Abstract
Objective
Endarterectomy and angioplasty with stenting have emerged as two alternative treatments for carotid artery stenosis. This study’s objective is to determine cost-effectiveness of carotid artery stenting compared to carotid endarterectomy in symptomatic subjects who are suitable for either intervention.
Methods
A Markov analysis of these two revascularization procedures was conducted using direct Medicare costs (2007$ USD) and characteristics of a symptomatic 70-year-old cohort over a lifetime.
Results
In the base case analysis, carotid stenting produced 8.97 quality adjusted life years compared to 9.64 quality adjusted life years for carotid endarterectomy. The incremental cost of stenting was $17,700 and thus, stenting was dominated by endarterectomy. Sensitivity analyses show that the long-term probabilities of major stroke or mortality influenced the results.
Conclusions
In the base case analysis, carotid endarterectomy for patients with symptomatic stenosis has a greater benefit than angioplasty with stenting with lower direct costs. With a 59% probability, CEA will be the optimal intervention when all of the model assumptions are varied simultaneously.
Keywords: carotid arteries, stenosis, cost-benefit analysis, stroke
Introduction
Stroke is the third leading cause of death in the United States and a leading cause of disability. Approximately 780,000 people annually have a new or recurrent stroke with an estimated financial burden of 62.7 billion dollars per year (US estimates). Among the various subtypes of stroke, patients with stroke due to carotid atheromatous disease had the highest odds of recurrence (OR=2.9) at 30 days and three months (17). Reducing the stroke rate in individuals with carotid artery stenosis could lead to substantial cost savings. Several randomized trials have demonstrated that carotid endarterectomy (CEA) in addition to medical therapy reduces morbidity and mortality compared to medical therapy alone. Also, CEA is cost-effective compared to medical treatment for both symptomatic and asymptomatic patients with carotid artery stenosis (4; 14; 21). Carotid angioplasty with stenting (CAS) has recently emerged as an alternative treatment for patients with carotid artery stenosis.
Kilaru et al (2003) used cost data and CEA outcomes from their institution and CAS outcomes from the literature to compare CAS to CEA over a lifetime in a standard-risk population (13). Surgery was cost-saving compared to stenting. Because CAS had a higher complication rate, CAS only became a cost-effective alternative when the CAS complication rates were comparable to the CEA complication rates (13). Recently, two meta-analyses comparing 30-day outcomes following CAS or CEA in those with symptomatic carotid artery disease showed a non-significant lower likelihood of death following CAS (9; 18; 19; 24; 27). The potential for life-year gains for CAS compared to CEA despite higher costs for CAS may generate a favorable incremental cost-effectiveness ratio (ICER).
Based on these findings, we sought to determine the cost-effectiveness of CAS versus CEA in a hypothetical cohort of symptomatic individuals. Creating a cost-effectiveness framework that incorporates mortality, stroke (all future events, not just ipsilateral events), and myocardial infarction (MI), permits an assessment of CEA and CAS in anticipation of data from ongoing trials including CREST, ACT 1, SPACE-2 and ACST-2 (SPACE-2: www.controlledtrials.com, rest: www.clinicaltrials.gov). When additional trial data are available, this model can be updated or adapted for an asymptomatic cohort.
Methods
A Markov model was designed to compare the costs and utilities of CAS against CEA. This analysis is based primarily on the results of a recent meta-analysis and other published trials (6; 9; 10; 20).
Target population
The model incorporates a 70-year old patient cohort with symptomatic carotid stenosis.
Perspective, boundaries and time horizon
The payer perspective includes US Medicare costs when available. At the decision node, symptomatic patients have undergone the necessary screening and diagnostic testing to reach the point where an intervention is recommended. Subjects must be suitable candidates for either procedure. The time horizon is the lifetime of the cohort.
Model description
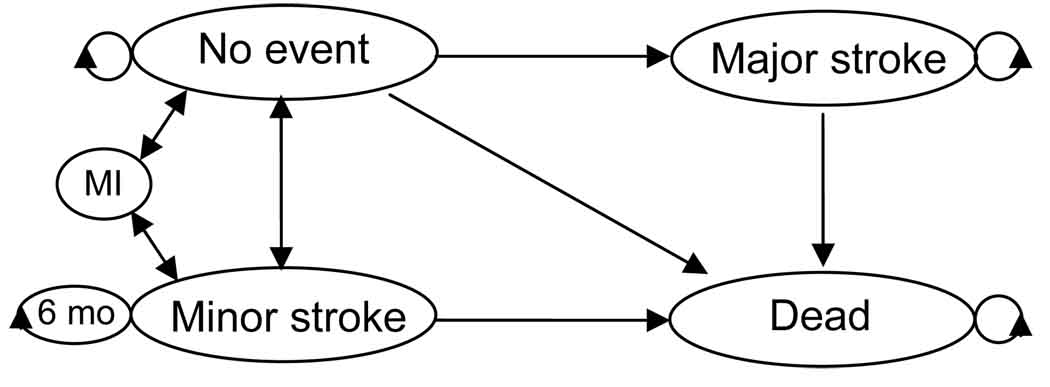
A Markov model was designed to reflect the outcomes of the clinical trials (Figure 1) using TreeAge Pro 2008 (Williamstown, MA). The one month cycle length allowed both higher 30-day peri-procedural risks and lower long-term risks to be incorporated into the model. Stroke included both ipsilateral and contralateral events. Major stroke, minor stroke, death and “continuing in the current state” were the allowed transitions from the well and minor stroke states. This symptomatic cohort started in the well or minor stroke branch based on a weighted average of those enrolled in EVA-3S or SPACE with transient ischemic attack or minor infarction as the qualifying event, respectively. Costs and disutilities for a minor stroke or recurrent transient ischemic attack accrued for six months (4; 13; 21). After six months, there were no additional costs, utility was restored to 1.0 and individuals were transitioned to the well state (2; 15). The major stroke state reflected a disabling stroke with a modified Rankin score of three or greater and no recovery was allowed. The transitions from major stroke were to continue in the current state or death.
Figure 1.
State transition diagram. Allowed transitions are identical for both the CAS and CEA branches. The terminal state is death.
Probability data
Probabilities for stroke reflect total events: ipsilateral plus contralateral stroke. The short-term probabilities for the first month (Markov cycle) following CAS or CEA were based on a meta-analysis of 30-day outcomes (Table 1; Supplementary Table 1 for references) (9). The probabilities for the subsequent months were a weighted average of the 2-year SPACE, 3-year SAPPHIRE or 4-year EVA-3S results (6; 10; 20). Only 30-day, peri-procedural MI was incorporated into the model because the long-term incidence of MI specifically related to CEA or CAS is not known. The incidence of MI beyond 30 days was assumed to be equal following CAS and CEA, thus an MI after 30 days would not contribute to differences in cost or utility between the two branches.
Table 1.
Inputs for the cost-utility model. Please see the supplemental material for additional reference material.
| Variables | CAS | CEA | Range | Distribution |
|---|---|---|---|---|
| base | base | |||
| case | case | |||
| Beginning as well (vs. minor stroke, %) | 46 | 46 | 0–1 | Beta |
| Transition rates (%): | ||||
| Death after a major stroke (case-fatality rate) |
13.9 | 13.9 | 10.5–17.4 | Beta |
| 30-day | ||||
| Death | 0.62 | 1.26 | 0–5.1 | Beta |
| Minor stroke | 3.81 | 2.66 | 0–12.8 | Beta |
| Major stroke | 3.21 | 2.02 | 0–7.3 | Beta |
| MI | 0.64 | 1.31 | 0–12.8 | Beta |
| Long-term | ||||
| Death, excess, per year | 1.5 | 0.96 | 0–44.6 | Beta |
| Any stroke, per year | 4.0 | 2.1 | 0–18 | Beta |
| Proportion major stroke | 0.3 | 0.3 | 0–0.75 | Beta |
| Costs (2007$): | ||||
| Hospitalization cost for MI | 9,100 | 9,100 | 5,000– 15,000 |
Triangular |
| minor stroke | 9,800 | 9,800 | 5,000– 15,000 |
Triangular |
| major stroke | 10,500 | 10,500 | 5,000– 15,000 |
Triangular |
| 15,000 | ||||
| Continuing (per yr) - MI | 4,500 | 4,500 | 3,500–5,500 | Triangular |
| minor stroke | 7,500 | 7,500 | 1,000– 15,000 |
Triangular |
| major stroke – first year | 66,500 | 66,500 | 40–90,000 | Triangular |
| major stroke – subsequent years | 33,900 | 33,900 | 15–55,000 | Triangular |
| Procedure | 10,400 | 9,170 | 6,000– 15,000 |
Triangular |
| Clopidogrel | 130 | |||
| Utilities: | ||||
| Myocardial infarction | 0.88 | 0.88 | 0.65–1.0 | Uniform |
| Minor stroke | 0.65 | 0.65 | 0.5–0.9 | Uniform |
| Major stroke | 0.15 | 0.15 | 0–0.5 | Uniform |
For the sensitivity analyses, the range of probabilities tested for the first month were chosen to reflect potential probabilities based on several of the high risk CAS registries, and a surgical cohort (5; 7; 8; 12; 25;26). The lower limit for the range of probabilities for long-term events reflected the best case scenario that all future events are avoided. The upper range for these probabilities reflected epidemiological studies of all persons after a stroke (11; 22). The upper and lower limits were inclusive of probabilities based on NASCET, ECST and ACST results. Deterministic sensitivity analyses were conducted for each variable (Table 1). Probabilistic sensitivity analysis was performed using a Monte Carlo simulation with 1000 iterations. Beta distributions were used for probabilities. Triangular distributions were used for cost estimates. Utilities were uniformly distributed over the ranges specified.
Utilities data
Quality of life for well and death were 1.0 and 0, respectively. The utility for a major stroke was 0.15 because this value approximates the Rankin score descriptions matched to EuroQol results (23). The range of utility after a major stroke was tested from 0–0.5. For minor stroke, the utility was 0.65 (23).
Utilization and cost data
All cost adjustments from different years are standardized to 2007$ (US) using the Consumer’s Price Index for medical goods. Cost data for clopidogrel were obtained from the average US prices of a 30-day supply (1). Clopidogrel costs were only added for the first 30 days immediately following CAS, thus reflecting clinical trial protocols (19; 24; 27). Because this analysis focused on the differences between CAS and CEA rather than building an all inclusive cost estimate of the procedures, follow-up clinic visits and ultrasound were assumed to be equal in the two groups and these costs were not incorporated into the model. All costs were direct medical costs; no indirect costs were included.
Analysis
CAS was the intervention and CEA was the comparator. CAS would be dominant if the health outcomes were not only better, but the lifetime costs were lower than costs associated with CEA. Future costs and utilities were discounted at 3%.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
In the base case analysis, the lifetime costs of CEA were $35,200 and the QALYs were 9.64. CAS was associated with lifetime costs of $52,900 and a QALY gain of 8.97. CAS was dominated by CEA because it was associated with fewer QALY gain at increased lifetime medical costs.
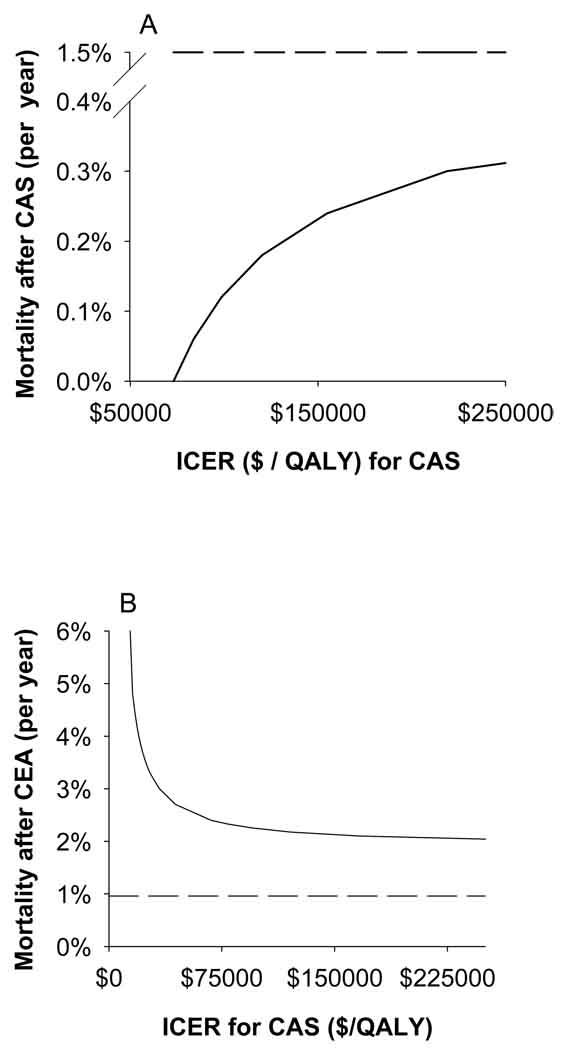
The assumptions in the model were tested with one-way deterministic sensitivity analyses. If the long-term stroke rate after CEA increased from 2.1% per year to 6.3% per year, CAS became dominant with respect to CEA. Long-term mortality after CEA or CAS created an ICER for CAS (Figure 2). Notably, varying the proportion of those starting in the “well” or “minor stroke” branches from 0–1 did not change the dominance of CEA. Varying the 30-day peri-operative or peri-procedural risks did not alter the results of the base case analysis. Finally, none of the procedural costs, other costs or utilities changed the conclusions of the base case analysis.
Figure 2.
One-way sensitivity analyses for the rate of death after CAS (A, solid line) and rate of death after CEA (B, solid line). Dashed lines are the base case mortality rates. The ICER for CAS drops sharply when post-CAS mortality is reduced by approximately 10-fold (A). The ICER for CAS decreases below $75,000/QALY as post-CEA mortality is increased 2–3 fold (B).
A two-way sensitivity analysis examined the influence of long-term stroke rates following CAS or CEA. In a net monetary benefits model, equal rates of stroke outside the 30-day peri-procedural window generally favor CEA (the area below the line in Supplementary Figure 1). A three-way sensitivity analysis was used to look at 30-day outcomes. Here, the combined effects of major stroke, minor stroke and death after CAS could be evaluated in relation to each other over a range of transition probabilities. In a net monetary benefits model, CEA is always the preferred option (data not shown).
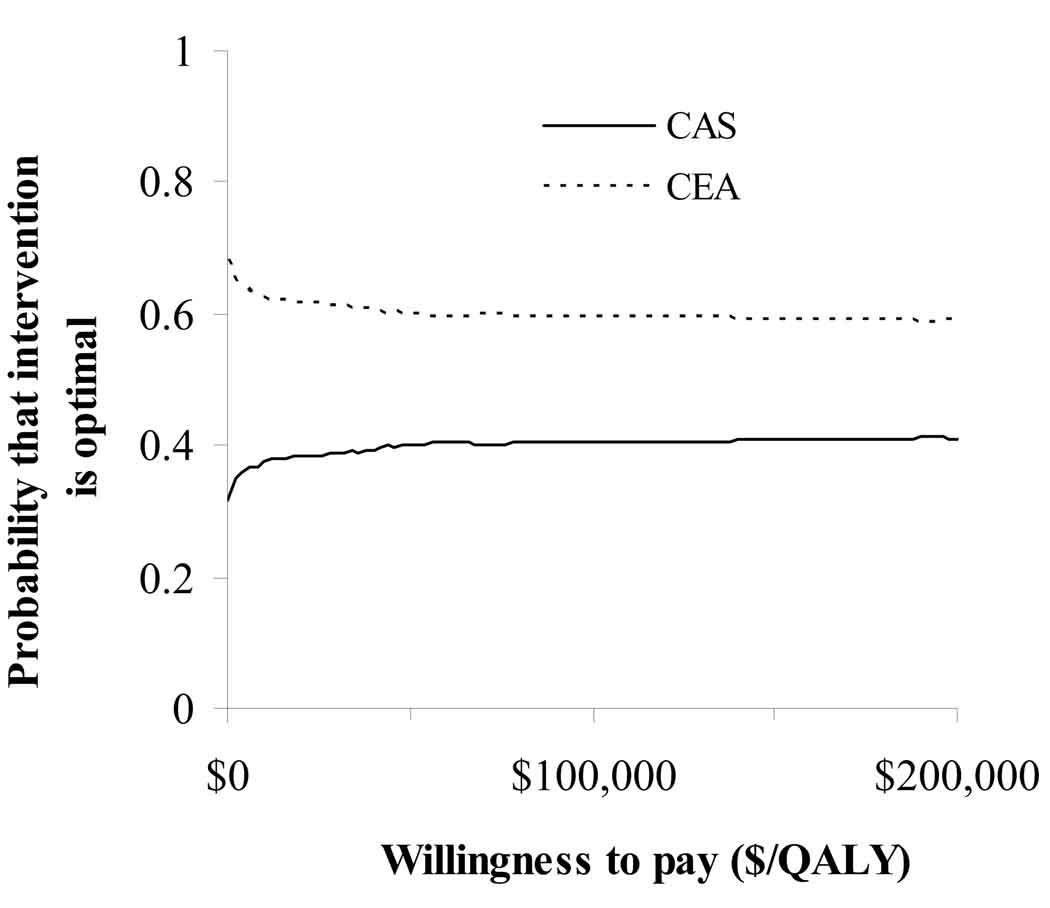
Probabilistic sensitivity analysis showed that CEA remains the optimal intervention 59% of the time over a wide range of economic values for a QALY (Figure 3).
Figure 3.
Probabilistic sensitivity analysis. After 1,000 iterations, CEA remains cost-effective 59% of the time over a range of economic values per QALY.
Discussion
In the base case analysis, CEA was the dominant option for the treatment of symptomatic carotid stenosis in a hypothetical cohort suitable for either CEA or CAS. CEA maximizes health benefits and cost savings. Deterministic sensitivity analyses showed that the assumptions regarding long-term stroke rates and mortality influence the suitability of CEA as the cost-effective option. With lower thresholds for cost-effectiveness, the probability that CEA is treatment of choice increases from 59% to 68%.
The recent follow-up data from EVA-3S, SPACE and SAPPHIRE fail to demonstrate a detectable difference in the annual rates of stroke or death. None of these studies shows non-inferiority on a yearly time scale. This analysis incorporated weighted averages (by trial size) for stroke or death beyond 30 days. These weighted averages do show a higher long-term risk following CAS. Aspects of clinical uncertainty surrounding the long-term performance of CEA and CAS are addressed by the probabilistic sensitivity analysis which randomly samples long-term probabilities.
To our knowledge, this is the first cost-effective analysis of CEA and CAS in symptomatic subjects incorporating meta-analyses for 30-day peri-procedural events along with weighted estimates of long-term events (6;9;10;20). Although the meta-analysis used for the base case analysis included both those at high risk and standard risk for CEA, a majority of the subjects represented a standard risk cohort. Surgical risk must be considered when applying these cost-effectiveness findings to a general population. Thus, if long-term stroke rates following CEA in high risk individuals are greatly increased, CAS may be an acceptable alternative.
MI was included as a health state in the model because it poses a major public health burden. If 30-day major and minor stroke risks are comparable, differences in the 30-day likelihood of MI could potentially make one procedure preferable over the other. However, our cost-effectiveness model is robust to a wide range of 30-day probabilities of MI.
The identification of death and major stroke rates as contributing factors to the cost-effectiveness analysis in our model is consistent with a previous study (13). Advantages to our model include incorporating outcomes from a meta-analysis with MI, major stroke and minor stroke as adverse events, the use of nationally based cost data, and consideration of both 30-day per-procedural and long-term events.
There are several limitations to this analysis. This study is a secondary data analysis that considers event probabilities for the base case analysis in a symptomatic cohort. Revascularization for asymptomatic subjects should have lower 30-day rates of adverse events which may limit the generalization of these results to an asymptomatic population. Follow-up in our model is limited to a timeframe of 2 to 4 years (6; 10; 20). Finally, the current model only considered major outcomes including death, major stroke, minor stroke and peri-procedural MI.
SPACE reported greater restenosis following CAS at 2 years, though restenosis rates following CAS may be artificially high (6). Residual stenosis after CAS may be subject to overestimation if traditional ultrasound criteria are used (3; 6). Also, there is no consensus for the treatment of post-CAS restenosis (16). Because of the possibility of overestimation and no data regarding defined treatment, restenosis was not included in our model, per se, unless it led to an event. Therefore, including restenosis as a health state would not change the results of our analysis. Treatment for restenosis, however, could increase CAS-associated costs relative to the costs of CEA. This increased CAS cost would not change the base case conclusions from cost-effectiveness analysis; CEA would still remain the least costly option and produce the most QALYs.
Although considerable uncertainty exists in the lifetime cost-effectiveness of CAS compared with CEA, our estimates suggest with nearly 60% probability that CAS may not be a cost-effective alternative. Given the uncertainty about the effectiveness and cost-effectiveness of CAS compared to CEA, CAS should remain limited to randomized trials or select populations of patients with carotid stenosis. Such trials will clarify the risks and benefits of CAS. This model provides a framework for the interpretation of ongoing trials that highlight the long-term benefits of carotid artery revascularization for secondary prevention of stroke.
Acknowledgements
KCY was supported in part by National Institutes of Health (NIH) T32HL007937 (to Dr. Thomas A Pearson, Department of Community and Preventive Medicine, University of Rochester). RGH was supported in part by K24NS42098 from the National Institute of Neurological Disorders. CGB is supported, in part, by NIH RO1HL080107. This publication was made possible, in part, by Grant Number 1UL1RR024160-01 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Disclosures: KCY, RGH, CGB: portion of salary covered by NIH funds (please see acknowledgements). WSB and CGB: clinical research grant support for ACT-1 (Abbott) and CREST (NIH).
Reference List
- 1.2006 Redbook. 2006 ed. Montvale: Thompson PDR; 2006. [Google Scholar]
- 2.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19:1497–1500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 3.Chahwan S, Miller MT, Pigott JP, et al. Carotid artery velocity characteristics after carotid artery angioplasty and stenting. J Vasc Surg. 2007;45:523–526. doi: 10.1016/j.jvs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Cronenwett JL, Birkmeyer JD, Nackman GB, et al. Cost-effectiveness of carotid endarterectomy in asymptomatic patients. J Vasc Surg. 1997;25:298–309. doi: 10.1016/s0741-5214(97)70351-3. [DOI] [PubMed] [Google Scholar]
- 5.Ecker RD, Lau T, Levy EI, Hopkins LN. Thirty-day morbidity and mortality rates for carotid artery intervention by surgeons who perform both carotid endarterectomy and carotid artery angioplasty and stent placement. J Neurosurg. 2007;106:217–221. doi: 10.3171/jns.2007.106.2.217. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein H-H, Ringleb PA, Allenberg J, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenosis at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 7.Gray WA, Hopkins LN, Yadav S, et al. Protected carotid stenting in high-surgical-risk patients: the ARCHeR results. J Vasc Surg. 2006;44:258–268. doi: 10.1016/j.jvs.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Gray WA, Yadav JS, Verta P, et al. The CAPTURE registry: results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007;69:341–348. doi: 10.1002/ccd.21050. [DOI] [PubMed] [Google Scholar]
- 9.Gurm HS, Nallamothu BK, Yadav J. Safety of carotid artery stenting for symptomatic carotid artery disease: a meta-analysis. Eur Heart J. 2007 doi: 10.1093/eurheartj/ehm362. [DOI] [PubMed] [Google Scholar]
- 10.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 11.Hankey GJ, Jamrozik K, Broadhurst RJ, et al. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29:2491–2500. doi: 10.1161/01.str.29.12.2491. [DOI] [PubMed] [Google Scholar]
- 12.Illig KA, Zhang R, Tanski W, et al. Is the rationale for carotid angioplasty and stenting in patients excluded from NASCET/ACAS or eligible for ARCHeR justified? J Vasc Surg. 2003;37:575–581. doi: 10.1067/mva.2003.79. [DOI] [PubMed] [Google Scholar]
- 13.Kilaru S, Korn P, Kasirajan K, et al. Is carotid angioplasty and stenting more cost effective than carotid endarterectomy? J Vasc Surg. 2003;37:331–339. doi: 10.1067/mva.2003.124. [DOI] [PubMed] [Google Scholar]
- 14.Kuntz KM, Kent KC. Is carotid endarterectomy cost-effective? An analysis of symptomatic and asymptomatic patients. Circulation. 1996;94(9 Suppl):II194–II198. [PubMed] [Google Scholar]
- 15.Lai SM, Duncan PW. Stroke recovery profile and the Modified Rankin assessment. Neuroepidemiology. 2001;20:26–30. doi: 10.1159/000054754. [DOI] [PubMed] [Google Scholar]
- 16.Lal BK. Recurrent carotid stenosis after CEA and CAS: diagnosis and management. Semin Vasc Surg. 2007;20:259–266. doi: 10.1053/j.semvascsurg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 18.Luebke T, Aleksic M, Brunkwall J. Meta-analysis of randomized trials comparing carotid endarterectomy and endovascular treatment. Eur J Vasc Endovasc Surg. 2007;34:470–479. doi: 10.1016/j.ejvs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 20.Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 21.Patel ST, Haser PB, Korn P, et al. Is carotid endarterectomy cost-effective in symptomatic patients with moderate (50% to 69%) stenosis? J Vasc Surg. 1999;30:1024–1033. doi: 10.1016/s0741-5214(99)70040-6. [DOI] [PubMed] [Google Scholar]
- 22.Petty GW, Brown RD, Jr, Whisnant JP, et al. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50:208–216. doi: 10.1212/wnl.50.1.208. [DOI] [PubMed] [Google Scholar]
- 23.Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke. 2001;32:1425–1429. doi: 10.1161/01.str.32.6.1425. [DOI] [PubMed] [Google Scholar]
- 24.Ringleb PA, Allenberg J, Bruckmann H, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 25.Safian RD, Bresnahan JF, Jaff MR, et al. Protected carotid stenting in high-risk patients with severe carotid artery stenosis. J Am Coll Cardiol. 2006;47:2384–2389. doi: 10.1016/j.jacc.2005.12.076. [DOI] [PubMed] [Google Scholar]
- 26.White CJ, Iyer SS, Hopkins LN, Katzen BT, Russell ME. Carotid stenting with distal protection in high surgical risk patients: the BEACH trial 30 day results. Catheter Cardiovasc Interv. 2006;67:503–512. doi: 10.1002/ccd.20689. [DOI] [PubMed] [Google Scholar]
- 27.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]