Abstract
We used a HPLC-MS/MS methodology for determination of a basic metabolomic profile (18:1,18:0 sphingoid backbone, C14-C26 N-acyl part) of “normal” sphingolipid levels in human serum and plasma. Blood was collected from healthy males and nonpregnant females under fasting and nonfasting conditions with and without anticoagulants. Sphingolipids analyzed included sphingoid bases, sphingosine and dihydrosphingosine, their 1-phosphates (S1P and dhS1P), molecular species (Cn-) of ceramide (Cer), sphingomyelin (SM), hexosylceramide (HexCer), lactosylceramide (LacCer), and Cer 1-phosphate (Cer1P). SM, LacCer, HexCer, Cer, and Cer1P constituted 87.7, 5.8, 3.4, 2.8, and 0.15% of total sphingolipids, respectively. The abundant circulating SM was C16-SM (64.0 µM), and it increased with fasting (100 µM). The abundant LacCer was C16-LacCer (10.0 µM) and the abundant HexCer was C24-HexCer (2.5 µM). The abundant Cer, C24-Cer (4.0 µM), was not influenced by fasting; however, levels of C16-C20 Cers were decreased in response to fasting. S1P levels were higher in serum than plasma (0.68 µM vs. 0.32 µM). We also determined levels of sphingoid bases and SM species in isolated lipoprotein classes. HDL3 was the major carrier of S1P, dhS1P, and Sph, and LDL was the major carrier of Cer and dhSph. Per particle, VLDL contained the highest levels of SM, Cer, and S1P. HPLC-MS/MS should provide a tool for clinical testing of circulating bioactive sphingolipids in human blood.
Keywords: sphingolipid metabolome, sphingomyelin, ceramide, sphingosine
Sphingolipids, once considered mainly structural components of cell membranes, have emerged as key signaling molecules involved in the regulation of a variety of cellular functions, including cell growth and differentiation, proliferation, and apoptotic cell death (1–4). Sphingolipids are distributed in the cytoplasmic and plasma membranes and interact with cholesterol to form membrane lipid microdomains that allow signal transduction. Complex membrane sphingolipids such as sphingomyelin (SM) and glycosphingolipids modulate the behavior of growth factor receptors and extracellular matrix proteins and also serve as binding sites for some microorganisms, toxins, and viruses (5). Maintenance of the membrane structure is crucial for mechanical stabilization, and any local or global changes in lipid asymmetry can induce a variety of cellular responses.
Sphingolipids are the most structurally diverse as well as complex category of lipids due to their numerous variations in the sphingoid bases, N-acyl linked fatty acids, and head groups (5). Several sphingolipid metabolites, especially ceramide (Cer), sphingosine (Sph), sphingosine 1-phosphate (S1P), and Cer 1-phosphate (Cer1P), have been identified as bioactive signaling molecules that control cell growth and death (1–10). Thus, it has become increasingly necessary to establish the metabolomic profile of sphingolipids to understand how sphingolipid biosynthesis and turnover regulate cell function under normal and abnormal conditions, how perturbations in one sphingolipid may enhance or interfere with the action of another, and where and how all these sphingolipids are made and removed.
Sphingolipids have been implicated in several diseases such as cancer, obesity, atherosclerosis, and sphingolipidoses (8, 9, 11–16); however, efforts addressing blood sphingolipid levels as biomarkers of disease are still in their infancy. Interest in sphingolipid biology in blood has also been enhanced by the recent development and clinical application of the new immunosuppressive agent FTY720, which causes lymphocyte sequestration by targeting S1P receptors (17). Plasma SM levels were shown to be closely related to the development of atherosclerosis (7), and plasma Cers were also proposed to serve as biomarkers for atherosclerosis (6). Abnormalities of glycolipid metabolism in Gaucher disease, Fabry disease, and metachromatic leukodystrophy have led to an interest in the composition, metabolic behavior, and physiological function of the glycosyl Cers in human blood (9). Farber and Niemann-Pick diseases related to dysfunction of acid ceramidase and acid sphingomyelinase showed elevated levels of Cer and SM (8).
Sphingolipids in the blood constitute a part of the circulating lipoprotein particles (VLDL, LDL, and HDL), are carried by serum albumin, and also are present in blood cells and platelets. The finding that plasma lipoproteins are S1P carriers and that more than 60% of the S1P in blood is associated with VLDL, LDL, and HDL is potentially relevant to vascular disorders (18). Numerous studies have shown that S1P regulates various functions of cells involved in vascular remodeling, including endothelial cells, smooth muscle cells, lymphocytes, monocytes, and platelets (10, 19–22). It also has been shown that all blood cells can remove plasma Sph, which is harmful or suppressive to cellular functions, and convert it into plasma S1P (23).
Studies addressing the sphingolipidome of blood are limited. HPLC-MS/MS methodology offers an efficient tool to monitor changes in the composition of sphingolipid species. Our group has recently reported a robust analytical HPLC-MS/MS procedure that can determine the composition of endogenous sphingolipids in varied biological materials (24). In the current paper, we describe a method for simultaneous analysis of the following blood sphingolipids: sphingoid bases (C18:1, C18:0): Sph, dihydrosphingosine (dhSph); sphingoid base phosphates: S1P, dhS1P; and molecular species (Cn-) of Cer (Cn-Cer), dihydroceramide (Cn-dhCer), Cer1P (Cn-Cer1P), SM (Cn-SM), hexosylceramide (Cn-HexCer), and lactosylceramide (Cn-LacCer) covering a basic metabolomic profiling of human blood serum and plasma using HPLC-MS/MS. The goal was to establish a profile of sphingolipids in “normal” human serum and plasma prepared with different anticoagulants and to determine a reference range for circulating sphingolipid species to be used for future translational research and clinical practices detecting disease biomarkers in both men and women.
MATERIALS AND METHODS
Subjects
The study was approved by the institutional review board at the Medical University of South Carolina (MUSC), and proper consent was obtained from each subject. Subjects aged 20–35 years were screened before participating in the study, and those with known hypertension, heart disease, kidney disease, diabetes, cancer, or serious current illness were excluded. Smokers and subjects who take daily multivitamins or antioxidants were also excluded. In addition, the candidate subjects were screened for healthy levels of conventional lipid panel [total cholesterol, HDL-cholesterol, LDL-cholesterol , VLDL-cholesterol, and triglycerides], glucose, C-reactive protein, complete blood count, platelet count, and comprehensive metabolic panel including liver and kidney function. Female subjects were also screened for pregnancy. Physical data (weight, height, blood pressure, and heart rate) and blood samples were obtained at the MUSC General Clinical Research Center (now Clinical and Translational Research Center), and the screening laboratory testing was performed at the clinical laboratories of MUSC Medical University Hospital. Subject characteristics are summarized in Table 1. Participating study subjects (n = 5/gender group) were asked to fast for 10 h overnight before collecting the fasting blood sample. Within 15 min of the fasting blood draw, subjects were provided with identical meals to be consumed within 30 min of first bite. The meal was composed of chicken, processed potatoes, and apple juice and contained 43% calories from fat as determined by a General Clinical Research Center nutritionist. The nonfasting blood sample was collected 2 h after the end of the meal.
TABLE 1.
Clinical profile of participating subjects
| Characteristic | Female | Male | Reference Range |
|---|---|---|---|
| (n) | 5 | 5 | |
| Age (years) | 26.0 ± 4.0 | 27.0 ± 2.0 | |
| Body mass index (BMI) (kg/m2) | 21.8 ± 3.0 | 21.8 ± 2.6 | |
| Weight (kg) | 59.9 ± 9.0 | 70.9 ± 9.0 | |
| Height (cm) | 165.5 ± 7.0 | 177.5 ± 9.0 | |
| Heart rate (bpm) | 74.4 ± 7.0 | 60.0 ± 14.0 | 60.0-90.0 |
| Blood pressure | |||
| Systolic (mmHg) | 110.0 ± 14.0 | 128.0 ± 19 | 100.0–119.0 |
| Diastolic (mmHg) | 66.0 ± 6.0 | 69.0 ± 9.0 | 60.0–79.0 |
| Lipid panel | |||
| Triglyceride (mg/dl) | 49.4 ± 16.0 | 50.8 ± 12.2 | ≤150.0 |
| Cholesterol (mg/dl) | 159.8 ± 28.0 | 146.8 ± 29.7 | ≤200.0 |
| HDL (mg/dl) | 53.8 ± 11.0 | 52.2 ± 8.6 | 40.0–59.0 |
| LDL (mg/dl) | 96.2 ± 20.0 | 84.2 ± 28.2 | ≤100.0 |
| VLDL (mg/dl) | 9.8 ± 3.1 | 10.4 ± 2.5 | ≤30.0 |
| Glucose (mg/dl) | 84.0 ± 7.2 | 93.0 ± 6.4 | 70.0–100.0 |
| C-reactive protein (mg/dl) | 0.25 ± 0.29 | 0.03 ± 0.01 | 0.000–1.000 |
| Comprehensive metabolic panel | |||
| Sodium (mmol/L) | 142.0 ± 3.0 | 140.0 ± 1.9 | 135.0–145.0 |
| Potassium (mmol/L) | 4.0 ± 1.0 | 4.0 ± 0.2 | 3.50–5.00 |
| Chloride (mmol/L) | 108.0 ± 2.0 | 105.0 ± 3.3 | 98.0–107.0 |
| CO2 content (mmol/L) | 28.0 ± 1.0 | 29.0 ± 2.4 | 22.0–32.0 |
| Anion gap (mmol/L) | 6.0 ± 2.0 | 6.0 ± 1.6 | 2.0–11.0 |
| Blood urea nitrogen (mg/dl) | 12.0 ± 5.0 | 16.0 ± 2.8 | 8.0–20.0 |
| Creatinine (mg/dl) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.6–1.3 |
| Calcium (mg/dl) | 9.0 ± 0.3 | 10.0 ± 0.3 | 8.4–10.2 |
| Bilirubin total (mg/dl) | 1.0 ± 0.3 | 2.0 ± 1.2 | 0.2–1.3 |
| Aspartate aminotransferase (IU/L) | 21.0 ± 4.2 | 27.0 ± 8.6 | 12.0–38.0 |
| Alanine aminotransferase (IU/L) | 16.0 ± 2.3 | 24.0 ± 8.5 | 10.0–45.0 |
| Alkaline phosphatase (IU/L) | 44.0 ± 11.7 | 56.0 ± 13.0 | 25.0–100.0 |
| Protein total (g/dl) | 6.0 ± 0.4 | 7.0 ± 0.5 | 6.0–8.0 |
| Albumin (g/dl) | 4.0 ± 0.3 | 4.0 ± 0.3 | 3.5–4.8 |
| Complete blood count | |||
| Mean platelet volume (fl) | 10.56 ± 0.9 | 10.80 ± 1.2 | 9.15–12.31 |
| White blood cells (103/µl) | 5.05 ± 0.5 | 4.20 ± 0.8 | 4.80–10.80 |
| RBC (106/µl) | 4.19 ± 0.3 | 5.10 ± 0.3 | 4.70–6.10 |
| Hemoglobin (gm/dl) | 12.44 ± 0.9 | 15.00 ± 0.8 | 14.0–18.0 |
| Hematocrit (%) | 37.24 ± 1.9 | 44.40 ± 2.7 | 42.0–52.0 |
| Mean corpuscular volume (fl) | 88.94 ± 5.1 | 87.30 ± 2.5 | 80.0–94.0 |
| Mean corpuscular hemoglobin (pg/cell) | 29.72 ± 2.3 | 29.60 ± 1.6 | 27.0–31.0 |
| Mean corpuscular hemoglobin concentration (gm/dl) | 33.38 ± 0.8 | 39.90 ± 1.7 | 30.7–34.4 |
| RBC distribution width (%) | 12.04 ± 0.6 | 12.90 ± 0.6 | 11.5–14.5 |
| Platelets (103/µl) | 236.0 ± 50.6 | 202.2 ± 31.5 | 140.0–440.0 |
Values are mean ± SD.
Blood sample collection
For serum samples, blood was collected in BD Vacutainer® glass serum tubes (#366430, red closure) (Becton Dickinson, Franklin Lakes, NJ) silicone coated with no additives. For plasma samples, blood was collected in BD Vacutainer® glass plasma tubes with either sodium heparin (#366480, green closure) or sodium citrate (#366415, blue closure) as anticoagulant. Blood was also collected in Monoject™ lavender stopper collection tubes (#8881311743) (COVIDIEN, Mansfield, MA) with EDTA as anticoagulant. With plasma collection, the need to properly fill the tubes during collection was important, because the anticoagulants were calibrated to provide the optimum blood-anticoagulant ratio. Therefore, the volume of blood collected in each tube and adequate mixing of the sample into the additive were critical (25). For serum samples, blood tubes were kept at room temperature for 20 min, allowing the coagulated blood to clot, and then centrifuged at 750 g for 15 min to yield the serum. For plasma samples, blood tubes were immediately placed on ice for approximately 5 min before they were centrifuged at 750 g for 15 min. After serum and plasma samples were separated, they were aliquoted into 0.5 ml aliquots and stored at −80°C until lipid extraction and analysis.
Lipid extraction
All solvents were analytical grade from Burdick and Jackson (Muskegon, MI) unless otherwise indicated. Sphingolipid standards were from MUSC Lipidomics Shared Resource or from a commercially available source (Avanti Polar Lipids, Alabaster, AL; and Matreya LLC, Pleasant Gap, PA), with purity of ≥98%.
The samples were removed from −80°C just before extraction and allowed to thaw at 4°C for 2 h. Duplicate serum and plasma samples (100 µL each), including those from freeze and thaw cycles, were extracted for analysis. Cellgro® RPMI 1640 culture media (Mediatech Inc., VA) (1900 µL) was first added to each sample in borosilicate glass tubes with screw caps (8 ml, 13 × 100 mm). The samples were also fortified with 50 µL of appropriate internal standards (1,000 pmol/ml each in methanol, 5,000 pmol/ml for SM). The samples were then extracted with 2 ml extraction solution consisting of isopropanol and ethyl acetate (15:85 v/v) using vortexing and centrifugation for 5 min at 3,000 rpm (2,500 g) on a Beckman Allegra 6R Centrifuge (Beckman Coulter, Brea, CA). The upper organic phase was then transferred to an 8 ml glass tube. The remaining diluted plasma or serum was then acidified with 100 μl formic acid (98%), and an additional 2 ml of extraction solution was added to further facilitate completion of extraction. The samples were then vortexed and centrifuged for 5 min at 3,000 rpm (2,500 g). The upper organic phase was then transferred and added to the glass tube containing the initial extract (total 4 ml extract) and mixed by vortexing. One milliliter extract was reserved for SM determination, and the remaining extract (3 ml) was evaporated to dryness with an N-Evap™ 112 Nitrogen Evaporator (Organomation Associates, Berlin, MA). The dried residues were reconstituted in 150 µL HPLC mobile phase B (see composition of mobile phases below). The reconstituted samples were then transferred to HPLC auto-sampler screw-cap vials with 200 µL insert. Samples in HPLC auto-sampler vials were placed temporarily at 4°C until ready for injection on the HPLC/MS/MS system
The 1 ml extract reserved for SM determination was evaporated to dryness under nitrogen. The dried extracts were reconstituted in 1 ml methanol and 20 µL of 1N NaOH (Sigma-Aldrich, St. Louis, MO) in methanol and vortexed. The samples were allowed to undergo mild alkaline hydrolysis at room temperature for 3 h. After hydrolysis, the following solvents were added sequentially to complete the extraction: chloroform (1 ml), methanol (1 ml), 1N NaCl (Sigma-Aldrich) (0.8 ml), chloroform (1 ml), and 1N NaCl (1 ml). Samples were vortexed after the addition of each solvent and then centrifuged for 5 min at 3,000 rpm (2,500 g). The upper (aqueous) phase was aspirated including any inter-phase observed, then the lower (organic) phase was evaporated to dryness under nitrogen. The dried residues were reconstituted in HPLC mobile phase B (150 µL) and transferred to HPLC auto-sampler vials. Samples in HPLC auto-sampler vials were temporarily placed at 4°C until ready for injection on the HPLC/MS/MS system.
Sphingolipid analyses by HPLC-MS/MS
Analyses of sphingolipids were performed by HPLC-MS/MS at MUSC Lipidomics Shared Resource. The equipment consisted of a Thermo Scientific Accela Autosampler and Quaternary Pump (Waltham, MA) coupled to a Thermo Scientific Quantum Access triple quadrupole mass spectrometer equipped with an ESI operating in multiple reaction monitoring positive ion mode. Chromatographic separations were obtained under a gradient elution, using mobile phase A consisting of 2 mM ammonium formate in 0.2% formic acid in water, and mobile phase B consisted of 1 mM ammonium formate in 0.2% formic acid in methanol, on a Peeke Scientific (Redwood City, CA) Spectra C8SR 150 × 3.0 mm 3 µm particle size column (24).
Sphingolipids analyzed included sphingoid bases (C18:1, C18:0); Sph and dhSph, their 1-phosphates (S1P and dhS1P), and Cn- of Cer, SM, HexCer, LacCer, and Cer1P. Quantitative analyses were based on eight-point calibration curves generated for each target analyte ranging from 2.5 to 400 pmol. Synthetic standards along with a set of internal standards (50 pmol each) were added to serum and plasma samples before extraction. The extracted standards were also analyzed by the HPLC-MS/MS system operating in positive multiple reaction monitoring mode employing a gradient elution. The percent recovery was 80–120% of internal standard added to analyzed samples. The analyte concentration was determined by plotting the analyte-internal standard peak area ratio on the analyte specific linear regression. Sphingolipids with no available standards were quantified using the calibration curve of its closest counterpart. A listing of the internal standards and sphingolipids with available calibration standards are provided in supplementary Data. The resulting data was then normalized to the volume of sample used.
Exposure of serum and plasma samples to freeze and thaw cycles
Fasting male serum and plasma EDTA samples were chosen to examine the effect of freeze and thaw cycles. Lipid extractions of serum and plasma samples exposed to freeze and thaw cycles were performed over three consecutive days. The samples for the first and second thaw cycle, T1 and T2, were done by removing the set of samples from −80°C and placing them at room temperature for 3 h prior to extraction. The third thaw cycle, T3, was performed by placing the samples at 4°C overnight after removing them from the −80°C freezer.
Preparation of lipoproteins
Blood was collected after a 12 h fast from healthy subjects who were normolipemic, not receiving prescription medication for any acute or chronic condition, and without family history of coronary artery disease, peripheral vascular disease, or stroke. None of the subjects were receiving antioxidant therapy. Each participant provided written informed consent, and the experimental protocol was approved by the MUSC institutional review board. The blood was pooled from 3 or 4 donors in the presence of a lipoprotein preservative cocktail consisting of EDTA (0.1% w/v), chloramphenicol (20 µg/ml), gentamycin sulfate (50 µg/ml), epsilon amino-caproic acid (0.13% w/v), and dithiobisnitrobenzoic acid (0.04% w/v) to inhibit the activity of lecithin-cholesterol acyltransferase, a major enzyme on HDL and LDL that converts free cholesterol into cholesteryl ester. Blood was then centrifuged and plasma was obtained.
VLDL (1.006<d<1.019 g/ml) were isolated from plasma with density adjusted with solid KBr by preparative ultracentrifugation at 50,000 rpm (200,000 g) for 18 h, 4°C, on a Beckman OptimaTM XL-100K ultracentrifuge, using a Ti70 rotor (Beckman Coulter). LDL (1.019<d<1.063 g/ml) was isolated after density adjustment with KBr by ultracentrifugation at 50,000 rpm for 18 h, 4°C. HDL2 (1.063<d<1.125 g/ml) and HDL3 (1.125<d<1.21 g/ml) were isolated by sequential ultracentrifugation of plasma with density adjusted with solid KBr in a Ti70 rotor spun at 70,000 rpm for 18 h at 4°C. The floating HDL subfractions were harvested after tube slicing, and each isolated HDL subfraction was washed and concentrated by ultracentrifugation at its isolation density in a SW41 rotor (Beckman Coulter) [41,000 rpm (207,000 g), 36 h, 4°C]. HDL subfractions were dialyzed against saline/EDTA (150 mM NaCl, 300 µM EDTA, pH 7.4), sterilized by filtering through a 0.22 µm membrane, and stored at 4°C until used. Protein levels in lipoprotein preparations were measured by the Lowry method.
Equal amounts (100 µg protein) of VLDL, LDL, and HDL subclasses (HDL2 and HDL3) were extracted and analyzed using HPLC-MS/MS as described above. The analysis of the Sph, dhSph, S1P, dhS1P, Cn-Cer, Cn-dhCer, and Cn-SM content of each lipoprotein was performed. To calculate the sphingolipid concentration per lipoprotein particle (nmol/nmol), we used the molecular weight of 10.0 × 106, 2.0 × 106, 4.0 × 105, and 2.0 × 105 for VLDL, LDL, HDL2, and HDL3, respectively, and the percentage protein composition in the lipoprotein particle as 10, 20, 40, and 55% for VLDL, LDL, HDL2, and HDL3, respectively (26–28). The reference range of lipoprotein particle concentration in plasma (nmol/ml) was as reported by Jeyarajah et al. (29). Because this reference range includes three subclasses of the HDL particle (small, medium, and large), the values for the medium HDL subclass were divided equally between the small and large HDL subclasses representing HDL3 and HDL2 subclasses, respectively.
Statistics
Significant differences between two groups were evaluated by Student's t-test and between more than two groups by one-way or two-way ANOVA followed by Tukey post hoc test for mean separation, or multiple comparisons versus one group test (Holm-Sidak method) (P < 0.05). Data are expressed as mean ± SD, except for sphingolipids in lipoprotein classes from pooled plasma samples data, which are presented as mean ± SE.
RESULTS
SM is the dominant circulating sphingolipid
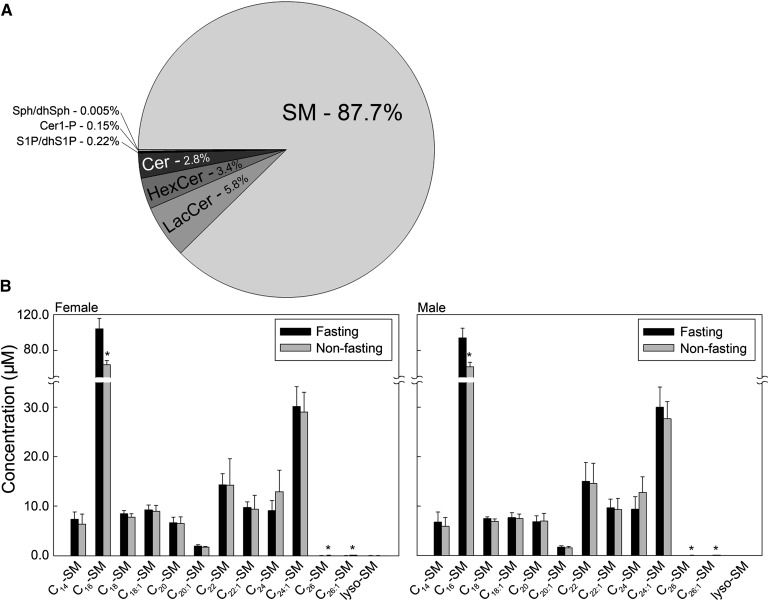
Compositional analysis of total sphingolipid content showed that SM was the dominant sphingolipid in serum and plasma, similarly in both males and females. Figure 1A and Table 2 show the average distribution of sphingolipids among all samples analyzed. Mean sphingolipid composition in serum and plasma was as follows: SM (87.7%) followed by LacCer (5.8%), HexCer (3.4%), Cer (2.8%), S1P and dhS1P (0.22%), Cer1P (0.15%), and Sph and dhSph (0.005%). The most prominent SM species was C16-SM, which was significantly higher in the serum of fasting compared with nonfasting subjects (100.0 µM vs. 64.0 µM) (Fig. 1B). The next most abundant SM was C24:1-SM (30 µM); however, C24:1-SM did not indicate the same response to fasting conditions as noted with C16-SM. Data displayed in Fig. 1B also correspond to the distribution of SM species in plasma prepared with the different anticoagulants, for which the data are not shown.
Fig. 1.
SM is the dominant circulating sphingolipid. Each target analyte (SM species) was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD, values for each serum sample are the average of duplicates extracted separately (100 µL each). Statistical differences detected between fasting and nonfasting groups are indicated with an asterisk (n = 5 for each group, P < 0.05).
TABLE 2.
Percentage of sphingolipid classes to total sphingolipid content
| Sphingolipids |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| SM | LacCer | HexCer | Cer | S1P/dhSph | Sph/dhSph | Cer1P | |||
| Male (n = 5) | Nonfasting | Serum | 85.3 ± 1.2 | 6.9 ± 1.4 | 3.8 ± 0.9 | 3.6 ± 0.9 | 0.40 ± 0.12 | 0.005 ± 0.001 | 0.08 ± 0.02 |
| Plasma EDTA | 85.7 ± 1.5 | 6.8 ± 1.2 | 3.8 ± 1.1 | 3.2 ± 0.7 | 0.21 ± 0.05* | 0.005 ± 0.001 | 0.24 ± 0.10* | ||
| Plasma citrate | 87.6 ± 1.2* | 5.6 ± 0.9 | 3.6 ± 0.9 | 2.9 ± 0.6 | 0.18 ± 0.03* | 0.005 ± 0.001 | 0.08 ± 0.04 | ||
| Plasma heparin | 86.8 ± 1.9 | 6.1 ± 1.2 | 3.6 ± 1.1 | 3.2 ± 0.6 | 0.20 ± 0.04* | 0.006 ± 0.000* | 0.06 ± 0.01* | ||
| Fasting | Serum | 88.5 ± 0.9† | 5.0 ± 0.5† | 3.2 ± 0.7 | 2.7 ± 0.5 | 0.38 ± 0.09 | 0.004 ± 0.001 | 0.18 ± 0.02† | |
| Plasma EDTA | 88.6 ± 1.0† | 5.1 ± 0.7† | 3.3 ± 0.7 | 2.7 ± 0.6 | 0.18 ± 0.04* | 0.004 ± 0.001 | 0.24 ± 0.01* | ||
| Plasma citrate | 89.8 ± 1.1† | 4.2 ± 0.6† | 3.4 ± 0.7 | 2.3 ± 0.5 | 0.15 ± 0.03* | 0.005 ± 0.001 | 0.14 ± 0.03† | ||
| Plasma heparin | 87.7 ± 1.3 | 4.9 ± 0.5 | 4.4 ± 1.0 | 2.7 ± 0.5 | 0.19 ± 0.02* | 0.006 ± 0.003 | 0.11 ± 0.02*† | ||
| Female (n = 5) | Nonfasting | Serum | 84.1 ± 1.7 | 8.6 ± 1.0 | 3.5 ± 0.3 | 3.2 ± 0.8 | 0.43 ± 0.13 | 0.004 ± 0.001 | 0.17 ± 0.04‡ |
| Plasma EDTA | 88.4 ± 1.6*‡ | 5.8 ± 1.2* | 2.9 ± 0.3* | 2.5 ± 0.6 | 0.20 ± 0.05* | 0.005 ± 0.001 | 0.18 ± 0.05 | ||
| Plasma citrate | 87.4 ± 1.4* | 6.4 ± 1.2* | 3.2 ± 0.5 | 2.7 ± 0.7 | 0.14 ± 0.01*‡ | 0.005 ± 0.001 | 0.07 ± 0.03* | ||
| Plasma heparin | 86.2 ± 2.2 | 7.4 ± 1.0 | 3.1 ± 0.7 | 2.9 ± 1.0 | 0.17 ± 0.03* | 0.006 ± 0.003 | 0.08 ± 0.02*‡ | ||
| Fasting | Serum | 88.6 ± 1.1† | 5.9 ± 1.2† | 2.8 ± 0.3† | 2.2 ± 0.5† | 0.29 ± 0.06† | 0.004 ± 0.001 | 0.23 ± 0.03†‡ | |
| Plasma EDTA | 89.8 ± 1.5 | 4.5 ± 0.8† | 2.7 ± 0.2 | 2.5 ± 0.6 | 0.14 ± 0.01*† | 0.004 ± 0.001 | 0.26 ± 0.03† | ||
| Plasma citrate | 89.3 ± 2.0 | 4.9 ± 1.3 | 3.4 ± 0.5* | 2.1 ± 0.4 | 0.13 ± 0.02* | 0.005 ± 0.001 | 0.15 ± 0.03*† | ||
| Plasma heparin | 88.7 ± 0.7† | 5.2 ± 0.4† | 3.4 ± 0.4* | 2.4 ± 0.7 | 0.15 ± 0.00*‡ | 0.005 ± 0.001 | 0.13 ± 0.04*† | ||
Values are mean ± SD. * Significantly different from serum within each fasting or nonfasting group, P < 0.05. † Significantly different from corresponding nonfasting serum, plasma-EDTA, -citrate, or -heparin within each gender group, P < 0.05. ‡ Significantly different from corresponding male fasting or nonfasting serum, plasma-EDTA, -citrate, or -heparin, P < 0.05.
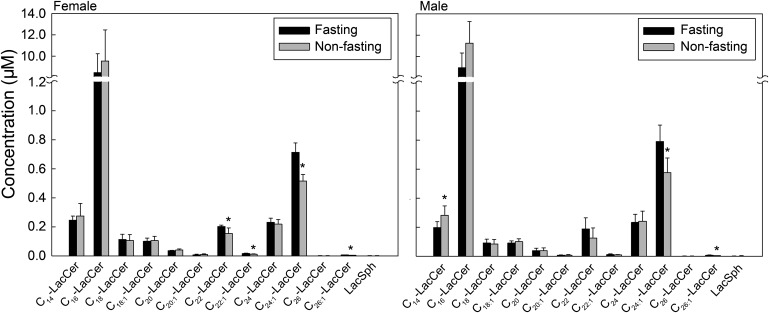
C16-LacCer is the dominant molecular species of LacCer
The next highest to SM was LacCer, but it was 15-fold less abundant than SM in serum and plasma (Fig. 1A). The most prominent LacCer species measured in both female and male subjects was C16-LacCer (11.0 µM) (Fig. 2). Among females, nonfasting subjects displayed significantly lower levels of long-chain LacCers (C22-LacCer, C22:1-LacCer, C24:1-LacCer, and C26:1-LacCer) compared with fasting subjects. Nonfasting males showed the same trend in long-chain LacCer levels as females but had statistically significant lower levels of only C24:1-LacCer and C26:1-LacCer than fasting males. Conversely, the short-chain C14-LacCer was significantly higher in nonfasting than fasting males, with the same trend in females (Fig. 2). Data displayed in Fig. 2 are for plasma prepared with EDTA anticoagulant and are representative for serum and plasma prepared with other anticoagulants.
Fig. 2.
C16-LacCer is the dominant molecular species of LacCer. Each target LacCer species was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD, values for each plasma EDTA sample are the average of duplicates extracted separately (100 µL each). Statistical differences detected between fasting and nonfasting groups are indicated with an asterisk (n = 5 for each group, P < 0.05).
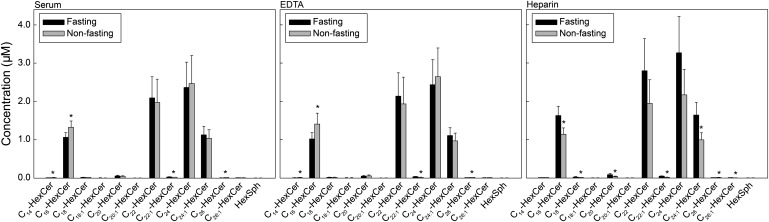
Heparin reveals higher variability in levels of molecular species of HexCer than other anticoagulants
We then analyzed the molecular species of HexCer, which comprise both isomeric glucosyl- and galactosyl-Cers. Figure 3 shows the HexCer species profile in serum and plasma of fasting and nonfasting male subjects. The highest HexCer species in both serum and plasma was C24-HexCer (2.5 µM), with no significant difference between fasting and nonfasting subjects. C16-HexCer was significantly higher (15%) in nonfasting than fasting subjects in both serum and plasma prepared using EDTA. However, when heparin was used as an anticoagulant, C16-HexCer was significantly lower in the plasma of nonfasting than fasting subjects (Fig. 3). Data displayed in Fig. 3 also correspond to the distribution of HexCer species in serum and plasma from female subjects (data not shown).
Fig. 3.
Heparin reveals higher variability in levels of molecular species of HexCer than other anticoagulants. Each target HexCer species was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD, values for each male donor are the average of duplicates extracted separately (100 µL each). Statistical differences detected between fasting and nonfasting groups are indicated with an asterisk (n = 5 for each group, P < 0.05).
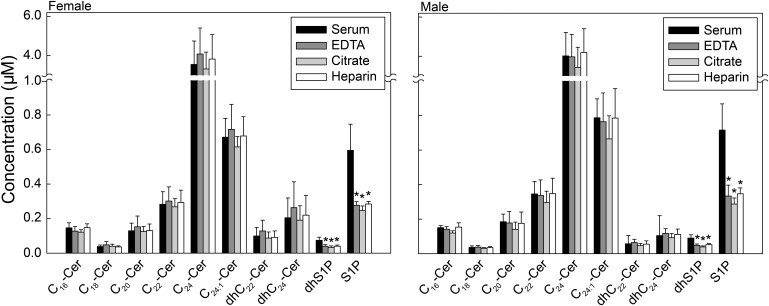
Levels of Cn-Cer are similar in plasma and serum
We then analyzed the molecular species of Cer, Sph and dhSph, and S1P and dhS1P under fasting conditions. As shown in Fig. 4, the dominant Cer species was C24-Cer (4.0 µM), with no significant differences in levels of Cer species between serum and plasma preparations (with EDTA, citrate, or heparin).
Fig. 4.
Levels of Cn-Cer are similar in plasma and serum and levels of S1P and dhS1P are higher in serum than plasma. Each target analyte (S1P and Cer species) was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD. Values for each fasting donor are the average of duplicates extracted separately (100 µL each). Statistical differences detected between each plasma group and serum are indicated with an asterisk (n = 5 for each group, P < 0.05).
Levels of S1P and dhS1P are higher in serum than plasma
As shown in Fig. 4, serum S1P levels were significantly higher than plasma S1P levels (0.68 µM vs. 0.31 µM, respectively). The higher levels for S1P in serum were mainly a result of platelet-activated release of S1P into the medium during clotting (30). Serum dhS1P levels were also significantly higher than plasma dhS1P levels (0.09 µM vs. 0.04 µM, respectively). There were no significant differences in Cer species or sphingoid bases and their 1-phosphates between male and female subjects (Fig. 4).
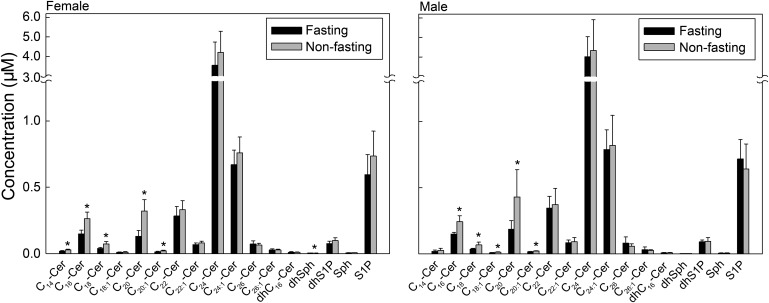
Levels of short- and medium-chain Cers but not C24-Cer decrease in response to fasting
We then examined the effect of fasting on the levels of Cer species and sphingoid bases and their 1-phosphates. There was no effect of fasting on the levels of the dominant C24-Cer in both males and females (Fig. 5). However, levels of short- and medium-chain Cers (C14-, C16-, C18- and C20-Cer) were lower under fasting than nonfasting conditions in both males and females. There were no significant differences in levels of sphingoid bases and S1P between fasting and nonfasting subjects (Fig. 5).
Fig. 5.
Levels of short- and medium-chain Cers but not C24-Cer decrease in response to fasting. Each target analyte (Sph, S1P, and Cer species) was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD, values for each serum sample are the average of duplicates extracted separately (100 µL each). Statistical differences detected between fasting and nonfasting groups are indicated with an asterisk (n = 5 for each group, P < 0.05).
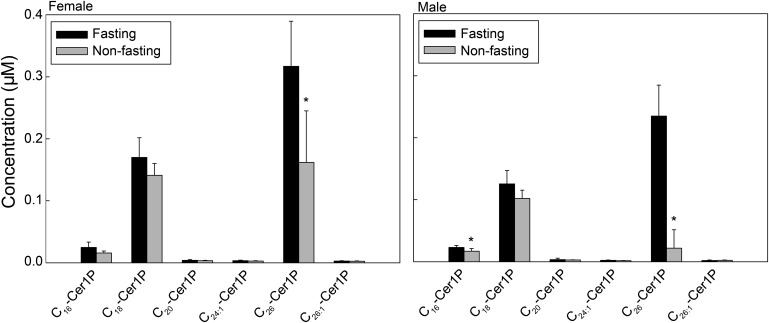
Levels of C26-Cer1P increase in response to fasting
Among the Cer1P species, C18-Cer1P and C26-Cer1P were the most abundant in both males and females (Fig. 6). Whereas fasting serum C26-Cer1P levels were lower than nonfasting levels, levels of C18-Cer1P did not change in response to fasting (Fig. 6). Data displayed in Fig. 6 are for serum; however, data are also representative for all plasma preparations.
Fig. 6.
Levels of C26-Cer1P increase in response to fasting. Each target Cer1P species was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD, values for each serum sample are the average of duplicates extracted separately (100 µL each). Statistical differences detected between fasting and nonfasting groups are indicated with an asterisk (n = 5 for each group, P < 0.05).
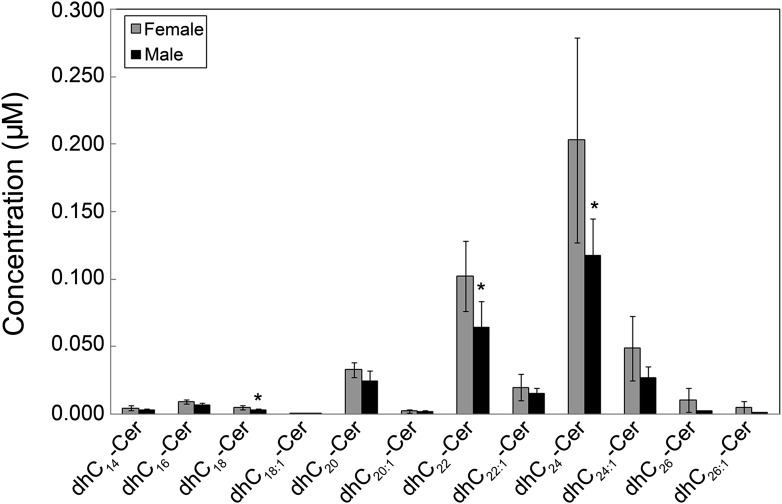
Gender differences in sphingolipids
The data showed gender differences in levels of serum and plasma sphingolipids. As shown in Table 3, higher levels of certain sphingolipids were observed in females than males. These sphingolipids included C18-SM, C18:1-SM, C18-Cer1P, and total dhCers. Figure 7 shows that levels of C18-, C22-, and C24-dhCer were significantly higher in females than males, with other dhCer species showing a trend of higher concentrations in females than males. Higher levels of HDL particle concentrations in females compared with males (29) could account at least in part for the higher sphingolipid levels in females.
TABLE 3.
Gender differences in levels of serum sphingolipids
| Sphingolipid (µM) | Male (n = 5) | Female (n = 5)* |
|---|---|---|
| C18-SM | 7.50 ± 0.32 | 8.47 ± 0.62 |
| C18:1-SM | 7.67 ± 0.99 | 9.28 ± 0.94 |
| C18-Cer1P | 0.126 ± 0.022 | 0.170 ± 0.032 |
| dhCers | 0.267 ± 0.064 | 0.444 ± 0.136 |
Values are mean ± SD. *Significantly different from male values under fasting conditions, P < 0.05.
Fig. 7.
Levels of Cn-dhCer are higher in females than males. Each target dhCer species was analyzed by reversed-phase HPLC-MS/MS and identified by its specific parent-daughter ion mass transition and retention time. Data are expressed as means ± SD, values for each serum sample under fasting conditions are the average of duplicates extracted separately (100 µL each). Statistical differences detected between males and females are indicated with an asterisk (n = 5 for each group, P < 0.05).
Effect of type of anticoagulant on sphingolipid levels
We analyzed the data using ANOVA to specifically test the effect of anticoagulants (EDTA, citrate, and heparin) compared with the absence of anticoagulant (serum) on sphingolipid levels in collected human blood. As shown in Table 4, EDTA generated the least variability from serum in sphingolipid levels compared with citrate and heparin. We also tested the effect of heparin and citrate compared with EDTA, which is the most commonly used anticoagulant in clinical settings. As shown in Table 4, the anticoagulants heparin and citrate had a tendency to vary recovery levels of sphingolipids compared with EDTA. This was particularly obvious when heparin revealed higher variability in levels of molecular species of HexCer than other anticoagulants (Fig. 3, Table 4). Furthermore, the effect of heparin on levels of LacCer, S1P, and dhS1P was statistically different from that of citrate (Table 4). As shown in Table 4 and also in Fig. 4, levels of S1P and dhS1P were higher in serum than plasma regardless to anticoagulant type.
TABLE 4.
Effect of type of anticoagulant on sphingolipid levels
| Sphingolipid (µM) | Serum | EDTA | Citrate | Heparin |
|---|---|---|---|---|
| SM | 194.88 ± 16.55 | 193.39 ± 18.97 | 176.90 ± 15.36 | 192.56 ± 15.44 |
| LacCer | 12.004 ± 2.143 | 10.381 ± 1.595* | 9.013 ± 1.934* | 11.023 ± 1.067‡ |
| HexCer | 6.567 ± 0.966 | 6.481 ± 1.259 | 6.724 ± 1.148 | 8.477 ± 1.920*†‡ |
| Cer | 5.400 ± 1.343 | 5.670 ± 1.447 | 4.334 ± 0.966† | 5.598 ± 1.480 |
| Sphingosine | 0.005 ± 0.001 | 0.006 ± 0.002 | 0.006 ± 0.001 | 0.008 ± 0.002* |
| dhSph | 0.003 ± 0.001 | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 |
| S1P | 0.655 ± 0.154 | 0.305 ± 0.054* | 0.242 ± 0.034*† | 0.315 ± 0.042*‡ |
| dhS1P | 0.082 ± 0.018 | 0.044 ± 0.009* | 0.033 ± 0.007*† | 0.046 ± 0.010*‡ |
| Cer1P | 0.456 ± 0.087 | 0.539 ± 0.055 | 0.290 ± 0.056*† | 0.262 ± 0.074*† |
| dhCer | 0.332 ± 0.175 | 0.406 ± 0.228 | 0.268 ± 0.128 | 0.338 ± 0.160 |
Values are mean ± SD, n = 10 combined males and females under fasting conditions. * Anticoagulant statistically different from serum. † Anticoagulant statistically different from EDTA. ‡ Heparin statistically different from citrate.
The effect of freeze and thaw cycles on alterations of sphingolipid levels
Biological samples including human serum and plasma are typically collected, stored frozen, and then thawed several times to perform analysis of various parameters. Therefore, we examined the effect of freeze and thaw cycles on the stability of sphingolipids in serum and plasma. Serum and plasma EDTA samples from fasting male subjects were used to test the effect of freeze and thaw cycles. There were no statistical differences in sphingolipid levels after the first thaw cycle (T1) (data not shown). As shown in Table 5, however, persistent freeze and thaw cycles affected recoveries of certain sphingolipids. As a result of freeze and thaw cycles, inconsistent recoveries of internal standards could also occur. For example, the variability in C16-Cer after the second cycle (T2) shown in Table 5 was a result of lower internal standard recovery, thus giving false elevated levels for C16-Cer recoveries. Most alterations of sphingolipid levels occurred in plasma EDTA samples after the third freeze and thaw cycles (T3).
TABLE 5.
Effect of freeze and thaw cycles on alterations of sphingolipid levels
| Serum |
Plasma EDTA |
|||
|---|---|---|---|---|
| Sphingolipid | T2 | T3 | T2 | T3 |
| C16-SM | P = 0.0384 | P = 0.0463 | ||
| C16-Cer | P = 0.0011 | |||
| Sphingosine | P = 0.0007 | |||
| dhSph | P = 0.0103 | P = 0.0282 | ||
| C18-Cer1P | P = 0.0153 | |||
| C18:1-Cer1P | P = 0.0038 | |||
| C26-Cer1P | P = 0.0016 | |||
| C16-LacCer | P = 0.0434 | |||
| C18:1-LacCer | P = 0.0377 | P = 0.0265 | ||
| C16-HexCer | P = 0.0322 | |||
| C22:1-HexCer | P = 0.0492 | |||
| Hexosylsphingosine | P = 0.0049 | |||
No P-value indicates absence of statistical difference from originally thawed sample (T0); no statistical differences in sphingolipid levels after T1.
Sphingolipid levels in lipoprotein particles
Mass spectrometric analysis was also used to determine sphingolipid composition of isolated lipoproteins VLDL, LDL, and subclasses of HDL, HDL2 and HDL3. The concentrations of SM, Cer, and S1P, and the ratios of SM:Cer, SM:S1P, and Cer:S1P in each lipoprotein particle are shown in Table 6. Among the lipoprotein particles analyzed, the VLDL particle contained the highest levels of SM, Cer, and S1P. The smallest lipoprotein particle, HDL3, contained the lowest levels of SM and Cer, but its S1P levels were higher than both HDL2 and LDL particles. As also shown in Table 6, HDL2 and HDL3 particles had similar SM:Cer ratios (72.9% and 78.9%, respectively) despite the difference in their particle size (8.5–13 and 7.3–8.5 nm, respectively). The SM:Cer ratio in the LDL particle (18–27 nm) was almost 2-fold higher than the VLDL particle (27 to >60 nm), mainly due to the lower content of Cer in the LDL particle (Table 6).
TABLE 6.
Levels of SM, Cer, and S1P in human lipoprotein particles
| Sphingolipid |
Ratio |
|||||
|---|---|---|---|---|---|---|
| Lipoprotein Classa | SM (nmol/nmol) | Cer (nmol/nmol) | S1P(nmol/nmol) | SM:Cer | S1P:SM | Cer:S1P |
| VLDL | 197.6 ± 23.30 | 8.86 ± 0.58 | 0.178 ± 0.032 | 22.3:1 | 1:1110 | 49.8:1 |
| LDL | 105.3 ± 10.87 | 2.07 ± 0.23 | 0.026 ± 0.009 | 50.9:1 | 1:4107 | 80.7:1 |
| HDL2 | 19.8 ± 1.50 | 0.27 ± 0.05 | 0.020 ± 0.002 | 72.9:1 | 1:977 | 13.4:1 |
| HDL3 | 6.55 ± 2.05 | 0.08 ± 0.02 | 0.037 ± 0.004 | 78.9:1 | 1:178 | 2.2:1 |
Values are mean ± SE, n = 3 pooled plasma samples, except for Cer and S1P in HDL2 and HDL3, n = 8 pooled plasma samples. Blood was pooled from 3 or 4 donors in the presence of EDTA, chloramphenicol, gentamycin sulfate, epsilon amino-caproic acid, and dithiobisnitrobenzoic acid.
To calculate molar values per lipoprotein particle, molecular weights for VLDL, LDL, HDL2, and HDL3 are 10.0 × 106, 2.0 × 106, 4.0 × 105, and 2.0 × 105, respectively; percentages of protein composition in the lipoprotein particles are 10, 20, 40, and 55% for VLDL, LDL, HDL2, and HDL3, respectively (26–28).
Levels of sphingolipids carried on lipoprotein particles in human plasma are shown in Table 7. Interestingly, 78.6% of lipoprotein-associated S1P was carried on HDL3 particles. Moreover, HDL3 particles were the major carriers of dhS1P and Sph, with 63.5% and 47.9% of total lipoprotein-associated Sph and dhS1P, respectively. The major carrier of Cer and dhSph was LDL, with 39.9% and 40.6% of total lipoprotein-associated Cer and dhSph, respectively (Table 7). The lipoprotein contributing the least to plasma sphingolipids was VLDL, mainly because its particle concentration in plasma is the lowest among lipoproteins (Table 7).
TABLE 7.
Levels of SM, Cer, Sph, dhSph, S1P, and dhS1P in human lipoproteins and plasma
| SM |
Cer |
Sph |
dhSph |
S1P |
dhS1P |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipoprotein Classa | nmol/ml | % | nmol/ml | % | pmol/ml | % | pmol/ml | % | pmol/ml | % | pmol/ml | % | Particle Concentration (nmol/ml)(29) |
| VLDL | 15.3 | 3.15 | 0.685 | 8.73 | 2.32 | 2.30 | 1.62 | 2.72 | 13.8 | 1.33 | 1.62 | 4.94 | 0.073 ± 0.06 |
| LDL | 159.4 | 32.85 | 3.134 | 39.94 | 32.39 | 32.06 | 24.17 | 40.63 | 38.8 | 3.73 | 4.46 | 13.59 | 1.514 ± 0.48 |
| HDL2 | 165.5 | 34.10 | 2.255 | 28.74 | 17.89 | 17.71 | 13.39 | 22.51 | 169.5 | 16.30 | 5.87 | 17.89 | 8.350 ± 4.60 |
| HDL3 | 145.1 | 29.90 | 1.772 | 22.59 | 48.42 | 47.93 | 20.31 | 34.14 | 817.6 | 78.64 | 20.86 | 63.58 | 22.10 ± 5.70 |
| Total | 485.3 | 100.0 | 7.846 | 100.0 | 101.02 | 100.0 | 59.49 | 100.0 | 1039.7 | 100.0 | 32.81 | 100.00 | |
Values are mean ± SE, n = 3 pooled plasma samples, except for Cer, Sph, dhSph, S1P, and dhS1P in HDL2 and HDL3, n = 8 pooled plasma samples. Blood was pooled from 3 or 4 donors in the presence of EDTA, chloramphenicol, gentamycin sulfate, epsilon amino-caproic acid, and dithiobisnitrobenzoic acid.
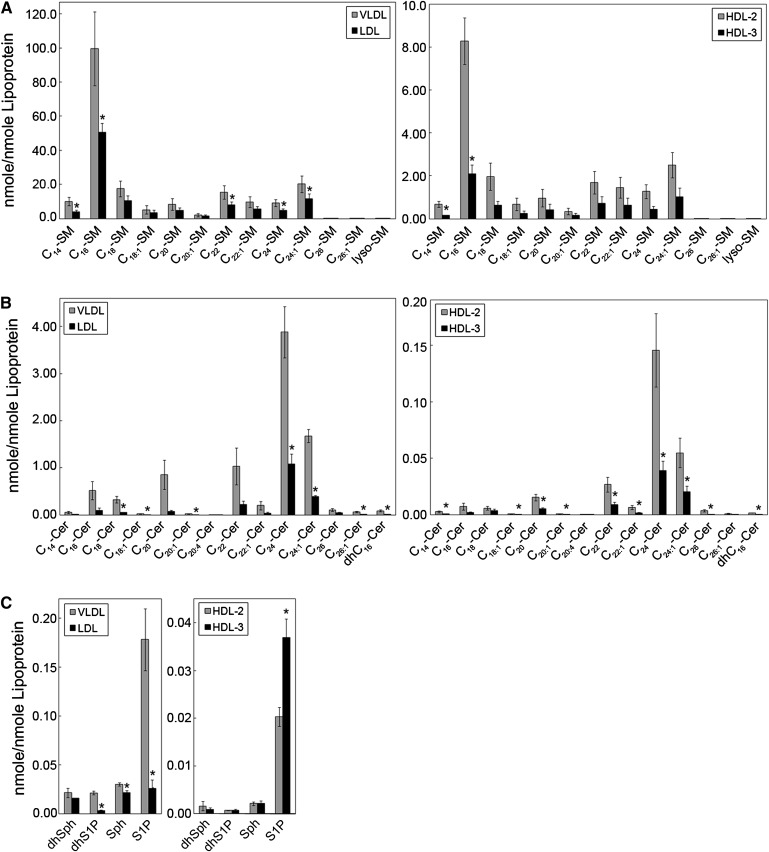
The results of the analysis of the molecular species of SM, Cer, and Sph and dhSph and their phosphates in lipoprotein particles are shown in Fig. 8. The levels of SM species in VLDL were higher than LDL (left panel), and levels of SM species in HDL2 were higher than HDL3 (right panel). Similarly, levels of Cer species in VLDL were higher than LDL (Fig. 8B, left panel), and levels of Cer species in HDL2 were higher than HDL3 (Fig. 8B, right panel). Whereas the most abundant SM species in all lipoprotein classes was C16-SM (Fig. 8A), C24-Cer was the Cer with the highest levels among Cer species in lipoproteins, followed by C24:1-Cer, C22-Cer, C20-Cer, C16-Cer, and C18-Cer (Fig. 8B). As shown in Fig. 8C, VLDL particles contained significantly higher levels of Sph, S1P, and dhS1P than LDL particles. Furthermore, levels of S1P, but not Sph, dhSph, or dhS1P, were significantly higher in the HDL3 than in the HDL2 particle.
Fig. 8.
Sphingolipid levels in lipoprotein particles. Blood was collected after 12 h of fasting from healthy normolipemic subjects and equal amount (100 µg) of VLDL, LDL, and HDL subclasses (HDL2 and HDL3) was extracted and analyzed using HPLC/MS/MS as described in “Methods.” A: Levels of molecular species of SM. B: Levels of molecular species of Cer. C: Levels of Sph, dhSph, S1P, and dhS1P. Levels of sphingolipid species were calculated per nmol lipoprotein as described in “Methods.” n = 3 pooled plasma samples, except for Cer, Sph, dhSph, S1P, and dhS1P in HDL2 and HDL3, n = 8 pooled plasma samples. Data are expressed as mean ± SE.
DISCUSSION
The major goal of this study was to establish a profile of sphingolipids in “normal” human serum and plasma prepared with different anticoagulants and determine a reference range for circulating sphingolipid species to be used for future translational research and clinical practices detecting disease biomarkers in both men and women. The structural diversity of sphingolipids, and their role in maintaining normal physiological functions and potential implication in diseases, dictated that analysis for these lipids be thoroughly evaluated.
Sphingolipids contain long-chain hydrocarbon groups in their molecules, are insoluble in water, and in many cases are soluble in organic solvents and have both hydrophobic and hydrophilic properties. Furthermore, most sphingolipids lack chromophores necessary for traditional HPLC UV and/or fluorescence detection, therefore limiting their detection. The application of HPLC coupled with MS/MS provides a comprehensive analytical profile of the actions of lipid molecules (lipidomics). In the current study, we describe a method for simultaneous analysis of major blood sphingolipids built on C18:1 and C18:0 sphingoid bases (Sph and dhSph), sphingoid base phosphates (S1P and dhS1P), and molecular species (Cn) of Cer, dhCer, Cer1P, SM, HexCer, and LacCer covering a basic metabolomic profiling of human blood serum and plasma using HPLC-MS/MS.
The results of this study showed that C16-SM and long-chain LacCers (C24:1-LacCer and C26:1-LacCer) increased, whereas levels of C16-C20 Cers, C14-LacCer, C16-HexCer, and C26-Cer1P, decreased in response to fasting. Thus, in this cross-sectional study, we were able to show significant differences in levels of certain sphingolipids in response to fasting. Future studies should address longitudinal changes in sphingolipid levels over postprandial periods and also over longer periods of fasting and starvation.
The data also showed gender differences in levels of serum and plasma sphingolipids. Higher levels of C18-SM, C18:1-SM, C18-Cer1P, and total dhCers, specifically C18-, C22-, and C24-dhCer, were observed in females than in males. Higher levels of HDL particle concentrations in females compared with males could account at least in part for the higher sphingolipid levels in females. However, other hormonal and metabolic factors could contribute to differences in sphingolipid levels between males and females.
There are multiple anticoagulants available for use in clinical settings. In this study, the anticoagulants heparin and citrate had a tendency to vary recovery levels of sphingolipids compared with EDTA. This was particularly obvious when heparin revealed higher variability in levels of molecular species of HexCer than other anticoagulants. EDTA generated the least variability from serum in sphingolipid levels compared with citrate and heparin. Thus, EDTA would be the preferred anticoagulant for sphingolipids analysis.
It was not surprising that serum S1P levels were 2-fold higher than plasma levels. Platelets lack S1P lyase, which is responsible for degradation of S1P; however, platelets possess a highly active Sph kinase, which converts Sph to S1P (30). As a result, platelets store and release S1P upon stimulation (23, 30). Thus, higher concentrations of S1P in serum could presumably be due to released S1P from activated platelets during clotting. Interestingly, no gender differences were detected in levels of sphingoid bases and their 1-phosphates in spite of the known differences in hematocrit between males and females. Because of careful handling of blood samples during collection and precipitation of red blood cells (RBC), blood hemolysis did not occur. Therefore, S1P that could have been generated from RBC rupture (31, 32) apparently did not contribute to our measurements.
Complex sphingolipids such as SM exist predominantly in the outer leaflet of the bilayer of cell membrane as well as the hydrophobic outer layer of the lipoprotein particle with free cholesterol and phospholipids (33). It has been shown that SM and phosphatidylcholine modulate the function of lipoproteins and serve as precursors for a variety of regulatory molecules, including lysophosphatidylcholine (34, 35) and Cer (36). Cer is the central molecule in the de novo pathway of sphingolipid metabolism (37). Cers also serve as the precursor for all major sphingolipids in eukaryotes such as SM and glucosylceramide (38). The breakdown of complex sphingolipids results in the formation of Cer through the action of either sphingomyelinases or glycosidases, and Cer has been proposed as a “coordinator” of eukaryotic stress responses (2, 39, 40). There is nonetheless little information regarding sphingolipids in the context of lipoprotein particles. In particular, information about the location and distribution of sphingolipid classes and species among lipoprotein particles is still obscure.
In this study, we determined the level of sphingolipid species of Cer and SM as well as levels of sphingoid bases and their 1-phosphates in VLDL, LDL, and the subclasses of HDL, HDL2, and HDL3. The data showed that the concentrations of SM and Cer species per lipoprotein particle reflected the size of the particle; the larger the size of the particles, the higher the content of SM and Cer species. Importantly, the data showed that the majority (78.6%) of lipoprotein-associated S1P was carried on HDL3 particles, and these particles were also the major carriers of dhS1P and Sph. Because of the role of HDL in the transport of cholesterol from peripheral tissues to the liver, the larger diameter HDL2 particles are viewed as more atheroprotective compared with the smaller sized HDL3 particles (41). A rapidly expanding literature suggests that HDL-associated S1P mediates many of the beneficial effects of HDL on the cardiovascular system, including the synthesis of potent antiatherogenic and antithrombotic molecules (e.g. nitric oxide and prostacyclin) (42, 43). There is emerging literature, however, to suggest that S1P also may be proatherogenic (44) because of its underlying involvement in inflammation (43, 45) and was even considered a biomarker of obstructive coronary artery disease (46). We have recently demonstrated that HDL3, which contains higher amounts of S1P than HDL2, significantly increases plasminogen activator inhibitor-1 secretion from adipocytes and thus may negatively modulate fibrinolysis in vivo (47).
It has been previously shown that modification of the core lipid content of HDL particles alters the conformation of apolipoprotein AI domains that are critical for HDL to act as lipid acceptor (48, 49). S1P, which is carried mainly on HDL, can be generated from membrane sphingolipids and their metabolites, Cer, Sph, and SM (50). Kontush et al. (51) showed that small HDL3 particles are enriched in S1P but poor in SM, with the S1P:SM molar ratio 4.3-fold higher in HDL3 than in HDL2. In the present study, we also showed that HDL3 contained higher levels of S1P than HDL2, with the S1P:SM molar ratio 5.5-fold higher in HDL3 than in HDL2.
In an analysis of long-chain SMs, Weisner et al. (52) showed that the major SM in lipoprotein classes isolated by fast performance liquid chromatography is SM 34:1, followed by SM 42:2, reported as SMs containing total 34 and 42 carbon atoms and one or two double bonds. SM 34:1 and SM 42:2 correspond to C16- and C24:1-SMs containing C18:1 sphingoid backbone, respectively. In the present study, we showed that in VLDL, LDL, HDL2, and HDL3 particles isolated by preparative ultracentrifugation, C16-SM is the major SM species, followed by C24:1-SM. Furthermore, the total SM concentration in lipoproteins in our study (485.3 nmol/ml) (Table 7) and that of Weisner et al. (52) (415 ± 141 µM) are in reasonable agreement.
It has been previously shown that the C24-Cer level in whole plasma increased in coronary artery disease and stroke patients compared with levels in control subjects without considerable changes of other Cer species (53). In this study, we showed that C24-Cer is the most abundant Cer species in all lipoprotein classes, including subclasses of HDL. Recently, it has been shown that in VLDL, LDL, and HDL isolated from serum of healthy subjects, the proportion of C24-Cer in total Cer is the highest among Cer species (52). Interestingly, although our study provided comparable values for total Cer concentrations in lipoproteins to those of Weisner et al. (52) (7.846 nmol/ml vs. 8.1 ± 3.4 µM, respectively) as well as comparable values for total SM concentrations (as mentioned in the previous paragraph), the percent distribution of Cer and SM in lipoprotein classes are not in agreement. A reason for this discrepancy may be the method by which concentrations of sphingolipids carried on lipoprotein particles in human plasma were deduced in our study (Table 7). Another reason could be the pooling of lipoprotein fractions separated by fast performance liquid chromatography in the study by Weisner et al. (52).
Efforts addressing the importance of determining blood sphingolipid levels as biomarkers of disease are still in their infancy. In a study designed to test the ability of serum sphingolipids to predict obstructive coronary artery disease, serum S1P was found to be a predictor of both the occurrence and severity of coronary stenosis (46). Using delayed extraction matrix-assisted laser desorption ionization time-of-flight MS to analyze sphingolipids in pericardial fluid, peritoneal fluid, and serum, it was found that the Cer monohexoside:SM ratio was increased in the Gaucher disease patients compared with controls (54). Nelson et al. (55) investigated whether plasma SM is an early atherogenic risk factor and found that plasma SM is associated with subclinical atherosclerotic disease. In a study examining the role of sphingolipids in the pathophysiology of sepsis, Drobnik et al. (56) showed that plasma levels of Cer and lysophosphatidylcholine have a highly predictive power in respect to sepsis-related mortality. In conclusion, we have provided benchmark data obtained from normal healthy subjects to enable future studies of sphingolipids to be used as biomarkers for clinical disease. Furthermore, a clinical laboratory testing of bioactive sphingolipids in blood could probably evolve to be analogous in importance to cholesterol and triglyceride determination in lipoprotein classes for diagnosis of lipid-related disorders.
Supplementary Material
Acknowledgments
The authors thank Andrea Semler and Charlyne Chassereau for assistance with lipoprotein isolation and special thanks to interested MUSC faculty who supported our study generously. Special acknowledgment is for the Lipidomics Shared Resource facility at MUSC.
Footnotes
Abbreviations:
- Cn-
- molecular species
- Cer
- ceramide
- Cer1P
- ceramide 1-phosphate
- dhCer
- dihydroceramide
- dhS1P
- dihydrosphingosine 1-phosphate
- dhSph
- dihydrosphingosine
- HexCer
- hexosylceramide
- LacCer
- lactosylceramide
- MUSC
- Medical University of South Carolina
- RBC
- red blood cell
- S1P
- sphingosine 1-phosphate
- Sph
- sphingosine
- Tn
- number of thaw cycles.
This work was supported by National Institutes of Health Grants HL-079274 and R0-1HL079274-04S1 (American Recovery and Reinvestment Act), The Southeastern Clinical and Translational Research Institute to S.M.H., the South Carolina Center of Biomedical Research Excellence (COBRE) in Lipidomics and Pathobiology (P20 RR17677 from National Center for Research Resources (NCCR) to S.M.H., Y.A.H., A.B., and J.B., the Department of Veterans Affairs Merit Review Program to R.L.K., and NCRR Grant CO6RR018823 for the facility. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Cuvillier O., Pirianov G., Kleuser B., Vanek P. G., Coso O. A., Gutkind S., Spiegel S. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 381: 800–803. [DOI] [PubMed] [Google Scholar]
- 2.Hannun Y. A. 1996. Functions of ceramide in coordinating cellular responses to stress. Science. 274: 1855–1859. [DOI] [PubMed] [Google Scholar]
- 3.Mathias S., Pena L. A., Kolesnick R. N. 1998. Signal transduction of stress via ceramide. Biochem. J. 335: 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel S., Merrill A. H., Jr 1996. Sphingolipid metabolism and cell growth regulation. FASEB J. 10: 1388–1397. [DOI] [PubMed] [Google Scholar]
- 5.Vesper H., Schmelz E. M., Nikolova-Karakashian M. N., Dillehay D. L., Lynch D. V., Merrill A. H., Jr 1999. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 129: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 6.Ichi I., Nakahara K., Kiso K., Kojo S. 2007. Effect of dietary cholesterol and high fat on ceramide concentration in rat tissues. Nutrition. 23: 570–574. [DOI] [PubMed] [Google Scholar]
- 7.Katsikas H., Wolf C. 1995. Blood sphingomyelins from two European countries. Biochim. Biophys. Acta. 1258: 95–100. [DOI] [PubMed] [Google Scholar]
- 8.Ozbayraktar F. B., Ulgen K. O. 2009. Molecular facets of sphingolipids: mediators of diseases. Biotechnol. J. 4: 1028–1041. [DOI] [PubMed] [Google Scholar]
- 9.Vance D. E., Sweeley C. C. 1967. Quantitative determination of the neutral glycosyl ceramides in human blood. J. Lipid Res. 8: 621–630. [PubMed] [Google Scholar]
- 10.Hammad S. M., Crellin H. G., Wu B. X., Melton J., Anelli V., Obeid L. M. 2008. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 85: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartke N., Hannun Y. A. 2009. Bioactive sphingolipids: metabolism and function. J. Lipid Res. 50 (Suppl.): S91–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowart L. A. 2009. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol Metab. 20: 34–42. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri S., Futerman A. H. 2007. The metabolism and function of sphingolipids and glycosphingolipids. Cell. Mol. Life Sci. 64: 2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liliom K., Sun G., Bunemann M., Virag T., Nusser N., Baker D. L., Wang D. A., Fabian M. J., Brandts B., Bender K., et al. 2001. Sphingosylphosphocholine is a naturally occurring lipid mediator in blood plasma: a possible role in regulating cardiac function via sphingolipid receptors. Biochem. J. 355: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill A. H., Jr, Stokes T. H., Momin A., Park H., Portz B. J., Kelly S., Wang E., Sullards M. C., Wang M. D. 2009. Sphingolipidomics: a valuable tool for understanding the roles of sphingolipids in biology and disease. J. Lipid Res. 50 (Suppl.): S97–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wymann M. P., Schneiter R. 2008. Lipid signaling in disease. Nat. Rev. Mol. Cell Biol. 9: 162–176. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann V., Lynch K. R. 2002. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr. Opin. Immunol. 14: 569–575. [DOI] [PubMed] [Google Scholar]
- 18.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. 2000. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352: 809–815. [PMC free article] [PubMed] [Google Scholar]
- 19.Bornfeldt K. E., Graves L. M., Raines E. W., Igarashi Y., Wayman G., Yamamura S., Yatomi Y., Sidhu J. S., Krebs E. G., Hakomori S., et al. 1995. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 130: 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura T., Watanabe T., Sato K., Kon J., Tomura H., Tamama K., Kuwabara A., Kanda T., Kobayashi I., Ohta H., et al. 2000. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem. J. 348: 71–76. [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M. J., Thangada S., Claffey K. P., Ancellin N., Liu C. H., Kluk M., Volpi M., Sha'afi R. I., Hla T. 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 99: 301–312. [DOI] [PubMed] [Google Scholar]
- 22.Yatomi Y., Yamamura S., Ruan F., Igarashi Y. 1997. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J. Biol. Chem. 272: 5291–5297. [DOI] [PubMed] [Google Scholar]
- 23.Yang L., Yatomi Y., Miura Y., Satoh K., Ozaki Y. 1999. Metabolism and functional effects of sphingolipids in blood cells. Br. J. Haematol. 107: 282–293. [DOI] [PubMed] [Google Scholar]
- 24.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. 2009. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579: 443–467. [DOI] [PubMed] [Google Scholar]
- 25.Tuck M. K., Chan D. W., Chia D., Godwin A. K., Grizzle W. E., Krueger K. E., Rom W., Sanda M., Sorbara L., Stass S., et al. 2009. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res. 8: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson G. J. 1972. Quantitative analysis of blood lipids. Blood Lipids and Lipoproteins: Quantitation, Composition and Metabolism. Nelson G. J., editor Wiley-Interscience, New York: 25–73. [Google Scholar]
- 27.Fisher W. R., Hammond M. G., Mengel M. C., Warmke G. L. 1975. A genetic determinant of the phenotypic variance of the molecular weight of low density lipoprotein. Proc. Natl. Acad. Sci. USA. 72: 2347–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betteridge D. J., Illingworth D. R., Shepherd J. 1999. Lipoproteins in health and disease. Arnold, London. [Google Scholar]
- 29.Jeyarajah E. J., Cromwell W. C., Otvos J. D. 2006. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 26: 847–870. [DOI] [PubMed] [Google Scholar]
- 30.Yatomi Y., Igarashi Y., Yang L., Hisano N., Qi R., Asazuma N., Satoh K., Ozaki Y., Kume S. 1997. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. 121: 969–973. [DOI] [PubMed] [Google Scholar]
- 31.Camerer E., Regard J. B., Cornelissen I., Srinivasan Y., Duong D. N., Palmer D., Pham T. H., Wong J. S., Pappu R., Coughlin S. R. 2009. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119: 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., et al. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 316: 295–298. [DOI] [PubMed] [Google Scholar]
- 33.Kumpula L. S., Kumpula J. M., Taskinen M. R., Jauhiainen M., Kaski K., Ala-Korpela M. 2008. Reconsideration of hydrophobic lipid distributions in lipoprotein particles. Chem. Phys. Lipids. 155: 57–62. [DOI] [PubMed] [Google Scholar]
- 34.Meyer zu Heringdorf D., Jakobs K. H. 2007. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim. Biophys. Acta. 1768: 923–940. [DOI] [PubMed] [Google Scholar]
- 35.Mueller R. B., Sheriff A., Gaipl U. S., Wesselborg S., Lauber K. 2007. Attraction of phagocytes by apoptotic cells is mediated by lysophosphatidylcholine. Autoimmunity. 40: 342–344. [DOI] [PubMed] [Google Scholar]
- 36.Tani M., Ito M., Igarashi Y. 2007. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell. Signal. 19: 229–237. [DOI] [PubMed] [Google Scholar]
- 37.Merrill A. H., Jr 2002. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277: 25843–25846. [DOI] [PubMed] [Google Scholar]
- 38.Kolter T., Proia R. L., Sandhoff K. 2002. Combinatorial ganglioside biosynthesis. J. Biol. Chem. 277: 25859–25862. [DOI] [PubMed] [Google Scholar]
- 39.Hannun Y. A., Obeid L. M. 2002. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277: 25847–25850. [DOI] [PubMed] [Google Scholar]
- 40.Hannun Y. A., Obeid L. M. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 41.Rader D. J. 2003. Regulation of reverse cholesterol transport and clinical implications. Am. J. Cardiol. 92: 42J–49J. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez C., Gonzalez-Diez M., Badimon L., Martinez-Gonzalez J. 2009. Sphingosine-1-phosphate: a bioactive lipid that confers high-density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thromb. Haemost. 101: 665–673. [PubMed] [Google Scholar]
- 43.Lucke S., Levkau B. 2010. Endothelial functions of sphingosine-1-phosphate. Cell. Physiol. Biochem. 26: 87–96. [DOI] [PubMed] [Google Scholar]
- 44.Okajima F. 2002. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim. Biophys. Acta. 1582: 132–137. [DOI] [PubMed] [Google Scholar]
- 45.Nixon G. F. 2009. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br. J. Pharmacol. 158: 982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deutschman D. H., Carstens J. S., Klepper R. L., Smith W. S., Page M. T., Young T. R., Gleason L. A., Nakajima N., Sabbadini R. A. 2003. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am. Heart J. 146: 62–68. [DOI] [PubMed] [Google Scholar]
- 47.Lee M. H., Hammad S. M., Semler A. J., Luttrell L. M., Lopes-Virella M. F., Klein R. L. 2010. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: the role of sphingosine-1-phosphate. J. Lipid Res 51: 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sparks D. L., Davidson W. S., Lund-Katz S., Phillips M. C. 1995. Effects of the neutral lipid content of high density lipoprotein on apolipoprotein A-I structure and particle stability. J. Biol. Chem. 270: 26910–26917. [DOI] [PubMed] [Google Scholar]
- 49.Curtiss L. K., Bonnet D. J., Rye K. A. 2000. The conformation of apolipoprotein A-I in high-density lipoproteins is influenced by core lipid composition and particle size: a surface plasmon resonance study. Biochemistry. 39: 5712–5721. [DOI] [PubMed] [Google Scholar]
- 50.Spiegel S., Milstien S. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4: 397–407. [DOI] [PubMed] [Google Scholar]
- 51.Kontush A., Therond P., Zerrad A., Couturier M., Negre-Salvayre A., de Souza J. A., Chantepie S., Chapman M. J. 2007. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 27: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 52.Wiesner P., Leidl K., Boettcher A., Schmitz G., Liebisch G. 2009. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 50: 574–585. [DOI] [PubMed] [Google Scholar]
- 53.Pfeiffer A., Bottcher A., Orso E., Kapinsky M., Nagy P., Bodnar A., Spreitzer I., Liebisch G., Drobnik W., Gempel K., et al. 2001. Lipopolysaccharide and ceramide docking to CD14 provokes ligand-specific receptor clustering in rafts. Eur. J. Immunol. 31: 3153–3164. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwaki T., Yamaguchi S., Tasaka M., Sakura N., Taketomi T. 2002. Application of delayed extraction-matrix-assisted laser desorption ionization time-of-flight mass spectrometry for analysis of sphingolipids in pericardial fluid, peritoneal fluid and serum from Gaucher disease patients. J. Chromatogr. B Analyt . Technol. Biomed Life Sci. 776: 115–123. [DOI] [PubMed] [Google Scholar]
- 55.Nelson J. C., Jiang X. C., Tabas I., Tall A., Shea S. 2006. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 163: 903–912. [DOI] [PubMed] [Google Scholar]
- 56.Drobnik W., Liebisch G., Audebert F. X., Frohlich D., Gluck T., Vogel P., Rothe G., Schmitz G. 2003. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 44: 754–761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.