Abstract
Group VIB Ca2+-independent phospholipase A2γ (iPLA2γ) is a membrane-bound iPLA2 enzyme with unique features, such as the utilization of distinct translation initiation sites and the presence of mitochondrial and peroxisomal localization signals. Here we investigated the physiological functions of iPLA2γ by disrupting its gene in mice. iPLA2γ-knockout (KO) mice were born with an expected Mendelian ratio and appeared normal and healthy at the age of one month but began to show growth retardation from the age of two months as well as kyphosis and significant muscle weakness at the age of four months. Electron microscopy revealed swelling and reduced numbers of mitochondria and atrophy of myofilaments in iPLA2γ-KO skeletal muscles. Increased lipid peroxidation and the induction of several oxidative stress-related genes were also found in the iPLA2γ-KO muscles. These results provide evidence that impairment of iPLA2γ causes mitochondrial dysfunction and increased oxidative stress, leading to the loss of skeletal muscle structure and function. We further found that the compositions of cardiolipin and other phospholipid subclasses were altered and that the levels of myoprotective prostanoids were reduced in iPLA2γ-KO skeletal muscle. Thus, in addition to maintenance of homeostasis of the mitochondrial membrane, iPLA2γ may contribute to modulation of lipid mediator production in vivo.
Keywords: cardiolipin, mitochondria, muscle, phospholipids
Phospholipase A2 (PLA2) enzymes catalyze the cleavage of the sn-2 ester bond of glycerophospholipids to yield free fatty acids and lysophospholipids, thereby playing critical roles in cellular lipid metabolisms linked to energy storage, membrane remodeling, and lipid mediator signaling. In the membrane remodeling reaction, fatty acyl groups are first removed (deacylated) by PLA2 and then replaced (reacylated) with different fatty acyl groups by acyltransferases, which allow membrane phospholipids to acquire a variation of molecular species. In the signaling reaction, polyunsaturated fatty acids [typically arachidonic acid (AA)] and lysophospholipids released by the action of PLA2s are metabolized to various lipid mediators, such as prostaglandins (PG), leukotrienes, and platelet-activating factor, which exert a variety of biological actions through their cognate receptors.
PLA2 enzymes have been classified into five major families: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), Ca2+-independent PLA2 (iPLA2), platelet-activating factor acetylhydrolase, and lysosomal PLA2, each of which occurs as multiple isoforms (1–3). Among these, the cPLA2 and iPLA2 families represent intracellular enzymes with a catalytic serine in their lipase consensus motif and are thought to diverge from a common ancestral gene (3). Currently, nine members of the iPLA2 family, also referred to as the patatin-like phospholipase-domain-containing family, have been identified. These iPLA2 isoforms share sensitivity to 4-bromoenol lactone, a mechanism-based irreversible inhibitor (4). Among them, two abundant isoforms of iPLA2 are thought to play important roles in the deacylation of cellular phospholipids. The first one is group VIA iPLA2 (also called iPLA2β) (5, 6), which was initially assumed to be the housekeeping enzyme responsible for phospholipid acyl group turnover and generation of the lysophospholipids necessary for AA incorporation (7). Subsequent studies employing iPLA2β-knockdown cells and knockout (KO) mice have provided evidence that iPLA2β plays roles not only in phospholipid homeostasis but also in signaling in diverse biological events (8–16). The second isoform of iPLA2, group VIB iPLA2 (also called iPLA2γ), was identified by a search of the expressed sequence tag database for sequences homologous to iPLA2β. The gene for iPLA2γ encodes a full-length 88 kDa protein, yet it can also be transcribed to N-terminally truncated 63, 74, and 77 kDa forms due to the utilization of distinct translation initiation sites (17). iPLA2γ has been reported to be localized in mitochondria, peroxisomes, and the endoplasmic reticulum. Because of this localization in organelles related to fatty acid β-oxidation and synthesis, iPLA2γ has been postulated to participate in the homeostatic lipid catabolism and turnover linked to bioenergic processes.
Recent gene-targeting studies of iPLA2γ have unveiled a particular role of this enzyme in the metabolism of cardiolipin (CL), a critical mitochondrial phospholipid that facilitates protein supercomplex formation in the mitochondrial inner membrane, thereby allowing optimal electron transport chain function (18, 19). Genetic ablation of iPLA2γ resulted in the generation of viable progeny that demonstrated decreased growth, cold intolerance due to impaired fat burning in brown adipose tissue, and defects in ascorbate-stimulated mitochondrial Complex IV function in the myocardium (18). Furthermore, these knockout mice also showed alterations in hippocampal CL content, mitochondrial degeneration, and cognitive dysfunction (19). These reports suggest that iPLA2γ, a mitochondrial iPLA2, regulates mitochondrial inner membrane lipid metabolism, perturbation of which may profoundly influence fatty acid β-oxidation, oxygen consumption, energy expenditure, and thus, tissue homeostasis.
In addition to its bioenergetic functions, the signaling role of iPLA2γ has been revealed by several in vitro studies. For instance, overexpression of iPLA2γ has been shown to promote spontaneous and agonist-stimulated release of AA, which is converted to PGE2 with preferred cyclooxygenase (COX)-1 coupling in HEK293 cells (20). The induction of group IIA sPLA2 by pro-inflammatory stimuli has been shown to require iPLA2γ through production of certain lipid metabolite(s) in rat fibroblastic 3Y1 cells (21). In addition, iPLA2γ could produce 2-arachidonoyl-lysphosphatidylcholine, a presumptive lipid mediator, through its PLA1 action (22). Although these observations suggest that iPLA2γ is able not only to regulate the remodeling of lipid membranes but also to modify the production of lipid mediator(s), no in vivo evidences for its role in such a signaling process have been obtained.
In this study, we established an additional line of iPLA2γ-deficient mice and found that the mice showed growth retardation and reduced exercise capacity, which had also been demonstrated for the initial line of null mice (18, 19). We found that this phenotype was caused by skeletal muscle atrophy. Analysis of the skeletal muscle showed decreased mitochondrial number, markedly enlarged and swollen mitochondria, and mitochondrial dysfunction accompanied by increasing oxidative stress in iPLA2γ-deficient mice. We further found that the compositions of CL and other phospholipid subclasses were altered, and the levels of PGF2α and PGD2 were reduced in iPLA2γ-knockout skeletal muscle. Therefore, in addition to its maintenance of homeostasis of the mitochondrial membrane, iPLA2γ may also contribute to modulation of lipid mediator production in vivo.
EXPERIMENTAL PROCEDURES
Materials
Collagenase type II was obtained from Worthington Biochemicals (Lakewood, NJ). Fetal calf serum (FCS) and horse serum (HS) were obtained from Bioserum (Middlesex, UK). Hank's balanced salt solution (HBSS), basic fibroblast growth factor, penicillin-streptomycin solution and HamF10 medium were obtained from Gibco/Invitrogen (Grand Island, NY). Dulbecco's modified Eagle's medium (DMEM) was obtained from Nissui Pharmaceutical (Tokyo, Japan). Rabbit polyclonal antibody against rat cytosolic PGE synthase (cPGES; p23) and rabbit polyclonal antibody against human iPLA2γ were prepared as described previously (21, 23). Mouse monoclonal antibody against human α-tubulin and horseradish peroxidase-conjugated anti-IgG antibodies were purchased from Zymed Laboratories. (South San Francisco, CA). Rabbit polyclonal antibody against human manganese superoxide dismutase (MnSOD/SOD2) and goat polyclonal antibody against human F1-ATPase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies against chicken α-actinin and indomethacin were purchased from Sigma Chemical (St. Louis, MO). Mouse monoclonal antibody against human porin and mouse skeletal muscle C2C12 myoblast cells were obtained from Prof. M. Shibanuma (Showa University, Tokyo, Japan), and the cells were maintained in DMEM with 10% FCS. All other reagents were from Sigma Chemical.
Mice
iPLA2γ-KO mice were produced by and obtained from TransGenic, Inc. (Kumamoto, Japan). The iPLA2γ gene was disrupted by gene-trapping methods (24), in which a gene cassette consisting of a splicing acceptor (SA)-βgeo-pA-pSP73 was integrated between exons 1 and 2 (supplementary Fig. IA). The SA contains three stop codons inframed with the ATG of the β-galactosidase/neomycin-resistance fusion gene (βgeo), which can function in promoter trapping. These mice were further backcrossed 10 generations onto a C57BL/6 background. All mice were housed in climate-controlled (21°C), specific pathogen-free facilities with a 12 h light/dark cycle, with free access to standard laboratory food (Picolab mouse diet 20; Laboratory Diet, Brentwood, MO) and water. All procedures involving animals were performed under approved institutional guidance. The genotypes of iPLA2γ+/+ and iPLA2γ−/− were confirmed by polymerase chain reaction (PCR) using tail DNA as a template and a set of the sense primer 5′-CCGTGACTACTGCCTGCGT-3′ and the antisense-1 primer 5′-CAAGCGATTGGGAGTGAGTTGG-3′, which amplified a 1409-bp fragment in iPLA2γ+/+ wild-type (WT) mice, or a set of the sense primer and the antisense-2 primer 5′-CTGGAGAAGGCCCGACCATC-3′, which amplified a 745-bp fragment in iPLA2γ−/− mice.
Computed tomography analysis
Muscle volume was analyzed in the four-month-old WT and iPLA2γ-KO mice using computed tomography (CT) systems (eXplore Locus; GE Healthcare, London, ON, Canada). Mice were anesthetized with 2% isoflurane (Dainippon Sumitomo Pharmaceutical Co. Ltd., Osaka, Japan) and scanned for 10 min under the following conditions: resolving power, 93 μm; view number, 400; voltage, 80 kVp; and electric current, 450 μA. CT images were analyzed using MicroView 2.0 software (GE Healthcare). For microCT analysis, mice were kept in a lateral position on a microCT SM90-CT (Shimadzu Co., Kyoto, Japan). The angles of spine curvature were determined by TRI/3D-BON software.
SDS-PAGE and Western blotting
Tissue homogenates or cell lysates (10 μg protein equivalents) were subjected to SDS-PAGE using 7.5% or 12% gels under reducing conditions. The separated proteins were electroblotted onto nitrocellulose membranes (Schleicher and Schuell, Keene, NH) with a semidry blotter (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. After blocking with 5% (w/v) skim milk in 10 mM Tris-HCl, pH 7.4, containing 150 mM NaCl and 0.05% Tween 20, the membranes were probed with the respective antibodies (1:5,000 dilution) for 2 h, followed by incubation with horseradish peroxidase-conjugated anti-mouse (1:5,000 for α-tubulin and porin), anti-rabbit (1:5,000 for SOD2, iPLA2γ, cPGES, and α-actinin), or anti-goat (1:10,000 for F1-ATPase) IgG. After being washed, the membranes were visualized with Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer Life Sciences, Boston, MA) as described previously (21).
Histology
For transmission electron microscopy, skeletal muscle tissue was fixed with 2.5% glutaraldehyde for 16 h at 4°C, postfixed with 2% OsO4 for 2 h at 4°C, dehydrated by an ascending ethanol series, passed through propylene oxide, and then embedded in Quetol812 resin (Nisshin EM, Tokyo, Japan). Ultrathin sections (90 nm thick) on mesh grids were stained with uranyl acetate and lead acetate and examined with an H-300 electron microscope (Hitachi, Tokyo, Japan). For histopathology, tissue sections were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin-eosin by a standard method. These paraffin sections were then additionally stained with Masson trichrome and Victoria blue (Wako) to examine the degree of organization and the fibrosis. Finally, the sections were stained with TUNEL to detect degenerating cells according to the manufacturer's instructions (Apoptag Plus Peroxidase In Situ Apoptosis Detection Kit; Chemicon International, Temecula, CA).
Primary myocytes culture
Primary myocytes were cultured from fore- and hindlimbs of iPLA2γ-KO and WT mice. The skeletal muscle was excised, minced, and then incubated at 37°C for 30 min with 2 ml of HBSS containing 0.2% (w/v) collagenase type II in HBSS per gram of tissue. Collagenase digestion was halted by the addition of ice-cold phosphate-buffered saline (PBS). The tissue slurry was then strained through a 100-μm filter and then a 40-μm filter (Becton Dickinson, Bedford, MA) and centrifuged at 500 g for 5 min. The pellet was resuspended in hypotonic solution [0.83% (w/v) NH4Cl: 0.17 M Tris-HCl pH 7.65 = 9:1], and after the reaction was stopped by the addition of PBS, it was centrifuged at 500 g for 5 min. The pellet was resuspended in growth medium (HamF10 medium containing 20% FCS, 2.5 ng/ml basic fibroblast growth factor, and 100 U/ml penicillin/100 μg/ml streptomycin), and the cells were plated in plastic dishes overnight at 37°C under 5% CO2. The unattached cells were seeded in collagen type-I-coated dishes (Iwaki Glass, Tokyo, Japan). The medium was changed every 2–3 days. After 1 or 2 weeks, when the adhering cells reached 70–80% confluence, they were dispersed by trypsinization and plated on collagen type-I-coated dishes in growth medium. After 2 or 3 days, myoblast fusion was induced by shifting the cells to a differentiation medium (DMEM supplemented with 5% HS and 100 U/ml penicillin/100 μg/ml streptomycin) for 3–6 days.
Behavioral testing
Each animal's grip was monitored by the wire hang test, also known as the wire-mesh test (13). The animals were placed on lattice covers held horizontally. The covers were first turned upright for 20 s and then upside down for an additional 120 s. Each animal was individually tested in two trials. The time at which the animal lost its grip was recorded.
Knockdown of iPLA2γ
siRNAs [Silencer predesigned siRNA iPLA2γ-specific (ID #295428) and Silencer control siRNA (Applied Biosystems, Cambridge, MA)] were transfected into C2C12 cells with LipofectamineTM RNAiMAX Reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Three days after transfection, the cells were used for the analyses.
Determination of tissue ATP content
Tissue or cell ATP content was determined using an ATP assay kit of tissues (TOYO Ink Co., Tokyo, Japan). Briefly, tissue pieces (100 mg) or cell lysate were homogenized in 10 ml homogenate buffer (0.25 M sucrose in 10 mM HEPES-NaOH, pH 7.4) and centrifuged at 1,000 g at 4°C for 10 min. Then 700 μl of homogenate buffer was added to 100 μl of the upper phase after the extraction of ATP and assayed using the ATP assay kit.
Quantitative RT-PCR
Total RNA was extracted from the thigh muscles of four-month-old WT and KO mice (n = 7) with TRIzol reagent (Invitrogen). First-strand cDNA synthesis was conducted by using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Then 100 ng of synthesized cDNA was used as a template for the quantitative RT-PCR (Q-PCR) reactions. Q-PCR was performed using a StepOne Real-time PCR System (Applied Biosystems) with SYBR Green Reagent (Applied Biosystems) according to the manufacturer's instructions.
The primer pairs were 5′-CTCTATCGAAAGTTGGGCTCAGA-3′ and 5′-TCCCACGTGTTACTGTCATAAAAC-3′ for mouse iPLA2γ (Pnpla8); 5′-CGGACGCCTCGTCAACA-3′ and 5′-CGGAATGGGTTCGAGAACAA-3′ for mouse iPLA2β (Pnpla9); 5′-CCCTGAGTAGTTTGAAGGAAAAGG-3′ and 5′-ACACGTGAAGAGAGGCAAAGG-3′ for mouse cPLA2α (pla2g4a); 5′-TTCGTATTGCGCCGCTAGA-3′ and 5′-CTTTCGCTCTGGTCCGTCTT-3′ for mouse 18s rRNA (rRNA); 5′-GGTCCTCTAAGCGTCACCAC-3′ and 5′-GAGCAGTTGGGGTCCATTC-3′ for mouse metallothionein-1 (Mt1); 5′-GCCCGCATGCAGATCCT-3′ and 5′-GGTCTCCTCCCAGACGGTTT-3′ for mouse NAD(P)H dehydrogenase, quinone 1 (Nqo1); 5′-GGTGATGCTGACAGAGGAACAC-3′ and 5′–TCTGACGAAGTGACGCCATCT-3′ for mouse heme oxygenase-1 (Hmox1); 5′-CCTGCCTGTGTGCTTACAACTG-3′ and 5′-GGTCCCGCCCGTCACT-3′ for mouse atrogin-1 (Fbxo32); 5′-TTGACGGACCCCAAAAGATG-3′ and 5′-TGGACAGCCCAGGTCAAAG-3′ for mouse interleukin (IL)-1β (Il1b); 5′-CCACGGCCTTCCCTACTTC-3′ and 5′-TTGGGAGTGGTATCCTCTGTGA-3′ for mouse IL-6 (Il6); and 5′-CAGCCGATGGGTTGTACCTT-3′ and 5′-GGCAGCCTTGTCCCTTGA-3′ for mouse tumor necrosis factor (TNF)-α (tnf).
Lipid peroxidation assay
The quantification of malondialdehyde (MDA) was performed according to the protocol for the BIOXYTECH LPO-586 kit (Oxis International, Portland, OR). A 20–30% (w/v) homogenate of skeletal muscle or C2C12 cells was prepared in 20 mM Tris buffer, pH 7.4, containing 5 mM butylated hydroxytoluene to prevent sample oxidation. Following centrifugation at 3,000 g at 4°C for 10 min, the LPO-586 R1 reagent, N-methyl-2-phenylindole in 25% methanol/75% acetonitrile, was added to the supernatants, followed by the addition of 12 N HCI and incubation at 45°C for 60 min. Following centrifugation at 15,000 g at 4°C for 10 min, the absorbance was read at 586 nm. The protein concentration was measured using a Bio-Rad protein assay according to the manufacturer's instructions.
Thin-layer chromatography
Lipids were extracted from tissue by the method of Bligh and Dyer (25). Thin-layer chromatography (TLC) plates (Silica gel 60A; Merck KGaA, Darmstadt, Germany) were washed twice with chloroform-methanol (1:1, v/v) and activated at 120°C before use. Total lipid extracts were separated on TLC silica gel plates (20 × 20 cm, layer thickness 0.2 mm). The plates were developed with a solvent system of chloroform-methanol-acetic acid-water (85:15:10:3.5, v/v). The developed TLC plates were then stained with iodine vapor to visualize the phospholipids. After scraping the silica in bands of phosphatidylcholine (PC), phosphatidylethanolamine (PE), CL, and phosphatidylglycerol from the plates, the lipids were extracted from the silica two times by the method of Bligh and Dyer. Individual phospholipids were quantified by a molybdenum blue method (26).
Mass spectrometric analysis
Lipids were extracted from tissue by the method of Bligh and Dyer. Before lipid extraction, PC with C28:0 (14:0-14:0; m/z = 678) was added to each sample as an internal standard (2 nmol per tissue). The ESI/MS analysis was performed using a 4000Q-TRAP quadrupole-linear ion trap hybrid mass spectrometer (Applied Biosystems/MDS Sciex) with an Ultimate 3000 HPLC system (Dionex, Sunnyvale, CA) combined with an HTC PAL autosampler (CTC Analytics, Zwingen, Switzerland). The extracted lipids were subjected to ESI/MS analysis by flow injection (3 nmol phosphorus equivalent) without liquid chromatography separation. The mobile phase composition was acetonitrile/methanol/water (6:7:2, v/v/v) (plus 0.1% ammonium formate, pH 6.8) at a flow rate of 10 µl/min. The scan range of the instrument was set at m/z 200–1000 at a scan speed of 1000 Da/s. The trap fill-time was set at 1 ms in the positive ion mode. The ion spray voltage and the declustering potential were set at 5,500 V and 100 V, respectively. Nitrogen was used as the curtain gas (setting of 10 arbitrary units) and the collision gas (set to “high”).
Assays for prostanoids
For measurement of prostanoids, mice were euthanized, and tissue pieces (100 mg) were homogenized with HBSS containing 10 μM indomethacin. The homogenates were adjusted to pH 3.0 with 1 N HCl, passed through Sep-Pak C18 cartridges (Waters, Milford, MA), and the retained PGs were eluted from the cartridges with 8 ml of methanol, as described previously (27). A trace amount of [3H] PGE2 (Cayman Chemical Co., Ann Arbor, MI) was added to the samples before passage through the cartridges to calibrate the recovery of PGs. The solvent of the samples was evaporated, and PGs were dissolved in an aliquot of buffer and assayed with commercial enzyme immunoassay kits for each prostanoid. The enzyme immunoassay kits for PGE2, 6-keto-PGF1α (a stable end product of PGI2), PGF2α, PGD2, and thromboxane B2 (TXB2) (a stable end product of TXA2) were purchased from Cayman Chemical Co.
Statistics
Data were statistically evaluated by unpaired Student's t–test, and values of P < 0.05 were considered to indicate statistical significance.
RESULTS
Generation of iPLA2γ-KO mice
The iPLA2γ gene was disrupted as shown in supplementary Fig. IA. Mice that were homozygous for the targeted mutation (KO, iPLA2γ−/−) were generated by the intercross of heterozygous animals (iPLA2γ+/−). Among the first 527 progenies of these heterozygous crosses, 145 (27.5%) were iPLA2γ+/+, 261 (49.5%) were iPLA2γ+/−, and 120 (23%) were iPLA2γ−/−. The number of iPLA2γ−/− animals corresponded to that expected for simple Mendelian inheritance, suggesting that the absence of iPLA2γ does not adversely affect intrauterine development or perinatal survival. To determine whether the skeletal muscle contains iPLA2γ and whether iPLA2γ was indeed knocked out in the null mice, its mRNA expression and protein in thigh muscles isolated from WT and iPLA2γ-KO mice was analyzed by Q-PCR and Western blotting, respectively. The expression of iPLA2γ mRNA (supplementary Fig. IB, left) and 88 kDa (as well as minor 77 and 63 kDa) immunoreactive iPLA2γ protein (supplementary Fig. IC) was detected in the muscle of WT mice but not in that of KO mice, verifying the presence of iPLA2γ (mainly as mitochondrial forms, judging from the molecular masses (20, 28)) in the skeletal muscle of WT mice and its successful ablation in KO mice. Conversely, the mRNA expression levels of other intracellular PLA2, cPLA2α and iPLA2β, were increased in iPLA2γ-KO muscle (supplementary Fig. IB, middle and right).
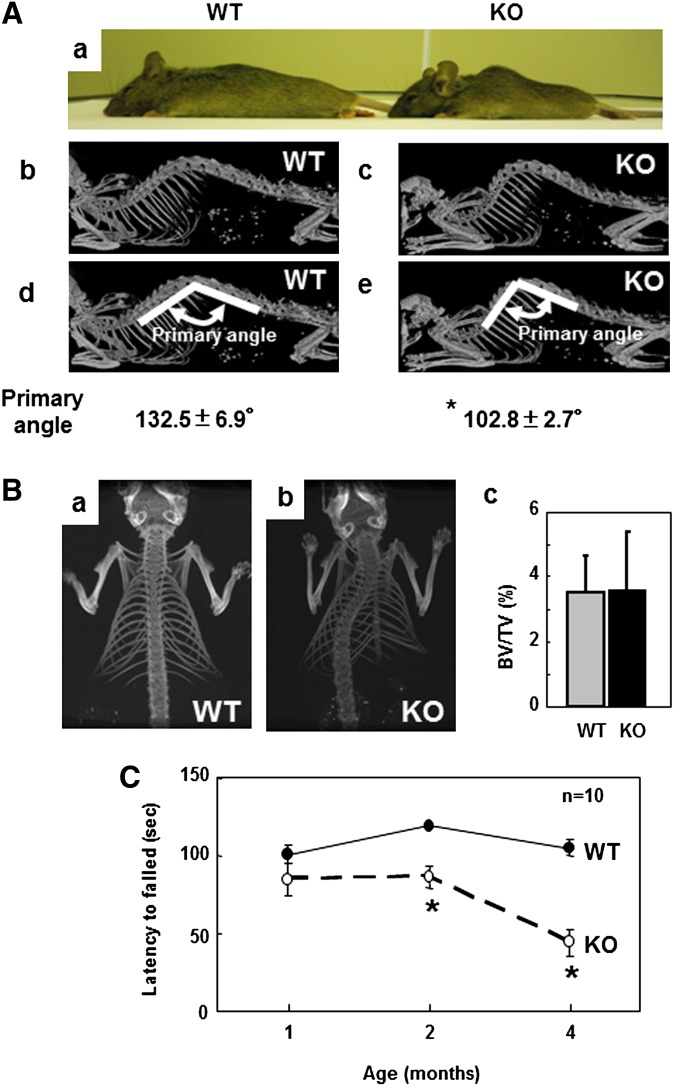
At birth, iPLA2γ-KO mice were indistinguishable from their WT littermates. However, the body weights of iPLA2γ-KO male mice after 10 weeks of age and those of female mice after 4 weeks of age were significantly lower than those of their WT littermates (supplementary Fig. ID, a and b). The weights of heterozygous iPLA2γ+/− mice were nearly equal to those of their WT littermates. We further found that, at two months (eight weeks) of age, the body lengths of iPLA2γ-KO mice, both male and female, were shorter than those of their WT littermates (supplementary Fig. IE, a and b). By four months of age, both male and female iPLA2γ-KO mice showed progressive kyphosis (curvature of the upper spine) (Fig. 1A). Whole-body microCT analysis revealed that iPLA2γ-KO mice exhibited a dorsal hump arising from an increased backward curvature of the spine (Fig. 1A, b and c). To quantify this morphological observation, the angles of spine curvature were determined. The angle formed in WT mice was approximately 132°, whereas it was reduced by greater than 20% in iPLA2γ-KO mice (Fig. 1A, d and e). Furthermore, CT analysis in a face-up position revealed that iPLA2γ-KO mice showed scoliosis (where the spine is curved from side to side) (Fig. 1B, a and b), which appeared by four weeks of age. Since kyphosis and scoliosis have often been found in mutant mice harboring bone defects (29–32), we further analyzed the bone density by microCT analysis; however, we found no obvious difference in bone volume/tissue volume (BV/TV) between WT and KO mice (Fig. 1B, c). Hence, we concluded that iPLA2γ-KO mice were smaller in size (both weight and length) than WT mice and had severe kyphosis and scoliosis without impairment of bone development.
Fig. 1.
Muscle weakness in iPLA2γ-KO mice. A: Direct photography of a lateral view of WT [left (a)] and KO [right (a)] mice after anesthesia. MicroCT of a lateral view of WT [(b) and (d)] and KO [(c) and (e)] mice. In (d) and (e), the primary angles were determined at the intersection of the two lines. Quantitative data are means ± SE. * P < 0.05 versus WT (n = 3). B: CT scans of a face-up view of WT (a) and KO (b) mice from a dorsal side. In (c), trabecular bone volume/tissue volume (BV/TV) of WT (gray bar) and KO (black bar) mice was evaluated (n = 3). Quantitative data are means ± SE. C: Hanging wire grip test. WT (closed circles, n = 10) and KO (open circles, n = 10) mice at one , two, and four months of age were tested. Quantitative data are means ± SE. *P < 0.001 versus WT. Abbreviations: CT, computed tomography; iPLA2, calcium-independent PLA2; KO, knockout; WT, wild-type.
By the age of four months, almost all of the iPLA2γ-KO mice showed abnormal movement of their hindlimbs. We assessed muscular strength in two different ways. First, each mouse was placed on an upside-down beaker. WT mice struggled to stay on their feet so as not to fall off the slippery beaker. In contrast, iPLA2γ-KO mice fell sooner because they could not stay on their feet (supplementary Video I). Second, we performed a hanging wire grip test, in which each mouse was placed on a wire net that was then turned upside down, and the latency time until the animals fell was recorded twice. Although one-month-old iPLA2γ-KO mice showed time scores indistinguishable from those of their WT littermates, the latency of iPLA2γ-KO mice gradually decreased afterwards and showed a greatly reduced time score at the age of four months (∼60% reduction relative to that of WT mice, which maintained a steady score over four months) (Fig. 1C and supplementary Video II). These findings indicate that iPLA2γ deficiency leads to a gradual loss of muscular strength after weaning, which appears to be a major cause of kyphosis and scoliosis.
Muscle atrophy and mitochondrial degeneration in iPLA2γ-deficient mice
Hematoxylin-eosin staining of the tissue sections of thigh muscles revealed some pathological characteristics in four-month-old iPLA2γ-KO mice compared with age-matched WT mice. In the KO mice, there was wide variation in the sizes of individual fibers, with a large number of aggregated nuclei (Fig. 2A, a and b). The sizes of individual myofibers in iPLA2γ-KO mice were reduced by more than 60% relative to those in WT mice, which had myofibers with a uniform size (Fig. 2B). On the other hand, at one month of age, no pathological characteristics, such as aggregated nuclei and variable sizes of muscle fibers, were observed in iPLA2γ-KO thigh muscle sections (supplementaryFig. IIA, a and b). These results suggested that the macroscopic muscle atrophy in the KO mice proceeded from one to four months of age, and then growth retardation (supplementary Fig. ID, E) and muscle weakness (Fig. 1C) became evident. In agreement with this idea, the volumes of the thigh and leg muscles, as evaluated by axial CT analysis, were smaller in four-month-old iPLA2γ-KO mice than in their WT littermates (Fig. 2C). Furthermore, the thigh muscle was significantly lighter in iPLA2γ-KO mice than in their WT littermates (Fig. 2D). However, neither TUNEL staining (Fig. 2A, c and d) nor Masson trichrome staining (Fig. 2A, e and f) provided increased signals in iPLA2γ-KO mice relative to WT mice, indicating that the observed muscle phenotypes in the KO mice were not due to increased apoptosis and fibrosis, respectively, of muscle fibers. In addition, the mRNA expression of several inflammatory cytokines, including IL-1β, IL-6, and TNF-α (33–36), in iPLA2γ-KO skeletal muscle was similar to that of WT mice (supplementary Fig. III), implying that the muscle atrophy in iPLA2γ-KO mice was not accompanied by an inflammatory response.
Fig. 2.
Histological analysis of thigh muscle from iPLA2γ-WT and -KO mice. A: Cross-sections of thigh muscle from WT (a) and KO (b) mice were stained with hematoxylin and eosin. TUNEL staining of thigh muscle from WT (c) and KO (d) mice. Masson trichrome staining of thigh muscle from WT (e) and KO (f) mice. Scale bar: 100 μm. B: Evaluation of the sizes of muscle fibers in iPLA2γ-WT and -KO mice. Values are means ± SE (n = 3). C: Volumes of thigh and leg muscles in iPLA2γ-WT (gray bar) and -KO (black bar) mice at four months of age by using CT scan analysis. Quantitative data are means ± SE. * P < 0.05 versus WT (n = 6). D: Weights of thigh muscles in iPLA2γ-WT (gray bar) and -KO (black bar) mice. Quantitative data are means ± SE. * P < 0.05 versus WT (n = 6). E: Electron microscopy of iPLA2γ-WT and -KO skeletal muscles. Skeletal muscles from four-month-old WT [(a) and (c)] and KO [(b) and (d)] mice were fixed in 2.5% (v/v) glutaraldehyde in phosphate buffer, and then analyzed by transmission electron microscopy. The micrographs show abnormal mitochondria and myofiber degeneration in the muscle from KO mice compared with that of WT mice. Arrows show mitochondria. Scale bar, 1 μm [(a) and (b)] and 100 nm [(c) and (d)]. Abbreviations: CT, computed tomography; iPLA2, calcium-independent PLA2; KO, knockout; WT, wild-type.
We further performed transmission electron microscopic analysis of the skeletal muscle (Fig. 2E). Compared with four-month-old WT skeletal muscle, in which individual myofilaments were aligned regularly (Fig. 2E, a and c), the age-matched iPLA2γ-KO muscle exhibited a reduction of mitochondrial number, increase of swollen mitochondria, and degeneration of myofilaments (Fig. 2E, b and d). In the KO mice, the mitochondria varied in size and many contained abnormal cristae (Fig. 2E, b and d). It was noteworthy that by electron microscopic examination, signs of myofilament degeneration as well as mitochondrial proliferation and swelling, typical features of mitochondrial stress, were observed in iPLA2γ-KO mice even at one month of age (supplementary Fig. IIB, a-d). Thus, mitochondrial stress had come out ahead of the onset of the muscle weakness in the KO mice.
We next assessed whether the observed muscle atrophy in iPLA2γ-KO mice was caused by abnormal myogenic differentiation. The primary myoblastic cells prepared from the thigh muscle of iPLA2γ-KO mice grew normally under high-FCS culture conditions. When placed under low-HS conditions, these cells were able to form myotubes normally, and the number of myotubes did not show any significant difference between WT and KO mice (supplementary Fig. IVA, B). On Western blot analysis, the expression of α-actinin, a microfilament protein that is necessary for the attachment of actin filaments to the Z-line membrane in muscle cells (37), was induced similarly in both WT- and iPLA2γ-KO-derived myoblasts during the process of differentiation (after 3–6 days of culture) (supplementary Fig. IVC). These results suggest that the deficiency of iPLA2γ does not affect myogenic differentiation per se.
Mitochondrial dysfunction and increased oxidative stress in iPLA2γ-KO muscle
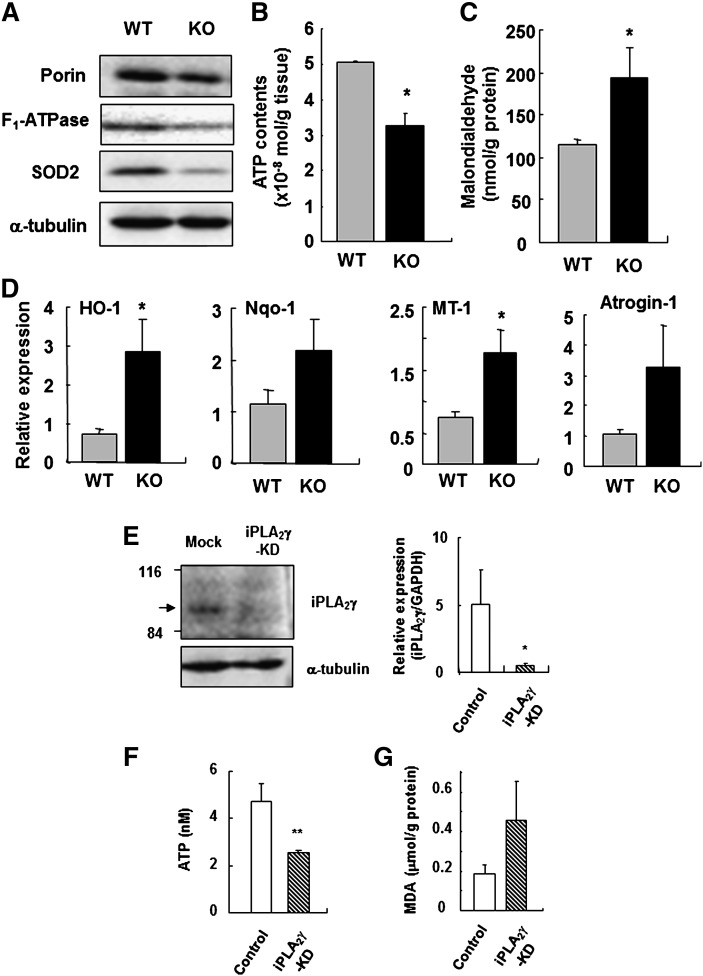
To further address the alterations in the mitochondria of iPLA2γ-KO mice, the expression of several mitochondrial proteins in homogenates of thigh muscles from WT and iPLA2γ-KO mice were examined by Western blotting. We found notable decreases in the expression levels of several mitochondrial markers, such as porin, F1-ATPase, and SOD2 (Mn-SOD), in four-month-old iPLA2γ-KO mice compared with WT mice (Fig. 3A), suggesting that the number and/or integrity of mitochondria was lower in iPLA2γ-KO muscle than in WT muscle. Furthermore, the ATP level in homogenates of the skeletal muscle was lower in iPLA2γ-KO mice than in WT mice (Fig. 3B), consistent with the reduction of F1-ATPase, an essential component of mitochondrial ATP synthesis, in the KO mice.
Fig. 3.
Mitochondrial dysfunction and increased lipid peroxidation by lacking of iPLA2γ expression. A: Western blot analysis of mitochondrial markers in iPLA2γ-WT and -KO muscles at four months of age. A blot for α-tubulin was used as a loading control. B: ATP contents in skeletal muscles from WT (gray bar) and KO mice (black bar) (n = 3). Quantitative data are means ± SE. *, P < 0.05 versus WT. C, lipid peroxidation assay of skeletal muscles in WT (gray bar) and KO mice (black bar) (n = 7). Quantitative data are means ± SE. *, P < 0.05 versus WT. D, Q-PCR analysis of the mRNA expression for antioxidant-defense enzymes in skeletal muscles from WT (gray bar) and KO mice (black bar) (n = 6-7). Quantitative data are means ± SE. *P < 0.05 versus WT. E: Reduction in iPLA2γ protein (left) and mRNA (right) expression after transfection of the C2C12 myoblast cells. Quantitative data are means ± SE. *P < 0.05 versus WT (n = 3). F: ATP contents in control (white bar) and iPLA2γ-KD C2C12 cells (striped bar). Quantitative data are means ± SE. **P < 0.01 versus control (n = 3). G: Lipid peroxidation assay of control (white bar) and iPLA2γ-KD C2C12 cells (striped bar) (n = 5). Quantitative data are means ± SE. *P < 0.05 versus WT. Abbreviations: CT, computed tomography; iPLA2, calcium-independent PLA2; KO, knockout; Q-PCR, quantitative RT-PCR; WT, wild-type.
Considering that mitochondria are the most important cellular source of reactive oxygen species (ROS), we next analyzed whether the skeletal muscle of iPLA2γ-KO mice would be exposed to oxidative stress resulting from mitochondrial dysfunction. The level of lipid peroxidation, as indicated by the accumulation of MDA, was significantly higher in iPLA2γ-KO muscle than in WT muscle (Fig. 3C). It has been shown that oxidative stress induces several enzymes involved in antioxidant defense to minimize oxidative damage (38–40). Q-PCR evaluation revealed that the expression levels of Hmox1 (heme oxygenase-1), Nqo1 (NAD(P)H dehydrogenase, quinine-1) and Mt1 (metallothionein-1) were higher in the skeletal muscle of iPLA2γ-KO mice than in that of WT mice (Fig. 3D). In addition, the mRNA level of Fbxo32 (atrogin-1 or muscle atrophy F-box), which encodes a muscle-associated, ROS-inducible E3 ubiquitin ligase, was substantially higher in the iPLA2γ-KO muscle (Fig. 3D).
To assess whether these phenotypes were caused directly by intrinsic mitochondrial defects or by the secondary effects of systemic growth abnormalities, we performed knockdown of iPLA2γ by siRNA in C2C12 cells, a mouse myoblast cell line. As shown in Fig. 3E, the expression of endogenous iPLA2γ was markedly reduced in cells transfected with iPLA2γ siRNA (iPLA2γ-KD) relative to those transfected with control siRNA (mock). The ATP content in the cell lysate was lower in iPLA2γ-KD C2C12 cells than in mock cells (Fig. 3F). The accumulation of MDA was substantially higher in iPLA2γ-KD cells than in mock cells (Fig. 3G). These results suggest that knockdown of iPLA2γ in C2C12 cells also leads to mitochondrial dysfunction, accompanied by increased oxidative stress.
Alterations of phospholipid compositions in skeletal muscle by iPLA2γ deficiency
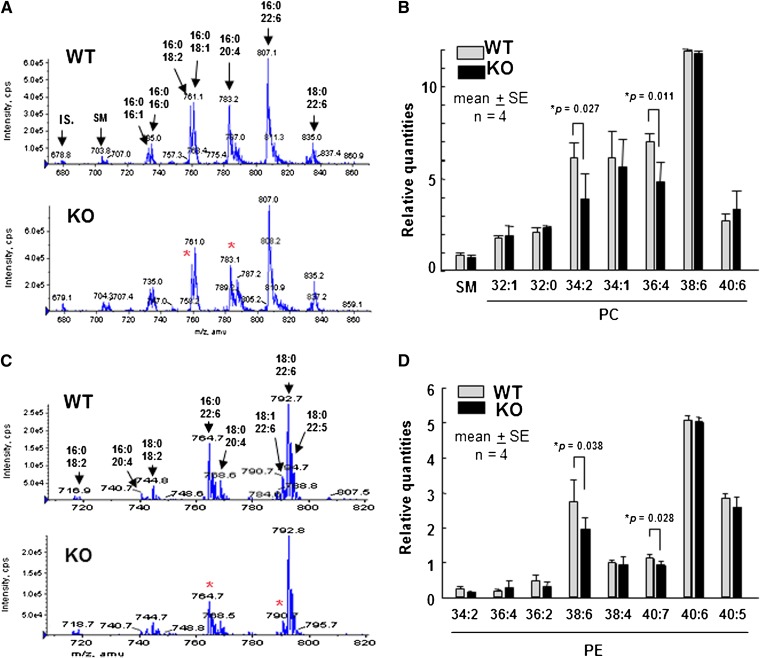
In the previous studies, the content of CL, a phospholipid that is mostly confined to mitochondrial membranes, was changed in the heart and brain of iPLA2γ-deficient mice (18, 19). We also investigated the phospholipid composition in the skeletal muscle from iPLA2γ-KO mice compared with that from WT mice. TLC analysis demonstrated a statistically significant decrease in CL content in iPLA2γ-deficient muscle compared with WT muscle (Table 1). The content of phosphatidylglycerol, a precursor of CL, in the skeletal muscle also showed a similar tendency of decrease in iPLA2γ-KO mice compared with WT mice, whereas the differences in the contents of PC and PE were only subtle and not statistically significant between the genotypes (Table 1). To examine whether there would be some alterations in individual molecular species of PC and PE between iPLA2γ-KO and WT muscles, we performed ESI/MS analyses of these phospholipids. Representative ESI/MS patterns of PC and PE species extracted from WT or iPLA2γ-KO muscles are shown in Fig. 4A and C, respectively. Significant reductions in some molecular species were observed in the KO mice compared with WT mice. Four independent trials of the ESI/MS analysis showed that PC subclasses with C34:2 (C16:0 and C18:2; m/z = 758.6) and C36:4 (C16:0 and C20:4, m/z = 782.6) (Fig. 4B) and PE subclasses with C38:6 (C16:0 and C22:6, m/z = 764.5) and C40:7 (C18:1 and C22:6, m/z = 790.5) (Fig. 4D) were significantly reduced in iPLA2γ-KO mice compared with WT mice. Phosphatidylinositol and phosphatidylserine did not show any significant difference between WT and iPLA2γ-KO mice (data not shown).
TABLE 1.
Phospholipid compositions in skeletal muscle by TLC analysis
| Phospholipid Class |
||||
|---|---|---|---|---|
| Genotype | CL | PE | PC | Phosphatidylglycerol |
| Mean ± SEM (%) | ||||
| WT | 1.89 ± 0.28 | 12.57 ± 2.67 | 34.25 ± 8.9 | 5.33 ± 2.44 |
| KO | 0.96 ± 0.21a | 11.08 ± 1.62 | 29.64 ± 4.71 | 1.75 ± 0.47 |
Total lipids were extracted from homogenates of thigh muscle from iPLA2γ+/+ (WT) and iPLA2γ−/− (KO) mice and separated on TLC silica gel plates. The TLC plates were stained by iodine vapor to visualize the phospholipids. After scraping the silica in bands of PC, PE, CL, and phosphatidylglycerol from the plates, the lipids were extracted from the silica two times with the method of Bligh and Dyer (25). Individual phospholipids were quantified by a molybdenum blue method. Quantitative data are means ± SE. CL, cardiolipin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; iPLA2, calcium-independent PLA2; KO, knockout; WT, wild-type.
P < 0.05 versus WT (n = 6).
Fig. 4.
ESI/MS analysis of PC and PE species in iPLA2γ-WT and -KO skeletal muscles. Total lipids were extracted from thigh muscle homogenates and then subjected to ESI/MS analysis of PC (A and B) and PE (C and D). Representative ESI/MS profile (A and C) and quantitative results of four independent experiments. Means ± SE. *P < 0.05 versus control (n = 4) are shown in (B) and (D). Asterisks in (A) and (B) show altered molecular species. Abbreviations: IS, internal standard; PC, phosphatidylcholine; PE, phosphatidylethanolamine; iPLA2, calcium-independent PLA2; KO, knockout; SM, sphingomyelin; WT, wild-type.
Alterations of prostanoid contents in skeletal muscle by iPLA2γ deficiency
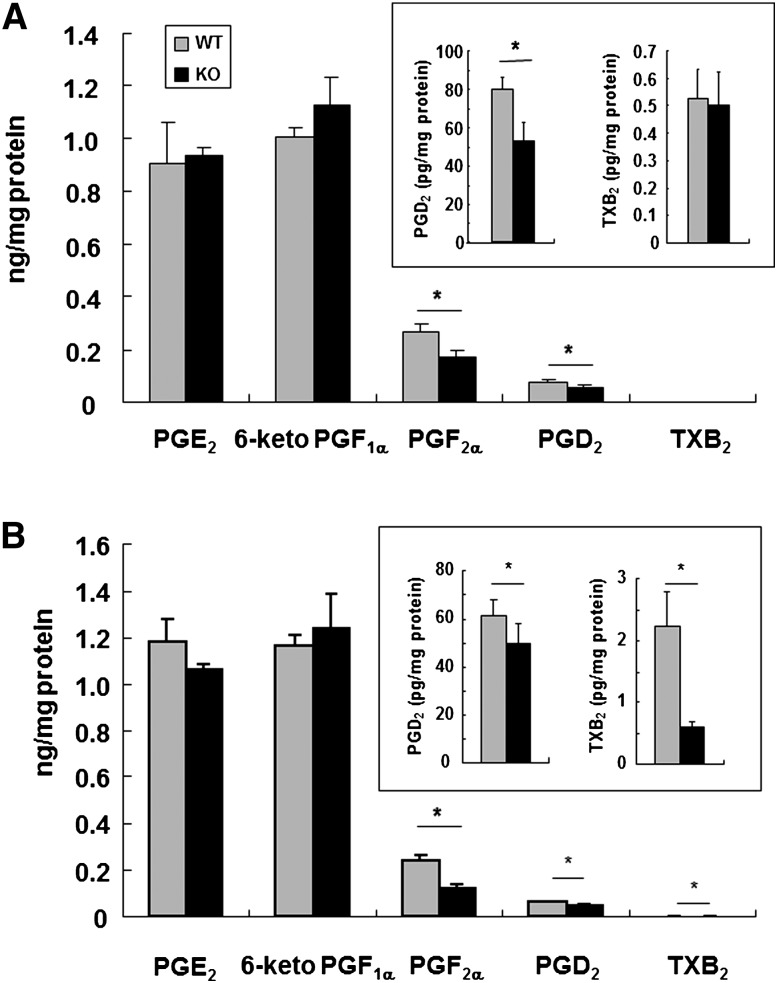
As described above, we previously demonstrated that iPLA2γ has a regulatory role in AA release and eicosanoid generation in vitro (20). As it has been reported that some prostanoids have myoprotective activities (41, 42), we here quantified the contents of PGE2, 6-ketoPGF1α, PGD2, PGF2α, and TXB2 in homogenates of the skeletal muscles from WT and iPLA2γ-KO mice. In the skeletal muscle of WT mice, PGE2 and 6-ketoPGF1α were much more abundant than PGF2α, PGD2, and TXB2 (Fig. 5A). Among these prostanoids, the levels of PGD2 and PGF2α were significantly lower in iPLA2γ-KO skeletal muscle than in WT skeletal muscle, although the 6-ketoPGF1α, PGE2 and TXB2 levels did not differ appreciably between the genotypes (Fig. 5A). Furthermore, the reduction of some, if not all, prostanoids in the KO mice was not limited to the skeletal muscle, as the levels of PGF2α, PGD2, and TXB2 but not of the major prostanoids PGE2 and 6-ketoPGF1α in the heart were also significantly reduced by iPLA2γ deficiency (Fig. 5B).
Fig. 5.
Prostanoid contents in skeletal muscle and heart. Contents of PGE2, 6-ketoPGF1α (a stable metabolite of PGI2), PGF2α, PGD2, and TXB2 (a stable metabolite of TXA2) in homogenates of skeletal muscle (A) and heart (B) were quantified by enzyme immunoassay kits. Quantitative data are means ± SE. *P < 0.05 versus control (n = 3–7). Abbreviations: KO, knockout; PGE2, prostaglandin E2; PGI2, prostaglandin I2; TXA2, thromboxane A2; WT, wild-type.
DISCUSSION
We found that genetic deletion of iPLA2γ in mice led to muscle atrophy and weakness. These findings could provide, at least in part, an explanation for the growth retardation, kyphosis, scoliosis, and reduced exercise capacity of the null mice. The muscle atrophy in iPLA2γ-KO mice was accompanied by mitochondrial degeneration, decreased CL and ATP levels, and elevated lipid peroxidation. These results are compatible with recent studies using another line of iPLA2γ-KO mice, in which impaired energy expenditure and oxygen consumption occurred in the brown adipose tissue, heart, and brain, most probably because of compromised mitochondrial CL homeostasis (18, 19). Furthermore, we found that the contents of PGF2α and PGD2 were significantly decreased in iPLA2γ-KO muscle compared with WT muscle. The latter finding is, to the best of our knowledge, the first demonstration that the absence of iPLA2γ is linked to reduced biosynthesis of lipid mediators in vivo.
In eukaryotes, CL is present exclusively in the membranes of mitochondria, where it interacts with a number of mitochondrial proteins and is essential for optimal mitochondrial functions (43–46). CL is synthesized de novo through condensation of phosphatidylglycerol with cytidine diphosphate-diacylglycerol (CDP-DAG) catalyzed by cardiolipin synthase (CLS). Following its biosynthesis, CL is actively remodeled to achieve its final acyl composition specific for cells and tissues. An important CL remodeling enzyme is tafazzin, a CL transacylase. Mutations in the tafazzin gene cause the X-linked recessive disorder Barth syndrome, which presents with dilated cardiomyopathy, skeletal myopathy, cyclic neutropenia, and growth retardation. Tafazzin-mediated transacylation of CL is particularly important in the heart and skeletal muscle, in which ∼80% of CL molecules are remodeled to be tetralinoleoyl-CL (47). Defective tafazzin function results in reduced reacylation of monolyso-CL and eventually in deficiency of CL (48–51); accordingly, patients with Barth syndrome present symptoms, such as cardioskeletal myopathy and exercise intolerance, which are commonly associated with mitochondrial diseases (52). It was very recently shown that genetic inactivation of the iPLA2β ortholog in Drosophila suppressed the phenotype caused by tafazzin deficiency (53). These findings suggested that iPLA2β or its related paralog(s) might be involved in the machinery of CL deacylation and remodeling in mammals. In this context, it is noteworthy that iPLA2β-KO mice develop age-dependent neurological impairment (13, 54), and mutations in the human iPLA2β gene (PLA2G6) have been identified in patients with infantile neuroaxonal dystrophy and neurodegeneration with iron accumulation in the brain (16, 55). However, there have been no reports demonstrating that iPLA2β-KO mice display cardioskeletal myopathy, exercise intolerance, and growth retardation, which are typical symptoms of mitochondrial diseases, including Barth syndrome. On the other hand, here we have demonstrated that iPLA2γ-KO mice showed age-dependent growth retardation and skeletal muscle loss, and that in these mice, mitochondrial degeneration and dysfunction preceded. The dramatic reduction of CL and its precursor phosphatidylglycerol in iPLA2γ-KO muscle compared with WT muscle further supports the impaired CL remodeling, which may lead to mitochondrial degeneration and thereby reduced ATP synthesis in iPLA2γ-KO mice. It has been reported that another line of iPLA2γ-KO mice also harbored myocardial and neuronal phenotypes with altered CL contents (18, 19). Moreover, neurodegeneration occurred at a much earlier stage in iPLA2γ-KO mice (19) than in iPLA2β-KO mice (13). Thus, in mice, iPLA2γ may cooperate with the transacylase tafazzin to control the remodeling of CL and thereby maintain homeostasis of mitochondria in tissues with high energy expenditure and high oxygen consumption (i.e., cardiac and skeletal muscles) under physiological conditions.
Mitochondria are the most important cellular source of ROS. If not adequately neutralized, ROS can damage cells by peroxidation of membrane phospholipids. In the present study, the loss of iPLA2γ resulted in increased lipid peroxidation in the skeletal muscle. Likewise, the knockdown of iPLA2γ in C2C12 myoblastic cells also caused elevation of lipid peroxidation and reduction of ATP synthesis, thus mirroring the in vivo effects of iPLA2γ deficiency. Kinsey et al. (56) have also reported that the knockdown of iPLA2γ expression in the primary cultures of rabbit renal proximal tubule cells induced elevation of lipid peroxidation and decrease in mitochondrial functions. Our ESI/MS analyses of phospholipids in iPLA2γ-KO and WT muscles revealed that some PE and PC molecular species containing linoleic acid, AA, and docosahexaenoic acid were decreased in iPLA2γ-KO muscle. As phospholipids bearing polyunsaturated fatty acids are particularly susceptible to peroxidation, these decreases may reflect the occurrence of oxidative modification of membranes. Among mitochondrial phospholipids, CL is regarded as a target of ROS attack, because it is particularly rich in linoleic acid and localizes in the inner mitochondrial membrane near the ROS-producing sites. Furthermore, peroxidation of CL in mitochondria has been suggested to initiate the mitochondria-mediated apoptotic signal (57). Thus, cells must replace peroxidized fatty acyl residues in phospholipids with native fatty acids by a sequential action of PLA2 and acyltransferase to keep mitochondrial membranes in an optimal state. From this point of view, iPLA2γ may be responsible for the repair of peroxidized CL in mitochondria. It has been proposed that the consecutive action of PLA2 and glutathione peroxidase (GPx) is required to reduce lipid peroxides in mitochondria (24). Like the gene disruption of iPLA2γ observed in the present study, gene disruption of cGPx, one of the GPx isozymes, has also been shown to result in growth retardation, presumably due in part to mitochondria disorders (58). Thus, iPLA2γ may play a role in removing peroxidized CL from the mitochondrial membrane, thereby preserving membrane integrity.
Oxidative stress induces the expression of antioxidant genes that protect against oxidative stress (59–61). In further support of the idea that oxidative stress is increased by the absence of iPLA2γ, we found that the mRNA levels of several antioxidant genes, including Hmox1, Nqo1, Mt1, and Fbxo32, were increased in iPLA2γ-KO muscle compared with WT muscle. These results suggest that, in the iPLA2γ-KO muscle, a set of antioxidant genes was induced in response to ROS following mitochondrial damage. Hmox1 and Nqo1 are induced through ROS-mediated activation of the transcription factor Nrf2 (60). Atrogin-1 (Fbxo32), an E3 ubiquitin ligase specifically expressed in cardiac and skeletal muscles, is dramatically upregulated by ROS in multiple atrophy models, as is muscle ring finger 1 (MuRF1), another E3 ubiquitin ligase (61–63). The increase in E3 ubiquitin ligases may facilitate ubiquitin/proteosome-dependent protein degradation, thereby contributing to muscular degeneration and atrophy. We further found that mRNA levels of iPLA2β and cPLA2α were increased in the iPLA2γ-KO muscle. Previous studies have shown that oxidative stress induced by exogenous adding of hydrogen peroxide or superoxide anion in macrophage cultures significantly increased iPLA2 and cPLA2 activities (64). It has been also shown that iPLA2β has an ability to repair oxidative modifications of mitochondrial lipids (65). In the iPLA2γ-KO muscle, oxidative stress may induce the expression of iPLA2β and cPLA2α as antioxidant genes.
In our previous report, overexpression of iPLA2γ was shown to facilitate cellular release of AA, which was converted to PGE2 with preferred COX-1 coupling (20). Studies of eicosanoid contents in the skeletal muscle indicated that PGF2α and PGD2 were noticeably reduced in iPLA2γ-KO muscle. Several PGs, such as PGE2, PGF2α, PGI2, and PGD2, have been shown to be increased in the damaged muscle tissue, where they exhibit muscle repair functions (41). PGF2α is produced by myoblasts and signals via the PGF receptor (FP) to increase myotube size by preventing myoblast apoptosis and by promoting muscular cell fusion and protein synthesis (42). Although the role of PGD2 in muscular cells is controversial, it appears to play both beneficial and detrimental roles. Tokudome et al. reported that PGD2 synthesis was protective against myocardial injury (66). In addition, 15-deoxy-delta-12, 14-PGJ2 (15d-PGJ2), a spontaneous dehydration product of PGD2, has the ability to affect ROS generation by covalently modifying cellular proteins, such as NF-κB, to activate peroxisome proliferator activating receptor γ (PPARγ) as a ligand (67) and to block myotube formation in a PPARγ-independent manner (68). Thus, besides the regulation of mitochondrial membrane homeostasis, iPLA2γ may protect the muscle from damages by regulating the production of these prostanoids.
It was noteworthy that in the iPLA2γ-KO mice, the production of major prostanoids, PGE2 and PGI2, was not affected, and only the synthesis of particular classes of prostanoid was suppressed. These results indicated that total AA supply from membrane phospholipids was not changed in the KO mice. AA from membrane phospholipids by iPLA2γ might be supplied specifically to particular downstream PG-biosynthetic enzymes (COXs and terminal PG synthases). It has been found that the biosynthesis of individual prostanoids shows distinct utilization of the two COX isoforms COX-1 and COX-2, probably because of the distinct functional coupling between COXs and terminal PG synthases (69). Interestingly, prostanoids that were affected by iPLA2γ deficiency (PGD2, PGF2α, and TXA2) in skeletal and cardiac muscles can be produced through COX-1 (69). iPLA2γ might be functionally coupled with COX-1 in preference to COX-2. However, even if AA supply accounts for the coupling of iPLA2γ with prostanoid synthesis, it still remains unclear how mitochondrial iPLA2γ could be coupled with COX enzymes that reside in the endoplasmic reticulum and nuclear envelope (70). In this regard, recent evidence suggests the presence of a mitochondria-endoplasmic reticulum tethering complex that connects inter-organelle calcium and lipid exchange (71). Alternatively, we could not rule out the possibility that the increased oxidative stress signaling in iPLA2γ-KO muscle might secondarily downregulate other PLA2, which in turn could supply AA to the PGF2α and PGD2-biosynthetic pathways, although cPLA2α and iPLA2β were upregulated in the iPLA2γ-KO muscle. Further details of the signaling role of iPLA2γ, together with its mechanistic insights, need to be clarified in a future study.
Supplementary Material
Acknowledgments
Professor Ichiro Kudo died April 27, 2008. We greatly miss him as a scientist and friend. We offer sincere thanks to all friends, colleagues, and former collaborators of Prof. Kudo who showed him kindness during his lifetime.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- CL
- cardiolipin
- COX
- cyclooxygenase
- CT
- computed tomography
- GPx
- glutathione peroxidase
- HBSS
- Hank's balanced salt solution
- HO
- heme oxigenase
- HS
- horse serum
- KO
- knockout
- MDA
- malondialdehyde
- MT
- methallothionein
- Nqo
- NAD(P)H dehydrogenase, quinine
- PAF
- platelet-activating factor
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- prostaglandin
- PGD2
- prostaglandin D2
- PGE2
- prostaglandin E2
- PGF2α
- prostaglandin F2α
- PGI2
- prostaglandin I2
- PLA2
- phospholipase A2
- cPLA2
- cytosolic PLA2
- iPLA2
- calcium-independent PLA2
- sPLA2
- secretory PLA2
- Q-PCR
- quantitative RT-PCR
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- TXA2
- thromboxane A2
- WT
- wild-type
This work was supported by a Showa University special grant-in-aid for Innovative Collaborative Research Projects and grants-in-aid for Scientific Research and High-Tech Research Center Project for Private Universities, with a matching fund subsidy (2004–2007) from the Japanese Ministry of Education, Science, Culture, Sports and Technology. M. Murakami was also supported by Precursory Research for Embryonic Science and Technology (PRESTO) of the Japan Science and Technology Agency.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures and four videos.
REFERENCES
- 1.Kudo I., Murakami M. 2002. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 68–69: 3–58. [DOI] [PubMed] [Google Scholar]
- 2.Murakami M., Kudo I. 2001. Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv. Immunol. 77: 163–194. [DOI] [PubMed] [Google Scholar]
- 3.Burke J. E., Dennis E. A. 2009. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50: S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. 2004. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279: 48968–48975. [DOI] [PubMed] [Google Scholar]
- 5.Ackermann E. J., Kempner E. S., Dennis E. A. 1994. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J. Biol. Chem. 269: 9227–9233. [PubMed] [Google Scholar]
- 6.Wolf M. J., Gross R. W. 1996b. Expression, purification, and kinetic characterization of a recombinant 80-kDa intracellular calcium-independent phospholipase A2. J. Biol. Chem. 271: 30879–30885. [DOI] [PubMed] [Google Scholar]
- 7.Balsinde J., Balboa M. A., Dennis E. A. 1997. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 272: 29317–29321. [DOI] [PubMed] [Google Scholar]
- 8.Wolf M. J., Wang J., Turk J., Gross R. W. 1997. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid mobilization. J. Biol. Chem. 272: 1522–1526. [DOI] [PubMed] [Google Scholar]
- 9.Roshak A. K., Capper E. A., Stevenson C., Eichman C., Marshall L. A. 2000. Human calcium-independent phospholipase A2 mediates lymphocyte proliferation. J. Biol. Chem. 275: 35692–35698. [DOI] [PubMed] [Google Scholar]
- 10.Atsumi G., Murakami M., Kojima K., Hadano A., Tajima M., Kudo I. 2000. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2α inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J. Biol. Chem. 275: 18248–18258. [DOI] [PubMed] [Google Scholar]
- 11.Smani T., Zakharov S. I., Csutora P., Leno E., Trepakova E. S., Bolotina V. M. 2004. A novel mechanism for the store-operated calcium influx pathway. Nat. Cell Biol. 6: 113–120. [DOI] [PubMed] [Google Scholar]
- 12.Bao S., Bohrer A., Ramanadham S., Jin W., Zhang S., Turk J. 2006. Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J. Biol. Chem. 281: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinzawa K., Sumi H., Ikawa M., Matsuoka Y., Okabe M., Sakoda S., Tsujimoto Y. 2008. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J. Neurosci. 28: 2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramanadham S., Yarasheski K. E., Silva M. J., Wohltmann M., Novack D. V., Christiansen B., Tu X., Zhang S., Lei X., Turk J. 2008. Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2β)-mull mice. Am. J. Pathol. 172: 868–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao S., Miller D. J., Ma Z., Wohltmann M., Eng G., Ramanadham S., Moley K., Turk J. 2004. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 279: 38194–38200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan N. V., Westaway S. K., Morton J. E., Gregory A., Gissen P., Sonek S., Cangul H., Coryell J., Canham N., Nardocci N., et al. 2006. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat. Genet. 38: 752–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancuso D. J., Jenkins C. M., Gross R. W. 2000. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J. Biol. Chem. 275: 9937–9945. [DOI] [PubMed] [Google Scholar]
- 18.Mancuso D. J., Sims H. F., Han X., Jenkins C. M., Guan S. P., Yang K., Moon S. H., Pietka T., Abumrad N. A., Schlesinger P. H., et al. 2007. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J. Biol. Chem. 282: 34611–34622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancuso D. J., Kotzbauer P., Wozniak D. F., Sims H. F., Jenkins C. M., Guan S. P., Han X., Yang K., Sun G., Malik I., et al. 2007. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy, and cognitive dysfunction. J. Biol. Chem. 284: 35632–35644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami M., Masuda S., Ueda-Semmyo K., Yoda E., Kuwata H., Takanezawa Y., Aoki J., Arai H., Sumimoto H., Ishikawa Y., et al. 2005. Group VIB Ca2+-independent phospholipase A2γ promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipase A2. J. Biol. Chem. 280: 14028–14041. [DOI] [PubMed] [Google Scholar]
- 21.Kuwata H., Fujimoto C., Yoda E., Shimbara S., Nakatani Y., Hara S., Murakami M., Kudo I. 2007. A novel role of group VIB calcium-independent phospholipase A2 (iPLA2γ) in the inducible expression of group IIA secretory PLA2 in rat fibroblastic cells. J. Biol. Chem. 282: 20124–20132. [DOI] [PubMed] [Google Scholar]
- 22.Yan W., Jenkins C. M., Han X., Mancuso D. J., Sims H. F., Yang K., Gross R. W. 2005. The highly selective production of 2-arachidonoyl lusophosphatidylcholine catalyzed by purified calcium-independent phospholipase A2γ. J. Biol. Chem. 280: 26669–26679. [DOI] [PubMed] [Google Scholar]
- 23.Tanioka T., Nakatani Y., Semmyo N., Murakami M., Kudo I. 2000. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 275: 32775–32782. [DOI] [PubMed] [Google Scholar]
- 24.Taniwaki T., Haruna K., Nakamura H., Sekimoto T., Oike Y., Imaizumi T., Saito F., Muta M., Soejima Y., Utoh A., et al. 2005. Characterization of an exchangeable gene trap using pU-17 carrying a stop codon-beta geo cassette. Dev. Growth Differ. 47: 163–172. [DOI] [PubMed] [Google Scholar]
- 25.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharmacol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 26.Rouser G., Siakotos A. N., Fleischer S. 1966. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1: 85–86. [DOI] [PubMed] [Google Scholar]
- 27.Kamei D., Yamakawa K., Takegoshi Y., Mikami-Nakanishi M., Nakatani Y., Oh-Ishi S., Yasui H., Azuma Y., Hirasawa N., Ohuchi K., et al. 2004. Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin E synthase-1. J. Biol. Chem. 279: 33684–33695. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso D. J., Jenkins C. M., Sims H. F., Cohen J. M., Yang J., Gross R. W. 2004. Complex transcriptional and translational regulation of iPLA2γ resulting in multiple gene products containing dual competing sites for mitochondrial or peroxisomal localization. Eur. J. Biochem. 271: 4709–4724. [DOI] [PubMed] [Google Scholar]
- 29.Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G., Ornitz D. M. 1996. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12: 390–397. [DOI] [PubMed] [Google Scholar]
- 30.Tourtellotte W. G., Milbrandt J. 1998. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat. Genet. 20: 87–91. [DOI] [PubMed] [Google Scholar]
- 31.Dabovic B., Chen Y., Colarossi C., Obata H., Zambuto L., Perle M. A., Rifkin D. B. 2002. Bone abnormalities in latent TGF-β binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF- β bioavailability. J. Cell Biol. 156: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baribault H., Danao J., Gupte J., Yang L., Sun B., Richards W., Tian H. 2006. The G-protein-coupled receptor GPR103 regulates bone formation. Mol. Cell. Biol. 26: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang N. P., Tonetti M. S., Suter J., Sorrell J., Duff G. W., Kornman K. S. 2000. Effect of interleukin-1 gene polymorphisms on gingival inflammation assessed by bleeding on probing in a periodontal maintenance population. J. Periodontal Res. 35: 102–107. [DOI] [PubMed] [Google Scholar]
- 34.Emery P., Salmon M. 1991. The immune response. 2. Systemic mediators of inflammation. Br. J. Hosp. Med. 45: 164–168. [PubMed] [Google Scholar]
- 35.Kishimoto T., Akira S., Taga T. 1992. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 258: 593–597. [DOI] [PubMed] [Google Scholar]
- 36.Birkedal-Hansen H. 1993. Role of cytokines and inflammatory mediators in tissue destruction. J. Periodontal Res. 28: 500–510. [DOI] [PubMed] [Google Scholar]
- 37.Sjöblom B., Salmazo A., Djinovi-Carugo K. 2008. α-actinin structure and regulation. Cell. Mol. Life Sci. 65: 2688–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang Y. J. 2007. Antioxidant defense against anthracycline cardiotoxicity by metallothionein. Cardiovasc. Toxicol. 7: 95–100. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T., Shimizu H., Morimatsu H., Inoue K., Akagi R., Morita K., Sassa S. 2007. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev. Med. Chem. 7: 745–753. [DOI] [PubMed] [Google Scholar]
- 40.Jaiswal A. K. 2000. Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic. Biol. Med. 29: 254–262. [DOI] [PubMed] [Google Scholar]
- 41.Velica P., Bunce C. M. 2008. Prostaglandins in muscle regeneration. J. Muscle Res. Cell Motil. 29: 163–167. [DOI] [PubMed] [Google Scholar]
- 42.Horsley V., Pavlath G. K. 2003. Prostaglandin F2α stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J. Cell Biol. 161: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlame M., Rua D., Greenberg M. L. 2000. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39: 257–288. [DOI] [PubMed] [Google Scholar]
- 44.Li G., Chen S., Thompson M. N., Greenberg M. L. 2007. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim. Biophys. Acta. 1771: 432–441. [DOI] [PubMed] [Google Scholar]
- 45.Houtkooper R. H., Vaz F. M. 2008. Cardiolipin, the heart of mitochondrial metabolism. Cell. Mol. Life Sci. 65: 2493–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlame M. 2008. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 49: 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlame M., Shanske S., Doty S., König T., Sculco T., DiMauro S., Blanck T. J. 1999. Microanalysis of cardiolipin in small biopsies including skeletal muscle from patients with mitochondrial disease. J. Lipid Res. 40: 1585–1592. [PubMed] [Google Scholar]
- 48.Xu Y., Condell M., Plesken H., Edelman-Novemsky I., Ma J., Ren M., Schlame M. 2006. A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. USA. 103: 11584–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Z., Valianpour F., Chen S., Vaz F. M., Hakkaart G. A., Wanders R. J., Greenberg M. L. 2004. Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol. Microbiol. 51: 149–158. [DOI] [PubMed] [Google Scholar]
- 50.Valianpour F., Wanders R. J., Overmars H., Vreken P., Van Gennip A. H., Baas F., Plecko B., Santer R., Becker K., Barth P. G. 2002. Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): a study in cultured skin fibroblasts. J. Pediatr. 141: 729–733. [DOI] [PubMed] [Google Scholar]
- 51.Vreken P., Valianpour F., Nijtmans L. G., Grivell L. A., Plecko B., Wanders R. J., Barth P. G. 2000. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 279: 378–382. [DOI] [PubMed] [Google Scholar]
- 52.Barth P. G., Valianpour F., Bowen V. M., Lam J., Duran M., Vaz F. M., Wanders R. J. 2004. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am. J. Med. Genet. A. 126A: 349–354. [DOI] [PubMed] [Google Scholar]
- 53.Malhotra A., Edelman-Novemsky I., Xu Y., Plesken H., Ma J., Schlame M., Ren M. 2009. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc. Natl. Acad. Sci. USA. 17: 2337–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malik I., Turk J., Mancuso D. J., Montier L., Wohltmann M., Wozniak D. F., Schmidt R. E., Gross R. W., Kotzbauer P. T. 2008. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am. J. Pathol. 172: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khateeb S., Flusser H., Ofir R., Shelef I., Narkis G., Vardi G., Shorer Z., Levy R., Galil A., Elbedour K., et al. 2006. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am. J. Hum. Genet. 79: 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kinsey G. R., Blum J. L., Covington M. D., Cummings B. S., McHowat J., Schnellmann R. G. 2008. Decreased iPLA2γ expression induces lipid peroxidation and cell death and sensitizes cells to oxidant-induced apoptosis. J. Lipid Res. 49: 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalvez F., Gottlieb E. 2007. Cardiolipin: setting the beat of apoptosis. Apoptosis. 12: 877–885. [DOI] [PubMed] [Google Scholar]
- 58.Esposito L. A., Kokoszka J. E., Waymire K. G., Cottrell B., MacGregor G. R., Wallace D. C. 2000. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic. Biol. Med. 28: 754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagawa Y. 2004. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann. N. Y. Acad. Sci. 1011: 177–184. [DOI] [PubMed] [Google Scholar]
- 60.Leung L., Kwong M., Hou S., Lee C., Chan J. Y. 2003. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J. Biol. Chem. 278: 48021–48029. [DOI] [PubMed] [Google Scholar]
- 61.Cao P. R., Kim H. J., Lecker S. H. 2005. Ubiquitin-protein ligases in muscle wasting. Int. J. Biochem. Cell Biol. 37: 2088–2097. [DOI] [PubMed] [Google Scholar]
- 62.Cai D., Frantz J. D., Tawa N. E., Jr, Melendez P. A., Oh B. C., Lidov H. G., Hasselgren P. O., Frontera W. R., Lee J., Glass D. J., et al. 2004. IKKβ/NF-kappaB activation causes severe muscle wasting in mice. Cell. 119: 285–298. [DOI] [PubMed] [Google Scholar]
- 63.Li Y. P., Chen Y., Li A. S., Reid M. B. 2003. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am. J. Physiol. Cell Physiol. 285: C806–C812. [DOI] [PubMed] [Google Scholar]
- 64.Martinez J., Moreno J. J. 2001. Role of Ca2+-independent phospholipase A2 on arachidonic acid release induced by reactive oxygen species. Arch. Biochem. Biophys. 392: 257–262. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Z., Zhang X., Zhao C., Choi J., Shi J., Song K., Turk J., Ma Z. A. 2010. Protection of pancreatic β-cells by group VIA phospholipase A2-mediated repair of mitochondrial membrane peroxidation. Endocrinology. 151: 3038–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokudome S., Sano M., Shinmura K., Matsuhashi T., Morizane S., Moriyama H., Tamaki K., Hayashida K., Nakanishi H., Yoshikawa N., et al. 2009. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J. Clin. Invest. 119: 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uchida K., Shibata T. 2008. 15-Deoxy-delta (12, 14)-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol. 21: 138–144. [DOI] [PubMed] [Google Scholar]
- 68.Hunter J. G., van Delft M. F., Rachubinski R. A., Capone J. P. 2001. Peroxisome proliferator-activated receptor γ ligands differentially modulate muscle cell differentiation and MyoD gene expression via peroxisome proliferator-activated receptor γ-dependent and -independent pathways. J. Biol. Chem. 276: 38297–38306. [DOI] [PubMed] [Google Scholar]
- 69.Ueno N., Takegoshi Y., Kamei D., Kudo I., Murakami M. 2005. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochem. Biophys. Res. Commun. 338: 70–76. [DOI] [PubMed] [Google Scholar]
- 70.Morita I., Schindler M., Reiger M. K., Otto J. C., Hori T., DeWitt D. L., Smith W. L. 1995. Different intracellular locations for prostaglandin endoperoxide H synthase-1 and -2. J. Biol. Chem. 270: 10902–10908. [DOI] [PubMed] [Google Scholar]
- 71.Kornmann B., Currie E., Collins S. R., Schuldiner M., Nunnari J., Weissman J. S., Walter P. 2009. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 325: 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.