Abstract
Niemann-Pick C1-Like 1 (NPC1L1) mediates intestinal cholesterol absorption. NPC1L1 knockout (L1-KO) mice were recently shown to be resistant to high-fat diet (HFD)-induced obesity in one study, which was contrary to several other studies. Careful comparison of dietary compositions in these studies implies a potential role of dietary cholesterol in regulating weight gain. To examine this potential, wild-type (WT) and L1-KO mice were fed one of three sets of diets for various durations: (1) a HFD without added cholesterol for 5 weeks; (2) a high-carbohydrate diet with or without added cholesterol for 5 weeks; or (3) a synthetic HFD with or without added cholesterol for 18 weeks. We found that L1-KO mice were protected against diet-induced weight gain only on a diet without added cholesterol but not on a diet containing 0.16% or 0.2% (w/w) cholesterol, an amount similar to a typical Western diet, regardless of the major energy source of the diet. Food intake and intestinal fat absorption were similar between the two genotypes. Intestinal cholesterol absorption was blocked, and fecal cholesterol excretion increased in L1-KO mice. Under all diets, L1-KO mice were protected from hepatosteatosis. In conclusion, increasing dietary cholesterol restores diet-induced weight gain in mice deficient in NPC1L1-dependent cholesterol absorption.
Keywords: obesity, intestinal fat absorption, ezetimibe, insulin resistance, hepatic steatosis
Elevated blood total and low-density lipoprotein cholesterol is an independent risk factor for atherosclerotic cardiovascular disease. In addition to de novo biosynthesis and hepatobiliary secretion, intestinal absorption of cholesterol is another major pathway by which the body regulates cholesterol homeostasis in response to fluctuations in cholesterol availability and utilization. Inhibiting this pathway by a cholesterol absorption inhibitor ezetimibe (trade name Zetia) (1) has been shown to significantly reduce plasma total and low-density lipoprotein cholesterol in all mammalian species tested, including humans (2–9).
Each day, large amounts of cholesterol enter the lumen of the small intestine. The daily dietary intake of cholesterol is ∼400 mg in a healthy human consuming a typical Western diet (10, 11). Additionally, ∼800 mg cholesterol is secreted into the gut lumen from bile each day (11). The fractional intestinal cholesterol absorption ranges from 29% to 80% in healthy individuals (12). To be efficiently absorbed, the hydrophobic cholesterol molecule has to cross the unstirred water layer lying between the bulk water phase and the intestinal mucosal cell membrane, which is achieved by its solubilization in mixed micelles containing bile acids, phospholipids, and hydrolytic products of fat (13). Whether free cholesterol in micelles is taken up passively or actively by absorptive enterocytes had remained elusive until the discovery of ezetimibe (13). The potency of low doses of ezetimibe in inhibiting intestinal cholesterol absorption strongly supports the notion that intestinal cholesterol absorption is an active and protein-mediated process. The search for this protein resulted in identification of Niemann-Pick C1-Like 1 (NPC1L1) as a cholesterol transporter essential for intestinal cholesterol absorption (14). NPC1L1 protein is highly expressed in the small intestine across species and localizes primarily at the apical membrane of absorptive enterocytes (14, 15). Although humans and nonhuman primates also express NPC1L1 in liver and perhaps other tissues, mouse NPC1L1 protein can only be detected in the small intestine (14, 16–18). NPC1L1 knockout (L1-KO) mice do not absorb cholesterol from the intestinal lumen and thus have a corresponding increase in fecal cholesterol excretion and compensatory upregulation of de novo cholesterol biosynthesis, similar to phenotypes observed in ezetimibe-treated animals (14, 19). Because inhibiting intestinal cholesterol absorption increases endogenous cholesterol synthesis, ezetimibe is often prescribed together with a statin that inhibits 3-hydroxy-3-methyl-glutaryl-CoA reductase, the rate-limiting enzyme of cholesterol synthesis (20, 21). Subsequent genetic, animal, and cell biology studies support the notion that NPC1L1 is the molecular target of ezetimibe (14, 17, 22–27).
Interestingly, ezetimibe treatment or genetic inactivation of NPC1L1 was recently shown to improve many aspects of metabolic syndrome, including insulin resistance and hepatic steatosis (28–34). For example, L1-KO mice on a pure C57BL/6 background or ezetimibe-treated wild-type (WT) C57BL/6 mice are resistant to weight gain on a high-fat diet (HFD) containing trace amounts of cholesterol (31, 33). L1-KO mice on 129/OlaHsd background, however, gain similar weight as WT controls after 24 weeks on a diet containing 40% calories from butter fat and 0.15% cholesterol (35). In another study examining the protective role of ezetimibe against the development of fatty liver disease, ezetimibe treatment for 4 weeks did not prevent weight gain in C57BL/6 mice fed a HFD containing 0.12% cholesterol (32). While these discrepancies among different studies may be attributable to differences in genetic background, gene-targeting strategies, experimental duration, and/or dietary fat composition, we noticed that resistance to diet-induced weight gain was found only in L1-KO mice or ezetimibe-treated mice fed a low (<0.02%, w/w) cholesterol-containing diet (31, 33) but not in those fed a high (0.15% or 0.12%, w/w) cholesterol diet (32, 35). This led us to hypothesize that dietary cholesterol modulates weight gain in NPC1L1-deficient mice.
To systemically determine if NPC1L1 deficiency prevents weight gain in a dietary cholesterol-dependent manner, we fed L1-KO mice and WT controls a diet containing either a low (<0.02%, w/w) or a high (∼0.2%, w/w) amount of cholesterol for 5 weeks or 18 weeks, and monitored body weight changes and other metabolic parameters in these animals. Our findings clearly demonstrate that dietary supplementation of cholesterol rescues weight gain in L1-KO mice on both HFD and high-carbohydrate diet (HCD). Future studies are needed to solve the conundrum of how increasing dietary cholesterol restores the diet-induced weight gain in mice deficient in intestinal cholesterol absorption.
MATERIALS AND METHODS
Animals
L1-KO mice were created using C57BL/6 embryonic stem cells, thus having a pure C57BL/6 background (28). These mice have been crossed to WT C57BL/6 mice for at least four generations before the use for this study. L1-KO mice and their controls were maintained on the pure C57BL/6 background for all the experiments. All mice were housed in a specific pathogen-free animal facility in plastic cages at 22°C, with a daylight cycle from 6 AM to 6 PM. The mice were provided with water and standard chow diet (Prolab RMH 3000; LabDiet, Brentwood, MO) ad libitum, unless stated otherwise. All animal procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University Health Sciences.
Diets
For the first short-term study, male L1-KO and WT C57BL/6 mice were fed a HFD containing a trace amount of cholesterol (∼0.007%, w/w) (HFD1-C) (TD.93075; Harlan Teklad, Madison, WI) for 5 weeks, starting at 8 weeks of age. This diet contains 54.8% calories from fat, 21.2% calories from protein, and 24% calories from carbohydrates. The fatty acid composition of total fat in this diet is included in supplementary Table I.
For the second short-term experiment, 8-week-old male L1-KO mice and WT controls were fed a synthetic high-carbohydrate diet (HCD) containing either <0.02% cholesterol (HCD-C) or ∼0.2% cholesterol (HCD+C) for 5 weeks. These synthetic diets were made at the Diet Core of Wake Forest University School of Medicine, which has more than 40 years of experience in preparing animal diets. Ingredients of these diets are presented in supplementary Table II. Both carbohydrate diets have identical ingredients except cholesterol, containing 11% calories from palm oil, 72% calories from carbohydrates, and 17% calories from protein. The fatty acid composition of the fat in this diet is included in supplementary Table I.
The third study was a long-term HFD study. Six-week-old male L1-KO mice and their WT controls were fed a synthetic HFD containing either a low (<0.02%, w/w, HFD-C) or a high (∼0.16%, w/w, HFD+C) amount of cholesterol for 18 weeks. These synthetic diets were made at the Diet Core of Wake Forest University School of Medicine. Ingredients of these diets are presented in supplementary Table II. The fatty acid composition of these diets is included in supplementary Table I. During the diet feeding period, body weight was monitored weekly. Food intake was monitored daily for 2 weeks after 16 weeks of diet feeding.
Blood biochemistries
Plasma insulin was measured by Ultra Sensitive Mouse Insulin Elisa Kit (Crystal Chem, Inc., Downers Grove, IL). Blood glucose was measured directly by Glucometer (Bayer Contour, Tarrytown, NY) via a tail nick. Plasma total cholesterol, free cholesterol, and triglyceride concentrations were analyzed by enzymatic assay as previously described (36). The amount of cholesteryl ester was calculated by subtracting free cholesterol from total cholesterol and multiplying by 1.67 to convert to cholesteryl ester mass. Plasma concentrations of nonesterified fatty acids were measured by colorimetric assay [HR Series NEFA-HR (2); Wako, Richmond, VA] following the manufacturer's instructions.
Liver lipids
At the end of diet studies, mice were euthanized after a 4 h fast. Livers were removed, weighed, and snap-frozen in liquid nitrogen for liver lipid analyses as we have described previously (18).
Intestinal cholesterol absorption and fecal cholesterol excretion
In week 18 of the long-term diet experiment, mice were individually housed for the determination of fractional cholesterol absorption and fecal neutral sterol excretion as described previously (37). Mass of dietary cholesterol absorption into the body was calculated by multiplying food intake, dietary cholesterol content, and percentage of cholesterol absorption (38).
Quantitative real-time PCR (qPCR)
Total RNAs were extracted from jejuna using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The qPCR was performed as described previously (39). All primers used for qPCR have been published previously (39, 40).
Intestinal fat absorption
After 16 weeks of diet feeding, mice were individually housed and fed a test diet for three days, which contained the same calories and fatty acid composition as the previous diet, except that 5% of fat was replaced by sucrose poly-behenate as a nonabsorbable marker. Fecal samples were collected for three days, and the fatty acid composition was measured in both fecal and diet samples by gas chromatography. Fractional absorption of total and individual fatty acids was calculated as previously described (41).
Glucose tolerance test and insulin tolerance test
For glucose tolerance test, mice were fasted for 10 h. After measuring the baseline blood glucose concentration from a tail cut by a Glucometer test strip (Bayer Ascensia Contour, Tarrytown, NY), mice were injected intraperitoneally with 20% glucose at 1.5 mg/g body weight. Blood glucose concentrations were then measured at 15, 30, 60, and 120 min after glucose injection. For insulin tolerance test, mice were fasted for 6 h. After measuring the baseline blood glucose concentration, mice were injected intraperitoneally with recombinant human insulin (Novo Nordisk, Inc., Princeton, NY) at 1.2 mU/g body weight. Blood glucose concentrations were then measured at 15, 30, 60, and 120 min after insulin administration.
Statistical analysis
All data are presented as mean ± SEM. The differences between the mean values of L1-KO mice and WT mice on the same diet, or between diet groups of mice of the same genotype, were tested for statistical significance by the two-tailed Student's t-test. Statistical significance was accepted at a value of P < 0.05.
RESULTS
Short-term study 1: L1-KO mice on the low-cholesterol HFD are resistant to weight gain
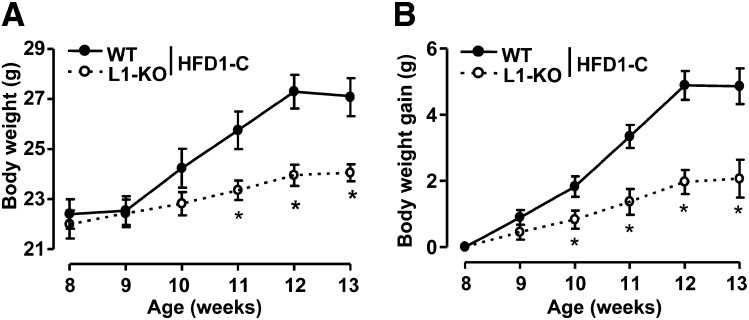
To determine the effect of HFD containing no added cholesterol on weight gain in L1-KO mice, mice were fed the HFD1-C as described in “Materials and Methods.” L1-KO and WT mice had similar weight at birth and at the time when the diet was started (Fig. 1A). Interestingly, L1-KO mice began to gain significantly less weight compared with WT mice after one week on this diet (Fig. 1B). After five weeks of diet feeding, L1-KO mice gained only ∼2 g of weight, whereas WT mice gained nearly 5 g of body weight (Table 1).
Fig. 1.
Body weight (A) and body weight gain (B) of L1-KO and WT mice on the low-cholesterol, high-fat diet (HFD1-C), starting at eight weeks of age. *P < 0.05 (n = 5–7). L1-KO, NPC1L1 knockout; WT, wild-type.
TABLE 1.
Plasma and hepatic parameters of WT and L1-KO mice fed HFD1-C diet for five weeks
| Low Cholesterol, High-Fat Diet |
||
|---|---|---|
| Parameter | WT Mice | L1-KO Mice |
| BW (g) | 26.8 ± 0.8 | 23.8 ± 0.2a |
| WAT weight (g) | 0.99 ± 0.04 | 0.34 ± 0.03b |
| WAT to BW ratio (%) | 3.74 ± 0.14 | 1.43 ± 0.13b |
| Liver weight (g) | 1.1 ± 0.05 | 0.9 ± 0.03a |
| Liver to BW ratio (%) | 4.2 ± 0.13 | 3.8 ± 0.12a |
| Plasma TC (mg/dl) | 129.8 ± 9.8 | 115.1 ± 8.1 |
| Plasma FC (mg/dl) | 33.9 ± 2.8 | 28.7 ± 1.5 |
| Plasma CE (mg/dl) | 160.1 ± 12.4 | 144.3 ± 11.8 |
| Plasma TG (mg/dl) | 49.1 ± 8.3 | 31.3 ± 3.1 |
| Plasma NEFA (mmol/l) | 0.37 ± 0.03 | 0.24 ± 0.05 |
| Hepatic TC (mg/g WW) | 2.1 ± 0.31 | 2.0 ± 0.08 |
| Hepatic TG (mg/g WW) | 24.9 ± 6.59 | 5.2 ± 0.79a |
Mice were fasted for 4 h prior to plasma and liver lipids analysis; n = 4–7 for liver lipids and n = 6–7 for other measurements. BW, body weight; CE, cholesteryl ester; FC, free cholesterol; HFD1-C, low-cholesterol, high-fat diet; L1-KO, NPC1L1 knockout; NEFA, nonesterified fatty acid; TC, total cholesterol; TG, triglyceride; WAT, white adipose tissue; WW, wet weight; WT, wild-type.
P < 0.05.
P < 0.001.
The reduced body weight gain in L1-KO mice was associated with a 66% decrease in epididymal fat weight and a modest but significant decrease in liver weight compared with WT mice (Table 1).
No differences were observed for plasma concentrations of total cholesterol, free cholesterol, cholesteryl ester, nonesterified fatty acids, or triglyceride between the two genotypes. Hepatic total cholesterol content did not differ between the two groups. Hepatic triglyceride content was ∼80% lower in L1-KO mice relative to WT mice (Table 1).
Short-term study 2: L1-KO mice on the low-cholesterol HCD are resistant to weight gain and addition of cholesterol to HCD rescues weight gain
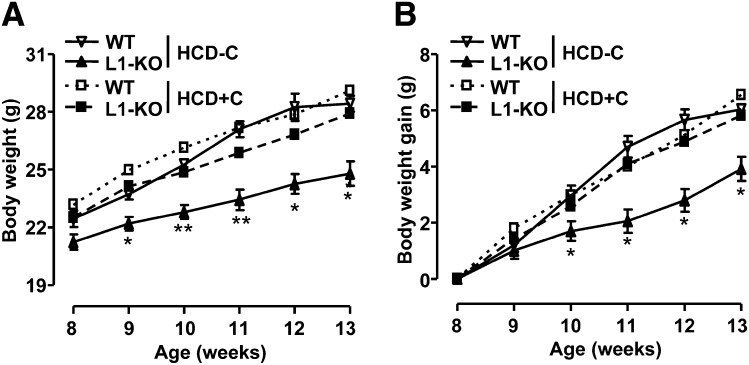
To determine whether dietary energy source influences cholesterol-dependent weight gain, mice were fed a synthetic sucrose-rich diet (HCD) with or without added cholesterol for five weeks as described in “Materials and Methods.”As shown in Fig. 2, the body weight was not significantly different between L1-KO and WT mice when the diet was started (Fig. 2A). WT mice gradually gained weight throughout the five-week period, regardless of the amount of cholesterol in the diet. Furthermore, increasing cholesterol content in the diet did not promote greater weight gain in WT mice. L1-KO mice, however, gained weight in a dietary cholesterol-dependent manner. On the high-carbohydrate diet containing a high amount of cholesterol (HCD+C), L1-KO mice gained weight similar to WT mice. On the high-carbohydrate diet containing a low amount of cholesterol (HCD-C), L1-KO mice began to gain significantly less weight after one week on diet, despite having similar food intake as WT controls (5.0 ± 0.1 g/day in L1-KO mice versus 5.1 ± 0.1 g/day in WT mice). At the end of the five-week diet period, L1-KO mice gained 2 g less than WT mice (Fig. 2).
Fig. 2.
Body weight (A) and body weight gain (B) of L1-KO and WT mice on the high-carbohydrate diet with a low (HCD-C) or a high (HCD+C) amount of cholesterol, starting at eight weeks of age. *P < 0.05; **P < 0.01 (n = 4–7). HCD, high-carbohydrate diet; L1-KO, NPC1L1 knockout; WT, wild-type.
On the low-cholesterol HCD, L1-KO relative to control WT mice also showed a significant reduction in epididymal fat weight, liver weight, and plasma concentrations of total cholesterol and cholesterol ester (Table 2). Hepatic contents of total cholesterol and triglyceride were ∼30% and ∼45% lower in L1-KO than WT mice, respectively (Table 2). Plasma concentrations of free cholesterol and triglyceride showed a trend toward decreases in L1-KO versus WT mice, while the plasma concentration of nonesterified fatty acids was similar between the two genotypes (Table 2).
TABLE 2.
Plasma and hepatic parameters of WT and L1-KO mice fed HCD containing either a low (HCD-C) or a high (HCD+C) amount of cholesterol for five weeks
| High-Carbohydrate Diet (HCD) |
||||
|---|---|---|---|---|
| HCD-C |
HCD+C |
|||
| Parameter | WT Mice | L1-KO Mice | WT Mice | L1-KO Mice |
| BW (g) | 27.4 ± 0.6 | 23.3 ± 0.7a | 28.6 ± 0.9 | 26.7 ± 1 |
| WAT weight (g) | 0.88 ± 0.05 | 0.60 ± 0.08a | 1.10 ± 0.10 | 0.96 ± 0.19 |
| WAT to BW ratio (%) | 3.20 ± 0.14 | 2.5 ± 0.28a | 3.80 ± 0.23 | 3.57 ± 0.57 |
| Liver weight (g) | 1.2 ± 0.06 | 0.9 ± 0.04a | 1.3 ± 0.08 | 1.1 ± 0.06 |
| Liver to BW ratio (%) | 4.4 ± 0.13 | 4.0 ± 0.08a | 4.4 ± 0.29 | 4.1 ± 0.07 |
| Plasma TC (mg/dl) | 140.4 ± 4.8 | 117.2 ± 7.0a | 145.0 ± 18.5 | 127.0 ± 4.57 |
| Plasma FC (mg/dl) | 35.9 ± 1.7 | 30.2 ± 2.0 | 36.5 ± 3.5 | 32.6 ± 1.7 |
| Plasma CE (mg/dl) | 174.6 ± 6.8 | 145.4 ± 8.89a | 181.1 ± 25.1 | 157.6 ± 5.3 |
| Plasma TG (mg/dl) | 78.7 ± 9.0 | 56.7 ± 7.7 | 25.7 ± 6.8 | 50.6 ± 13.6 |
| Plasma NEFA (mmol/l) | 0.47 ± 0.04 | 0.51 ± 0.11 | 0.55 ± 0.05 | 0.44 ± 0.03 |
| Hepatic TC (mg/g WW) | 2.7 ± 0.22 | 1.9 ± 0.06a | 12.0 ± 1.65b | 3.2 ± 0.15a,b |
| Hepatic TG (mg/g WW) | 24.6 ± 1.79 | 13.6 ± 2.48a | 157.8 ± 29.27b | 24.8 ± 4.38b |
Mice were fasted for 4 h prior to the analysis of plasma and hepatic parameters; n = 4–7 for live lipid and n = 6–7 for other measurements. BW, body weight; CE, cholesteryl ester; FC, free cholesterol; HCD, high-carbohydrate diet; HCD-C, HCD containing a low amount cholesterol; HCD+C, HCD containing a high amount cholesterol; L1-KO, NPC1L1 knockout; NEFA, nonesterified fatty acid; TC, total cholesterol; TG, triglyceride; WAT, white adipose tissue; WW, wet weight; WT, wild-type.
Significantly different from WT mice on the same diet, P < 0.05.
Significantly different from mice of the same genotype on different diets, P < 0.001.
On the high-cholesterol HCD, no differences were found in epididymal fat pad weight, liver weight, plasma concentrations of total cholesterol, free cholesterol, cholesteryl ester, triglyceride, or nonesterified fatty acid between the two genotypes (Table 2). However, hepatic contents of total cholesterol and triglyceride were ∼73% and ∼84% lower, respectively, in L1-KO versus WT mice (Table 2).
Long-term HFD study: addition of cholesterol to HFD rescues weight gain in L1-KO mice
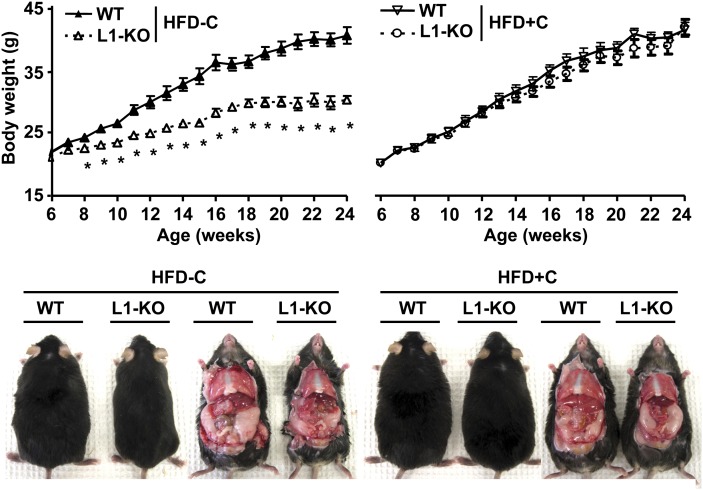
To determine whether dietary cholesterol alters weight gain and obesity induced by long-term HFD feeding, mice were fed a synthetic HFD with or without added cholesterol for 18 weeks as described in “Materials and Methods.”Regardless of dietary cholesterol contents, WT mice continuously gained weight during 18 weeks of diet feeding (Fig. 3). At the end of the diet period, there were ∼76% and ∼79% increases in body weight in WT mice fed the low-cholesterol HFD (HFD-C) and the high-cholesterol HFD (HFD+C), respectively (Table 3). L1-KO mice on the high-cholesterol HFD gained weight similar to WT mice on either diet. On the low-cholesterol HFD, however, L1-KO mice began to gain less weight during the second week of diet feeding (Fig. 3) and were protected against HFD-induced obesity with only an ∼39% increase in body weight at the end of the diet period (Table 3). Given the slight differences in dietary compositions, we made HFD with identical compositions, except the amounts of cholesterol (supplementary Table III), and fed our mice with these diets for 5 weeks. Consistently, L1-KO mice were resistant to weight gain only on the low-cholesterol HFD, but not on the high-cholesterol HFD (supplementary Fig. I).
Fig. 3.
Growth curves and gross appearance of L1-KO and WT mice on the low-cholesterol (0.02%, w/w), high-fat diet (HFD-C) or on the high-cholesterol (0.16%, w/w), high-fat diet (HFD+C) for 18 weeks. *P < 0.05 (n = 5–7). HFD, high-fat diet; L1-KO, NPC1L1 knockout; WT, wild-type.
TABLE 3.
Plasma and hepatic parameters of WT and L1-KO mice fed HFD containing either a low (HFD-C) or a high (HFD+C) amount of cholesterol for 18 weeks
| High-Fat Diet (HFD) | ||||
|---|---|---|---|---|
| HFD-C | HFD+C | |||
| Parameter | WT Mice | L1-KO Mice | WT Mice | L1-KO Mice |
| Initial BW (g) | 21.9 ± 0.4 | 21.1 ± 0.4 | 20.3 ± 0.5 | 20.3 ± 0.4 |
| Final BW (g) | 38.6 ± 1.3 | 29.4 ± 0.5a | 36.3 ± 1.9 | 35.0 ± 1.4 |
| WAT weight (g) | 1.86 ± 0.09 | 0.94 ± 0.08a | 1.89 ± 0.09 | 1.92 ± 0.10 |
| WAT to BW ratio (%) | 4.88 ± 0.33 | 3.19 ± 0.24a | 5.29 ± 0.41 | 5.47 ± 0.11 |
| Liver weight (g) | 1.55 ± 0.11 | 1.04 ± 0.02a | 1.91 ± 0.23 | 1.19 ± 0.04a,b |
| Liver to BW ratio (%) | 4.0 ± 0.17 | 3.54 ± 0.06a | 5.20 ± 0.34b | 3.39 ± 0.0a |
| Plasma TC (mg/dl) | 183.9 ± 8.5 | 152.5 ± 3.7a | 247.8 ± 9.0b | 188.3 ± 14.7a,b |
| Plasma FC (mg/dl) | 41.0 ± 2.2 | 36.2 ± 0.4a | 63.6 ± 2.1b | 46.1 ± 3.6a,b |
| Plasma CE (mg/dl) | 238.6 ± 13.6 | 194.4 ± 5.9a | 307.7 ± 12.9b | 237.5 ± 19.1a,b |
| Plasma TG (mg/dl) | 46.8 ± 6.5 | 47.4 ± 4.57 | 27.3 ± 6.0 | 35.0 ± 6.0 |
| Plasma insulin (ng/ml) | 1.69 ± 0.21 | 0.96 ± 0.20a | 1.35 ± 0.35 | 1.89 ± 0.65 |
| Blood glucose (mg/dl) | 131 ± 8 | 152 ± 4 | 136 ± 20 | 153 ± 7 |
| Hepatic TC (µg/mg protein) | 20.4 ± 3.6 | 10.0 ± 0.3a | 90.3 ± 7.4b | 14.9 ± 0.9a,b |
| Hepatic FC (µg/mg protein) | 7.3 ± 0.5 | 5.3 ± 0.2a | 15.8 ± 1.3b | 6.6 ± 0.8a |
| Hepatic CE (µg/mg protein) | 22.0 ± 5.8 | 7.8 ± 0.5a | 124.3 ± 14.2b | 13.9 ± 0.8a,b |
| Hepatic TG (µg/mg protein) | 704 ± 132 | 99 ± 12a | 1432 ± 235b | 589 ± 238a,b |
Mice were fasted for 4 h prior to the analysis of plasma and hepatic parameters; n = 5–7 for each group. BW, body weight; CE, cholesteryl ester; FC, free cholesterol; HFD, high-fat diet; HFD-C, HFD containing a low amount cholesterol; HFD+C, HFD containing a high amount cholesterol; L1-KO, NPC1L1 knockout; NEFA, nonesterified fatty acid; TC, total cholesterol; TG, triglyceride; WAT, white adipose tissue; WT, wild-type.
Significantly different from WT mice on the same diet, P < 0.05.
Significantly different from mice of the same genotype on different diets, P < 0.05.
Consistent with weight changes, epididymal fat pad weights were similar between L1-KO and WT mice fed the high-cholesterol HFD for 18 weeks, but they were significantly reduced in L1-KO relative to WT mice fed the low-cholesterol HFD for 18 weeks (Table 3). Regardless of dietary cholesterol content, liver weights and liver-to-body weight ratios were significantly reduced in L1-KO mice compared with WT mice (Table 3).
While the high-cholesterol HFD feeding raised plasma concentrations of total cholesterol, free cholesterol, and cholesteryl ester in WT mice, we surprisingly found that this was also the case in L1-KO mice, although to a lesser extent when compared with WT mice (Table 3). In the liver, increasing dietary cholesterol dramatically raised the contents of total cholesterol and cholesteryl ester in WT mice. This was also observed in L1-KO mice, though L1-KO mice versus WT mice showed significantly reduced hepatic contents of these lipids (Table 3). The high-cholesterol HFD versus the low-cholesterol HFD dramatically raised the hepatic triglyceride content by ∼100% in WT mice and, to our surprise, by ∼500% in L1-KO mice. Despite this remarkable increase, the hepatic triglyceride content was still significantly lower in L1-KO relative to WT mice, regardless of dietary cholesterol (Table 3). These findings together suggest that a small proportion of dietary cholesterol may have entered the body in the absence of NPC1L1 under our experimental conditions.
Long-term HFD study: L1-KO mice have undetectable intestinal cholesterol absorption and increased fecal cholesterol excretion
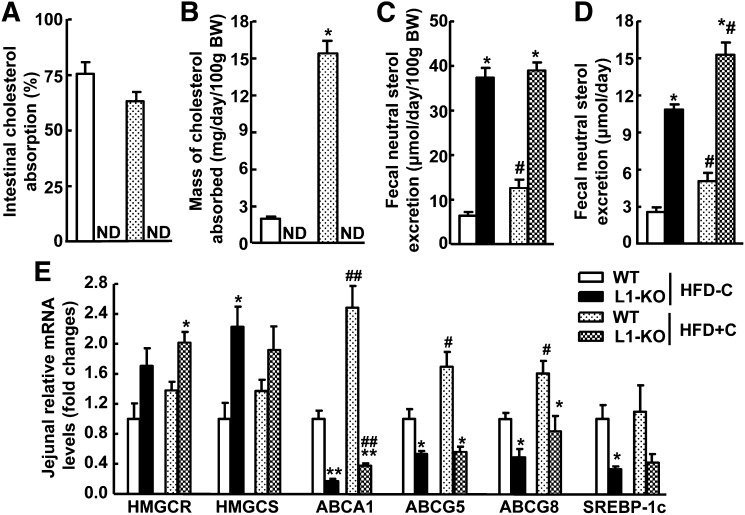
To examine cholesterol balance in L1-KO and WT mice on our synthetic diets, fractional intestinal cholesterol absorption and fecal cholesterol excretion were measured. As expected, genetic deletion of NPC1L1 in mice blocked intestinal cholesterol absorption to an undetectable level when the dual-isotope method was used (Fig. 4A, B), which was associated with a dramatic increase in fecal cholesterol loss (Fig. 4C, D). To compensate for the reduced cholesterol absorption, intestinal levels of 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA) reductase (HMGCR) and HMG-CoA synthase (HMGCS) were upregulated in L1-KO mice (Fig. 4E). Cholesterol is known to increase intestinal mRNAs for ABC transporter A1 (ABCA1), ABCG5, ABCGG8, and sterol regulatory element-binding protein (SREBP)-1c via activating liver X receptor (LXR) (42–45). Consistently, intestinal expression levels of these genes were substantially reduced in L1-KO mice. Surprisingly, under our experimental conditions, WT mice on the high-cholesterol HFD compared with WT mice on the low-cholesterol HFD did not show a suppression in cholesterol synthetic genes and an increase in LXR target gene SREBP-1c in the jejuna (Fig. 4E), suggesting the involvement of other factors in the transcription regulation of these genes (46, 47). Alternatively, long-term HFD feeding may attenuate cholesterol-dependent transcriptional regulation of these genes. As expected, intestinal mRNA levels of ABCA1, ABCG5, and ABCG8 were significantly increased in WT mice on the high-cholesterol HFD compared with the low-cholesterol HFD. Interestingly, addition of cholesterol to HFD significantly increased intestinal ABCA1 expression even in the L1-KO mice (Fig. 4E), implying a small amount of cholesterol may enter the absorptive enterocytes in the absence of NPC1L1.
Fig. 4.
Effects of NPC1L1 deficiency on (A) intestinal cholesterol absorption, (B) mass of cholesterol absorbed into the body, (C) fecal neutral sterol excretion normalized to body weight (BW), and (D) fecal neutral sterol excretion without body weight normalization. Measurements were done in L1-KO and WT mice fed the low-cholesterol (0.02%, w/w), high-fat diet (HFD-C) or the high-cholesterol (0.16%, w/w), high-fat diet (HFD+C) for 18 weeks (n = 5–7). E: Jejunal mRNA levels of genes involved in cholesterol synthesis and regulation. The total RNA was extracted from the proximal second segment of the small intestine (equally divided into 5 segments) in individual mice in each group (n = 4). The mRNA level for each gene in each sample was quantified by qPCR. Cyclophilin was used as an internal control. *P < 0.05, **P < 0.01, significantly different between L1-KO and WT mice on the same diet; #P < 0.05, ##P < 0.05, significantly different between mice of the same genotype on different diets. HFD, high-fat diet; HMGCR, 3-hydroxy-3-methyl-glutaryl CoA (HMG-CoA) reductase; HMGCS, HMG-CoA synthase; L1-KO, NPC1L1 knockout; ND, not detectable; WT, wild-type.
Long-term HFD study: L1-KO and WT mice have similar food intake and fat absorption
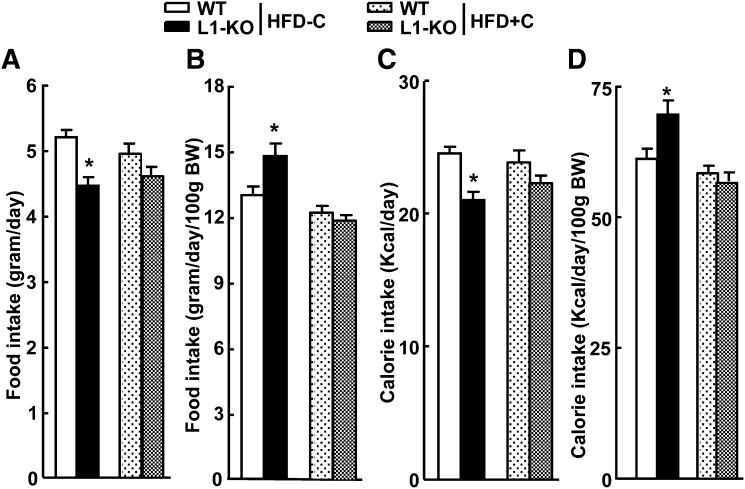
Food intake and intestinal fat absorption were determined to explore potential mechanisms underlying the protective role of NPC1L1 deficiency in diet-induced obesity. On the low-cholesterol HFD, WT and L1-KO mice ate 5.2 ± 0.1 and 4.5 ± 0.1 g/day of food, respectively (Fig. 5A). When food intake was normalized to body weight, L1-KO mice, however, ate 14% more than WT mice (Fig. 5B) because L1-KO mice were leaner on this diet (Fig. 3). On the high-cholesterol HFD, L1-KO and WT mice had similar food intake (Fig. 5A, B).
Fig. 5.
Daily food and calorie intake of WT and L1-KO mice on HFD-C or HFD+C diet. A: Food intake not normalized to body weight (BW). B: Food intake normalized to body weight. C: Calorie intake not normalized to body weight. D: Calorie intake normalized to body weight. *Significantly different from WT mice on the same diet; P < 0.05 (n = 5–7). HFD, high-fat diet; HFD-C, HFD containing a low amount cholesterol; HFD+C, HFD containing a high amount cholesterol; L1-KO, NPC1L1 knockout; WT, wild-type.
Calorie intake was also calculated based on the calories in 100 g of each diet and food intake results. On the low-cholesterol HFD, L1-KO mice had slightly but significantly reduced calorie intake (Fig. 5C). However, when calorie intake was normalized to body weight, L1-KO mice consumed 14% more calories than WT mice (Fig. 5D). On the high-cholesterol HFD, L1-KO and WT consumed similar calorie amounts (Fig. 5C, D).
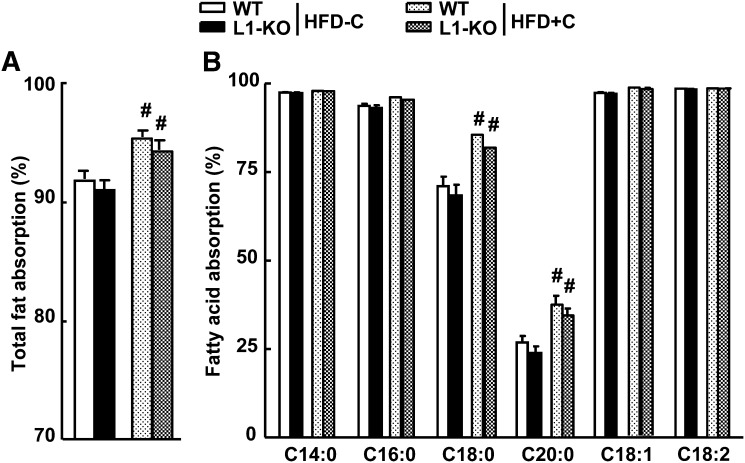
Intestinal fat absorption is very efficient in general. It was reported that L1-KO compared with WT mice had similar food intake but a small (∼5.5%) yet significant reduction in intestinal absorption of total fatty acids with more dramatic decreases seen in absorption of saturated fatty acids (33). Although this modest reduction in fat absorption may account for the significantly lower weight gain over a long period, in the current study using the same method, we found that L1-KO and WT mice had similar intestinal absorption of total fatty acids and specific fatty acid species (Fig. 6A, B) on either the low-cholesterol HFD or the high-cholesterol HFD. Therefore, weight gain differences seen in mice on the low-cholesterol HFD cannot be attributable to changes in intestinal fat absorption. Interestingly, intestinal absorption of total fatty acids and saturated fatty acids (C18:0 and C20:0) were significantly higher in mice fed the high-cholesterol HFD compared with those on the low-cholesterol HFD, suggesting that dietary cholesterol may facilitate intestinal absorption of specific fatty acids.
Fig. 6.
Fractional intestinal fat absorption in L1-KO and WT mice on HFD-C or HFD+C diet. A: Absorption of total fat. B: Absorption of specific fatty acids. #Significantly different from mice of the same genotype on different diets; P < 0.05 (n = 5–7). HFD, high-fat diet; HFD-C, HFD containing a low amount cholesterol; HFD+C, HFD containing a high amount cholesterol; L1-KO, NPC1L1 knockout; WT, wild-type.
Long-term HFD study: L1-KO mice on the low-cholesterol HFD have improved glucose tolerance and insulin sensitivity
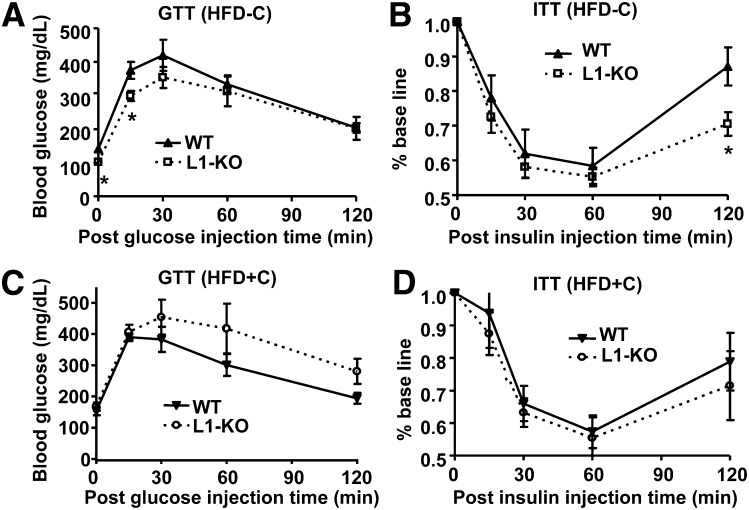
Obesity and hepatic steatosis are often associated with insulin resistance. We assessed systemic insulin sensitivity in mice fed a synthetic HFD with or without added cholesterol. Consistent with the positive correlation between obesity/hepatic steatosis and insulin resistance, L1-KO versus WT mice fed the low-cholesterol HFD showed a reduced blood glucose level at time zero (after a 10 h fast) and improved glucose tolerance 15 min after glucose injection during glucose tolerance test (Fig. 7A). They also showed increased insulin sensitivity 120 min after insulin injection during insulin tolerance test (Fig. 7B). On the other hand, glucose tolerance and insulin sensitivity were similar between the two genotypes on the high-cholesterol HFD (Fig. 7C, D). Consistently, the plasma insulin concentration was ∼43% lower in L1-KO relative to WT mice on the low-cholesterol HFD, but it did not differ between the two genotypes on the high-cholesterol HFD (Table 3). The plasma glucose concentrations were not significantly different between L1-KO and WT mice fasted for 4 h during the daytime cycle, regardless of dietary cholesterol (Table 3).
Fig. 7.
Glucose tolerance test (GTT) and insulin tolerance test (ITT) in L1-KO and WT mice fed the low-cholesterol, high-fat diet (HFD-C) or the high-cholesterol, high-fat diet (HFD+C). *P < 0.05 for L1-KO versus WT at the same time point (n = 5–7). HFD, high-fat diet; HFD-C, HFD containing a low amount cholesterol; HFD+C, HFD containing a high amount cholesterol; L1-KO, NPC1L1 knockout; WT, wild-type.
DISCUSSION
The major finding in this study is that NPC1L1 deficiency prevents diet-induced weight gain in a dietary cholesterol-dependent manner. Increasing dietary cholesterol content to a level similar to that in a typical Western diet is sufficient to rescue diet-induced weight gain in L1-KO mice on either HFD or HCD. Intriguingly, our data also show that increasing dietary cholesterol can raise plasma and liver cholesterol in mice deficient in intestinal cholesterol absorption, such as L1-KO mice, though to a much lesser extent compared with WT mice (Table 3). Further, addition of cholesterol to a HFD can significantly raise hepatic triglyceride content (Table 3) and intestinal ABCA1 mRNA levels (Fig. 4E) in both WT and L1-KO mice. These observations suggest that there is an NPC1L1-independent and perhaps passive pathway for intestinal cholesterol absorption. The relative contribution of this pathway to overall cholesterol absorption may be minor under normal physiological conditions, but it may play a critical role in rescuing weight gain in L1-KO mice. Perhaps the cholesterol deficit in the body has to be below a critical threshold to prevent diet-induced weight gain, and dietary supplementation of cholesterol somehow exceeds this threshold, even in the absence of the major NPC1L1-dependent cholesterol absorption pathway, thereby maintaining normal diet-induced weight gain in L1-KO mice. Alternatively, increased dietary cholesterol can substantially raise cholesterol content in the gut lumen of L1-KO mice because their intestinal cholesterol absorption is inhibited. During the movement to the feces for disposal (Fig. 4), this large amount of cholesterol is exposed to intestinal mucosa as well as luminal microbes, which may have rescued weight gain in L1-KO mice indirectly by modulating intestinal physiology, such as incretin secretion by intestinal endocrine cells, bile acid absorption, and colonization of gut microbes, all of which have been implicated in regulation of weight gain (48–51).
The weight gain difference was not observed in L1-KO and WT mice of both genders on mixed genetic background (75% C57BL/6 and 25% 129/OlaHsd) fed a Western-type diet containing 0.15% (w/w) cholesterol and 40% calorie from butter fat for 24 weeks (35). In the same study, NPC1L1 deletion also did not alter weight gain in male and female apolipoprotein E knockout mice on the same Western diet. Additionally, ezetimibe treatment did not reduce weight gain in mice and hamsters on a diet containing high amounts of cholesterol (2, 4, 32). All of these observations are consistent with our current data showing that L1-KO and WT mice on high-cholesterol diet gain weight similarly.
In this study, in addition to showing that L1-KO mice are protected against obesity induced by a low-cholesterol-containing HFD, which is consistent with previous observations (33, 34), we also demonstrated that L1-KO mice are protected against weight gain induced by a low-cholesterol diet enriched with carbohydrates, suggesting that the weight gain difference between L1-KO and WT mice is independent of dietary energy sources.
There are two published reports that appear to be contrary to our observations: (1) L1-KO male mice of mixed background (75% C57BL/6 and 25% 129/OlaHsd) on a standard chow diet containing <0.02% (w/w) cholesterol did not show reduced weight gain compared with WT males of the same genetic background (35); and (2) NPC1L1 inhibition by ezetimibe failed to reduce weight gain in C57BL/6 apolipoprotein E knockout mice fed a semisynthetic cholesterol-free diet (10% calorie as corn oil) (2). Although genetic background and gene-targeting strategy differences may have played a role in these discrepancies, a chow diet was used in the first study, which has different ingredients and does not usually promote adiposity. In the second study, hypercholesterolemic apolipoprotein E knockout mice were used, and the increased blood cholesterol concentration in these animals may have been above the aforementioned cholesterol deficit “threshold” needed for prevention of diet-induced weight gain.
In humans, NPC1L1 gene sequence variations are associated with intestinal cholesterol absorption efficiency, but they are not associated with body mass index in African Americans (22). The majority of clinical studies do not show an effect of NPC1L1 inhibition by ezetimibe on weight gain, body mass index, or waist circumference (7, 52, 53). In these studies, many factors may have confounded the results, including genetic background, ethnic groups, and exercise. According to our study, dietary cholesterol intake may be another important factor. Recently, a small clinical study reported significantly reduced weight gain after ezetimibe treatment in 38 nonobese Japanese males with hypercholesterolemia. Ezetimibe treatment for 4–8 weeks at 5 mg/day significantly reduced body weight, body mass index, and waist circumference in these subjects (34). Unfortunately, whether these individuals were under a dietary cholesterol control regimen is unclear, although cholesterol content is generally low in typical Japanese diets, and a low-cholesterol diet is commonly recommended for hypercholesterolemic patients.
It is currently unknown how NPC1L1 deficiency or ezetimibe treatment reduces weight gain in mice on a low-cholesterol diet. L1-KO mice have been reported to consume the same amount of food as WT controls on a diet containing 58% fat from coconut and no added cholesterol (33). Consistently, the food intake was similar between L1-KO and WT mice on the low-cholesterol HCD in this study. Although L1-KO mice versus WT mice on the low-cholesterol HFD appeared to eat less, the food intake normalized to body weight was even higher in L1-KO mice than WT mice (Fig. 5). Thus, decreased food intake does not seem to account for reduced weight gain in L1-KO mice or ezetimibe-treated mice on a low-cholesterol diet.
Another important factor profoundly influencing energy intake is fractional intestinal fat absorption. In one study, NPC1L1 deficiency or ezetimibe treatment was shown to slightly but significantly decrease intestinal absorption of total fat and saturated fatty acids in mice consuming a diet containing 58% fat from coconut (33). In the present study, however, despite a remarkably reduced weight gain in L1-KO mice relative to WT mice on our low-cholesterol HFD, the fractional intestinal absorption of total fat and all of the specific fatty acids examined did not differ between the two genotypes (Fig. 6). This discrepancy in fat absorption between the two studies may be attributable to differences in dietary fatty acid composition (coconut oil in the previous study versus lard in the present study). Nonetheless, our data demonstrated that reduced weight gain in L1-KO mice on our low-cholesterol HFD is not a result of decreased intestinal fat absorption.
In the present study, L1-KO versus WT mice on our low-cholesterol HFD showed reduced plasma concentrations of glucose and insulin (Fig. 7A and Table 3), and appeared to have improved systemic glucose tolerance and insulin sensitivity (Fig. 7A, B), which is consistent with a previous study (33). The molecular mechanism for improved insulin sensitivity in L1-KO mice is currently unknown, but it may be related to reduced hepatic steatosis and weight gain in these animals.
In conclusion, increasing dietary cholesterol rescues diet-induced obesity in NPC1L1-deficient mice. NPC1L1 may have evolved to promote fat storage in addition to intestinal cholesterol absorption. Dietary cholesterol has been shown to protect liver X receptor α and liver X receptor β double KO mice from weight gain (54). Although this observation is opposite to ours, it also suggests that dietary cholesterol regulates energy metabolism. Future studies are required to define how dietary cholesterol increases weight gain when intestinal cholesterol absorption is inhibited, and how NPC1L1 deficiency or ezetimibe treatment influences energy expenditure, particularly in animals on a low-cholesterol-containing diet.
Supplementary Material
Acknowledgments
The authors thank Drs. Yiannis A. Ioannou and Joanna P. Davies at Mount Sinai School of Medicine in New York for providing L1-KO mice. The authors also thank Dr. Jenna L. Betters for editing the manuscript.
Footnotes
Abbreviations:
- ABC
- ATP-binding cassette transporter
- HCD
- high-carbohydrate diet
- HFD
- high-fat diet
- L1-KO
- NPC1L1 knockout
- LXR
- liver X receptor
- NPC1L1
- Niemann-Pick C1-Like 1
- SREBP-1c
- sterol regulatory element-binding protein 1c
- WT
- wild-type
This work was supported in part by an American Heart Association Scientist Development Grant (L.Y.) and by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01 DK-085176 (L.Y.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and three tables.
REFERENCES
- 1.Rosenblum S. B., Huynh T., Afonso A., Davis H. R., Jr, Yumibe N., Clader J. W., Burnett D. A. 1998. Discovery of 1-(4-fluorophenyl)-(3R)-[3-(4-fluorophenyl)-(3S)-hydroxypropyl]-(4S)-(4 -hydroxyphenyl)-2-azetidinone (SCH 58235): a designed, potent, orally active inhibitor of cholesterol absorption. J. Med. Chem. 41: 973–980. [DOI] [PubMed] [Google Scholar]
- 2.Davis H. R., Jr, Compton D. S., Hoos L., Tetzloff G. 2001. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 21: 2032–2038. [DOI] [PubMed] [Google Scholar]
- 3.Davis H. R., Jr, Pula K. K., Alton K. B., Burrier R. E., Watkins R. W. 2001. The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor, ezetimibe, in combination with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors in dogs. Metabolism. 50: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 4.van Heek M., Austin T. M., Farley C., Cook J. A., Tetzloff G. G., Davis H. R. 2001. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes. 50: 1330–1335. [DOI] [PubMed] [Google Scholar]
- 5.van Heek M., Compton D. S., Davis H. R. 2001. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur. J. Pharmacol. 415: 79–84. [DOI] [PubMed] [Google Scholar]
- 6.van Heek M., Farley C., Compton D. S., Hoos L., Davis H. R. 2001. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br. J. Pharmacol. 134: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagne C., Bays H. E., Weiss S. R., Mata P., Quinto K., Melino M., Cho M., Musliner T. A., Gumbiner B. 2002. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am. J. Cardiol. 90: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 8.Davidson M. H., McGarry T., Bettis R., Melani L., Lipka L. J., LeBeaut A. P., Suresh R., Sun S., Veltri E. P. 2002. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J. Am. Coll. Cardiol. 40: 2125–2134. [DOI] [PubMed] [Google Scholar]
- 9.Sudhop T., Lutjohann D., Kodal A., Igel M., Tribble D. L., Shah S., Perevozskaya I., von Bergmann K. 2002. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 106: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 10.Salen G., Ahrens E. H., Jr, Grundy S. M. 1970. Metabolism of beta-sitosterol in man. J. Clin. Invest. 49: 952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D. Q. 2007. Regulation of intestinal cholesterol absorption. Annu. Rev. Physiol. 69: 221–248. [DOI] [PubMed] [Google Scholar]
- 12.Bosner M. S., Lange L. G., Stenson W. F., Ostlund R. E., Jr 1999. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 40: 302–308. [PubMed] [Google Scholar]
- 13.Turley S. D., Dietschy J. M. 2003. Sterol absorption by the small intestine. Curr. Opin. Lipidol. 14: 233–240. [DOI] [PubMed] [Google Scholar]
- 14.Altmann S. W., Davis H. R., Jr, Zhu L. J., Yao X., Hoos L. M., Tetzloff G., Iyer S. P., Maguire M., Golovko A., Zeng M., et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 15.Sane A. T., Sinnett D., Delvin E., Bendayan M., Marcil V., Menard D., Beaulieu J. F., Levy E. 2006. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J. Lipid Res. 47: 2112–2120. [DOI] [PubMed] [Google Scholar]
- 16.Davies J. P., Levy B., Ioannou Y. A. 2000. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 65: 137–145. [DOI] [PubMed] [Google Scholar]
- 17.Yu L., Bharadwaj S., Brown J. M., Ma Y., Du W., Davis M. A., Michaely P., Liu P., Willingham M. C., Rudel L. L. 2006. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J. Biol. Chem. 281: 6616–6624. [DOI] [PubMed] [Google Scholar]
- 18.Temel R. E., Tang W., Ma Y., Rudel L. L., Willingham M. C., Ioannou Y. A., Davies J. P., Nilsson L. M., Yu L. 2007. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Invest. 117: 1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis H. R., Jr, Zhu L. J., Hoos L. M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S. P., Lam M. H., Lund E. G., et al. 2004. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279: 33586–33592. [DOI] [PubMed] [Google Scholar]
- 20.Tobert J. A. 2003. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2: 517–526. [DOI] [PubMed] [Google Scholar]
- 21.Endo A. 1992. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 33: 1569–1582. [PubMed] [Google Scholar]
- 22.Cohen J. C., Pertsemlidis A., Fahmi S., Esmail S., Vega G. L., Grundy S. M., Hobbs H. H. 2006. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc. Natl. Acad. Sci. USA. 103: 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegele R. A., Guy J., Ban M. R., Wang J. 2005. NPC1L1 haplotype is associated with inter-individual variation in plasma low-density lipoprotein response to ezetimibe. Lipids Health Dis. 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J., Williams C., Hegele R. 2005. Compound heterozygosity for two non-synonymous polymorphisms in NPC1L1 in a non-responder to ezetimibe. Clin. Genet. 67: 175–177. [DOI] [PubMed] [Google Scholar]
- 25.Simon J. S., Karnoub M. C., Devlin D. J., Arreaza M. G., Qiu P., Monks S. A., Severino M. E., Deutsch P., Palmisano J., Sachs A. B., et al. 2005. Sequence variation in NPC1L1 and association with improved LDL-cholesterol lowering in response to ezetimibe treatment. Genomics. 86: 648–656. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Calvo M., Lisnock J., Bull H. G., Hawes B. E., Burnett D. A., Braun M. P., Crona J. H., Davis H. R., Jr, Dean D. C., Detmers P. A., et al. 2005. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA. 102: 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinglass A. B., Kohler M., Schulte U., Liu J., Nketiah E. O., Thomas A., Schmalhofer W., Williams B., Bildl W., McMasters D. R., et al. 2008. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl. Acad. Sci. USA. 105: 11140–11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies J. P., Scott C., Oishi K., Liapis A., Ioannou Y. A. 2005. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 280: 12710–12720. [DOI] [PubMed] [Google Scholar]
- 29.Assy N., Grozovski M., Bersudsky I., Szvalb S., Hussein O. 2006. Effect of insulin-sensitizing agents in combination with ezetimibe, and valsartan in rats with non-alcoholic fatty liver disease. World J. Gastroenterol. 12: 4369–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deushi M., Nomura M., Kawakami A., Haraguchi M., Ito M., Okazaki M., Ishii H., Yoshida M. 2007. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett. 581: 5664–5670. [DOI] [PubMed] [Google Scholar]
- 31.Nozaki Y., Fujita K., Yoneda M., Wada K., Shinohara Y., Takahashi H., Kirikoshi H., Inamori M., Kubota K., Saito S., et al. 2009. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J. Hepatol. 51: 548–556. [DOI] [PubMed] [Google Scholar]
- 32.Zheng S., Hoos L., Cook J., Tetzloff G., Davis H., Jr, van Heek M., Hwa J. J. 2008. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur. J. Pharmacol. 584: 118–124. [DOI] [PubMed] [Google Scholar]
- 33.Labonte E. D., Camarota L. M., Rojas J. C., Jandacek R. J., Gilham D. E., Davies J. P., Ioannou Y. A., Tso P., Hui D. Y., Howles P. N. 2008. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G776–G783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagi S., Akaike M., Aihara K., Iwase T., Ishikawa K., Yoshida S., Sumitomo-Ueda Y., Kusunose K., Niki T., Yamaguchi K., et al. 2010. Ezetimibe ameliorates metabolic disorders and microalbuminuria in patients with hypercholesterolemia. J. Atheroscler. Thromb. 17: 173–180. [DOI] [PubMed] [Google Scholar]
- 35.Davis H. R., Jr, Hoos L. M., Tetzloff G., Maguire M., Zhu L. J., Graziano M. P., Altmann S. W. 2007. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler. Thromb. Vasc. Biol. 27: 841–849. [DOI] [PubMed] [Google Scholar]
- 36.Tang W., Ma Y., Jia L., Ioannou Y. A., Davies J. P., Yu L. 2008. Niemann-Pick C1-like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler. Thromb. Vasc. Biol. 28: 448–454. [DOI] [PubMed] [Google Scholar]
- 37.Tang W., Ma Y., Jia L., Ioannou Y. A., Davies J. P., Yu L. 2009. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J. Lipid Res. 50: 293–300. [DOI] [PubMed] [Google Scholar]
- 38.Repa J. J., Buhman K. K., Farese R. V., Jr, Dietschy J. M., Turley S. D. 2004. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 40: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 39.Yang J., Goldstein J. L., Hammer R. E., Moon Y. A., Brown M. S., Horton J. D. 2001. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. USA. 98: 13607–13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang W., Ma Y., Yu L. 2006. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 44: 1259–1266. [DOI] [PubMed] [Google Scholar]
- 41.Jandacek R. J., Heubi J. E., Tso P. 2004. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology. 127: 139–144. [DOI] [PubMed] [Google Scholar]
- 42.Repa J. J., Turley S. D., Lobaccaro J. A., Medina J., Li L., Lustig K., Shan B., Heyman R. A., Dietschy J. M., Mangelsdorf D. J. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 289: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 43.Repa J. J., Liang G., Ou J., Bashmakov Y., Lobaccaro J. M., Shimomura I., Shan B., Brown M. S., Goldstein J. L., Mangelsdorf D. J. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14: 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Repa J. J., Berge K. E., Pomajzl C., Richardson J. A., Hobbs H., Mangelsdorf D. J. 2002. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 277: 18793–18800. [DOI] [PubMed] [Google Scholar]
- 45.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775. [DOI] [PubMed] [Google Scholar]
- 46.Deng X., Yellaturu C., Cagen L., Wilcox H. G., Park E. A., Raghow R., Elam M. B. 2007. Expression of the rat sterol regulatory element-binding protein-1c gene in response to insulin is mediated by increased transactivating capacity of specificity protein 1 (Sp1). J. Biol. Chem. 282: 17517–17529. [DOI] [PubMed] [Google Scholar]
- 47.Vock C., Doring F., Nitz I. 2008. Transcriptional regulation of HMG-CoA synthase and HMG-CoA reductase genes by human ACBP. Cell. Physiol. Biochem. 22: 515–524. [DOI] [PubMed] [Google Scholar]
- 48.Majumdar I. D., Weber H. C. 2010. Gastrointestinal regulatory peptides and their effects on fat tissue. Curr. Opin. Endocrinol. Diabetes Obes. 17: 51–56. [DOI] [PubMed] [Google Scholar]
- 49.Badman M. K., Flier J. S. 2005. The gut and energy balance: visceral allies in the obesity wars. Science. 307: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., et al. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 439: 484–489. [DOI] [PubMed] [Google Scholar]
- 51.Turnbaugh P. J., Gordon J. I. 2009. The core gut microbiome, energy balance and obesity. J. Physiol. 587: 4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bays H. E., Ose L., Fraser N., Tribble D. L., Quinto K., Reyes R., Johnson-Levonas A. O., Sapre A., Donahue S. R. 2004. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin. Ther. 26: 1758–1773. [DOI] [PubMed] [Google Scholar]
- 53.Feldman T., Davidson M., Shah A., Maccubbin D., Meehan A., Zakson M., Tribble D., Veltri E., Mitchel Y. 2006. Comparison of the lipid-modifying efficacy and safety profiles of ezetimibe coadministered with simvastatin in older versus younger patients with primary hypercholesterolemia: a post hoc analysis of subpopulations from three pooled clinical trials. Clin. Ther. 28: 849–859. [DOI] [PubMed] [Google Scholar]
- 54.Kalaany N. Y., Gauthier K. C., Zavacki A. M., Mammen P. P., Kitazume T., Peterson J. A., Horton J. D., Garry D. J., Bianco A. C., Mangelsdorf D. J. 2005. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 1: 231–244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.