Abstract
Genetic variation at the ABCG5/G8 locus has been associated with markers of cholesterol homeostasis. As data originate from small-scale studies, we performed a meta-analysis to study these associations in a large dataset. We first investigated associations between five common ABCG5/G8 polymorphisms (p.Q604E, p.D19H, p.Y54C, p.T400K, and p.A632V) and plasma sterol levels in 245 hypercholesterolaemic individuals. No significant associations were found. Subsequently, our data were pooled into a meta-analysis that comprised 3,364 subjects from 16 studies (weighted mean age, 46.7 ± 10.5 years; BMI, 23.9 ± 3.5 kg/m2). Presence of the minor 632V allele correlated with reduced LDL-C concentrations (n = 367) compared with homozygosity for the 632A variant [n = 614; −0.11 mmol/l (95% CI, range: −0.20 to −0.02 mmol/l); P = 0.01]. The remaining polymorphisms were not associated with plasma lipid levels. Carriers of the 19H allele exhibited lower campesterol/TC (n = 83; P < 0.001), sitosterol/TC (P < 0.00001), and cholestanol/TC (P < 0.00001), and increased lathosterol/TC ratios (P = 0.001) compared with homozygous 19D allele carriers (n = 591). The ABCG8 632V variant was associated with a clinically irrelevant LDL-C reduction, whereas the 19H allele correlated with decreased cholesterol absorption and increased synthesis without affecting the lipid profile. Hence, associations between frequently studied missense ABCG5/G8 polymorphisms and markers of cholesterol homeostasis are modest at best.
Keywords: polymorphism, LDL-cholesterol, plant sterol
Intestinal cholesterol absorption is an active and selective process, mediated by several transporter proteins located at the intestinal brush border membrane. In fact, cholesterol and plant sterols are taken up by the enterocyte through the Niemann-Pick C1 Like 1 (NPC1L1) transporter (1). The ATP-binding cassette (ABC) transporters G5 and G8 actively efflux plant sterols and, to lesser extent cholesterol, back into the intestinal lumen. In addition, ABCG5 and ABCG8 are located at the canalicular membranes of hepatocytes, where they facilitate efflux of cholesterol and plant sterols into bile (2).
The ABCG5 and ABCG8 genes are located in a head-to-head configuration on chromosome 2p21 (3). Mutations in either of these genes can cause sitosterolemia, a rare autosomal, recessively inherited disorder, characterized by xanthomas, arthralgia, anemia, and premature atherosclerosis (3). The underlying mechanism involves increased intestinal absorption of plant sterols and concomitantly decreased biliary secretion of sterols due to the absence of a functional ABCG5/G8 transporter protein (4). In addition to rare sequence changes causing sitosterolemia, several more common sequence variants in ABCG5/G8 were identified in both healthy and hypercholesterolaemic individuals. These single nucleotide polymorphisms (SNP) were found to be associated with plasma cholesterol and non-cholesterol sterol levels (5–8), as well as with the response to various cholesterol-lowering strategies, including diet interventions (7, 9), consumption of plant sterols or stanols (10, 11), and treatment with statins (12).
We evaluated associations of five frequently tested missense polymorphisms in ABCG5/G8 and plasma cholesterol and non-cholesterol sterol levels in 245 mildly hypercholesterolaemic subjects, as a replication dataset for results found in a comparable cohort (13). Plasma levels of campesterol, sitosterol, and cholestanol serve as markers of cholesterol absorption, and plasma lathosterol levels as markers of cholesterol synthesis (14). To obtain a large cohort for replication of signals observed in studies with limited subjects (reviewed in Ref. 15), we performed a systematic review and meta-analysis, in which we quantitatively assessed associations between five common ABCG5/G8 polymorphisms and plasma cholesterol and non-cholesterol sterol levels. We used a combined dataset, consisting of our present study results pooled with data from 15 other studies that met our inclusion criteria. Based on these results, we conclude that associations between ABCG5/G8 polymorphisms and markers of cholesterol homeostasis are modest at best. The use of small cohorts for such genetic association studies does not yield reproducible findings and is therefore not considered appropriate.
METHODS
Observational study
Subjects and study design.
Our study was a cross-sectional study, consisting of a single visit to our hospital for a blood draw and a physical examination. Recruitment took place via advertisements in local newspapers. Each subject gave written, informed consent.
Caucasian subjects of 18 years or older, with plasma low-density lipoprotein cholesterol (LDL-C) concentrations between 3.5 and 5.0 mmol/l were included. Exclusion criteria were a history of arterial disease, including unstable angina, myocardial infarction, transient ischemic attack, or a cerebrovascular accident; diabetes mellitus; uncontrolled hypertension; familial hypercholesterolemia (FH), diagnosed either by genotyping or by WHO diagnostic criteria; plasma triglyceride (TG) concentrations greater than 4.0 mmol/l; BMI greater than 30 kg/m2 or excessive alcohol consumption. Subjects were not treated with cholesterol-lowering medication and were not consuming sterol- or stanol-enriched food products six weeks prior to the study visit. Blood was collected after an overnight fast of at least 12 h. Plasma was isolated by centrifugation and stored at −80°C. Genomic DNA was prepared from blood leukocytes according to standard procedures. This study was approved by the institutional review board of our hospital.
Analysis of genetic variation in ABCG5/G8.
We genotyped five nonsynonymous polymorphisms in ABCG5/G8 [ABCG5: p.Q604E (rs6720173); ABCG8: p.D19H (rs11887534), p.Y54C (rs4148211), p.T400K (rs4148217), and p.A632V (rs6544718)] using allelic discrimination. Primers were obtained from Applied Biosystems (Foster City, CA), and analyses were performed on a Biorad C1000 Thermal Cycler CFX96 RT System, using VIC® and FAMTM dyes (Applied Biosystems). PCR was performed according to the manufacturer's protocol using Taqman PCR mix (Applied Biosystems).
Plasma analyses.
Plasma TC, HDL-C, and TG levels were measured with standard automated methods; LDL-C levels were calculated using the Friedewald formula (16). Plasma non-cholesterol sterol concentrations were analyzed from nonsaponifiable plasma material with gas-chromatography mass-spectrometry (GCMS) as described previously (17), with minor modifications. Sample size was reduced to 60 μl of serum for analysis in a ZB-1 GC column (60m × 0.25 mm, 0.25 μm, Phenomenex, Torrance, CA) with deuterated campesterol, β-sitosterol, and lathosterol as internal standards.
Statistical analyses.
Baseline non-cholesterol sterol levels are presented as cholesterol-adjusted ratios (non-cholesterol sterol/TC), which is generally performed to eliminate the influence of lipoprotein concentrations on these levels (18). Data that were normally distributed are presented as means and standard deviations (SD). Skewed data were log-transformed prior to testing and are presented as median with the range.
Each genotype was tested for Hardy-Weinberg equilibrium (HWE) by use of the χ2 test. Cross-sectional differences in baseline parameters between genotype variants (carriers versus noncarriers) were evaluated by use of unpaired Student's t-tests, with a Bonferroni-corrected significance of P < 0.001 (0.05/40) (SPSS version 15.0, Chicago, IL).
Systematic review and meta-analysis
Data sources and search strategy.
We performed a systematic literature search for reports assessing associations between ABCG5/G8 polymorphisms and plasma lipid profiles and/or non-cholesterol sterols in healthy or hypercholesterolaemic subjects. Potentially eligible studies were retrieved via electronic databases (MEDLINE, EMBASE, and the Science Citation Index). The following combinations of medical subject headings and free text keywords were used: “polymorphism, single nucleotide”; “snp(s)”; “dna sequence variation”; “ genetic polymorphism”; “dna polymorphism”; “genotype”; “ABCG5 protein, human”; “ABCG8 protein, human”;“ ABCCG5/G8”; “ATP binding cassette transporter(s) G5”; “ATP binding cassette transporter(s) G8”; “ATP binding cassette transporter(s) G5/G8”; and “ATP binding cassette protein(s)G5/G8”. Additional studies were retrieved by a manual search through reference lists of relevant publications and recent reviews.
Study selection and data extraction.
Two reviewers independently determined the eligibility of the studies, according to the following inclusion criteria: subjects aged 18 years or older, availability of genotyping data for one or more of the five ABCG5/G8 polymorphisms, and available TC, LDL-C, HDL-C, TG, lathosterol, campesterol, sitosterol, or cholestanol data. Studies were excluded if subjects had FH or were using lipid-lowering drugs or plant sterols at baseline of the study. No restrictions regarding study design, ethnicity of the subjects, or language of the original articles were imposed. This also applies to possible deviations from HWE, as it was shown that there is no benefit in excluding these studies from genetic meta-analyses (19). Duplicate and preliminary reports were excluded. To obtain the most complete set of data, study authors were approached for additional information by e-mail. The two reviewers independently extracted data of the selected studies, according to a predetermined form. These data concern means and standard deviations of plasma lipoprotein and non-cholesterol sterols. When necessary, TC, LDL-C, HDL-C, and triglyceride data were converted to mmol/l and non-cholesterol sterol data to μg/mg cholesterol.
Statistical analyses.
Data of heterozygous and homozygous minor allele carriers were combined and compared with carriers of the common variant. This was done in the majority of the reviewed studies to balance the groups within each study more effectively. In case the data were not combined in the original report, we averaged means of each minor allele group. Standard deviations were combined according to: √(SD12 · df1) + (SD22 · df2)/(df1+df2), where SD1 represents the mean SD of the subjects with the heterozygous variant, and SD2 the mean SD of subjects with the homozygous variant. Df1 represents the degrees of freedom in the heterozygous group, and df2 the degrees of freedom in the homozygous group.
We calculated differences in means between the common and minor allele groups and the combined variance for each study. If study results for a certain outcome were homogeneous, we used a fixed model that considers consistency in effects over individual studies with natural variation around a constant effect. Then, the pooled mean difference was calculated by using the weighted sum of these differences, with the weight being the reciprocal of the combined variance for each study. Heterogeneity of study results was evaluated separately with the χ2 test and the I2 test for each outcome measure. When the probability value for the χ2-test was <0.10 and/or I2 was >50%, data were considered as heterogeneous. In case of significant heterogeneity, data from the studies were combined using a random effects model according to the method of DerSimonian and Laird (20). A Z-test was performed to test the overall effect.
Associations were evaluated in Caucasian, Asian, and other populations separately by pooling data of eligible studies according to ethnicity. In addition, data of all eligible studies were analyzed, regardless of ethnicity. Statistical analyses were performed using Review Manager 4.2.10 (The Cochrane Collaboration).
RESULTS
Observational study
A total of 245 subjects were included in our study. Baseline data are displayed in Table 1. Frequency distributions of the polymorphisms were similar to those reported previously in European-American populations (supplementary Table I). All genotype distributions were in HWE, and there were no gender differences (data not shown). No significant associations were found with plasma lipid, lipoprotein, and non-cholesterol sterol levels (Table 2).
TABLE 1.
Baseline characteristics of observational study
| Characteristic | Value |
|---|---|
| Subjects, n | 245 |
| Age, years | 58.2 ± 8.0 |
| Male, n (%) | 196 (80%) |
| BMI, kg/m | 25.7 ± 3.1 |
| Lipids, mmol/l | |
| Total cholesterol | 6.18 ± 0.75 |
| LDL-cholesterol | 4.07 ± 0.62 |
| HDL-cholesterol | 1.53 ± 0.40 |
| Triglycerides | 1.10 [0.26–3.93] |
| Non-cholesterol sterols, μg/mg | |
| Campesterol/TC | 1.46 ± 0.71 |
| Sitosterol/TC | 1.13 ± 0.56 |
| Cholestanol/TC | 1.48 ± 0.34 |
| Lathosterol/TC | 1.25 ± 0.51 |
Values are means ± SD. Triglycerides are shown as median [range]. BMI, body mass index; TC, total cholesterol.
TABLE 2.
Associations between ABCG5/G8 polymorphisms and plasma lipids and non-cholesterol sterol ratios
| Lipids and Lipoproteins (mmol/l) |
Non-Cholesterol Sterol Ratios (μg/mg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | N | TC | LDL-C | HDL-C | Triglycerides | Campesterol/TC | Sitosterol/TC | Cholestanol/TC | Lathosterol/TC |
| Q604E | |||||||||
| 171 | 6.16 ± 0.76 | 4.05 ± 0.61 | 1.54 ± 0.40 | 1.09 [0.29–3.56] | 1.45 ± 0.64 | 1.12 ± 0.53 | 1.49 ± 0.33 | 1.24 ± 0.49 | |
| QE/EE | 72 | 6.23 ± 0.73 | 4.11 ± 0.63 | 1.49 ± 0.36 | 1.19 [0.47–3.93] | 1.47 ± 0.88 | 1.16 ± 0.63 | 1.46 ± 0.38 | 1.28 ± 0.57 |
| D19H | |||||||||
| DD | 219 | 6.19 ± 0.75 | 4.08 ± 0.61 | 1.52 ± 0.40 | 1.10 [0.29–3.93] | 1.49 ± 0.69 | 1.16 ± 0.55 | 1.49 ± 0.34 | 1.24 ± 0.49 |
| DH/HH | 24 | 6.04 ± 0.81 | 3.90 ± 0.63 | 1.60 ± 0.38 | 1.15 [0.47–1.97] | 1.24 ± 0.82 | 0.92 ± 0.53 | 1.38 ± 0.34 | 1.35 ± 0.64 |
| Y54C | |||||||||
| YY | 73 | 6.32 ± 0.73 | 4.14 ± 0.64 | 1.58 ± 0.37 | 1.09 [0.45–3.93] | 1.59 ± 0.76 | 1.22 ± 0.60 | 1.53 ± 0.33 | 1.24 ± 0.53 |
| YC/CC | 171 | 6.12 ± 0.76 | 4.03 ± 0.61 | 1.51 ± 0.41 | 1.11 [0.29–3.56] | 1.41 ± 0.68 | 1.09 ± 0.53 | 1.46 ± 0.34 | 1.26 ± 0.50 |
| T400K | |||||||||
| TT | 183 | 6.16 ± 0.75 | 4.03 ± 0.60 | 1.55 ± 0.40 | 1.10 [0.29–3.93] | 1.48 ± 0.69 | 1.14 ± 0.55 | 1.49 ± 0.35 | 1.25 ± 0.48 |
| TK/KK | 61 | 6.24 ± 0.76 | 4.19 ± 0.66 | 1.47 ± 0.37 | 1.14 [0.47–2.61] | 1.38 ± 0.76 | 1.09 ± 0.59 | 1.45 ± 0.31 | 1.27 ± 0.59 |
| A632V | |||||||||
| AA | 139 | 6.22 ± 0.72 | 4.12 ± 0.60 | 1.51 ± 0.37 | 1.12 [0.33–2.90] | 1.49 ± 0.72 | 1.16 ± 0.58 | 1.51 ± 0.37 | 1.21 ± 0.49 |
| AV/VV | 105 | 6.14 ± 0.79 | 3.99 ± 0.63 | 1.57 ± 0.43 | 1.07 [0.29–3.93] | 1.42 ± 0.70 | 1.09 ± 0.52 | 1.44 ± 0.30 | 1.31 ± 0.52 |
Values shown are means ± SD. Triglycerides are shown as median [range]. No significant differences between groups were found. Triglyceride data were log-transformed prior to testing. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SNP, single nucleotide polymorphism; TC, total cholesterol.
Meta-analysis: characteristics of included studies
The literature search generated 62 publications, 22 of which investigated the polymorphisms of interest. Eight studies were excluded, due to the inclusion of children (21), FH patients (22), subjects treated with cholesterol-lowering medication (6, 23), unavailable cholesterol data (10, 24, 25), or a duplicate report (26).
Fifteen studies were included in the meta-analysis, next to our present study (Table 3). The number of study participants ranged from 26 to 1,046. Five studies were conducted in a population of Caucasians (5, 7, 11, 13, 27), three studies investigated Asian populations (8, 28, 29), two studies were conducted in a Hispanic population (30), and two in a mixed racial population (31, 32). The remaining three studies did not report the ethnicity of the study subjects (9, 12, 33). The majority of the studies were conducted in mildly hypercholesterolaemic, otherwise healthy populations (5, 12, 13, 27, 28, 31–33). Five studies evaluated normocholesterolaemic subjects (9, 11, 29, 30); two studies evaluated both (8, 30). Most studies included normoweighted subjects; however, one study selected overweight, mildly hypercholesterolaemic women (31), and one study was conducted in obese, insulin-resistant men without type 2 diabetes mellitus (T2DM) (33). Allele frequencies in all individual studies were in line with those reported previously (https://www.ncbi.nlm.nih.gov/SNP/). Each of the studies reported that genotype distribution was in HWE for the investigated SNPs, with the exception of three studies that did not comment on HWE (5, 27, 33). From eight authors, we received additional data, which were not described in the original studies.
TABLE 3.
Characteristics of studies included in the meta-analysis
| Reference | Number of Subjects | Age | Male/Female | Body Mass Index | Ethnicity | Single Nucleotide Polymorphisms | Lipids | Non-Cholesterol Sterols |
|---|---|---|---|---|---|---|---|---|
| n | years | n | kg/m2 | |||||
| Berge, 2002 (5) | 148 | 55 ± 11 | 74/74 | Not reported | Caucasian | D19H, T400K, Y54C, Q604E, A632V | TC | CAMP, SITO, CHOLST, LATHO |
| Weggemans, 2002 (7) | 486 | 26.3 ± 11.6 | 257/229 | 21.7 ± 3.0 | Caucasian | Q604E | TC | — |
| Gylling, 2004 (13) | 262 | 53.1 ± 8.1 | 143/119 | 26.4 ± 6.5 | Caucasian | D19H, T400K, Y54C, Q604E, A632V | TC, LDL-C, HDL-C, TG | CAMP, SITO, CHOLST, LATHO |
| Plat, 2005 (11)a | 112 | 33 ± 16 | 41/71 | 22.9 ± 3.6 | Caucasian | T400K, Q604E, A632V | LDL-C, HDL-C, TG | CAMP, SITO, LATHO |
| Acalovschi, 2006 (27) | 72e | 56.3 (36–80) | 30/42 | 30.1 ± 4.9 | Caucasian | D19H, T400K, Y54C, Q604E, A632V | TC, HDL-C, TG | — |
| Jakulj, et al.b | 245 | 58.4 ± 7.5 | 189/48 | 25.8 ± 3.0 | Caucasian | D19H, T400K, Y54C, Q604E, A632V | TC, LDL-C, HDL-C, TG | CAMP, SITO, CHOLST, LATHO |
| Miwa, 2005 (28) | 100 | 62.4 ± 12.1 | 48/52 | 23.0 ± 3.5 | Asian | T400K, Y54C, Q604E | — | SITO, LATHO |
| Wang, 2007 (29)a,c | 134 | 54.1 ± 8.1 | 134/0 | 23.2 ± 2.3 | Asian | T400K, Y54C, Q604E | TC, LDL-C, HDL-C, TG | — |
| Chen, 2008 (8)a | 1046 | 47.0 ± 9.3 | 894/152 | 24.9 ± 2.4 | Asian | D19H, T400K, Y54C, Q604Ei | TC, LDL-C, HDL-C, TG | — |
| Caamano, 2008 (30)d | 104 | 40 ± 10 | 58/46 | 25.5 ± 3.3 | Hispanic | Y54C, Q604E | TC, LDL-C, HDL-C, TG | — |
| 118 | 44 ± 7 | 71/47 | 27.6 ± 4.9 | Hispanic | Y54C, Q604E | TC, LDL-C, HDL-C, TG | — | |
| Santosa, 2007 (31)a | 35 | 49.4 ± 6.7 | 0/35 | 31.4 ± 2.8 | Mixedf | D19H, T400K, Y54C, Q604E | TC, LDL-C, HDL-C, TG | — |
| Rudkowska, 2008 (32) | 26 | 59.6 ± 9.6 | 15/11 | 26.4 ± 2.7 | Mixedf | D19H, T400K, Y54C, Q604E | TC, LDL-C, HDL-C, TG | — |
| Chan, 2004 (33)a | 47 | 54.5 ± 8.4 | 47/0 | 32 ± 3.6 | Not reportedg | D19H, T400K | TC, LDL-C, HDL-C, TG | CAMP, SITO, LATHO |
| Kajinami, 2004 (12)a | 338 | 58 ± 11 | 203/135 | 27.0 ± 3.0 | Not reportedh | D19H, T400K, Y54C, Q604E, A632V | TC, LDL-C, HDL-C, TG | — |
| Herron, 2006 (9)a | 91 | 31.1 ± 9.2 | 40/51 | 24.8 ± 4.7 | Not reportedh | Q604E | TC, LDL-C, HDL-C | — |
| Total (WM) | 3364 | 46.7 ± 10.5 | 2251/ 1113 | 23.9 ± 3.5 |
Number and characteristics of studies included in the meta-analysis. CAMP, campesterol/TC ratio; CHOLST, cholestanol/TC ratio; HDL-C, HDL-cholesterol; LATHO, lathosterol/TC ratio; LDL-C, LDL-cholesterol; SITO, sitosterol/TC ratio; TC, total cholesterol; TG, triglyceride; WM, weighted mean.
Additional data received after contacting the authors.
Present study.
Population included 287 gallstone patients and 219 gallstone-free controls, both men and women. Lipid data distributed over the genotypes were not stated in the original report; Additional data were received by request, only for 134 males, both gallstone patients and controls.
Study described two SNPs in a separate population of subjects with and without hypercholesterolemia. Data of these two groups were separately included into the meta-analysis.
Plasma TC, HDL-C, and TG levels were studied in 68 siblings with gallstones, however, data of 72 subjects are presented and 23 out of 72 (31%) had T2DM.
Study conducted in Montreal, Canada.
Study conducted in Australia.
Study conducted in USA.
A632V was initially studied as well, but not further analyzed as the first 500 subjects were monomorphic.
Meta-analysis: pooled effects of polymorphisms on lipids and non-cholesterol sterols
We evaluated associations between the ABCG5/G8 polymorphisms and plasma lipid and non-cholesterol sterol concentrations by pooling data according to ethnicity, as well as by pooling data of all studies regardless of ethnicity. When performing the latter, there was little heterogeneity of study results, as evaluated with the χ2 test and the I2 test. In other words, a fixed model applied to almost all study parameters. Hence, all eligible studies were included into the meta-analysis, thereby increasing power (supplementary Table II).
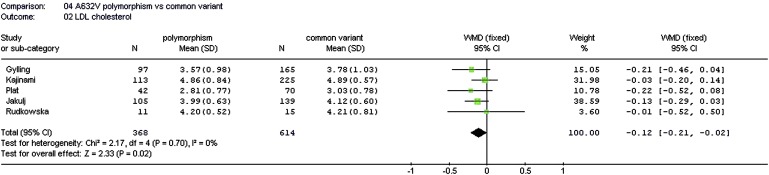
Of the five tested polymorphisms, only the p.A632V variant was associated with baseline lipid concentrations. In pooled data of five studies, 368 subjects carrying the minor 632V allele had significantly lower baseline LDL-C levels compared with 614 subjects with the common 632A variant, with a weighted mean difference (WMD) of −0.11 mmol/l (95% CI, range: −0.20 to −0.03 mmol/l; P = 0.01) (Fig. 1).
Fig. 1.
Association between the ABCG8 p.A632V variant and plasma LDL-C levels. LDL-C, low-density lipoprotein cholesterol; WMD, weighted mean difference.
Six studies evaluated associations between ABCG5/G8 polymorphisms and non-cholesterol sterol concentrations (5, 11, 13, 28, 33). Analysis of the pooled data revealed that the minor allele of the p.D19H variant was associated with significantly reduced markers of cholesterol absorption and increased markers of cholesterol synthesis (Fig. 2); 83 subjects carrying the 19H allele showed decreased campesterol/TC, sitosterol/TC, and cholestanol/TC ratios, respectively, compared with 591 subjects with the common variant [WMD −0.50 μg/mg (95% CI, range: −0.80 to −0.20 μg/mg; P = 0.001); WMD −0.36 μg/mg (95% CI, range: −0.45 to −0.27 μg/mg; P < 0.00001); and WMD −0.24 μg/mg (95% CI, range: −0.31 to −0.17 μg/mg; P < 0.00001), respectively). Concomitantly, 79 subjects carrying the minor 19H allele expressed higher lathosterol/TC ratios compared with 541 subjects carrying the common variant [WMD 0.26 μg/mg (95% CI, range: 0.10 to 0.41 μg/mg; P = 0.001)]. The p.T400K polymorphism showed similar trends with borderline significance, whereas the remaining three polymorphisms were not associated with baseline non-cholesterol sterol levels (supplementary Table II).
Fig. 2.
Associations between the ABCG8 p.D19H variant and plasma non-cholesterol sterol levels. WMD, weighted mean difference.
DISCUSSION
This meta-analysis is the first to evaluate associations between frequently studied missense ABCG5/G8 variants and plasma cholesterol and non-cholesterol levels in a large dataset, which consists of 3,364 individuals from 16 studies. We found no substantial associations with plasma cholesterol levels. The p.D19H variant was associated with changes in cholesterol absorption and cholesterol synthesis markers without affecting plasma cholesterol levels.
To date, multiple studies have investigated associations between missense ABCG5/G8 variants and markers of baseline cholesterol metabolism (reviewed in Ref. 15). Several studies showed positive associations with plasma lipoprotein levels (5, 7, 8), which we and others were not able to replicate. The majority of these studies were small-scaled. Hence, pooling data of these studies allowed us to evaluate these associations in the largest possible number of individuals. The single significant association concerned the p.A632V polymorphism (i.e., carriers of the minor 632V allele exhibited an average 0.11 mmol/l decrease in plasma LDL-C levels, compared with carriers of the common variant). This association was not found in any of the individual studies, and although statistically significant in our meta-analysis, its clinical relevance is modest at best. It was previously shown that a 10% reduction of plasma LDL-C levels is associated with a 20% decrease in major cardiovascular events (34). Based on such epidemiological data, subjects carrying the minor 632V allele would carry a reduced CVD risk of merely 5%. Interestingly, several genome-wide association studies (GWAS) have also shown significant associations between ABCG5/G8 variants and plasma LDL-C levels (35–38). Although these studies mostly include common variants with a minor allele frequency (MAF) greater than 0.1, two studies described a significant association between the lesser common p.D19H variant and plasma LDL-C concentrations (36, 38), although associations were relatively weak.
We did not find any association between the p.D19H variant and baseline LDL-C levels in 155 subjects with the minor allele versus 1,842 subjects carrying the common variant (supplementary Table II). This is in line with previous findings in a cohort of 2,012 heterozygous familial hypercholesterolemia (FH) patients (22). However, the 19H allele was significantly associated with decreased sitosterol/TC, campesterol/TC, and cholesterol/TC ratios, with concomitantly increased lathosterol/TC ratios. This corroborates findings by others (5, 6, 13, 38) and might imply that subjects carrying the 19H allele excrete higher amount of sterols in bile and intestine, possibly mediated by a gain-of-function of the ABCG8 transporter. This possibility is supported by the previously described association of this variant with cholesterol gallstones in several large studies (25, 39, 40). However, in silico analysis by polyphen predicts p.D19H as a benign variant, and no relation was found between this SNP and ABCG8 mRNA expression levels in human liver tissue samples (38). Of note, in silico analyses of the remaining four variants predicted only the p.Y54C polymorphism to be damaging. However, this SNP was not associated with plasma lipid or non-cholesterol sterol levels. To the best of our knowledge, direct functional analyses of the studied variants, by means of in vitro studies or knock-in models, have not been reported thus far.
Studies on ABCG5/G8 polymorphisms and their associations with CVD risk are scarce. One study evaluated associations between p.T400K and p.D19H polymorphisms and CVD risk in a cohort of 2,012 heterozygous FH patients (22). Neither of the two variants was independently associated with total CVD risk; however, when combined p.T400K and p.D19H gene scores were calculated, a positive correlation was found. In contrast, two other studies reported that none of the five missense ABCG5/G8 polymorphisms was associated with CAD (41, 42), although the latter two studies were not primarily designed nor powered to draw substantial conclusions. During the preparation of this article, a large GWAS report convincingly showed that two common ABCG8 variants associated with elevated plasma phytosterol levels were also associated with increased CAD risk, whereas one variant, associated with decreased phytosterol levels, was associated with reduced CAD risk (38). This GWAS did not report associations between the five missense variants of our meta-analysis and CAD risk; however, the variant associated with reduced CAD risk was a SNP in close linkage disequilibrium with the p.D19H variant. Finally, although this GWAS provides important novel evidence that ABCG8 variants associated with elevated serum phytosterol levels are also associated with increased CAD risk, it is not clear whether these associations are causally linked, because the identified variants were also associated with concomitantly increased plasma cholesterol levels. Hence, it remains to be established whether associations between ABCG8 variants and CVD risk are mediated by plasma phytosterol concentrations alone.
Finally, our meta-analysis has a number of limitations. First, although we performed our analyses in a large population by pooling data of 3,364 subjects, associations between the polymorphisms and individual sterol parameters could only be performed in smaller subsets. Hence, some of our analyses might still lack statistical power. Furthermore, we were not able to investigate gender-specific associations, which have been reported in some of the studies (6, 29, 31). This also applies to possible gene-diet or gene-environment interactions, which have been suggested to be of importance (23). In addition, due to their skewed distribution, triglyceride data could not be pooled. Twelve studies reported significant associations with baseline triglyceride levels, which mostly involved the p.T400K polymorphism (13, 29, 33); however, this was not consistent throughout all included studies (8, 11, 12, 27, 30–32). Finally, in our analyses, we assumed a dominant genetic model, as most of the included studies presented their data according to this model. However, recent studies suggest a genetic model-free approach, because the assumption of a certain genetic model might introduce bias (43). We did not have sufficient data to perform these analyses. Furthermore, the added value of the genetic model in this study is questionable, given the low minor allele frequencies of the variants studied.
In conclusion, this meta-analysis shows a positive association of the ABCG8 p.D19H polymorphism with decreased plasma plant sterol levels and concomitantly increased plasma lathosterol levels. We did not observe any substantial associations between ABCG5/G8 polymorphisms and plasma lipid levels. Hence, the study of small cross-sectional cohorts for genetic association studies does not yield reproducible insights and is, therefore, considered inappropriate.
Supplementary Material
Acknowledgments
The authors are grateful to M. Brousseau, D.C. Chan, M.L. Fernandez, T-Q Han, P.J. Hsiao, P.J.H. Jones, K. Kajinami, M. Fernandez, J. Plat, I. Rudkowska, and S. Santosa for kindly providing the requested additional data for the meta-analysis.
Footnotes
Abbreviations:
- ABC
- adenosine tri phosphate binding cassette
- BMI
- body mass index
- CAD
- coronary artery disease
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- FH
- familial hypercholesterolemia
- GWAS
- genome-wide association study
- HDL-C
- high-density lipoprotein cholesterol
- HWE
- Hardy-Weinberg equilibrium
- LD
- linkage disequilibrium
- LDL-C
- low-density lipoprotein cholesterol
- MAF
- minor allele frequency
- NPC1L1
- Niemann-Pick C1 Like 1
- SNP
- single nucleotide polymorphism
- TC
- total cholesterol
- T2DM
- type 2 diabetes mellitus
- TG
- triglyceride
- WMD
- weighted mean difference
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables.
REFERENCES
- 1.Davies J. P., Levy B., Ioannou Y. A. 2000. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 65: 137–145. [DOI] [PubMed] [Google Scholar]
- 2.Graf G. A., Yu L., Li W. P., Gerard R., Tuma P. L., Cohen J. C., Hobbs H. H. 2003. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 278: 48275–48282. [DOI] [PubMed] [Google Scholar]
- 3.Berge K. E., Tian H., Graf G. A., Yu L., Grishin N. V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H. H. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290: 1771–1775. [DOI] [PubMed] [Google Scholar]
- 4.Yu L., Li-Hawkins J., Hammer R. E., Berge K. E., Horton J. D., Cohen J. C., Hobbs H. H. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berge K. E., von Bergmann K., Lutjohann D., Guerra R., Grundy S. M., Hobbs H. H., Cohen J. C. 2002. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 43: 486–494. [PubMed] [Google Scholar]
- 6.Hubacek J. A., Berge K. E., Stefkova J., Pitha J., Skodova Z., Lanska V., Poledne R. 2004. Polymorphisms in ABCG5 and ABCG8 transporters and plasma cholesterol levels. Physiol. Res. 53: 395–401. [PubMed] [Google Scholar]
- 7.Weggemans R. M., Zock P. L., Tai E. S., Ordovas J. M., Molhuizen H. O., Katan M. B. 2002. ATP binding cassette G5 C1950G polymorphism may affect blood cholesterol concentrations in humans. Clin. Genet. 62: 226–229. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z. C., Shin S. J., Kuo K. K., Lin K. D., Yu M. L., Hsiao P. J. 2008. Significant association of ABCG8:D19H gene polymorphism with hypercholesterolemia and insulin resistance. J. Hum. Genet. 53: 757–763. [DOI] [PubMed] [Google Scholar]
- 9.Herron K. L., McGrane M. M., Waters D., Lofgren I. E., Clark R. M., Ordovas J. M., Fernandez M. L. 2006. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J. Nutr. 136: 1161–1165. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H. L., Houweling A. H., Vanstone C. A., Jew S., Trautwein E. A., Duchateau G. S., Jones P. J. 2008. Genetic variation in ABC G5/G8 and NPC1L1 impact cholesterol response to plant sterols in hypercholesterolemic men. Lipids. 43: 1155–1164. [DOI] [PubMed] [Google Scholar]
- 11.Plat J., Bragt M. C., Mensink R. P. 2005. Common sequence variations in ABCG8 are related to plant sterol metabolism in healthy volunteers. J. Lipid Res. 46: 68–75. [DOI] [PubMed] [Google Scholar]
- 12.Kajinami K., Brousseau M. E., Nartsupha C., Ordovas J. M., Schaefer E. J. 2004. ATP binding cassette transporter G5 and G8 genotypes and plasma lipoprotein levels before and after treatment with atorvastatin. J. Lipid Res. 45: 653–656. [DOI] [PubMed] [Google Scholar]
- 13.Gylling H., Hallikainen M., Pihlajamaki J., Agren J., Laakso M., Rajaratnam R. A., Rauramaa R., Miettinen T. A. 2004. Polymorphisms in the ABCG5 and ABCG8 genes associate with cholesterol absorption and insulin sensitivity. J. Lipid Res. 45: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 14.Miettinen T. A., Tilvis R. S., Kesaniemi Y. A. 1990. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am. J. Epidemiol. 131: 20–31. [DOI] [PubMed] [Google Scholar]
- 15.Rudkowska I., Jones P. J. 2008. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr. Rev. 66: 343–348. [DOI] [PubMed] [Google Scholar]
- 16.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 17.Ahmida H. S., Bertucci P., Franzo L., Massoud R., Cortese C., Lala A., Federici G. 2006. Simultaneous determination of plasmatic phytosterols and cholesterol precursors using gas chromatography-mass spectrometry (GC-MS) with selective ion monitoring (SIM). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 842: 43–47. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen T. A., Gylling H., Lindbohm N., Miettinen T. E., Rajaratnam R. A., Relas H. 2003. Serum noncholesterol sterols during inhibition of cholesterol synthesis by statins. J. Lab. Clin. Med. 141: 131–137. [DOI] [PubMed] [Google Scholar]
- 19.Minelli C., Thompson J. R., Abrams K. R., Thakkinstian A., Attia J. 2008. How should we use information about HWE in the meta-analyses of genetic association studies? Int. J. Epidemiol. 37: 136–146. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R., Laird N. 1986. Meta-analysis in clinical trials. Control. Clin. Trials. 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21.Viturro E., de Oya M., Lasuncion M. A., Gorgojo L., Moreno J. M., Benavente M., Cano B., Garces C. 2006. Cholesterol and saturated fat intake determine the effect of polymorphisms at ABCG5/ABCG8 genes on lipid levels in children. Genet. Med. 8: 594–599. [DOI] [PubMed] [Google Scholar]
- 22.Koeijvoets K. C., van der Net J. B., Dallinga-Thie G. M., Steyerberg E. W., Mensink R. P., Kastelein J. J., Sijbrands E. J., Plat J. 2009. ABCG8 gene polymorphisms, plasma cholesterol concentrations, and risk of cardiovascular disease in familial hypercholesterolemia. Atherosclerosis. 204: 453–458. [DOI] [PubMed] [Google Scholar]
- 23.Junyent M., Tucker K. L., Smith C. E., Garcia-Rios A., Mattei J., Lai C. Q., Parnell L. D., Ordovas J. M. 2009. The effects of ABCG5/G8 polymorphisms on plasma HDL cholesterol concentrations depend on smoking habit in the Boston Puerto Rican Health Study. J. Lipid Res. 50: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Z. Y., Parini P., Eggertsen G., Davis M. A., Hu H., Suo G. J., Zhang S. D., Rudel L. L., Han T. Q., Einarsson C. 2008. Increased expression of LXR alpha, ABCG5, ABCG8, and SR-BI in the liver from normolipidemic, nonobese Chinese gallstone patients. J. Lipid Res. 49: 464–472. [DOI] [PubMed] [Google Scholar]
- 25.Kuo K. K., Shin S. J., Chen Z. C., Yang Y. H., Yang J. F., Hsiao P. J. 2008. Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br. J. Surg. 95: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 26.Kajinami K., Brousseau M. E., Ordovas J. M., Schaefer E. J. 2004. Interactions between common genetic polymorphisms in ABCG5/G8 and CYP7A1 on LDL cholesterol-lowering response to atorvastatin. Atherosclerosis. 175: 287–293. [DOI] [PubMed] [Google Scholar]
- 27.Acalovschi M., Ciocan A., Mostean O., Tirziu S., Chiorean E., Keppeler H., Schirin-Sokhan R., Lammert F. 2006. Are plasma lipid levels related to ABCG5/ABCG8 polymorphisms? A preliminary study in siblings with gallstones. Eur. J. Intern. Med. 17: 490–494. [DOI] [PubMed] [Google Scholar]
- 28.Miwa K., Inazu A., Kobayashi J., Higashikata T., Nohara A., Kawashiri M., Katsuda S., Takata M., Koizumi J., Mabuchi H. 2005. ATP-binding cassette transporter G8 M429V polymorphism as a novel genetic marker of higher cholesterol absorption in hypercholesterolaemic Japanese subjects. Clin. Sci. (Lond.). 109: 183–188. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Jiang Z. Y., Fei J., Xin L., Cai Q., Jiang Z. H., Zhu Z. G., Han T. Q., Zhang S. D. 2007. ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin. Chim. Acta. 384: 80–85. [DOI] [PubMed] [Google Scholar]
- 30.Caamano J. M., Pacheco A., Lanas F., Salazar L. A. 2008. Single nucleotide polymorphisms in ABCG5 and ABCG8 genes in Chilean subjects with polygenic hypercholesterolemia and controls. Clin. Chem. Lab. Med. 46: 1581–1585. [DOI] [PubMed] [Google Scholar]
- 31.Santosa S., Demonty I., Lichtenstein A. H., Ordovas J. M., Jones P. J. 2007. Single nucleotide polymorphisms in ABCG5 and ABCG8 are associated with changes in cholesterol metabolism during weight loss. J. Lipid Res. 48: 2607–2613. [DOI] [PubMed] [Google Scholar]
- 32.Rudkowska I., AbuMweis S. S., Nicolle C., Jones P. J. 2008. Association between non-responsiveness to plant sterol intervention and polymorphisms in cholesterol metabolism genes: a case-control study. Appl. Physiol. Nutr. Metab. 33: 728–734. [DOI] [PubMed] [Google Scholar]
- 33.Chan D. C., Watts G. F., Barrett P. H., Whitfield A. J., van Bockxmeer F. M. 2004. ATP-binding cassette transporter G8 gene as a determinant of apolipoprotein B-100 kinetics in overweight men. Arterioscler. Thromb. Vasc. Biol. 24: 2188–2191. [DOI] [PubMed] [Google Scholar]
- 34.Law M. R., Wald N. J., Thompson S. G. 1994. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 308: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aulchenko Y. S., Ripatti S., Lindqvist I., Boomsma D., Heid I. M., Pramstaller P. P., Penninx B. W., Janssens A. C., Wilson J. F., Spector T., et al. 2009. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 41: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kathiresan S., Willer C. J., Peloso G. M., Demissie S., Musunuru K., Schadt E. E., Kaplan L., Bennett D., Li Y., Tanaka T., et al. 2009. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma L., Yang J., Runesha H. B., Tanaka T., Ferrucci L., Bandinelli S., Da Y. 2010. Genome-wide association analysis of total cholesterol and high-density lipoprotein cholesterol levels using the Framingham heart study data. BMC Med. Genet. 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teupser D., Baber R., Ceglarek U., Scholz M., Illig T., Gieger C., Holdt L. M., Leichtler A., Greiser K. H., Huster D., et al. 2010. Genetic regulation of serum phytosterol levels and risk of coronary artery disease. Circ. Cardiovasc. Genet. Epub ahead of print. June 7, 2010; doi:10.1161/CIRCGENETICS.109.907873. [DOI] [PubMed] [Google Scholar]
- 39.Grunhage F., Acalovschi M., Tirziu S., Walier M., Wienker T. F., Ciocan A., Mosteanu O., Sauerbruch T., Lammert F. 2007. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 46: 793–801. [DOI] [PubMed] [Google Scholar]
- 40.Buch S., Schafmayer C., Volzke H., Becker C., Franke A., von Eller-Eberstein H., Kluck C., Bassmann I., Brosch M., Lammert F., et al. 2007. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat. Genet. 39: 995–999. [DOI] [PubMed] [Google Scholar]
- 41.Gylling H., Hallikainen M., Rajaratnam R. A., Simonen P., Pihlajamaki J., Laakso M., Miettinen T. A. 2009. The metabolism of plant sterols is disturbed in postmenopausal women with coronary artery disease. Metabolism. 58: 401–407. [DOI] [PubMed] [Google Scholar]
- 42.Matthan N. R., Pencina M., LaRocque J. M., Jacques P. F., D'Agostino R. B., Schaefer E. J., Lichtenstein A. H. 2009. Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD. J. Lipid Res. 50: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minelli C., Thompson J. R., Abrams K. R., Thakkinstian A., Attia J. 2005. The choice of a genetic model in the meta-analysis of molecular association studies. Int. J. Epidemiol. 34: 1319–1328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.