid imaging via PET with PIB ([N-methyl-[11C]]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole)34 was performed as described previously.12 Approximately 12 mCi of [11C]PIB was administered intravenously simultaneous with initiation of a 60-minute dynamic PET scan in three-dimensional mode. Measured attenuation factors and a ramp filter were used to reconstruct dynamic PET images. Three-dimensional regions-of-interest were then created for each participant based on their individual MRI scans (T1-weighted 1×1×1.25mm MPRAGE). A binding potential (BP) for each region-of-interest was calculated to express regional binding values in a manner proportional to number of binding sites. BP values from prefrontal cortex, gyrus rectus, lateral temporal, and precuneus regions-of-interest were averaged to calculate a mean cortical binding potential (MCBP) value based on brain regions known to have high PIB uptake among participants with AD.12
APOE genotyping
TaqMan assays (Applied Biosystems, Foster City, USA) for both rs429358 (ABI#C_3084793_20) and rs7412 (ABI#C_904973_10) were used for APOE genotyping. Allele calling was performed using the allelic discrimination analysis module of ABI Sequence Detection Software. Positive controls for each of six possible APOE genotypes were included on the genotyping plate. Individuals were then classified as ε4+ (44, 34, 24) or ε4− (33, 23, 22) depending on whether or not at least one ε4 allele was present.
Diagnostic biomarker thresholds
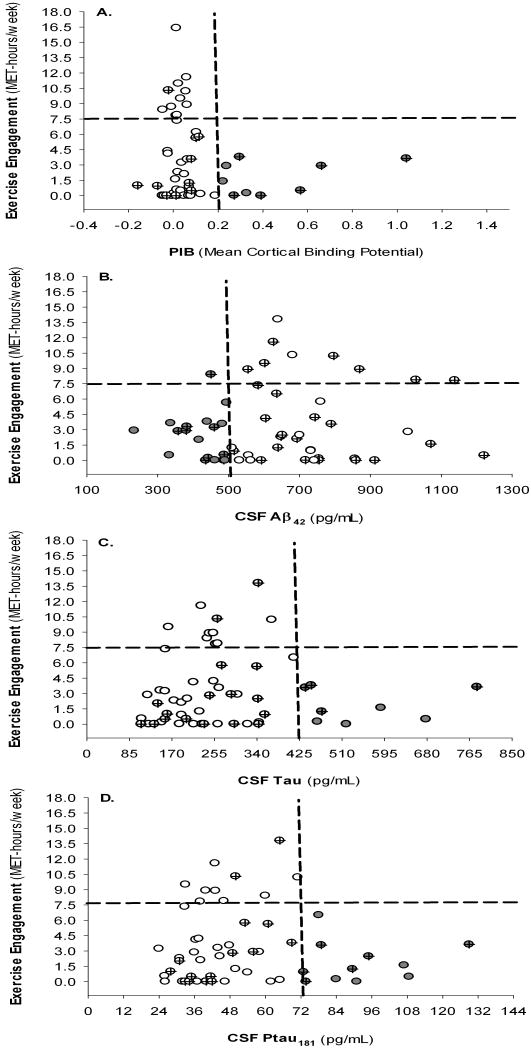
Although use of CSF biomarker thresholds in determining risk of AD is prevalent, standard diagnostic cut-off values are lacking as noted previously.35 This study used a PIB cut-off of 0.20 MCBP because regional brain atrophy in cognitively normal individuals with PIB levels at or above this level has been demonstrated. 13 Individuals with MCBP≥0.20 were considered PIB+ (“at risk” phenotype); those below this cut-off were considered PIB- (“normal” phenotype). For CSF Aβ42, 500 pg/mL was chosen as this level is indicative of brain amyloid as assessed by PET-PIB8, 36 and is associated with whole brain and hippocampal atrophy in cognitively normal individuals37. For CSF tau and ptau181, there was less precedent in existing literature for predetermined cut-offs as a wide range of values are reported to distinguish AD from controls with no clear guidelines.9,35 Therefore, we plotted the data, and as apparent in Fig. 1C-D, there was a behavior that seemed to be best expressed with tau and ptau181 thresholds of 425 pg/mL and 72 pg/mL, respectively; which are within the range of diagnostic values reported previously.35,36 Individuals with low CSF Aβ42 (<500pg/mL, “at risk”) were considered Aβ42+; those with high Aβ42 (≥500pg/mL, “normal”) were considered Aβ42-. Similarly, those with CSF tau and ptau181 values above the cut-offs were classified tau+ and ptau181+ (“at risk”), respectively; those below the cut-offs were classified tau- and ptau181- (“normal”).
Figure 1.
Associations between exercise engagement and biomarker levels. (A) Mean cortical binding potential; (B) CSF Aβ42; (C) CSF tau; (D) CSF ptau181. Quadrants are formed by crossing biomarker thresholds with AHA recommended level of exercise; note the L-shaped distributions in which individuals with positive biomarker status (gray-filled circles) are all sedentary with one exception in (B). Crossed circles represent ε4+ individuals.
Statistical analyses
All analyses were conducted using SPSS/PASW 17.0 (SPSS Inc., Chicago, Illinois). All statistical significance tests were 2-tailed with α=0.05. We first tested for group differences in demographic variables, APOE status, and medical conditions using Student's t tests for continuous variables and χ2 or Fisher's exact test for dichotomous variables (Tables 1 and 2). With two exceptions (see Table 1 and Results section), there were no significant differences between biomarker groups or between exercise groups.
Table 1.
Participant characteristics for biomarker groups.
| MCBP | Aβ42 | Tau | Ptau181 | |||||
|---|---|---|---|---|---|---|---|---|
| At risk | Normal | At risk | Normal | At risk | Normal | At risk | Normal | |
| N | 9 | 45 | 18 | 38 | 8 | 48 | 11 | 45 |
| Mean age (SD), years | 73 (8) | 70 (10) | 71 (8) | 65 (8)* | 70 (10) | 66 (8) | 68 (9) | 67 (8) |
| Gender (F/M) | 5/4 | 35/10 | 12/6 | 31/7 | 7/1 | 36/12 | 10/1 | 33/12 |
| Mean education (SD), years | 16 (6) | 16 (3) | 17 (4) | 15 (3) | 16 (4) | 16 (3) | 16 (4) | 16 (3) |
| BMI (normal/overweight/obese) | 3/6/0 | 17/19/9 | 3/10/5 | 17/12/9 | 4/3/1 | 16/19/13 | 5/3/3 | 15/19/11 |
| Diabetes (-/+) | 9/0 | 43/2 | 17/1 | 36/2 | 8/0 | 45/3 | 11/0 | 42/3 |
| Hypertension (-/+) | 7/2 | 24/21 | 8/10 | 23/15 | 4/4 | 27/21 | 6/5 | 25/20 |
| Heart problems (-/+) | 7/2 | 35/10 | 13/5 | 29/9 | 4/4 | 38/10 | 6/5 | 36/9 |
| Depression (-/+) | 9/0 | 43/2 | 18/0 | 36/2 | 8/0 | 46/2 | 10/1 | 44/1 |
| APOE ε4 (-/+) | 3/6 | 35/10* | 9/9 | 26/12 | 4/4 | 31/17 | 5/6 | 30/15 |
| AHA exercise group (-/+) | 9/0 | 34/11 | 17/1 | 29/9 | 8/0 | 38/10 | 11/0 | 35/10 |
| Mean Exercise Score (SD) | 1.7 (1.6) | 3.7 (4.3)* | 2.4 (2.3) | 3.7 (4.0) | 1.8 (1.6) | +3.5 (3.8)* | 1.9 (2.0) | +3.6 (3.8)* |
p < .05.
Non-significant trend only after controlling for age, gender and education.
BMI=Body Mass Index; AHA exercise group represents whether individuals met the American Heart Association's recommended exercise levels (see text for details) with + indicating those who met recommendations.
Table 2.
Participant characteristics for exercise groups.
| PIB sample | CSF sample | |||
|---|---|---|---|---|
| Exerciser | Non-exerciser | Exerciser | Non-exerciser | |
| N | 11 | 43 | 10 | 46 |
| Mean age (SD), years | 68 (10) | 71 (10) | 64 (7) | 67 (9) |
| Gender (F/M) | 6/5 | 34/9 | 6/4 | 37/9 |
| Mean education (SD), years | 17 (2) | 16 (4) | 16 (3) | 16 (4) |
| BMI (normal/overweight/obesity) | 4/6/1 | 16/19/8 | 4/5/1 | 16/17/13 |
| Diabetes (-/+) | 11/0 | 41/2 | 10/0 | 43/3 |
| Hypertension (-/+) | 7/4 | 24/19 | 8/2 | 23/23 |
| Heart problems (-/+) | 10/1 | 32/11 | 9/1 | 33/13 |
| Depression (-/+) | 11/0 | 41/2 | 10/0 | 44/2 |
| APOE ε4 (-/+) | 10/1 | 28/15 | 8/2 | 27/19 |
| Mean MCBP (SD) | .02 (.03) | +.10 (.16)** | -- | -- |
| Mean Aβ42 (SD), pg/mL | -- | -- | 739 (217) | +600 (185)* |
| Mean Tau (SD), pg/mL | -- | -- | 263 (58) | 282 (152) |
| Ptau181 (SD), pg/mL | -- | -- | 49 (13) | 54 (25) |
p < .05;
p<.01.
Data with one outlier removed (see text for details).
Note: BMI=Body Mass Index. Exercise groups are based on the American Heart Association's recommended exercise levels (see text for details).
Primary questions regarding associations between exercise and biomarkers were examined with Student's t-test for groups with equal variance; unequal variance t-tests were used when the assumption of equal variances was unmet. Although age, gender and education were not significantly associated with dependent variables (with two exceptions noted above), analyses were also conducted controlling for these potentially confounding factors. In these analyses, residuals of the dependent variable after adjustment for age, gender and education were submitted to t-tests. Values ≥3 STD from the mean were considered outliers. Unless otherwise specified in the Results section, results were unchanged when outliers were removed.
Results
Individuals with “at-risk” biomarker levels evidence less exercise engagement
Examination of graphs plotting exercise engagement against biomarker levels (Fig 1) suggests that individuals with elevated PIB, CSF tau or ptau181, or decreased CSF Aβ42 (“at risk” phenotypes) consistently exercised less. Based on categorization of individuals into PIB+ and PIB- groups, there was significantly greater exercise in the PIB- group (t(34.4)=2.393, p=.022; covariates controlled: t(22.0)=2.314, p=.030). For Aβ42, there was a non-significant trend for greater exercise in the Aβ42- group (t(52.1)=-1.518, p=.135; covariates controlled: t(50.8)=1.310, p=.196). Individuals in the negative tau and ptau181 groups evidenced significantly greater exercise engagement (t(22.8)=2.175, p=.040 and t(29.9)=2.104, p=.044, respectively). However, these effects were reduced to non-significant trends with covariates controlled (tau: t(24.2)=1.598, p=.115; ptau181: t(21.7)=1.641, p=.123).
AHA exercise engagement levels are associated with reduced amyloid deposition
We also assessed whether biomarker levels differed between individuals who did or did not satisfy AHA guidelines of 7.5 MET-hours/week of exercise (Table 2). Individuals who met or exceeded the recommended exercise had significantly lower MCBP (t(48.7)=2.854, p=.006) than those not meeting recommendations. The difference was not significant after controlling for age, gender, education and significant biomarker group differences in APOE status (t(52)=1.106, p=.274). However, when an outlier value was removed, there was a significant difference in MCBP between exercise groups (covariates controlled: t(46.5)=3.477, p=.001) and APOE status no longer differed between groups. There was a non-significant trend for exercisers to have higher Aβ42 levels (t(54)=-1.739, p=.088; covariates controlled: t(54)=1.680, p=.099). When an outlier value was removed, however, there was a significant difference in Aβ42 levels between exercise groups (t(53)=-2.082, p=.042; covariates controlled; t(53)=2.040, p=.046). There were no significant differences in tau (t(39.0)=0.662, p=.512; covariates controlled: t(37.3)=.140, p=.890) or ptau181 (t(27.5)=0.948, p=.351; covariates controlled: t(28.7)=.332, p=.743) levels between exercise groups.
Exercise effects by APOE genotype
There were significant differences in Aβ42 levels depending on APOE genotype with ε4+ individuals having lower Aβ42 levels (ε4+: 564 pg/mL; ε4-: 679 pg/mL; t(54)=2.024, p=.048). There was also a non-significant trend for ε4+ individuals to have higher MCBP (ε4+: 0.214, ε4: 0.048; t(15.8)=-2.070, p=.055). There were, however, no significant differences in tau or ptau181 levels depending on APOE genotype (tau - ε4+: 303 pg/mL, ε4-: 264 pg/mL, t(54)=-1.021, p=.312; and ptau181 - ε4+: 58 pg/mL, ε4-: 50 pg/mL, t(54)=-1.337, p=.187). Therefore, we examined the relationship between exercise and amyloid deposition separately for ε4+ and ε4- groups (see Tables 1-2 for sample sizes).
For the ε4- group, there was a non-significant trend for PIB- individuals to report greater exercise (t(7.417)=2.231, p=.059; covariates controlled: t(34.91)=1.993, p=.054). There was a non-significant trend for MCBP levels to be lower in exercisers (t(35.9)=1.707, p=.096; covariates controlled: t(35.1)=1.600, p=.119). For the ε4+ group, there were no significant biomarker group differences in exercise (t(14)=0.714, p=.487; covariates controlled: t(13)=.887, p=.391). Notably, only one ε4+ individual exercised at or above the recommended level, although mean exercise scores did not differ between ε4+ and ε4- groups (t(42.2)=1.236, p=.223) in the PIB sample.
In terms of Aβ42, for ε4- individuals there were no significant differences in exercise between Aβ42, groups (t(20.3)=-1.310, p=0.205; covariates controlled: t(20.3)=1.023, p=.319) nor were there significant differences in Aβ42 levels between exercise groups (t(33)=-1.184, p=0.245; covariates controlled: t(33)=.944, p=.352). When an outlier was removed, there was a non-significant trend for higher Aβ42 levels in the exercise group (t(32)=-1.584; p=0.123; covariates controlled: t(32)=1.319, p=.197). For the ε4+ group, there was not a significant difference between Aβ42 groups (t(19)=-.484, p=0.634; covariates controlled: t(19)=.238, p=.814). There were only two ε4+ individuals who met AHA recommendations, although exercise did not differ between ε4+ and ε4- groups (t(54)=0.632, p=0.530).
Discussion
Emerging evidence from human structural imaging and AD transgenic mouse studies suggests physical exercise may have beneficial effects on AD-related pathology and/or cognition.16, 17, 23-27 The purpose of the current investigation was to extend this knowledge base to include human findings regarding exercise engagement and biomarkers including amyloid deposition measured with PET-PIB and CSF levels of Aβ42, tau, and ptau181. We observed novel associations between exercise engagement and biomarker levels in cognitively normal older adults.
Visual inspection of graphs plotting exercise engagement against biomarker levels suggests strong associations with individuals dispersed in an L-shaped distribution (Figure 1). Most notable is that at one end of the distribution, individuals with greater physical exercise have lower MCBP, CSF tau and ptau181, or higher CSF Aβ42, whereas individuals with “at-risk” levels of biomarkers at the other end of the distribution are all relatively sedentary. This observation was somewhat confirmed by comparing exercise scores of those with “at-risk” biomarker levels against those with “normal” levels. Participants with elevated MCBP, tau, or ptau181 exercised significantly less, and there was a non-significant trend for lower exercise in those with decreased Aβ42. However, associations for tau and ptau181 were reduced to non-significant trends with age, gender and education controlled suggesting these variables accounted for a portion of the variance despite the lack of significant group differences in these variables. Past research indicates that cognitively normal individuals who are PIB+ exhibit regional brain atrophy and longitudinal cognitive decline compared to PIB- individuals13. In addition, reduced CSF Aβ42 in cognitively normal individuals has been associated with whole brain and hippocampal atrophy in cognitively normal individuals37 and elevated MCBP8, 36. Collectively, results are suggestive of differing levels of exercise across biomarker status such that individuals potentially at greater risk for AD engaged in less exercise.
Comparing biomarker levels of those who did or did not meet AHA exercise guidelines provided further insights into associations between exercise and AD pathology. Active individuals exhibited significantly lower MCBP and higher CSF Aβ42 levels. However, no differences in tau and ptau181 levels were found between exercise groups. This may be attributable to alterations in tau and ptau181 potentially occurring subsequent to amyloid plaque formation later in the course of AD disease progression37-39, thereby resulting in less evident exercise effects on these biomarkers in a cognitively normal population. Past research is suggestive that beneficial effects of exercise on tau levels in a transgenic mouse model may be observed at the time at which tau is typically expressed.24 Current findings for MCBP and Aβ42 do converge to suggest that greater exercise engagement is associated with healthier amyloid profiles.
Previous investigations have observed elevated levels of amyloid and tau as measured by PET-PIB and PET-FDDNP in cognitively normal adults with an APOE ε4 allele.40, 41 In this study, ε4+ individuals displayed elevated MCBP and lowered Aβ42 but no differences in tau or ptau181. In separate analyses of associations between amyloid and exercise for e4+ and e4-groups, results for ε4-, but not for ε4+, individuals essentially mirrored findings in the full sample for MCBP. Considering past literature indicates greater exercise-related benefits for cognition for ε4+ individuals6, 42-44, a larger exercise effect on biomarkers in this group might be expected. It is notable that in the current study, although mean exercise scores did not significantly differ between ε4+ and ε4- individuals, there were fewer ε4+ individuals exercising at AHA recommended levels (see Table 2). Therefore, there is some indication that presence of this risk factor for AD (and altered biomarker levels) may lead to reduced exercise levels. Beneficial effects of exercise on cognition in ε4+ individuals observed previously may relate to mechanisms other than direct effects on neuropathology, such as increased brain-derived neurotrophic factor and synaptogenesis.45 Current analyses are limited by differential sample sizes between the ε4+ and ε4- groups. It would be of particular interest to determine whether gene×environment interactions exist such that exercise actually moderates associations between ε4+ status and greater amyloid deposition in larger samples.
Although current results suggest a relatively robust relationship between exercise engagement and examined AD biomarkers, particularly in terms of amyloid levels, the direction of causality between exercise and human AD pathology remains uncertain considering our cross-sectional design. Furthermore, the current self-report measure of exercise engagement is significantly but not perfectly related to cardiorespiratory fitness and may also be limited by the ability of older adults to accurately recall and report their exercise over a 10-year span. Furthermore, although our exercise measure captured engagement at the PET-PIB or CSF assessment for most participants, it is possible that exercise behavior changes occurred for individuals whose PET-PIB or CSF assessment followed measurement of exercise engagement. The study is also limited by the lack of well-established, research-guided thresholds for determining “at-risk” levels of biomarkers, particularly for tau and ptau181, and also by the relatively small sample size. To further elucidate whether physical exercise directly deters AD pathology, an interventional study in cognitively normal and AD individuals is needed. Future investigations might also include cognitive and brain atrophy outcome measures while comparing combinations of exercise, social, and cognitive interventions.46 The novel findings of this study, in conjunction with positive results in transgenic AD mouse models, suggest a further rationale for clinicians to prescribe exercise regimens as not only symptomatic relief for AD patients, but as potentially preventive care as well.
Acknowledgments
We thank our lumbar puncture physicians for obtaining our CSF samples, Ms. Aarti Shah for processing and analyzing the CSF samples and Ms. Sushila Sathyan for scheduling the lumbar punctures. We thank Martha Storandt for helpful comments on this paper. We thank the Clinical Core of the Alzheimer Disease Research Center for participant assessments and the Genetics Core for APOE genotyping. This work was supported by NIH grants P50 AG05861, P01 AG03991 and P01 AG026276. J.M.B. was supported by NIA 5T32AG00030. The authors and their institution have no conflicts of interest related to this work.
References
- 1.Sabbagh MN. Drug development for Alzheimer's disease: where are we now and where are we headed? Am J Geriatr Pharmacother. 2009;7:167–185. doi: 10.1016/j.amjopharm.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulette CM, Welsh-Bohmer KA, Murray MG, et al. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 4.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 5.Guideline for Alzheimer's disease management. Chicago, IL: Alzheimer's Association; 2008. [Google Scholar]
- 6.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Middleton L. Physical activity and the maintenance of cognitive function. Alzheimers Dement. 2007;3:S38–44. doi: 10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell AJ. CSF phosphorylated tau in the diagnosis and prognosis of mild cognitive impairment and Alzheimer's disease: a meta-analysis of 51 studies. J Neurol Neurosurg Psychiatry. 2009;80:966–975. doi: 10.1136/jnnp.2008.167791. [DOI] [PubMed] [Google Scholar]
- 10.Hampel H, Burger K, Teipel SJ, et al. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 12.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 13.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss are signatures of cerebral Abeta deposition identified with. PIB Arch Neurol. 66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2009.03.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 16.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honea RA, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berardi N, Braschi C, Capsoni S, et al. Environmental enrichment delays the onset of memory deficits and reduces neuropathological hallmarks in a mouse model of Alzheimer-like neurodegeneration. J Alzheimers Dis. 2007;11:359–370. doi: 10.3233/jad-2007-11312. [DOI] [PubMed] [Google Scholar]
- 19.Costa DA, Cracchiolo JR, Bachstetter AD, et al. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007;28:831–844. doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Arendash GW, Garcia MF, Costa DA, et al. Environmental enrichment improves cognition in aged Alzheimer's transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15:1751–1754. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- 22.Jankowsky JL, Xu G, Fromholt D, et al. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:1220–1227. doi: 10.1093/jnen/62.12.1220. [DOI] [PubMed] [Google Scholar]
- 23.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leem YH, Lim HJ, Shim SB, et al. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009;87:2561–2570. doi: 10.1002/jnr.22075. [DOI] [PubMed] [Google Scholar]
- 25.Um HS, Kang EB, Leem YH, et al. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- 26.Pietropaolo S, Sun Y, Li R, et al. The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Wolf SA, Kronenberg G, Lehmann K, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Richter H, Ambree O, Lewejohann L, et al. Wheel-running in a transgenic mouse model of Alzheimer's disease: protection or symptom? Behav Brain Res. 2008;190:74–84. doi: 10.1016/j.bbr.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- 31.Bowles HR, FitzGerald SJ, Morrow JR, Jr, et al. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 33.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 34.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 35.Hort J, Bartos A, Pirttila T, Scheltens P. Use of cerebrospinal fluid biomarkers in diagnosis of dementia across Europe. Eur J Neurol. 2009 doi: 10.1111/j.1468-1331.2009.02753.x. [DOI] [PubMed] [Google Scholar]
- 36.Fagan AM, Roe CM, Xiong C, et al. Cerebrospinal fluid tau/β-amyloid42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 37.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Small GW, Siddarth P, Burggren AC, et al. Influence of cognitive status, age, and APOE-4 genetic risk on brain FDDNP positron-emission tomography imaging in persons without dementia. Arch Gen Psychiatry. 2009;66:81–87. doi: 10.1001/archgenpsychiatry.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeny SP, Poeppel D, Zimmerman JB, et al. Exercise, APOE, and working memory: MEG and behavioral evidence for benefit of exercise in epsilon4 carriers. Biol Psychol. 2008;78:179–187. doi: 10.1016/j.biopsycho.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etnier JL, Caselli RJ, Reiman EM, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39:199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 44.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 45.Nichol K, Deeny SP, Seif J, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cracchiolo JR, Mori T, Nazian SJ, et al. Enhanced cognitive activity--over and above social or physical activity--is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem. 2007;88:277–294. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]