Abstract
Uremic patients have increased plasma lipoprotein(a) [Lp(a)] levels and elevated risk of cardiovascular disease. Lp(a) is a subfraction of LDL, where apolipoprotein(a) [apo(a)] is disulfide bound to apolipoprotein B-100 (apoB). Lp(a) binds oxidized phospholipids (OxPL), and uremia increases lipoprotein-associated OxPL. Thus, Lp(a) may be particularly atherogenic in a uremic setting. We therefore investigated whether transgenic (Tg) expression of human Lp(a) increases atherosclerosis in uremic mice. Moderate uremia was induced by 5/6 nephrectomy (NX) in Tg mice with expression of human apo(a) (n = 19), human apoB-100 (n = 20), or human apo(a) + human apoB [Lp(a)] (n = 15), and in wild-type (WT) controls (n = 21). The uremic mice received a high-fat diet, and aortic atherosclerosis was examined 35 weeks later. LDL-cholesterol was increased in apoB-Tg and Lp(a)-Tg mice, but it was normal in apo(a)-Tg and WT mice. Uremia did not result in increased plasma apo(a) or Lp(a). Mean atherosclerotic plaque area in the aortic root was increased 1.8-fold in apo(a)-Tg (P = 0.025) and 3.3-fold (P = 0.0001) in Lp(a)-Tg mice compared with WT mice. Plasma OxPL, as detected with the E06 antibody, was associated with both apo(a) and Lp(a). In conclusion, expression of apo(a) or Lp(a) increased uremia-induced atherosclerosis. Binding of OxPL on apo(a) and Lp(a) may contribute to the atherogenicity of Lp(a) in uremia.
Keywords: uremia, atherogenesis, Lp(a), OxPL
Chronic kidney disease markedly accelerates atherosclerosis and is a potent risk factor of cardiovascular death (1). Recent clinical trials have shown that lowering low-density lipoprotein (LDL) with statins does not reduce cardiovascular risk in dialysis patients (2, 3). This underscores the need to investigate unique mechanisms causing accelerated atherosclerosis in this patient group. In hypercholesterolemic apolipoprotein E knockout (apoE−/−) mice, moderate uremia, as induced by 5/6 nephrectomy (NX), markedly accelerates atherosclerosis (4–7). Hence, the 5/6 NX mouse model is a valuable tool for dissecting mechanisms of uremic atherosclerosis. We have recently documented that the pro-atherogenic effect of uremia in 5/6 NX mice includes increased levels of oxidized phospholipids (OxPL) on mouse apoB particles and increased formation of autoantibodies to oxidized LDL (OxLDL) (8, 9). OxPL have a number of pro-atherogenic effects on all vascular wall cells, including macrophages, endothelial cells, and smooth muscle cells (10) and could be an essential mediator of uremic atherosclerosis.
Lipoprotein(a) [Lp(a)] consists of apolipoprotein(a) [apo(a)], which is bound to apoB-100 through a single disulfide bond, and elevated plasma Lp(a) is a risk factor for cardiovascular disease in humans (11–13). The atherogenic potential of Lp(a) may exceed that of LDL because Lp(a) has unique pro-atherogenic and pro-inflammatory properties (14). Recent results suggest that the pro-atherogenic potential of Lp(a) includes the ability to bind pro-inflammatory OxPL (15). Hence, compared with other lipoproteins, Lp(a) was recently demonstrated to be selectively enriched in OxPL, as detected by murine monoclonal antibody E06, in humans and in Lp(a) transgenic (Tg) mice expressing a mini-apo(a) construct containing eight kringle IV repeats (15–18). Lp(a) is selectively trapped in atherosclerotic lesions through a lysine binding site on kringle IV-10 (19, 20, 21) and may deliver apo(a) with its prothrombotic potential, cholesterol, lipids, and pro-inflammatory OxPL, into atherosclerotic lesions. Most, but not all, previous studies indicate a pro-atherogenic role of apo(a) and Lp(a) in transgenic (Tg) mice and rabbits (19–25).
Plasma Lp(a) is increased in uremic patients (26–28). The mechanism may include an increased turnover-time of plasma Lp(a) (29), and the uremia-induced increase of plasma Lp(a) may be isoform-dependent (30–32). It has been suggested that the increase of Lp(a) in uremic individuals reflects decreased clearance in the kidney (28, 33, 34), although kinetic studies in rabbits and mice using radiolabeled Lp(a) indicate that the liver is the major organ for clearance of plasma Lp(a) in nonuremic animals (35, 36). Elevated Lp(a) has been associated with increased risk of cardiovascular disease in relatively small studies of uremic patients (37, 38). The putative pro-atherogenic ability of Lp(a) to bind OxPL may be particularly important in conditions associated with increased oxidative stress, such as uremia. Nevertheless, it remains to be established whether Lp(a) plays a causal role in development of uremic atherosclerosis.
To address this issue, we used the 5/6 NX mouse model, which develops moderate uremia but not end-stage renal failure. Mice do not express apo(a), and human apo(a) cannot bind covalently to mouse apoB (39). Thus, co-expressing human apo(a) and human apoB is needed to study bona fide Lp(a) in Tg mice (40, 41). We used wild-type (WT), human apo(a)-Tg, human apoB-Tg, and Lp(a)-Tg mice to assess the impact of 5/6 NX on plasma Lp(a) and the development of uremic atherosclerosis.
MATERIALS AND METHODS
Mice
Two types of apo(a) transgenic mice were used in this study. The cDNA-apo(a)-Tg mice were originally produced by Chiesa et al. (42) and were bred onto a C57BL/6 background for the study. They express a natural human apo(a) with 17 kringle (K) IV repeats, followed by KV and a protease-like domain from a cDNA transgene controlled by the mouse transferrin promoter. The yeast artificial chromosome (YAC)-apo(a)-Tg mice were made with a piece of genomic DNA, including the endogenous apo(a) promoter region (43), and express 12 kringle IV repeats. They were a generous gift from Knut Eliassen and Kåre Berg, Institute of Medical Genetics, University of Oslo, Norway. The YAC-apo(a)-Tg mice were only used to assess the impact of 5/6 NX on plasma apo(a).
Hemizygous cDNA-apo(a)-Tg mice were bred with hemizygous human apoB-Tg mice ([B6.SJL-Tg(APOB)1102Sgy] backcrossed to the C57/Bl6 background more than 23 times) to generate WT, cDNA-apo(a)-Tg, apoB-Tg, and cDNA-apo(a) + apoB double-Tg mice (Lp(a)-Tg). Mice were phenotyped using human apo(a) and human apoB ELISAs (described below). All mice were kept on a 12 h light/dark cycle in a temperature-controlled room at 21–23°C with free access to water and mouse chow (Altromin 1314 without soy and alfalfa, Altromin, Lage, Germany). Upon induction of uremia [immediately after the first operation (see below)], all mice in the atherosclerosis study were switched to a diet containing 42% fat and 0.2% cholesterol (Harlan Teklad, TD.88137).
Uremia was induced by 5/6 nephrectomy in two operations as previously described (6). At age 12–16 weeks, both poles of the right kidney were removed. Two weeks later, the entire left kidney was removed. Anesthesia was achieved subcutaneously with a mixture of fentanyl (0.079 mg/ml), fluanisone (2.5 mg/ml), and midazolam (1.25 mg/ml) (Hypnorm/Dormicum) at a dose of 0.1 ml per 10 g body weight. After the surgical procedures, analgesia (buprenorphine 0.001 mg per 10 g body weight) was given subcutaneously for 2–3 days. In studies of the impact of 5/6 NX on plasma apo(a) levels, mice were euthanized 9–10 weeks after the second operation. In the study of uremic atherosclerosis, cDNA-apo(a)-Tg, apoB-Tg, Lp(a)-Tg, and WT littermate control mice were euthanized 35 weeks after the second operation (age range at termination 47–51 weeks).
The experiments were performed according to the principles stated in the Danish law on animal experiments and were approved by the Animal Experiments Inspectorate, Ministry of Justice, Denmark.
Plasma biochemistry
Blood was collected from the retro-orbital venous plexus in heparinized microtubes (Capiject; Terumo Medical, Elkton, MD) and centrifuged at 4000 rpm for 10 min at 4°C. Plasma cholesterol, urea, creatinine, alanine aminotransferase, and alkaline phosphatase were measured with a Modular P Hitachi automatic analyzer (Roche A/S, Hvidovre, Denmark) and reagents from Roche A/S. Plasma triglycerides were measured with the GPO-Trinder method (Sigma Diagnostics Inc., St. Louis, MO).
Apo(a), Lp(a), apoB, and OxPL measurements
The effect of 5/6 NX on plasma levels of apo(a) was assessed with an apo(a)-specific double sandwich ELISA using the monoclonal antibody MAb a-6 as the capture antibody and the monoclonal antibody MAb a-1-1 labeled with horse radish peroxidase (HRP) as the detection antibody (44). Control human serum with known Lp(a) concentration was used as the standard. Human apoB was determined with an ELISA employing monoclonal antibody MB47 (45) for capture and the monoclonal antibody 1D1 (46) labeled with HRP for detection.
A chemiluminescence ELISA was used to measure the amount of OxPL present on human apoB-100 particles (OxPL/h-apoB) using E06 as previously described in detail (18). For this assay, plasma was added (1:100 dilution) to microtiter plates precoated with MB47, which binds human but not mouse apoB. The presence of OxPL was detected with biotinylated E06; and values were expressed as relative light units (RLU). To determine the amount of h-apoB-100 captured on each well, MB47 was coated on parallel plates; plasma (1:100 dilution) was added; and then biotinylated goat anti-h-apoB-100 was added to determine the amount of apoB-100 (in RLUs) captured. The E06 RLUs were then divided by the apoB RLUs, and the OxPL/apoB ratio was derived. A similar methodology was used to measure the content of OxPL on apo(a) particles [OxPL/apo(a)], except LPA4 was used as the capture antibody, followed by E06 as the detecting antibody. To determine the amount of apo(a) captured on each well, LPA4 was coated on parallel plates, plasma (1:100 dilution) was added, and then a goat-anti-apo(a) antibody (Diasorin, Inc., Stillwater, MN) was added, followed by an alkaline phosphatase labeled anti-goat antibody to quantify the amount of apo(a) (in RLUs). The E06 RLUs were then divided by the apo(a) RLUs to derive the OxPL/apo(a) ratio. To determine OxPL on mouse apoB-100 particles (OxPL/ m-apoB), we used the same methodology as above except that plates were coated with murine monoclonal antibody LF5, which only binds mouse apoB; plasma (1:100 dilution) was added; and then OxPL/m-apoB was determined by biotinylated E06. To determine the amount of m-apoB captured, parallel plates were coated with LF5, plasma (1:100 dilution) was added, and the amount of m-apoB captured was determined by LF3. The E06 RLUs were then divided by the LF3 RLUs to determine the OxPL/m-apoB ratio (18).
At the termination of the atherosclerosis study, plasma Lp(a) levels [i.e., apoB-100 covalently bound to apo(a)] were measured as previously described (18), where apoB from plasma (1:400 dilution) was captured on a plate with monoclonal antibody MB47 (5 µg/ml) and apo(a) was detected with biotinylated murine monoclonal antibody LPA4. A standard curve was generated with human plasma of known Lp(a) mass and used to determine Lp(a) levels. Apo(a) values (which reflect both free apo(a) and apo(a) bound covalently to human apoB or noncovalently to mouse apoB) were measured by plating LPA4 (5 µg/ml), adding plasma (1:1000 dilution), and then detecting apo(a) with a goat anti-apo(a) antibody (Diasorin, Inc.), followed by an alkaline phosphatase labeled anti-goat antibody (in RLUs).
Western blot
Proteins were separated under denaturing, nonreducing or reducing conditions in 3–8% NuPAGE Tris-acetate gels (Invitrogen, Taastrup, Denmark) and transferred to Hybond-P 0.45-µm PVDF membranes using a semidry electroblotter. After blocking (5% skimmed milk in 10 mM Tris, 100 mM NaCl, 0.02% Tween-20), membranes were incubated with polyclonal rabbit anti-human Lp(a) antibody (1:10.000, Dakocytomation, Q0023, Glostrup, Denmark) followed by HRP-conjugated goat-anti-rabbit antibody (1:2000, Dakocytomation, P0448). Western blots were developed with Supersignal West Pico chemiluminiscent Substrate (Pierce Chemicals, Copenhagen, Denmark), and a chemiluminiscence reader (FujiFilm LAS-1000, Trorod, Denmark) was used to detect signals.
Size exclusion chromatography
To separate lipoproteins according to size, pooled plasma samples (150 µl) from eight randomly selected male or female mice were subjected to fast-phase liquid chromatography on a Superose 6 10/300 GL FPLC column (Amersham Pharmacia Biotech, Hoersholm, Denmark) using PBS with Na2EDTA (0.1 g/l) as running buffer. The column was calibrated with human plasma lipoproteins. Cholesterol concentrations in eluted fractions were determined with the CHOD-PAP reagent (Roche).
Histological analyses of aortic root atherosclerosis
The heart with 1–2 mm of the aortic root was embedded in Tissue-Tek (Sakura Finetek Inc., Vaerloese, Denmark) and serial 10 µm sections of the aortic sinus were cut in a cryostate as previously described (47). Sections were collected in series of three microscope slides (A-C) with three sections on each slide. Slide A contained section numbers 1, 4, and 7, slide B section numbers 2, 5, and 8, and slide C section numbers 3, 6, and 9. In this manner, one A-C series spans an area of 90 µm, and each tissue section is separated by 30 µm on each slide. To quantify atherosclerosis, sections were stained with oil-red-O (Sigma-Aldrich, O08625) and counterstained with Mayer's hematoxylin (Sigma-Aldrich, MHS-16). The microscope slides were chosen based on two criteria: i) the presence of a defined aortic wall and ii) the presence of three aortic valves and/or commissures in each tissue section. The ORO-stained area was quantified on three sections from each mouse using the image analysis software IM50 (Leica, Copenhagen, Denmark).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 4 (GraphPad software Inc., San Diego, CA). One-way ANOVA or Mann-Whitney test were used as specified in the figure and table legends. P < 0.05 was considered significant.
RESULTS
No effect of reducing kidney mass on plasma apo(a) or Lp(a) levels in mice
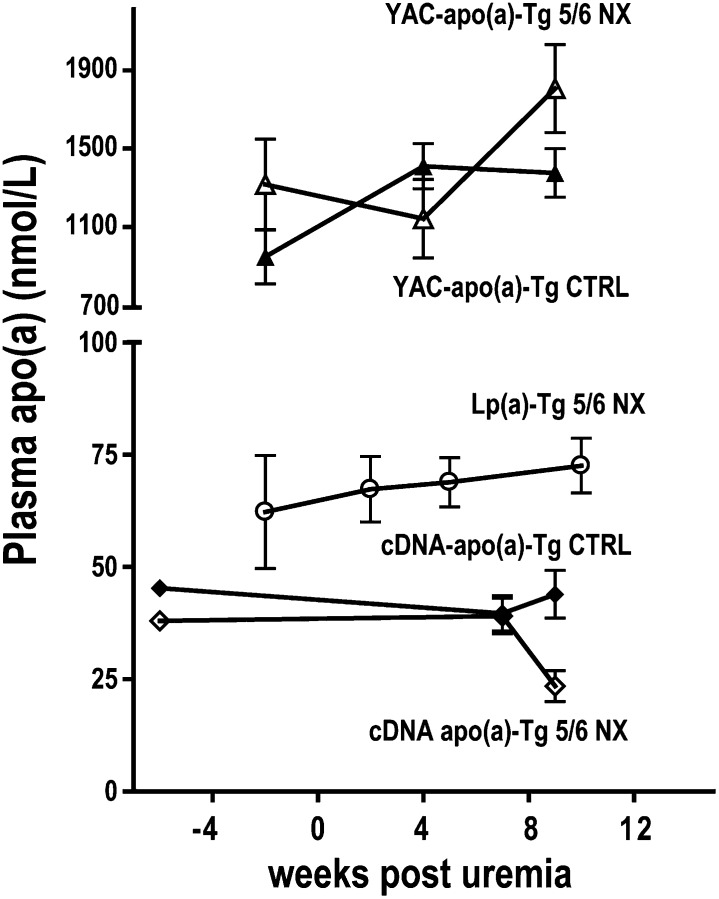
The kidney has been proposed to be an important site of Lp(a) catabolism (29, 33, 34). If this hypothesis is true, a marked reduction of kidney mass should increase plasma apo(a) and Lp(a) levels. We removed more than 80% of the kidney mass by 5/6 NX in cDNA-apo(a)-Tg and Lp(a)-Tg mice, where the apo(a) transgene was controlled by the transferrin promoter. As previously observed, 5/6 NX increased plasma creatinine and urea concentrations ∼2–3-fold (data not shown) and caused moderate uremia (5, 6). Plasma apo(a) concentrations were unaffected by 5/6 NX in cDNA-apo(a)-Tg and Lp(a)-Tg mice (Fig. 1). Notably, hepatic expression of the cDNA apo(a) transgene was not affected by 5/6 NX as analyzed by RT-PCR: the apo(a) mRNA content in liver biopsies from 5/6 NX mice was 94 ± 8% (n = 6) of the level in nonuremic controls (n = 7). We also assessed plasma apo(a) concentrations in YAC-apo(a)-Tg mice, where apo(a) expression is conferred by a genomic transgene spanning the entire apo(a) coding region and large 5′ and 3′ flanking sequences (43). The plasma apo(a) concentrations in YAC-apo(a)-Tg mice are markedly higher than in the cDNA-apo(a)-Tg mice (Fig. 1), probably due to differences in integration sites and/or activity of the two different promoter regions. Like in cDNA-apo(a)-Tg mice, 5/6 NX did not increase plasma apo(a) levels in YAC apo(a)-Tg mice (Fig. 1).
Fig. 1.
Plasma apo(a) is not affected by moderate uremia in apo(a)-Tg mice. Plasma levels of apo(a) in uremic (5/6 NX) (open symbols) and nonuremic (CTRL) (closed symbols) mice. Mice were transgenic for cDNA-apo(a) [diamonds, n = 11 (5 NX, 6 CTRL)], both cDNA-apo(a) and human apoB (Lp(a)-Tg mice, circles, n = 4), or YAC-apo(a) [triangles, n = 14 (7 NX, 7 CTRL)]. Apo, apolipoprotein; Lp(a), lipoprotein(a); Tg, transgenic.
Apo(a) promotes development of uremic atherosclerosis
To study the impact of Lp(a) on development of uremic atherosclerosis, we performed 5/6 NX in WT, cDNA-apo(a)-Tg, human apoB-Tg, and Lp(a)-Tg mice. Subsequently, the mice were fed a high-cholesterol, high-fat diet without cholic acid for 35 weeks. The plasma urea and creatinine concentrations were 2–3-fold elevated above the concentration in normal mice [i.e., ∼10 mmol/l and ∼14 µmol/l, respectively (8)], illustrating that the 5/6 NX mice were moderately uremic (Table 1). Plasma markers of kidney (creatinine and urea) and liver (alkaline phosphatase and alanine aminotransferase) function were not affected by apo(a) or human apoB expression (Table 1).
TABLE 1.
Effect of apo(a) and apoB expression on plasma biochemical parameters
| Genotype | WT | Apo(a) | ApoB | Lp(a) |
|---|---|---|---|---|
| Female mice |
||||
| Number of mice | 15 | 10 | 12 | 7 |
| Creatinine (µmol/l) | 23.1 ± 2.0 | 24.3 ± 1.5 | 23.5 ± 2.7 | 25.7 ± 1.9 |
| Urea (mmol/l) | 20.6 ± 2.4 | 19.1 ± 1.9 | 24.4 ± 4.7 | 19.0 ± 3.1 |
| ALAT (U/l) | 40.5 ± 7.3 | 42.7 ± 6.7 | 51.8 ± 8.5 | 66.9 ± 27.7 |
| Alkaline phosphatase (U/l) | 117.6 ± 9.3 | 110.3 ± 13.4 | 144.3 ± 16.7 | 141.4 ± 15.9 |
| Triglycerides (mmol/l) | 0.53 ± 0.04 | 0.63 ± 0.07 | 0.78 ± 0.07a | 0.87 ± 0.11a |
| Male mice |
||||
| Number of mice | 6 | 9 | 8 | 8 |
| Creatinine (µmol/l) | 18.6 ± 3.0 | 15.3 ± 1.8 | 18.4 ± 1.7 | 19.9 ± 1.6 |
| Urea (mmol/l) | 21.5 ± 2.4 | 19.7 ± 1.7 | 26.0 ± 4.5 | 19.7 ± 1.8 |
| ALAT (U/l) | 75.6 ± 39.7 | 25.0 ± 2.8 | 38.3 ± 7.9 | 73.5 ± 26.1 |
| Alkaline phosphatase (U/l) | 58.2 ± 10.9 | 49.7 ± 3.2 | 74.6 ± 9.5 | 74.6 ± 9.6 |
| Triglycerides (mmol/l) | 0.44 ± 0.08 | 0.54 ± 0.04 | 0.81 ± 0.10 | 1.01 ± 0.18a,b |
Values are mean ± SEM two months after induction of uremia and initiation of high-fat, high-cholesterol diet. ALAT, alanine aminotransferase; apo, apolipoprotein; Lp(a), lipoprotein(a); WT, wild-type.
P < 0.05 compared with WT (one way ANOVA).
P < 0.05 compared with apo(a) (one way ANOVA).
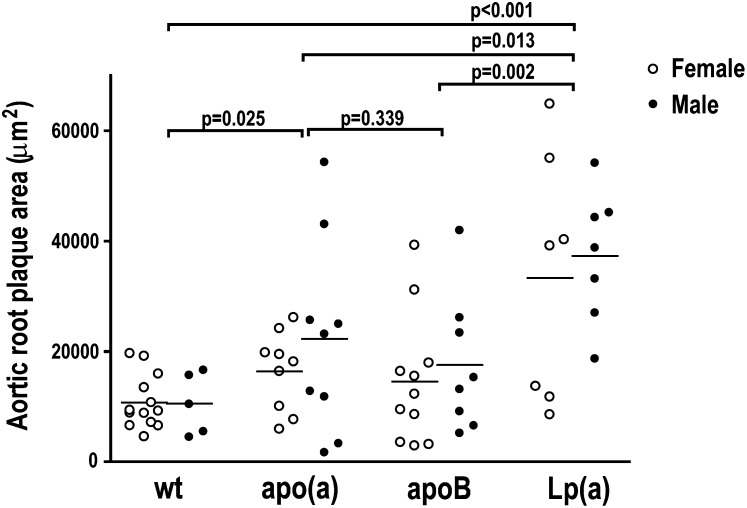
Irrespective of genotype, male and female mice developed similarly sized atherosclerotic lesions and were therefore analyzed together (Fig. 2). Lp(a)-Tg mice had on average 3.3-fold larger atherosclerotic lesions in the aortic root than WT mice (35,296 ± 17,397 versus 10,681 ± 4,895 μm2; P < 0.001, Fig. 2). cDNA-apo(a)-Tg mice had 1.8-fold larger lesions (19,239 ± 13,331 μm2) than WT mice (P = 0.025), whereas there was no significant difference in lesion areas between apoB-Tg (15,809 ± 11,712 μm2) and WT mice (P = 0.09, Fig. 2) or between cDNA-apo(a)-Tg and apoB-Tg mice (P = 0.3). There was no visible atherosclerosis in the aortic arch en face.
Fig. 2.
Expression of Lp(a) increases uremic atherosclerosis. Atherosclerotic plaque areas in the aortic root in female (open symbols) and male (closed symbols) WT (n = 18), cDNA-apo(a)-Tg (n = 18), apoB-Tg (n = 19), and Lp(a)-Tg (n = 14) mice 35 weeks after induction of uremia. Black lines represent mean values. P values are from Mann-Whitney tests. Apo, apolipoprotein; Lp(a), lipoprotein(a); Tg, transgenic; WT, wild-type.
Effect of human apoB and apo(a) expression on plasma lipids, lipoproteins, and apolipoproteins
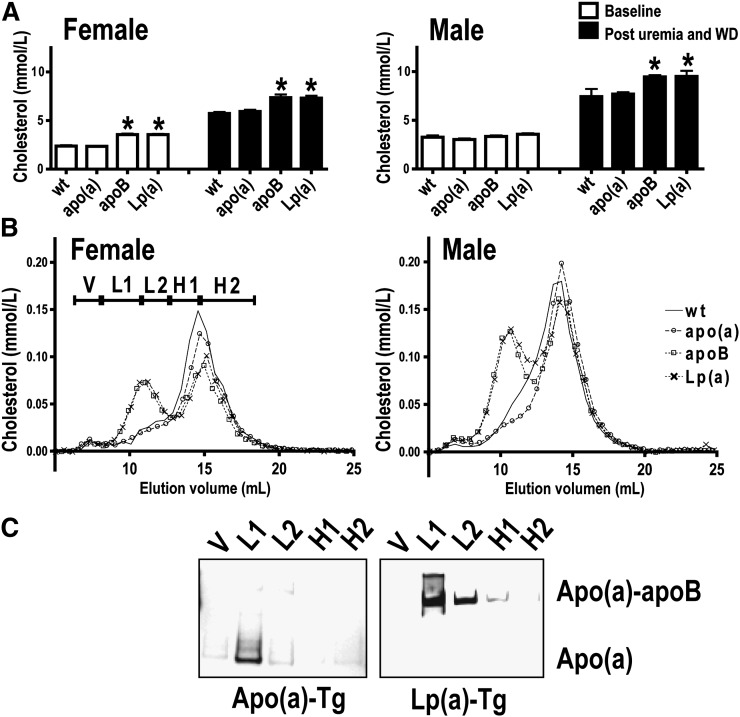
Total plasma cholesterol concentrations at baseline and after 5/6 NX and initiation of the high-fat, high-cholesterol diet are shown in Fig. 3A, B. In the uremic mice, total plasma cholesterol (Fig. 3A) concentrations were higher in apoB-Tg and Lp(a)-Tg mice compared with WT mice. Also, plasma triglycerides had a tendency to be higher in apoB-Tg and Lp(a)-Tg versus WT mice (Table 1). The increase in total cholesterol in apoB-Tg and Lp(a)-Tg mice caused by the combination of uremia and the high-fat diet reflected increased LDL-cholesterol, as judged from the gel filtration analyses of pooled plasma samples (Fig. 3B). In female mice, transgenic expression of apoB and Lp(a) [and to a lesser extent, apo(a)] appeared to lower HDL-cholesterol (Fig. 3B). We cannot rule out that such a decline in HDL-cholesterol may accelerate atherosclerosis. However, this cannot solely explain the accelerated atherosclerosis in uremic Lp(a)-Tg compared with apoB-Tg mice (Fig. 2) as the HDL-cholesterol levels were similar in female apoB-Tg and Lp(a)-Tg mice (Fig. 3B). Also, Lp(a) promoted atherogenesis in Lp(a)-Tg male mice without affecting HDL-cholesterol (Figs. 2 and 3B). Total plasma cholesterol and triglyceride concentrations and plasma lipoprotein profiles were similar in WT and cDNA-apo(a)-Tg uremic mice; i.e., the majority of plasma cholesterol was recovered in HDL-sized lipoproteins (Table 1 and Fig. 3A, B).
Fig. 3.
Plasma cholesterol levels and profiles in uremic mice. A: Plasma cholesterol levels were measured at baseline (1–2 weeks before induction of uremia) (open bars) and two months after induction of uremia and ingestion of a high-fat, high-cholesterol Western-type diet (WD) (closed bars) in female and male mice. For a given genotype and gender, post uremia and WD levels were higher than baseline levels. *P < 0.05 compared with WT and cDNA-apo(a)-Tg mice at the given time point (one way ANOVA). B: Cholesterol gel filtration profiles in female (left) and male (right) nontransgenic WT (solid line), cDNA-apo(a)-Tg (circles), apoB-Tg (squares), and Lp(a)-Tg (crosses) uremic mice two months after induction of uremia. The elution of VLDL (V), LDL (L1 and L2), and HDL (H1 and H2) sized lipoproteins is indicated in the left panel. C: Apo(a) Western blot analyses of gel filtration fractions V, L1, L2, H1, and H2 [as specified in (B)] was performed under denaturing, nonreducing conditions to separate noncovalently bound “free” apo(a) from apoB-bound apo(a) in Lp(a). Apo, apolipoprotein; Lp(a), lipoprotein(a); Tg, transgenic; WT, wild-type.
Western blot analyses of gel filtration fractions showed that apo(a) coeluted with LDL-sized lipoproteins both in cDNA-apo(a)-Tg and Lp(a)-Tg mice (Fig. 3C). SDS-PAGE was done under nonreducing, denaturing conditions to separate covalently bound apo(a)/human apoB complexes from noncovalently bound apo(a)/mouse apoB complexes. The LDL-associated apo(a) was recovered as noncovalently bound apo(a) in cDNA-apo(a)-Tg mice but as apoB-bound apo(a) in Lp(a)-Tg mice (Fig. 3C). These results are in accordance with previous findings in nonuremic mice (18, 48). They illustrate that human apoB but not mouse apoB contains the crucial cysteine involved in bona fide Lp(a) formation (49).
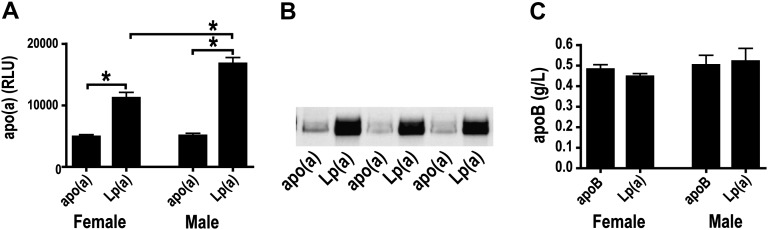
The plasma apo(a) concentration was higher in Lp(a)-Tg than in cDNA-apo(a)-Tg mice as judged by ELISA measurements (Fig. 4A) and Western blotting with a polyclonal antibody (Fig. 4B). The mean plasma concentration of human apoB was similar in apoB-Tg and Lp(a)-Tg mice (Fig. 4C). Previous studies have shown that feeding a high-fat diet increases the plasma levels of apo(a) and human apoB in transgenic mice (22). Thus, we could not ascertain the effect of uremia on plasma apo(a)/Lp(a) in the atherosclerosis study, as the mice were switched to a high-fat, high-cholesterol diet concomitantly with induction of uremia.
Fig. 4.
Plasma levels of apo(a) and apoB in uremic mice. A: Plasma apo(a) in female (left) and male (right) cDNA-apo(a)- and Lp(a)-Tg uremic mice. *P < 0.05 (one-way ANOVA). B: Representative apo(a) Western blot of plasma from cDNA-apo(a)-Tg and Lp(a)-Tg uremic mice. Each lane shows data from one mouse. The gel was run under denaturing, reducing conditions. C: Plasma human apoB in female (left) and male (right) apoB-Tg and Lp(a)-Tg uremic mice. Apo, apolipoprotein; Lp(a), lipoprotein(a); Tg, transgenic.
Oxidized phospholipids in cDNA-apo(a)-Tg and Lp(a)-Tg mice
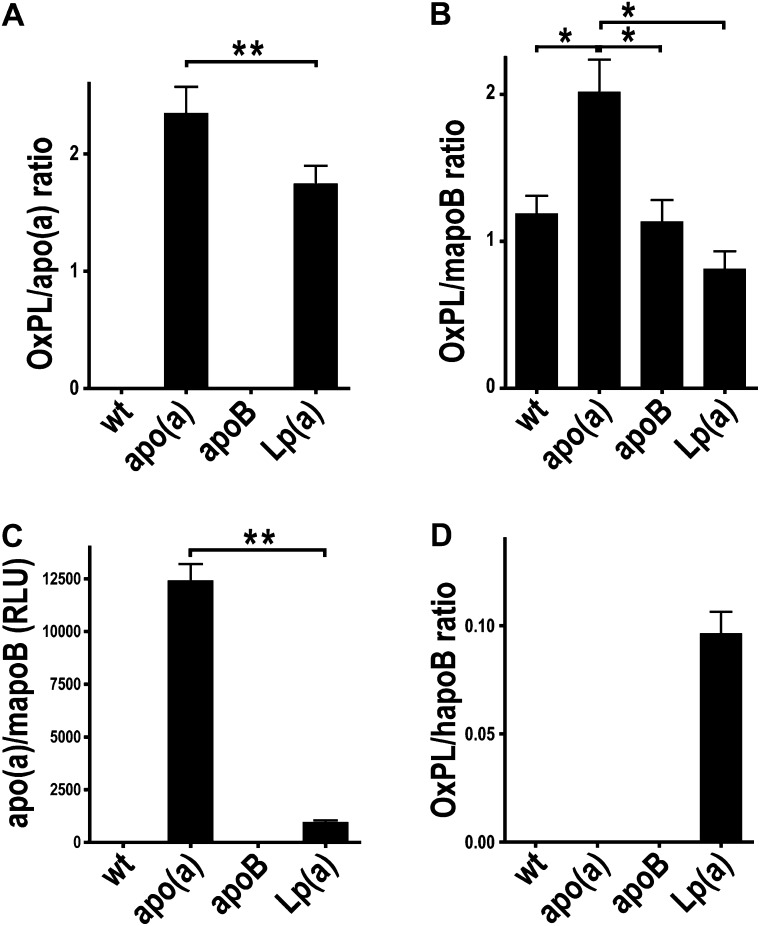
Previous studies in 5/6 NX apoE−/− mice suggest that increased formation of pro-atherogenic OxPL on mouse apoB particles may contribute to accelerated uremic atherogenesis (8). E06-detectable OxPL in human plasma and in transgenic mice can be bound both by apo(a) and by Lp(a), wherein some OxPL are extractable (16–18). Thus, the ability of apo(a) to bind OxPL provides an attractive explanation for the increased lesion formation in uremic mice that express apo(a).
We assessed the association of OxPL with apo(a), mouse apoB, and human apoB in plasma from the various mouse models by capturing apo(a) and mouse (or human) apoB on microtiter well plates and assessing the content of OxPL with the E06 antibody. As expected, OxPL/apo(a) was not present in WT and apoB mice as they do not express apo(a) (Fig. 5A). cDNA-apo(a)-Tg and Lp(a)-Tg mice had a ratio of ∼2 for the amount of OxPL per apo(a), with a significantly higher OxPL content on apo(a) in cDNA-apo(a)-Tg mice. Fig. 5B evaluates OxPL on mouse apoB. Note that all mouse models had evidence of OxPL on mouse apoB, suggesting that uremia alone may induce increased levels of plasma oxidized phospholipids which then bind to mouse apoB particles. The apo(a) mice had the highest amount of OxPL/mapoB, likely reflecting the fact that free apo(a) and its associated OxPL associates with mouse apoB noncovalently (Fig. 5C), as previously shown (16, 18, 42). In the Lp(a)-Tg mice (Fig. 5C), most of the apo(a) binds covalently to human apoB, and thus, there is less apo(a) available to bind to mouse apoB, and correspondingly less OxPL on mouse apoB. Finally, OxPL on human apoB was only present in Lp(a)-Tg mice, but not in apoB-Tg mice, reflecting the fact that E06-detectable OxPL are mainly present on Lp(a) and not human apoB.
Fig. 5.
OxPL associate with apo(a) and apoB in uremic transgenic mice. Plasma E06-reactive OxPL associated with apo(a) (A) or mouse apoB (B) in the various models. Note that apo(a) associates noncovalently with mouse apoB, and in the format of this assay, most of the OxPL on mouse apoB is actually on apo(a), as shown in panel C and as previously shown in Ref. 16. OxPL associated with human apoB particles (D) in WT mice and mice expressing human apoB, cDNA-apo(a), and Lp(a). The data in panels A (OxPL/apo(a)), B (OxPL/mapoB), and D (OxPL/hapoB) are presented as a ratio, and the data in panel C as relative light units (RLU). P < 0.05 as determined by one-way ANOVA (*) or Mann-Whitney tests (**). Apo, apolipoprotein; Lp(a), lipoprotein(a); OxPL, oxidized phospholipid; WT, wild-type.
We did not detect any significant correlations in univariate linear regression analyses of plasma apo(a), Lp(a), OxPL/apo(a), OxPL/mapoB, OxPL/hapoB, or apo(a)/mapoB as predictors of plaque area (data not shown).
DISCUSSION
The major finding in the present study is that expression of Lp(a) and apo(a) leads to increased atherosclerosis in uremic mice. Furthermore, moderate uremia was not associated with increased apo(a) or Lp(a) levels in these transgenic mouse models. It is noteworthy that the 5/6 NX mice overexpressing apo(a) or Lp(a) developed much larger atherosclerotic lesions on a high-fat diet compared with apoB and C57Bl/6 WT mice (50, 51), supporting the pronounced pro-atherogenic effect of apo(a) and Lp(a) in the setting of uremia. Overexpression of human apoB, despite raising (LDL-) cholesterol, did not significantly increase atherosclerosis compared with uremic C57Bl/6 WT mice. In contrast, uremic Lp(a)-Tg mice with similarly elevated plasma cholesterol levels developed significantly larger lesions than both apoB-Tg and WT uremic mice and also developed larger lesions than cDNA-apo(a)-Tg mice. The present results cannot be used to determine whether it is the higher plasma levels of Lp(a), apo(a), or the combination of higher Lp(a) and high LDL levels that results in more atherosclerosis in Lp(a)-Tg compared with cDNA-apo(a)-Tg mice. Five previous studies have shown that transgenic expression of apo(a) in cDNA-apo(a)-Tg mice increases classical atherosclerosis in the aortic root (supplementary Table I). It is important to stress that C57Bl/6 WT mice essentially do not develop atherosclerosis, even on a high-fat diet. Thus, nonuremic WT mice only develop significant lesions when fed a hepatoxic cholic acid-enriched (Paigen) diet. In contrast, as demonstrated in the present study, WT mice on a cholic acid-free diet develop substantial lesions upon induction of moderate uremia by 5/6 NX. This implies (although not proves) that we have been studying a uremia-specific process rather than classical atherosclerosis. The results thus support the idea of a causal role of apo(a) and Lp(a) in uremic atherosclerosis that has not previously been demonstrated.
What is the relevance of moderately uremic mouse models to atherosclerosis in uremic patients? Based on the high risk of cardiovascular death and the lack of effective treatment modalities, it is important to understand the biological mechanisms responsible for accelerating atherosclerosis in a uremic setting. For that purpose, the 5/6 NX mouse model provides an easily accessible genetic model system, in which atherosclerotic lesions resemble those seen in humans. As such, data obtained with the mouse model may direct further studies in humans. Nevertheless, it should be emphasized that the mouse model only develops very early lesions and that the model as such fails to provide insights into the impact of Lp(a) and uremia in advanced atherosclerosis and plaque rupture.
Surprisingly, a >80% reduction of the kidney mass had little effect on plasma apo(a) in cDNA-apo(a)-Tg and YAC-apo(a)-Tg mice expressing apo(a) with 17 or 12 apo(a) kringle IV repeats, respectively. A small cross-sectional study showed that the increase of Lp(a) in uremic patients was confined to small apo(a) isoforms (32). However, larger prospective studies suggest that the increase mainly occurs for larger isoforms (>22 kringles) (30, 31). It was further demonstrated that a decrease of Lp(a) occurs following kidney transplantation and that this is exclusively caused by changes in the expression of high molecular weight apo(a) isoforms (52). Thus, even though the present results argue against the kidney as a major site for removal of apo(a), at least in these mice with 5/6 nephrectomy, we cannot exclude that this may be due to the fact that the currently studied Tg mice only express relatively small apo(a) isoforms. Also, the 5/6 NX mice are only moderately uremic, whereas the elevation of Lp(a) mainly has been described in patients with end-stage renal disease. Thus, there may be a threshold glomerular filtration rate (which was not reached in this study) at which Lp(a) starts to accumulate in plasma.
Is the pro-atherogenic effect of Lp(a) in uremic mice related to an ability of Lp(a) to transport pro-inflammatory OxPL (10, 15)? Uremia increases oxidative stress and formation of OxPL (8, 53). The oxidative stress in uremia may in part relate to increased activity of the renin-angiotensin system (8) and activation of the receptor for advanced glycation end products (RAGE) (9) by advanced glycation end product (AGE)-modified proteins. Thus, inhibition of angiotensin II or RAGE attenuates OxPL formation and reduces atherosclerosis in uremic mice (8, 9). Moreover, the antioxidant N-acetylcysteine retards development of uremic atherosclerosis in the 5/6 NX mouse model (54). We therefore speculate that Lp(a) may be particularly pro-atherogenic in a uremic setting with increased formation of OxPL, although we cannot exclude additional effect(s) of Lp(a) [e.g., inhibition of fibrinolysis or retention of the cholesterol moiety in the arterial wall (55)] that may also play a role. Future studies focusing on removing the OxPL component of Lp(a) may be useful in addressing the mechanism through which Lp(a) is pro-atherogenic.
The current data are compatible with the notion that Lp(a) is a major lipoprotein carrier of OxPL in a uremic setting. In support of Lp(a) binding OxPL in uremic mice, E06-reactive OxPL were associated with apo(a) in both cDNA-apo(a)- and Lp(a)-Tg uremic mice. Interestingly, OxPL were also associated with mouse apoB in WT mice, consistent with prior data that uremia increases oxidative stress. Despite this, the Lp(a)-Tg mice had even more atherosclerosis than the WT mice, consistent with the fact that Lp(a) and its attendant OxPL are pro-atherogenic. Overexpression of apo(a) also increased the amount of OxPL in mouse apoB-containing lipoproteins, although this appears to be mainly due to the fact that apo(a) noncovalently attaches to mouse apoB, as shown previously (16, 18, 42), and that the assay format detects the OxPL on the apo(a) component of these mouse apoB particles. This is further supported by the fact that human apoB-Tg mice have almost no OxPL on their apoB particles. Accordingly, previous studies using apo(a)-Tg and Lp(a)-Tg mice have shown that practically all plasma OxPL associated with lipoproteins is carried by apoB-containing particles to which apo(a) is covalently or noncovalently attached (16). Notably, we found less OxPL associated with Lp(a) in the currently studied Lp(a)-Tg mice than in previous studies of Lp(a) mice overexpressing an eight kringle IV apo(a) mini isoform (16, 18). This likely reflects the fact that the plasma Lp(a) levels in the currently studied cDNA-apo(a)-Tg mice are lower (∼50 mg/l) than in the formerly described Lp(a)-Tg mice (∼700 mg/dl) (16). However, the ratio of OxPL/apo(a) was approximately 2 in both animal models, suggesting that this more likely reflects differences in the number of Lp(a) particles in these models [with the eight kringle IV model having significantly more Lp(a) particles] where the apo(a) isoforms are relatively small, rather than the increased capacity of Lp(a) to carry OxPL.
Prevention of cardiovascular disease is a challenge in patients with renal disease. Hence, conventional LDL-lowering therapy with statins has little or no beneficial effect on cardiovascular risk in end-stage renal disease patients (2, 3). On the other hand, treatment with the anti-oxidant vitamin E [which has little or no effect on cardiovascular risk in nonuremic patients (56)] may be beneficial in hemodialysis patients (57). Thus, oxidative stress, perhaps involving formation of Lp(a)-associated OxPL, may indeed be particularly important for the increased cardiovascular risk in the uremic patient.
In conclusion, the present data suggest that Lp(a) accelerates uremia-induced atherosclerosis. Further studies are warranted to assess the anti-atherogenic effect of Lp(a) lowering in uremic patients. As such, it is promising that apoB-antisense therapies currently in clinical development, as opposed to conventional LDL-lowering drugs, might reduce both LDL and Lp(a), as well as Lp(a)-associated OxPL (18).
Supplementary Material
Acknowledgments
The authors acknowledge the expert technical assistance of Tina Estrup Axen, Charlotte Wandel, and Elizabeth Miller, and we thank Emil Daniel Bartels for valuable help with mice. Knut Eliassen and Kåre Berg generously provided YAC-apo(a)-Tg mice.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- Lp(a)
- lipoprotein(a)
- NX
- nephrectomy
- OxLDL
- oxidized LDL
- OxPL
- oxidized phospholipid
- Tg
- transgenic
- WT
- wild-type
This work was supported by grants from the Danish Medical Research Council, the Danish Heart Foundation, the Leo Dannin Award (L.B.N.), and the Fondation Leducq (S.T.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table.
REFERENCES
- 1.Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. 2004. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2.Fellstrom B. C., Jardine A. G., Schmieder R. E., Holdaas H., Bannister K., Beutler J., Chae D. W., Chevaile A., Cobbe S. M., Gronhagen-Riska C., et al. 2009. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360: 1395–1407. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C., Krane V., Marz W., Olschewski M., Mann J. F., Ruf G., Ritz E. 2005. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353: 238–248. [DOI] [PubMed] [Google Scholar]
- 4.Massy Z. A., Ivanovski O., Nguyen-Khoa T., Angulo J., Szumilak D., Mothu N., Phan O., Daudon M., Lacour B., Drueke T. B., et al. 2005. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J. Am. Soc. Nephrol. 16: 109–116. [DOI] [PubMed] [Google Scholar]
- 5.Bro S., Bentzon J. F., Falk E., Andersen C. B., Olgaard K., Nielsen L. B. 2003. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J. Am. Soc. Nephrol. 14: 2466–2474. [DOI] [PubMed] [Google Scholar]
- 6.Bro S., Moeller F., Andersen C. B., Olgaard K., Nielsen L. B. 2004. Increased expression of adhesion molecules in uremic atherosclerosis in apolipoprotein-E-deficient mice. J. Am. Soc. Nephrol. 15: 1495–1503. [DOI] [PubMed] [Google Scholar]
- 7.Buzello M., Tornig J., Faulhaber J., Ehmke H., Ritz E., Amann K. 2003. The apolipoprotein E knockout mouse: a model documenting accelerated atherogenesis in uremia. J. Am. Soc. Nephrol. 14: 311–316. [DOI] [PubMed] [Google Scholar]
- 8.Bro S., Binder C. J., Witztum J. L., Olgaard K., Nielsen L. B. 2007. Inhibition of the renin-angiotensin system abolishes the proatherogenic effect of uremia in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 9.Bro S., Flyvbjerg A., Binder C. J., Bang C. A., Denner L., Olgaard K., Nielsen L. B. 2008. A neutralizing antibody against receptor for advanced glycation end products (RAGE) reduces atherosclerosis in uremic mice. Atherosclerosis. 201: 274–280. [DOI] [PubMed] [Google Scholar]
- 10.Berliner J. A., Leitinger N., Tsimikas S. 2009. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 50(Suppl): S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danesh J., Collins R., Peto R. 2000. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 102: 1082–1085. [DOI] [PubMed] [Google Scholar]
- 12.Erqou S., Kaptoge S., Perry P. L., Di A. E., Thompson A., White I. R., Marcovina S. M., Collins R., Thompson S. G., Danesh J. 2009. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 302: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., Nordestgaard B. G. 2009. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen L. B. 1999. Atherogenecity of lipoprotein(a) and oxidized low density lipoprotein: insight from in vivo studies of arterial wall influx, degradation and efflux. Atherosclerosis. 143: 229–243. [DOI] [PubMed] [Google Scholar]
- 15.Tsimikas S., Witztum J. L. 2008. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr. Opin. Lipidol. 19: 369–377. [DOI] [PubMed] [Google Scholar]
- 16.Schneider M., Witztum J. L., Young S. G., Ludwig E. H., Miller E. R., Tsimikas S., Curtiss L. K., Marcovina S. M., Taylor J. M., Lawn R. M., et al. 2005. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J. Lipid Res. 46: 769–778. [DOI] [PubMed] [Google Scholar]
- 17.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E. R., Shin M. J., Binder C. J., Horkko S., Krauss R. M., Chapman M. J., et al. 2008. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 49: 2230–2239. [DOI] [PubMed] [Google Scholar]
- 18.Merki E., Graham M. J., Mullick A. E., Miller E. R., Crooke R. M., Pitas R. E., Witztum J. L., Tsimikas S. 2008. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 118: 743–753. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen L. B., Stender S., Jauhiainen M., Nordestgaard B. G. 1996. Preferential influx and decreased fractional loss of lipoprotein(a) in atherosclerotic compared with nonlesioned rabbit aorta. J. Clin. Invest. 98: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen L. B., Stender S., Kjeldsen K., Nordestgaard B. G. 1996. Specific accumulation of lipoprotein(a) in balloon-injured rabbit aorta in vivo. Circ. Res. 78: 615–626. [DOI] [PubMed] [Google Scholar]
- 21.Hughes S. D., Lou X. J., Ighani S., Verstuyft J., Grainger D. J., Lawn R. M., Rubin E. M. 1997. Lipoprotein(a) vascular accumulation in mice. In vivo analysis of the role of lysine binding sites using recombinant adenovirus. J. Clin. Invest. 100: 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancini F. P., Newland D. L., Mooser V., Murata J., Marcovina S., Young S. G., Hammer R. E., Sanan D. A., Hobbs H. H. 1995. Relative contributions of apolipoprotein(a) and apolipoprotein-B to the development of fatty lesions in the proximal aorta of mice. Arterioscler. Thromb. Vasc. Biol. 15: 1911–1916. [DOI] [PubMed] [Google Scholar]
- 23.Fan J., Shimoyamada H., Sun H., Marcovina S., Honda K., Watanabe T. 2001. Transgenic rabbits expressing human apolipoprotein(a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arterioscler. Thromb. Vasc. Biol. 21: 88–94. [DOI] [PubMed] [Google Scholar]
- 24.Sun H., Unoki H., Wang X., Liang J., Ichikawa T., Arai Y., Shiomi M., Marcovina S. M., Watanabe T., Fan J. 2002. Lipoprotein(a) enhances advanced atherosclerosis and vascular calcification in WHHL transgenic rabbits expressing human apolipoprotein(a). J. Biol. Chem. 277: 47486–47492. [DOI] [PubMed] [Google Scholar]
- 25.Berg K., Svindland A., Smith A. J., Lawn R. M., Djurovic S., Alestrom A., Alestrom P., Eliassen K. 2002. Spontaneous atherosclerosis in the proximal aorta of LPA transgenic mice on a normal diet. Atherosclerosis. 163: 99–104. [DOI] [PubMed] [Google Scholar]
- 26.Parra H. J., Mezdour H., Cachera C., Dracon M., Tacquet A., Fruchart J. C. 1987. Lp(a) lipoprotein in patients with chronic renal failure treated by hemodialysis. Clin. Chem. 33: 721. [PubMed] [Google Scholar]
- 27.Yilmaz F. M., Yilmaz G., Duranay M., Parpucu H., Senes M., Tekeli N., Yucel D. 2005. Cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Scand. J. Clin. Lab. Invest. 65: 739–745. [DOI] [PubMed] [Google Scholar]
- 28.Kronenberg F., Utermann G., Dieplinger H. 1996. Lipoprotein(a) in renal disease. Am. J. Kidney Dis. 27: 1–25. [DOI] [PubMed] [Google Scholar]
- 29.Frischmann M. E., Kronenberg F., Trenkwalder E., Schaefer J. R., Schweer H., Dieplinger B., Koenig P., Ikewaki K., Dieplinger H. 2007. In vivo turnover study demonstrates diminished clearance of lipoprotein(a) in hemodialysis patients. Kidney Int. 71: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 30.Dieplinger H., Lackner C., Kronenberg F., Sandholzer C., Lhotta K., Hoppichler F., Graf H., Konig P. 1993. Elevated plasma concentrations of lipoprotein(a) in patients with end-stage renal disease are not related to the size polymorphism of apolipoprotein(a). J. Clin. Invest. 91: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kronenberg F., Konig P., Neyer U., Auinger M., Pribasnig A., Lang U., Reitinger J., Pinter G., Utermann G., Dieplinger H. 1995. Multicenter study of lipoprotein(a) and apolipoprotein(a) phenotypes in patients with end-stage renal disease treated by hemodialysis or continuous ambulatory peritoneal dialysis. J. Am. Soc. Nephrol. 6: 110–120. [DOI] [PubMed] [Google Scholar]
- 32.Sechi L. A., Zingaro L., Catena C., Perin A., De M. S., Bartoli E. 1999. Lipoprotein(a) and apolipoprotein(a) isoforms and proteinuria in patients with moderate renal failure. Kidney Int. 56: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 33.Albers J. J., Koschinsky M. L., Marcovina S. M. 2007. Evidence mounts for a role of the kidney in lipoprotein(a) catabolism. Kidney Int. 71: 961–962. [DOI] [PubMed] [Google Scholar]
- 34.Reblin T., Donarski N., Fineder L., Brasen J. H., Dieplinger H., Thaiss F., Stahl R. A., Beisiegel U., Wolf G. 2001. Renal handling of human apolipoprotein(a) and its fragments in the rat. Am. J. Kidney Dis. 38: 619–630. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen L. B., Juul K., Nordestgaard B. G. 1998. Increased degradation of lipoprotein(a) in atherosclerotic compared with nonlesioned aortic intima-inner media of rabbits: in vivo evidence that lipoprotein(a) may contribute to foam cell formation. Arterioscler. Thromb. Vasc. Biol. 18: 641–649. [DOI] [PubMed] [Google Scholar]
- 36.Cain W. J., Millar J. S., Himebauch A. S., Tietge U. J., Maugeais C., Usher D., Rader D. J. 2005. Lipoprotein [a] is cleared from the plasma primarily by the liver in a process mediated by apolipoprotein [a]. J. Lipid Res. 46: 2681–2691. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi H., Oda H., Ohno M., Watanabe S., Sakata S. 1999. Lipoprotein(a) as a risk factor for coronary artery disease in hemodialysis patients. Kidney Int. Suppl. 71: S242–S244. [DOI] [PubMed] [Google Scholar]
- 38.Tzanatos H. A., Tseke P. P., Pipili C., Retsa K., Skoutelis G., Grapsa E. 2009. Cardiovascular risk factors in non-diabetic hemodialysis patients: a comparative study. Ren. Fail. 31: 91–97. [DOI] [PubMed] [Google Scholar]
- 39.Cheesman E. J., Sharp R. J., Zlot C. H., Liu C. Y., Taylor S., Marcovina S. M., Young S. G., McCormick S. P. 2000. An analysis of the interaction between mouse apolipoprotein B100 and apolipoprotein(a). J. Biol. Chem. 275: 28195–28200. [DOI] [PubMed] [Google Scholar]
- 40.Linton M. F., Farese R. V., Jr, Chiesa G., Grass D. S., Chin P., Hammer R. E., Hobbs H. H., Young S. G. 1993. Transgenic mice expressing high plasma concentrations of human apolipoprotein B100 and lipoprotein(a). J. Clin. Invest. 92: 3029–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callow M. J., Stoltzfus L. J., Lawn R. M., Rubin E. M. 1994. Expression of human apolipoprotein B and assembly of lipoprotein(a) in transgenic mice. Proc. Natl. Acad. Sci. USA. 91: 2130–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiesa G., Hobbs H. H., Koschinsky M. L., Lawn R. M., Maika S. D., Hammer R. E. 1992. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a). J. Biol. Chem. 267: 24369–24374. [PubMed] [Google Scholar]
- 43.Frazer K. A., Narla G., Zhang J. L., Rubin E. M. 1995. The apolipoprotein(a) gene is regulated by sex hormones and acute-phase inducers in YAC transgenic mice. Nat. Genet. 9: 424–431. [DOI] [PubMed] [Google Scholar]
- 44.Marcovina S. M., Albers J. J., Gabel B., Koschinsky M. L., Gaur V. P. 1995. Effect of the number of apolipoprotein(a) kringle 4 domains on immunochemical measurements of lipoprotein(a). Clin. Chem. 41: 246–255. [PubMed] [Google Scholar]
- 45.Pease R. J., Milne R. W., Jessup W. K., Law A., Provost P., Fruchart J. C., Dean R. T., Marcel Y. L., Scott J. 1990. Use of bacterial expression cloning to localize the epitopes for a series of monoclonal antibodies against apolipoprotein B100. J. Biol. Chem. 265: 553–568. [PubMed] [Google Scholar]
- 46.Young S. G., Witztum J. L., Casal D. C., Curtiss L. K., Bernstein S. 1986. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 6: 178–188. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen T. X., Bro S., Andersen M. H., Etzerodt M., Jauhiainen M., Moestrup S., Nielsen L. B. 2009. Effect of treatment with human apolipoprotein A-I on atherosclerosis in uremic apolipoprotein E- deficient mice. Atherosclerosis. 202: 372–381. [DOI] [PubMed] [Google Scholar]
- 48.Sanan D. A., Newland D. L., Tao R., Marcovina S., Wang J., Mooser V., Hammer R. E., Hobbs H. H. 1998. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc. Natl. Acad. Sci. USA. 95: 4544–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormick S. P., Ng J. K., Taylor S., Flynn L. M., Hammer R. E., Young S. G. 1995. Mutagenesis of the human apolipoprotein B gene in a yeast artificial chromosome reveals the site of attachment for apolipoprotein(a). Proc. Natl. Acad. Sci. USA. 92: 10147–10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paigen B. 1995. Genetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am. J. Clin. Nutr. 62: 458S–462S. [DOI] [PubMed] [Google Scholar]
- 51.Bang C. A., Bro S., Bartels E. D., Pedersen T. X., Nielsen L. B. 2007. Effect of uremia on HDL composition, vascular inflammation, and atherosclerosis in wild-type mice. Am. J. Physiol. Renal Physiol. 293: F1325–F1331. [DOI] [PubMed] [Google Scholar]
- 52.Kronenberg F., Lhotta K., Konig P., Margreiter R., Dieplinger H., Utermann G. 2003. Apolipoprotein(a) isoform-specific changes of lipoprotein(a) after kidney transplantation. Eur. J. Hum. Genet. 11: 693–699. [DOI] [PubMed] [Google Scholar]
- 53.Himmelfarb J. 2005. Relevance of oxidative pathways in the pathophysiology of chronic kidney disease. Cardiol. Clin. 23: 319–330. [DOI] [PubMed] [Google Scholar]
- 54.Ivanovski O., Szumilak D., Nguyen-Khoa T., Ruellan N., Phan O., Lacour B., Descamps-Latscha B., Drueke T. B., Massy Z. A. 2005. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 67: 2288–2294. [DOI] [PubMed] [Google Scholar]
- 55.Scanu A. M. 2003. Lipoprotein(a) and the atherothrombotic process: mechanistic insights and clinical implications. Curr. Atheroscler. Rep. 5: 106–113. [DOI] [PubMed] [Google Scholar]
- 56.Heart Protection Study Collaborative Group. 2002. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 360: 23–33.12114037 [Google Scholar]
- 57.Boaz M., Smetana S., Weinstein T., Matas Z., Gafter U., Iaina A., Knecht A., Weissgarten Y., Brunner D., Fainaru M., et al. 2000. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 356: 1213–1218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.