Abstract
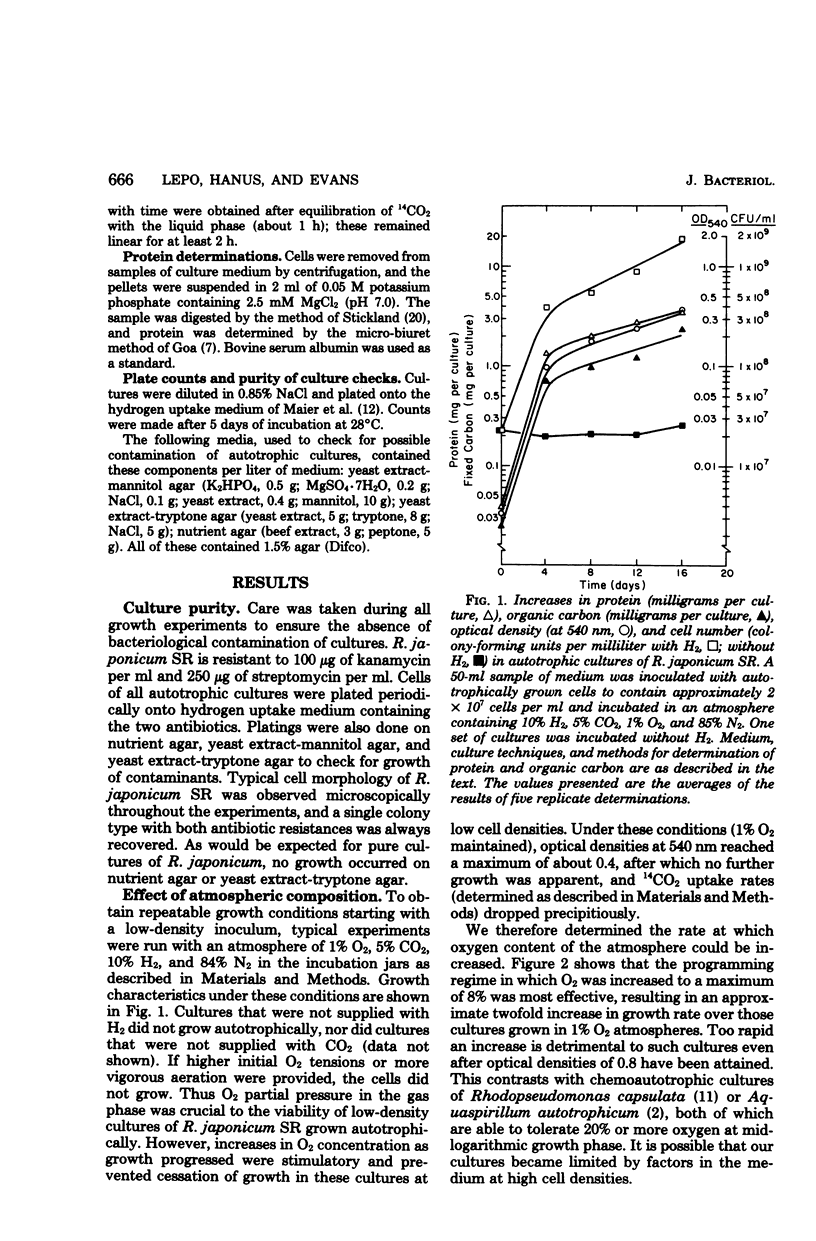
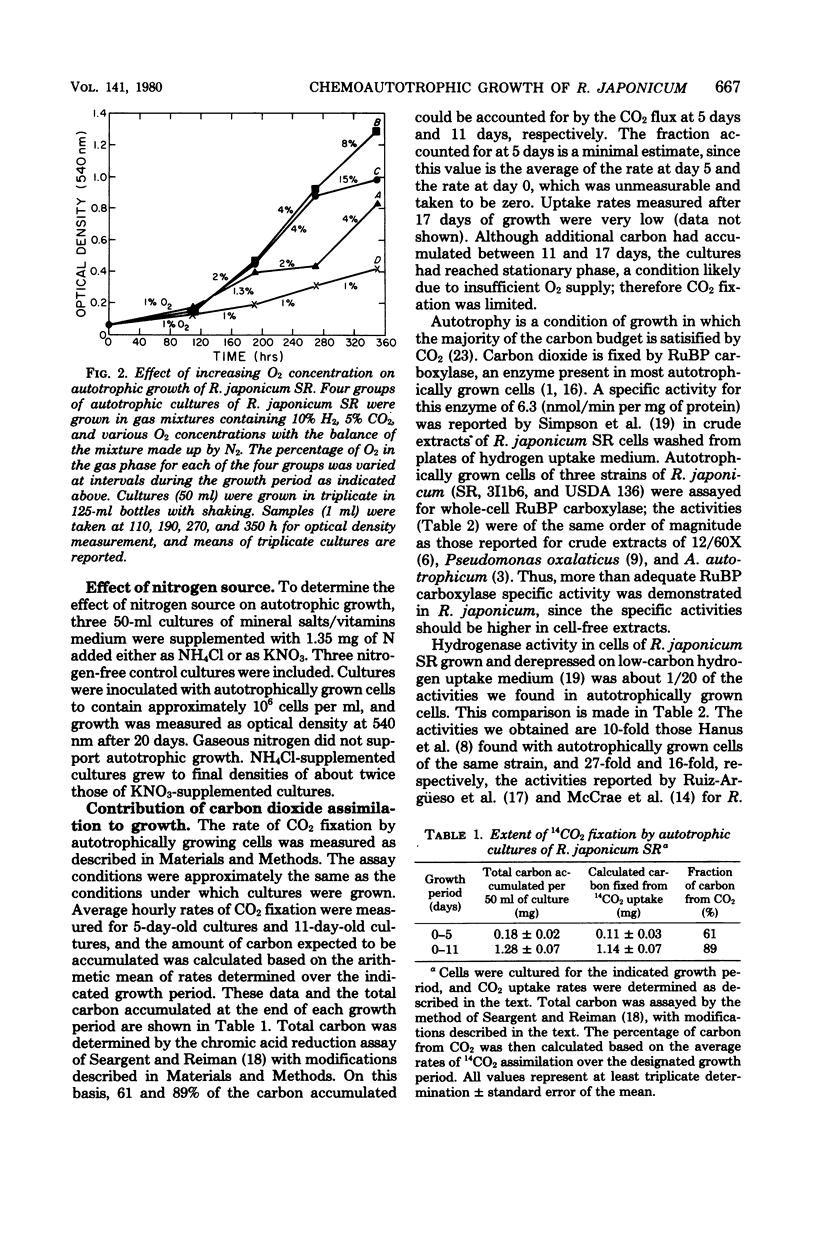
Recently reported research from this laboratory has demonstrated the autotrophic growth of certain hydrogen-uptake-positive strains of Rhizobium japonicum and defined minimal conditions for such growth. Ribulose 1,5-bisphosphate carboxylase has been detected in autotrophically growing cells, but at low specific activity. Moreover, growth rates were low, and growth ceased at low cell densities. We report here improved autotrophic growth rates of R. japonicum SR through the use of a modified mineral salts/vitamins medium and a programmed increase in oxygen tension as autotrophic growth proceeds. Under these conditions, ribulose, 1,5-biphosphate carboxylase activity increased greater than 10-fold and crude-extract-uptake-hydrogenase activities were from 20 to 47 times those heretofore reported for free-living R. japonicum. It is likely that previous assays for these enzymes were done on preparations of cells in which their synthesis had been partially repressed. The contribution of CO2 fixation to organic carbon accumulation in autotrophic cells was assessed as sufficient to support observed growth. Enzymological determination of the product of carbon fixation has established a stoichiometric ratio of 1.9 mol of 3-phosphoglycerate per mol of CO2 fixed and unequivocally assigns the role of carbon fixation catalysis to ribulose 1,5-bisphosphate carboxylase. Ammonium served best as a nitrogen source, nitrate was less effective, and gaseous nitrogen would not support autotrophic growth. Ecological, evolutionary, and practical considerations of autotrophy in the rhizobia are briefly discussed in the light of our findings.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E. Ribulose-1, 5-diphosphate carboxylase from Rhadospirillum rubrum. Methods Enzymol. 1975;42:457–461. doi: 10.1016/0076-6879(75)42151-6. [DOI] [PubMed] [Google Scholar]
- Bowien B., Mayer F., Codd G. A., Schlegel H. G. Purification, some properties and quaternary structure of the D-ribulose 1,5-diphosphate carboxylase of Alcaligenes eutrophus. Arch Microbiol. 1976 Nov 2;110(23):157–166. doi: 10.1007/BF00690223. [DOI] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt U. On chemolithotrophy and hydrogenase of a gram-positive knallgas bacterium. Arch Mikrobiol. 1969;66(1):91–104. doi: 10.1007/BF00414667. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlis V. B., Jr, Gordon G. L., McFadden B. A. Ribulose 1,5-bisphosphate carboxylase/oxygenase from Pseudomonas oxalacticus. J Bacteriol. 1979 Jul;139(1):287–298. doi: 10.1128/jb.139.1.287-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol. 1979 Jan;137(1):524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Tabita F. R., Kuehn G. D. Ribulose-diphosphate carboxylase from the hydrogen bacteria and Rhodospirillum rubrum. Methods Enzymol. 1975;42:461–472. doi: 10.1016/0076-6879(75)42152-8. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Evans H. J. Phosphoenolpyruvate carboxylase from soybean nodule cytosol. Evidence for isoenzymes and kinetics of the most active component. Biochim Biophys Acta. 1979 Apr 12;567(2):445–452. doi: 10.1016/0005-2744(79)90130-x. [DOI] [PubMed] [Google Scholar]
- Quayle J. R., Ferenci T. Evolutionary aspects of autotrophy. Microbiol Rev. 1978 Jun;42(2):251–273. doi: 10.1128/mr.42.2.251-273.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Caruso P., Whitman W. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal Biochem. 1978 Feb;84(2):462–472. doi: 10.1016/0003-2697(78)90064-7. [DOI] [PubMed] [Google Scholar]
- Wang R., Healey F. P., Myers J. Amperometric measurement of hydrogen evolution in chlamydomonas. Plant Physiol. 1971 Jul;48(1):108–110. doi: 10.1104/pp.48.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]



