Abstract
N-acylethanolamines (NAEs) are a group of lipid mediators synthesized in response to a number of physiological and pathological stimuli. Because of the low tissue concentrations of NAEs, analyses often include liquid extraction followed by solid-phase extraction and subsequent quantitation by LC/MS or GC/MS. Reported levels of NAEs vary considerably, however, and often no explanation is given for these discrepancies. Brought on by difficulties encountered during method development, the effects of using four different brands of silica-containing solid phase extraction (SPE) columns and five different brands of chloroform for sample preparation were investigated. Considerable variation in the retention and recoveries of seven NAEs and 2-arachidonoylglycerol existed between the SPE columns. Furthermore, it was found that some chloroforms contained quantifiable amounts of N-palmitoylethanolamine and N-stearoylethanolamine. Finally, it was found that use of one of the chloroforms resulted in a loss of N-oleoylethanolamine from solution due to addition of chlorine to the ω-9 bond. The identity of this reaction product was confirmed by LC-MS/MS and NMR. It is recommended that these aspects of sample preparation and analysis should be thoroughly validated during method development and the relevant information on specific brands used be reported in future communications in order to better estimate the validity of reported quantitative data.
Keywords: anandamide, artefact, chloroform, contamination, extraction, oleoylethanolamide, palmitoylethanolamide, stearoylethanolamide
N-acylethanolamines (NAEs) are a group of lipid mediators synthesized in the organism in response to a variety of physiological and pathological stimuli, e.g., food intake, obesity, inflammation, and cell injury. N-arachidonoylethanolamine (AEA, anandamide) and N-oleoylethanolamine (OEA, Fig. 1) are among the most intensively investigated NAEs, particularly in relation to obesity and appetite regulation (1–3). In addition, N-palmitoylethanolamine (PEA, Fig. 1) and N-stearoylethanolamine (SEA, Fig. 1) have attracted attention in relation to antiinflammatory and apoptotic properties (4–6).
Fig. 1.
Structures of the key compounds in this study: PEA, SEA, and OEA.
NAEs are present in biological tissues in concentrations in the pmol per gram scale (7–9). The ability to measure such low concentrations imposes high demands with regard to recovery of the analytes following sample preparation and the sensitivity of the analytical method used for measuring. MS coupled to GC (10–14) or HPLC (15–19) separation techniques are the current standard technologies used in NAE research, often incorporating deuterium-labeled analogs as internal standards (ISTD). The choice of technique is mostly dependent on instrumentation available, because the techniques are almost equal in performance. The major difference is that GC/MS requires derivatization to make the NAEs sufficiently volatile for analysis, making the GC/MS technique more laborious. The standard sample preparation procedures are modifications of Folchs method (20), which consists of a homogenization and lipid extraction step using organic solvents to isolate the NAEs from the biological sample of interest, most often followed by a sample purification or concentration step using solid phase extraction (SPE) columns packed with silica or C18 material (16, 17, 21).
A review of reports from the last decade on quantification of NAEs in various biological samples reveals large discrepancies among the published tissue concentrations found under comparable conditions. In most cases, no plausible explanation for the observed differences is given. In case an explanation is stated, it often refers to differences in the in vivo/in vitro methodologies (e.g., procedure for tissue collection or dissection) or in the analytical procedure (e.g., extraction procedure, chromatographic principles, use of ISTD or derivatization procedures). During our studies of the effect of dietary fatty acids on tissue levels of different NAEs (7, 22, 23), we observed some artifacts during the assay development, using solid-phase extraction and MS with ISTDs. Occasionally, the OEA standard disappeared and sometimes unrealistic levels of PEA and SEA were obtained. This led to investigations of the cause of these spurious effects.
Thus far, little attention has been given to the quality of the solvents used for the sample preparation procedures as a potential interference factor, which may explain our experiences during method development and also some of the observed differences among published NAE tissue levels. Also, although the choice of packing materials used for the SPE procedures have been discussed by Hardison et al. (21), little interest has been paid to possible performance differences among manufactures of the same type of packing material. Here, for the first time, we report on discrepancies found in the performance among commercially available silica packing materials. Furthermore, we find that PEA and SEA may be present as contaminants in some standard chloroform brands and that free chlorine in chloroform may react with the double-bond in OEA, and potentially other unsaturated NAEs, which may result in underestimation of concentrations or false negatives when measured by MS.
MATERIALS
A number of different chloroforms were used: Sigma-Aldrich ACS spectrophotometric grade [product no. 154733, stabilized with amylene (two different batches); product no. 366919, stabilized with 0.5–1.0% ethanol (EtOH) (three different batches)], Sigma-Aldrich CHROMASOLV®plus (product no. 650498, stabilized with amylene; product no. 650471, stabilized with 0.5–1.0% EtOH), Fluka purum [product no. 25693, stabilized with 0.006% amylene (three different batches)], Fluka p.a. ACS reagent [product no. 25690, stabilized with 1% EtOH (three different batches)], Merck LiChrosolv for chromatography (product no. 1.022444, stabilized with amylene), Merck EMSURE p.a. [product no. 1.02445, stabilized with 0.6–1.0% EtOH (two different batches)] and Alfa-Aesar HPLC grade (product no. 043685, stabilized with 80–160 ppm amylene; product no. 022920, stabilized with 0.5–1.0% EtOH).
Methanol (MeOH) LiChrosolv HPLC-grade, ethyl acetate GR p.a. ACS grade, n-hexane EMSURE p.a., and formic acid GR p.a. ACS grade were from Merck KGaA Chemicals (Darmstadt, Germany). MeOH HiPerSolv LC-grade and acetonitrile HiPerSolv CHROMANORM LC-grade were from BDH prolabo (VWR, Herlev, Denmark).
Ultrapure Milli-Q water was tapped from an in-house Direct-Q 3 UV system (Millipore s.a.s., Molsheim, France).
Synthetic standards of PEA, SEA, OEA, AEA, and arachidonoylglycerol (2-AG) were bought from Cayman Chemical (Ann Arbor, MI). Linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid were bought from Cayman Chemical (Ann Arbor, MI). Oxalylchloride, N,N-dimethylforamide, and ethanolamine were bought from Sigma Aldrich Chemie GmbH (Schnelldorph, Germany). Manganese oxide [Mn(IV)O2] p.a. was from Riedel-de-Häen A.G. (Berlin, Germany) and concentrated hydrochloric acid (37%) p.a. and reduced iron powder p.a. were from Merck KGaA chemicals (Darmstadt, Germany).
N-linoleoylethanolamine (LEA), N-eicosapentatenoylethanolamine (EPEA), and N-docosahexaenoylethanolamine (DHEA) were synthesized as described previously (24). In brief, a fatty acid chloride intermediate was synthesized by reacting the individual fatty acid with oxalylchloride and N,N-dimethylformamide in a molar ratio of 2 to 1, respectively. The final NAE product was then synthesized by letting the respective fatty acid chlorides react with 10 molar equivalents of ethanolamine. Purity of all NAEs was verified by thin-layer chromatography using chloroform: MeOH:ammonium hydroxide (80:20:2) as eluent (purities were found to be >95% by TLC with iodine-staining). Authenticities of the compounds were verified by HPLC coupled to LC-MS.
EXPERIMENTAL PROCEDURES
Validation of commercial SPE columns
Four commercial silica SPE columns [Strata Si-1 Silica from Phenomenex (SupWare, Birkerød, Denmark); Supelco DSC-Si (Sigma-Aldrich, Brøndby, Denmark); Waters Sep-Pak Silica (Waters, Hedehusene, Denmark); Isolute Silica (IST, Microlab-Aarhus, Højbjerg, Denmark)] were tested for recoveries of 2-AG and NAEs. The SPE cartridges contained between 100 and 300 mg sorbent (1–3 cc). A solution of 500 pmol/ml of PEA, SEA, OEA, LEA, AEA, EPEA, DHEA, and 2-AG in chloroform was made. Then 1.0 ml of this solution was applied to each of the SPE columns to be tested (n = 5 for each brand of SPE column) and washed with 3 × 1.0 ml of chloroform (Wash-fraction = 4 ml). The compounds were eluted from the SPE column using increasing concentrations of MeOH in chloroform. First elution was by 4 × 1.0 ml of 3% MeOH (E 3% fraction), followed by 4 × 1.0 ml of 25% MeOH (E 25% fraction) and finally by 4 × 1.0 ml 100% MeOH (E 100% fraction). All fractions were evaporated to dryness under N2, reconstituted in 100 μl chloroform, before being transferred to injection vials and analyzed by LC-MS. Three individual 1.0 ml aliquots of the NAE solution were evaporated to dryness under N2, reconstituted in 100 µL chloroform, and used as references for recovery calculations. Samples were analyzed by LC-MS using an Agilent 1100 series LC-MSD single quadrupole mass spectrometer (Agilent Technologies Inc, Santa Clara, CA) equipped with a Phenomenex HyperClone ODS(C18) (3 μm, 120 Å pore size, 150 × 2.00 mm i.d.) column (Supware, Birkerød, Denmark). The mobile phases consisted of 15% MeOH (BDH prolabo HiPerSolv) in milli-Q water (mobile phase A) and 100% MeOH (BDH prolabo HiPerSolv) (mobile phase B), both containing 0.05% formic acid. Elution was performed using a flow of 200 μl/min and running a linear gradient of 80% to 100% MeOH (25% A for 2 min, 15% A for 3 min, 5% A for 20 min, 0% A for 5 min). The column compartment was kept at 25°C. Quantitative analysis was performed by positive electrospray ionization in selected ion monitoring (SIM) mode, detecting protonated or sodium-adducted molecules of m/z 300.3 ([PEA + H]+), m/z 328.3 ([SEA + H]+), m/z 326.3 ([OEA + H]+), m/z 324.3 ([LEA + H]+), m/z 348.3 ([AEA + H]+), m/z 368.3 ([EPEA + Na]+), m/z 372.3 ([DHEA + Na]+), and m/z 401.3 ([2-AG + Na]+). The single quadrupole ionization settings were: capillary voltage, 4.0 kV; fragmentor voltage, 100 V; drying gas (N2) flow rate, 12 l/min; drying gas temperature, 350°C; vaporizer temperature, 200°C; and nebulizer pressure, 60 psi.
Identification of SEA and PEA in solvents used for sample preparation
Sample preparation.
Fifteen, 30, and 60 ml of chloroform, ethyl acetate, or hexane was evaporated to dryness under N2, reconstituted in 100 μl MeOH, and analyzed by LC-MS in SIM mode. All solvents were obtained from sealed new bottles. All glassware, used for the evaporation step, was washed twice with the respective solvent before addition of the amount of solvent to be evaporated. Washing solvents were discarded. All evaporation experiments were performed in duplicate.
LC-MS and LC-MS/MS.
LC-MS and LC-MS/MS were performed on a Thermo-Finnigan Surveyor HPLC–system (Thermo-Finnigan, San Jose, CA) using a Phenomenex Luna C18(2)-HST column (100 × 2.0 mm i.d.) with 2.5 µm particles (Supware, Birkerød, Denmark). The mobile phases consisted of milli-Q water with 0.05% formic acid (mobile phase A) and acetonitrile (MeCN) with 0.05% formic acid (mobile phase B). Elution was performed using a flow of 200 µl/min and running a gradient of 70% B to 100% B over 5 min, followed by 1 min at 100% B before returning to 70% B over 0.5 min. The column compartment was kept at 40°C. The outlet from the column was connected to a Thermo Finnigan TSQ Quantum ultra AM triple-quadrupole MS (Thermo Finnigan, San Jose, CA), running ESI in positive ionization mode. The ionization settings were as follows: Spray voltage 4000 V, sheath gas pressure 55 (arb), ion Sweep gas pressure 5.0 (arb), aux gas pressure 20 (arb), capillary temp. 350°C, capillary offset 1 (arb), skimmer offset 6 (arb). For analyses using SIM, the MS was set to scan on m/z 300.250 (PEA) and m/z 328.250 (SEA) in Q1 with a scan width of 0.50 u and resolution of 0.5 FWHM. For verification purposes, MS/MS analyses were run on the masses of PEA and SEA in the samples from solvents where either PEA or SEA had been found. MS/MS settings were (PEA): isolation, m/z 300.25; collision energy, 22 (arb); q2 gas pressure, 2.0 (arb); Q1 = 0.5 FWHM and Q3 = 0.5 FWHM; Q3-scan m/z 30–325. MS/MS (SEA): isolation, m/z 328.250; collision energy, 22 (arb); Q1 = 0.50 FWHM and Q3 = 0.5 FWHM; Q3-scan, m/z 30–350.
Reaction between OEA and five brands of CHCl3 with either EtOH or amylene as stabilizer
A stock solution of 1 mM OEA in MeCN was prepared fresh, and 10 µl of this solution was added to 490 µl of each of the five brands of chloroform stabilized with EtOH and to each of the five brands of chloroform stabilized with amylene to give a final concentration of 20 µM OEA. Similarly, samples were made of 20 µM OEA in MeCN and of blank (without OEA) MeCN and blank CHCl3 (Merck LiChroSolv). All samples were made in triplicate. The samples were allowed to stand covered from light at ambient temperature for 30 min, after which they were evaporated to dryness under N2. The samples were reconstituted in MeCN before being analyzed by LC-MS in SIM mode.
LC-MS.
For LC-MS analyses in SIM mode, the same instrumentation and conditions as stated above for PEA and SEA were used. The MS was set to scan on m/z 326.250 (OEA) and m/z 396.300 (9,10-dichloro-SEA) in Q1 with a scan width of 0.50 u and resolution of 0.5 FWHM.
Identification of reaction product between OEA and chloroform, Merck LiChrosolv
Purification of OEA reaction product from Merck LiChrosolv.
The reaction product between OEA and chloroform of Merck LiChroSolv quality was synthesized and purified by dissolving approximately 50 mg of OEA in 250 ml Merck LiChroSolv chloroform from a new, previously unopened bottle. The solution was allowed to stand at room temperature in the dark for 30–45 min before being evaporated to dryness in a rotary evaporator. The residual was redissolved in 50 ml 70% MeCN with 0.05% HCOOH.
The resulting solution was subjected to SPE, using a Strata-X 33 µm polymeric sorbent (500 mg, 6 cc) from Phenomenex (Supware, Allerød, Denmark). The SPE cartridge was conditioned with 3 ml MeCN followed by 3 ml 70% MeCN with 0.05% HCOOH, before 1 ml sample solution was applied. The cartridge was washed with 2 ml 70% MeCN with 0.05% HCOOH before elution with 2 ml 90% MeCN with 0.05% HCOOH. The eluate was evaporated to dryness on a rotary evaporator, and the residue was reconstituted in 5 ml 60% MeOH. This solution was further purified by preparative HPLC on an Agilent 1100-series HPLC-system with fraction collector and UV-detector, using a Luna C18(2) column (5 µm particles, 150 × 4.6 mm i.d.) from Phenomenex (SupWare, Allerød, Denmark). The mobile phase consisted of 70% MeCN with 0.05% HCOOH. The column was kept at 40°C and the UV-detector was set to measure at 220 nm, 230 nm, 260 nm, and 280 nm. Time windows for collection of fractions from the preparative column was based on previous knowledge of using the same system for LC-MS analysis, to determine which fractions contained compounds of interest (i.e., 9,10-dichloro-SEA).
Synthesis of 9,10-dichloro-SEA.
The identified reaction product from OEA in Merck LiChroSolv chloroform was synthesized by an alternate route by dissolving 12.8 mg OEA in 4 ml dichloromethane. To this solution, chlorine gas was lead from a round bottomed flask containing Mn(IV)O2, into which was added concentrated hydrochloric acid. The produced chlorine gas was led through a glass tube into a washing bottle containing water, in order to trap fine particulate manganese compounds and hydrochloric acid produced, and from there into the reaction vessel containing the OEA solution and reduced iron powder. The reaction vessel was kept in an ice bath (0°C). Samples were taken from the reaction vessel at 0 min (before addition of chlorine gas), 15 min, and 60 min, before being diluted 400 times with MeCN and subjected to LC-MS analysis for the expected reaction product (9,10-dichloro-SEA) and remaining OEA.
LC-MS and LC-MS/MS.
LC analyses of samples were performed on a Thermo Finnigan Surveyor HPLC-system coupled to a TSQ Quantum ultra AM triple quadrupole (Thermo Finnigan, San Jose, CA). The mobile phases consisted of milli-Q water with 0.05% formic acid (mobile phase A) and MeCN with 0.05% formic acid (mobile phase B). Elution was performed using a flow of 200 µl/min and running a gradient of 60% B to 100% B over 6 min, followed by 1 min at 100% B before returning to 60% B over 0.5 min. The column compartment was kept at 40°C. All MS analyses were run using ESI in the positive ionization mode. The ionization settings were as follows: spray voltage, 4000 V; sheath gas pressure, 50 (arb); ion sweep gas pressure, 0 (arb); Aux gas pressure, 20 (arb); capillary temperature, 350°C; capillary offset, 1 (arb); and skimmer offset, 5 (arb).
For isotopic pattern spectra of OEA and the reaction product with m/z 396 (9,10-dichloro-SEA), the MS was set to perform a full scan in Q1 with a scan speed of 0.7 s, resolution of 0.25 FWHM, and scan ranges of m/z 320–335 or m/z 390–405 for OEA and the reaction product, respectively. Simulated isotopic pattern spectra were calculated using Xcalibur Qual Browser software version 2.0 (Thermo Electron Corporation).
For MS/MS analyses of OEA and 9,10-dichloro-SEA, the settings were: OEA: isolation m/z, 326.250; collision energy, 25 (arb); q2 gas pressure, 2.0 (arb); Q1 = 0.50 FWHM and Q3 = 0.5 FWHM; Q3-scan m/z, 30–335. Settings for 9,10-dichloro-SEA: isolation m/z, 396.250; collision energy, 25 (arb); q2 gas pressure, 2.0 (arb); Q1 = 0.50 FWHM and Q3 = 0.5 FWHM; Q3-scan m/z, 30–405.
NMR analyses.
1H-NMR spectra were acquired using a Bruker Avance 400 WB (Bruker Biospin, Karlsruhe, Germany) operating at 400.13 MHz for 1H, equipped with a 1 mm TXI (1H observe, 13C, 77Se decouple). Samples were dissolved in d5-pyridine in a standard 1 mm NMR tube at T = 310.2. Sixty-four transients for OEA or 128 for 9,10-dichloro-OEA were added. The 90° pulse was 5.75 µs at 9 dB dampening. A relaxation delay of 1.0 s was used, and the FID was collected using 32,768 data points in the time domain. A 1 Hz exponential line broadening was applied, and the FID was Fourier transformed using 131,072 data points for the real part of the transformed spectrum. For 9,10-dichloro-OEA, a phase-sensitive COSY spectrum was acquired using a standard pulse program from the Bruker library (cosyph). The 90° pulse was 5.75 µs at 9 dB dampening. A relaxation delay of 1.0 s was used. A total of 512 increments in t1 each of 96 added transients were acquired using 1,024 points in t2. A squared unshifted sinebell (QSINE, SSB = 0) was applied in both dimensions before 2D Fourier transformation. Then 2,048 points (real part of the transformed spectrum) were used for both dimensions allowing cross peaks for small couplings to be observed. (All NMR spectra, except the H-NMR spectrum of 9,10-dichloro-SEA, are presented in the supplementary data section).
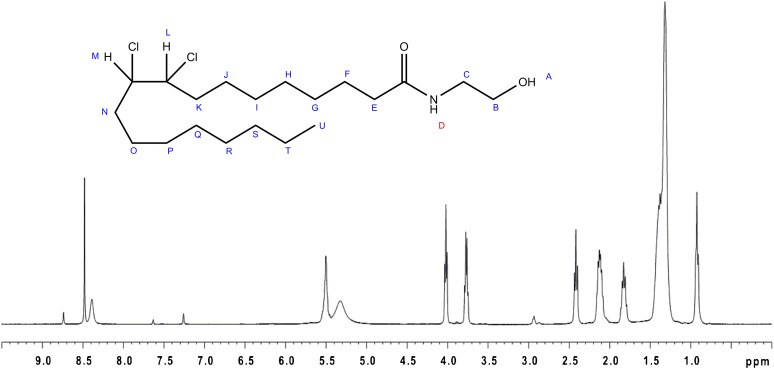
1H-NMR of OEA gave the following result: δ 0.9 (3 H, s, HU); δ 1.31–1.40 (20 H, broad s, HG-J+O-T); δ 1.83 (2 H, tt, J= 6.8 Hz and J= 7.2 Hz, HF); δ 2.12 (4 H, tt, J= 6.4 Hz and J= 6.8 Hz, HN+K); δ 2.42 (2 H, t, J= 7.4 Hz, HE); δ 3.75 (2 H, apparent dt, J= 5.6 Hz, HC); δ 4.02 (2 H, t, J= 5.6 Hz, HB); δ 5.50 (2 H, broad s, HL+M); δ 8.39 (1 H, broad s, HD). Minor peaks at δ 2.93, δ 7.26, δ 7.63, δ 8.48, and δ 8.74 and a broad peak at δ 5.33 were attributed to the solvent.
1H-NMR of 9,10-dichloro-SEA gave the following result: δ 0.91 [3 H, apparent t, J= 6.8 Hz (average value), HU]; δ 1.28–1.64 [18 H, s(m), HG-I + P-T + O or J]; δ 1.61 (2 H, m, HJ or O); δ 1.81 (2 H, tt, J= 7.2 Hz and 7.6 Hz, HF); δ 1.97 (4 H, m, HN + K); δ 2.41 [2 H, apparent t, J= 7.4 Hz (average value), HE]; δ 3.78 (2 H, dt, J= 5.6, HC); δ 4.03 (2 H, t, J= 5.6 Hz, HB); δ 4.31 (2 H, m, J= 5.6-7.2, HL + M); δ 8.43 (1 H, broad s, HD). As for the spectrum of OEA, minor peaks at δ 2.93, δ 7.26, δ 7.63, δ 8.48, and δ 8.74 and a broad peak at δ 5.33 were attributed to the solvent.
RESULTS
Validation of commercial SPE columns
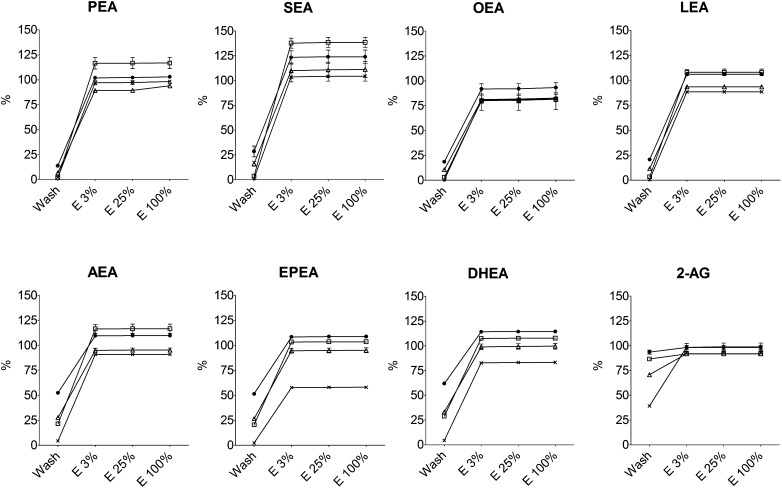
Four commercially available SPE columns with silica sorbents were investigated with respect to recoveries of 2-AG and seven different NAEs at clinically relevant concentrations (Fig. 2). The results showed significant differences in retention and recoveries between SPE columns despite the fact that the columns were filled with the same type of sorbent (i.e., silica). For most of the compounds, the columns showed average recoveries of 95–120%. However, the recoveries were lower than 100% for EPEA and DHEA on the Phenomenex columns (58% and 83%, respectively) and generally for OEA on all columns (81–93%). When looking at overall retention during the wash procedure, the Phenomenex columns showed the best performance for all the studied compounds, whereas the poorest performance was with the Isolute columns that lost between 14% (PEA) and 93% (2-AG) during the wash procedure. For AEA, EPEA, and DHEA, the losses during the wash step were considerable on the Supelco, Waters, and Isolute columns (average losses: 34% for AEA; 33% or EPEA; 41% for DHEA). None of the columns were able to satisfyingly retain 2-AG, because most of the compound was lost in the wash step on all columns (losses: 39% on Phenomenex; 71% on Waters; 86% on Supelco; 93% on Isolute). The results for 2-AG notwithstanding, the average results for the investigated compounds on the respective SPE columns were (wash loss % ± SD; total recovery % ± SD): Supelco (11.7% ± 11.4%; 110.3% ± 17.1%), Waters (18.9% ± 10.2%; 95.9% ± 8.4%), Isolute (35.3% ± 19.4%; 108.4% ± 9.5%), and Phenomenex (2.2% ± 1.6%; 86.5% ± 14.8%).
Fig. 2.
Evaluation of SPE columns from Supelco (open square), Waters (open triangle), Isolute (closed circle), and Phenomenex (x) on the recoveries of PEA, SEA, OEA, and LEA (top row, left to right) and AEA, EPEA, DHEA, and 2-AG (bottom row, left to right; values are mean with SEM, n = 5).
Most interesting were the results showing the abilities, or lack thereof, of the individual columns to retain the polyunsaturated compounds, which are normally present in low concentrations in biological tissues [e.g., brain (7, 8)], i.e., AEA, EPEA, DHEA, and LEA. For these compounds, the Isolute SPE columns already lost on average 55% during the wash step (range 21–62%), whereas the average losses on the other columns for these compounds during the wash step were 24% (Supelco; range 3–29%), 27% (Waters; range 12–33%), and 4% (Phenomenex; range 1–4%).
Identification of SEA and PEA in solvents used for sample preparation
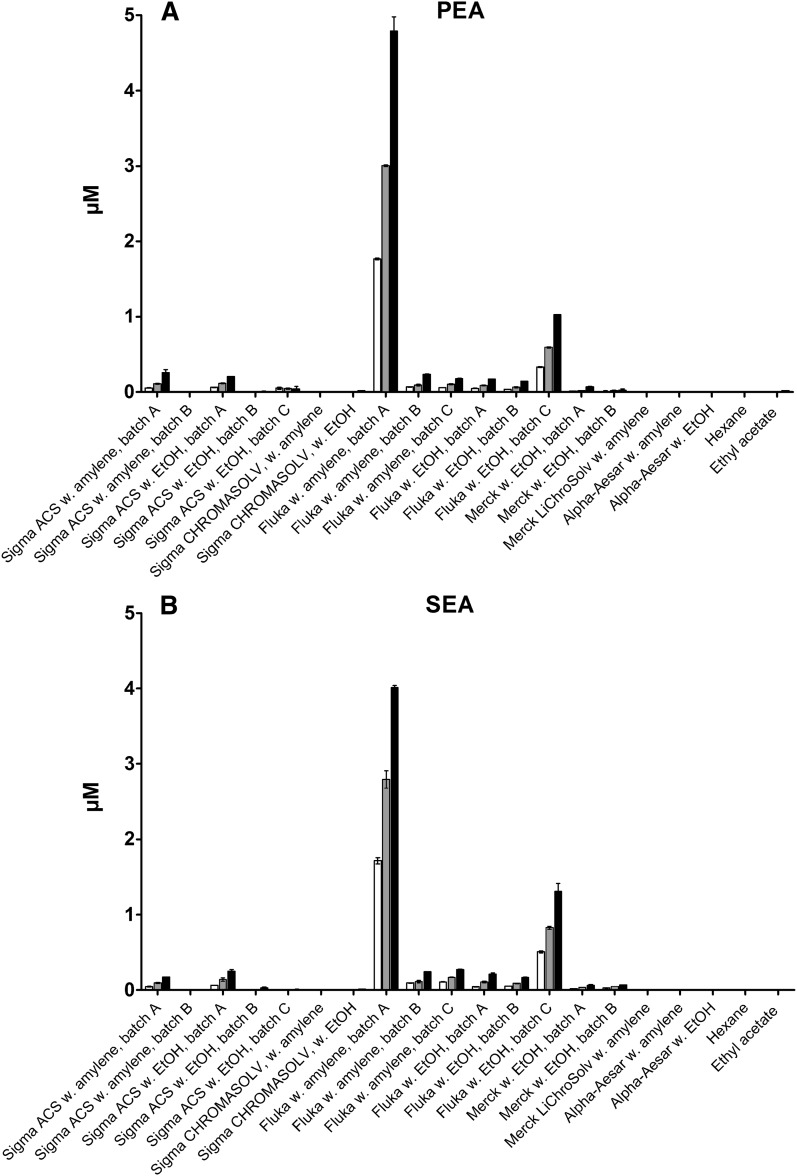
Based on previous, unpublished experiments, where PEA and SEA were found in allegedly blank samples, it was investigated whether PEA and SEA could be found in blank CHCl3, ethyl acetate, or hexane used for sample preparation. After evaporation of increasing volumes (15, 30, and 60 ml) of the solvents and subsequent reconstitution in 100 µl MeOH (Merck LiChrosolv), the samples were analyzed for the presence of PEA or SEA by LC-MS in SIM mode. The analyses showed no presence of either PEA or SEA in ethyl acetate or hexane. In several of the chloroforms, however, substantial amounts of both PEA and SEA were found, based on the retention times and MS data of the chromatographic peaks, which were identical to those of the authentic standards of PEA and SEA. Interestingly, PEA and SEA were identified simultaneously in a number of chloroform brands, while they were both absent in the remaining chloroform brands, i.e., complete cooccurrence. The highest levels of both PEA and SEA were found in batch A of the chloroform from Fluka stabilized with amylene, while minor amounts were detected in batch A and C of the Fluka chloroform stabilized with EtOH and in batch A of the two chloroforms from Sigma-Aldrich of ACS spectrophotometric grade (Fig. 3). However, there was considerable batch-to-batch variation in the chloroforms where PEA and SEA could be measured, and in some batches the two compounds were not detected. Trace amounts could be detected in Merck EMSURE chloroform stabilized with EtOH. Neither PEA nor SEA could be detected at significant levels in the remaining chloroforms. Calculating from injections of a 1 µM standard of PEA and SEA in MeCN, the concentrations of the two NAEs in the chloroform samples were estimated to be in the high picomolar range to low nanomolar range [highest: 9.9 (±1.7) nM PEA and 9.1 (± 2.1) nM SEA (Fluka w. Amylene, batch A); lowest: 90 (±24) pM PEA and 110 pM (±16) SEA (Merck EMSURE with EtOH, batch A)]. Please refer to supplementary Table IA, B for calculated concentrations in the individual solvents.
Fig. 3.
Levels of PEA (A) and SEA (B) found in different brands of chloroform (calculated concentration of µM in injected sample; mean with range; n = 2).
For verification purposes, MS/MS analyses were performed on samples from solvents where PEA and SEA had been identified (please refer to supplementary Figs. IA, B and IIA, B for MS/MS spectra of PEA and SEA standards along with examples of spectra from the chloroform samples). The MS/MS spectra from the identified peaks were compared with spectra from the authentic standards of PEA and SEA. MS/MS spectra of m/z 300.25 (PEA) from chloroform samples of Fluka chloroform with amylene, Fluka chloroform with EtOH, Sigma-Aldrich ACS grade chloroform with amylene, Sigma-Aldrich ACS grade chloroform with EtOH, and Merck EMSURE chloroform with EtOH all showed fragmentation patterns similar to those of the standard PEA with characteristic fragments of m/z 44.1 ([C2H4O]+), m/z 62.1 ([ethanolamine + H]+), m/z 282.2 (PEA – H2O + H]+), and m/z 283.0 ([PEA – OH + H]+). In addition, several minor fragments spaced 14 u apart were indicative of the presence of the palmitoyl-chain, yielding spectra similar to ones previously observed for PEA by ESI-MS/MS (25, 26).
MS/MS spectra of SEA from the same chloroforms showed fragmentation patterns similar to those of the standard SEA, with characteristic fragments of m/z 44.1 ([C2H4O]+), m/z 62.1 ([ethanolamine + H]+), m/z 310.1 (SEA – H2O + H]+), and m/z 311.1 ([SEA – OH + H]+). In addition, several minor fragments spaced 14 u apart were indicative of the presence of the stearoyl-chain, in agreement with what has previously been observed for SEA (11, 27).
Reaction between OEA and five brands of CHCl3 with either EtOH or amylene as stabilizer
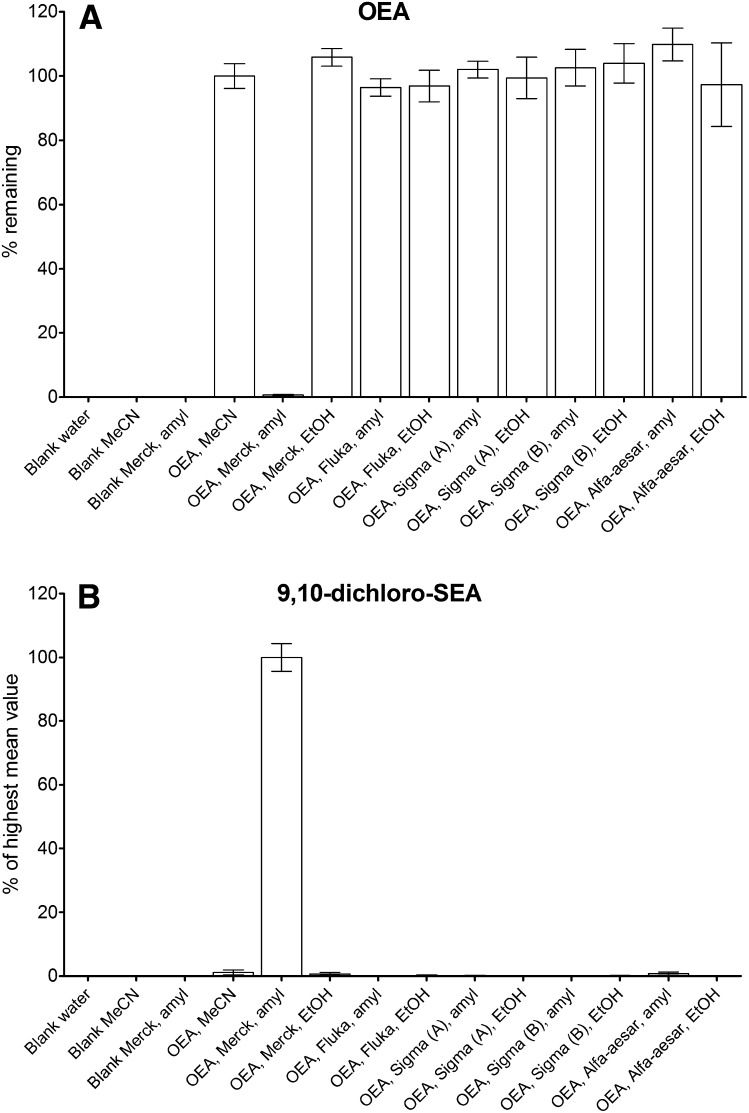
Due to problems encountered during method development for sample purification of OEA using Merck LiChroSolv chloroform stabilized with amylene, where the OEA standard disappeared, it was investigated if this was a general problem with using chloroform stabilized with amylene. Incubating OEA with five different brands of CHCl3 stabilized with either EtOH or amylene, it was found that almost complete disappearance of OEA happened in Merck LiChroSolv chloroform stabilized with amylene (Fig. 4A). No disappearance of OEA from the other chloroform brands was found. Performing a full scan survey (m/z 50–500; data not shown) by LC-MS on the incubations of OEA in the Merck LiChroSolv chloroform revealed the occurrence of a number of chromatographic peaks in the total ion chromatogram. The most intense peak was found to have a base-peak in the mass spectrum of m/z 396. Peaks with this mass were neither found in any of the other chloroforms incubated with OEA nor in Merck LiChrosolv chloroform without OEA added (Fig. 4B).
Fig. 4.
Results from incubation of OEA in five different chloroforms stabilized with either EtOH or amylene (mean with SEM; n = 6, from two separate experiments). A: Percent OEA remaining, calculated relative to the standard solution of OEA in MeCN. B: Formation of m/z 396 (9,10-dichloro-SEA) in the respective solvents, calculated as percent relative to Merck LiChrosolv with amylene.
Identification of reaction product between OEA and Merck LiChroSolv
Because data from incubations with OEA in Merck LiChroSolv stabilized with amylene showed almost complete disappearance of OEA from the solution, with the apparent major reaction product being identified as having an m/z of 396, it was decided to elucidate the identity of this reaction product.
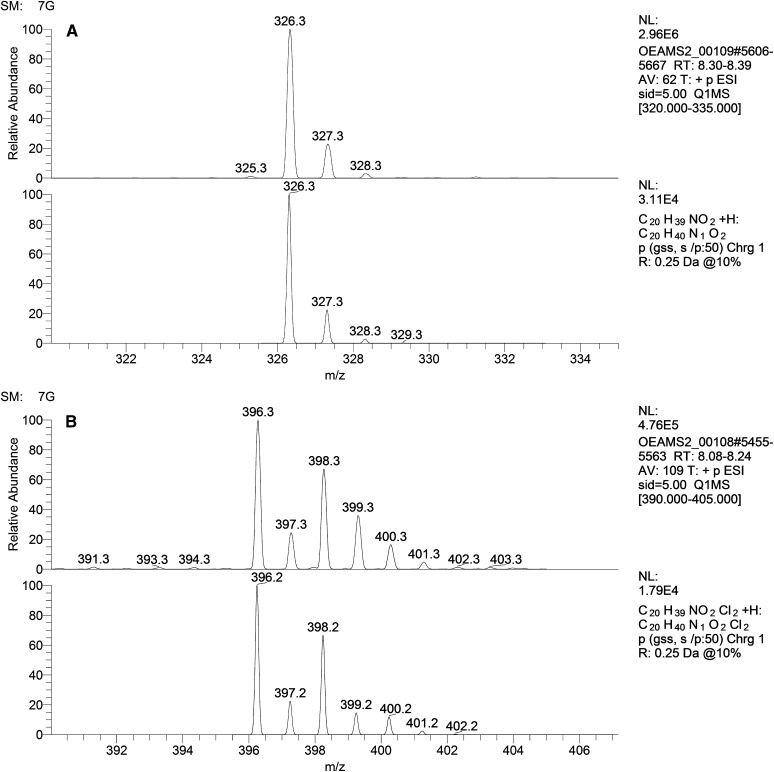
To this end, analyses of the isotope patterns of OEA and of the reaction product were performed (Fig. 5A, B). The isotope pattern of OEA was as expected for a compound with an empirical formula of C20H40NO2, corresponding to [OEA + H]+ (Fig. 5A, top), with a protonated molecule of m/z 326.3 and isotope peaks at [M + 1 + H]+ and [M + 2 + H]+ with relative abundances of approximately 22% and 3%, respectively. The pattern was similar to the simulated isotope pattern for a molecule with this empirical formula (Fig. 5A, bottom).
Fig. 5.
A: Profile spectrum showing the isotope pattern of OEA in MeCN (top) (full scan m/z 320–335, FWHM 0.25) and simulated Gaussian profile spectrum of C20H40NO2+ (bottom). B: Profile spectrum showing the isotope pattern of the reaction product between OEA and Merck LiChroSolv chloroform stabilized with amylene (top) (full scan m/z 390–405, FWHM 0.25), and simulated Gaussian profile spectrum of C20H40NO2Cl2+ (bottom).
The isotope pattern for the reaction product revealed what appeared to be a molecule containing two chlorine atoms, based on the 9:6:1 ratio of the [M + H]+, [M + 2 + H]+, and [M + 4 + H]+ m/z peaks. Simulation of the isotope pattern of the empirical formula C20H40NO2Cl2 corroborated this assumption, as these spectra were very similar (Fig. 5B). The only major difference was the larger relative abundance of M+3 in the experimental spectrum compared with the simulated spectrum (33% vs. 15%, respectively).
To further verify the identity of the reaction product, 9,10-dichloro-SEA was synthesized by chlorination of the double bond of OEA. This was achieved by bubbling chlorine gas through a solution of OEA in dichloromethane in the presence of reduced iron dust. This resulted in formation of a product with a retention time and MS/MS spectrum similar to that of the reaction product between OEA and Merck LiChroSolv chloroform (refer to supplementary Fig. IIIA, B for LC-MS chromatograms and MS/MS spectra).
MS/MS of OEA and 9,10-dichloro-SEA
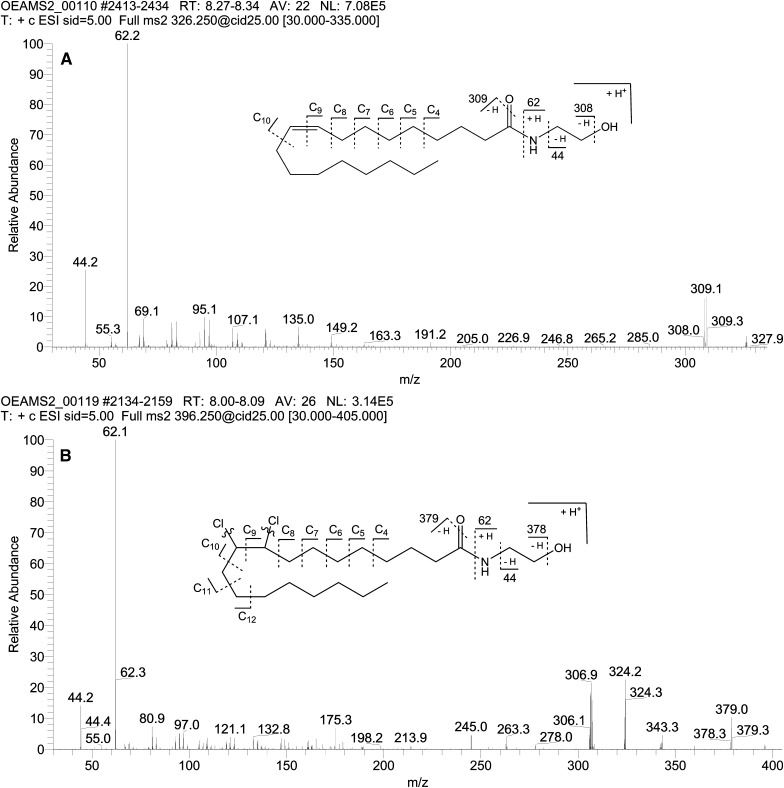
MS/MS fragmentation of m/z 326.25 gave the following characteristic fragment ions: m/z 44.2 ([C2H4O]+); m/z 62.2 ([ethanolamine + H]+); m/z 308.2 ([OEA − H2O + H]+); m/z 309.2 ([OEA − OH + H]+). In addition to these fragments, a number of minor fragments, verifying the presence of the oleoyl chain, were observed. These fragments appeared in groups of two to three, with the groups spaced 14 u apart (nominal masses along with number of carbons from oleoyl-chain retained are given; refer to Fig. 6A for explanation): m/z 67/69/71 (C4); m/z 79/81/83 (C5); m/z 93/95/97 (C6); m/z 107/109/111 (C7); m/z 121/123 (C8); m/z 135 (C9); and m/z 149 (C10).
Fig. 6.
A: MS2 of m/z 326.25, 10 µM OEA standard in MeCN. B: MS2 of m/z 396.25 from incubation of OEA with Merck LiChroSolv chloroform. Curved lines indicate that chlorines are lost in most fragments.
MS/MS fragmentation of m/z 396.25 gave the following characteristic fragment ions: m/z 44.2 ([C2H4O]+); m/z 62.2 ([ethanolamine + H]+); m/z 306.2 ([9,10-dichloro-SEA − 2Cl − 2H − H2O + H]+); m/z 307.0 ([9,10-dichloro- SEA − 2Cl − 2H − OH + H]+); m/z 324.2 ([9,10-dichloro-SEA − 2Cl − 2H]+); m/z 343.3 ([9,10-dichloro-SEA − H2O − Cl + H]+); m/z 378.3 ([9,10-dichloro-SEA − H2O + H]+); m/z 379.0 ([9,10-dichloro-SEA − OH + H]+). In addition to these fragments, a number of minor fragments, verifying the presence of the stearoyl chain, were observed. These fragments appeared in groups of two to three, with the groups spaced 14 u apart, in line with what was observed for OEA: m/z 67/69/71 (C4); m/z 79/81/83 (C5); m/z 93/95/97 (C6); m/z 107/109/111 (C7); m/z 121/123 (C8); m/z 135/137/139 (C9); m/z 149/151 (C10); m/z 161/163/165 (C11); and m/z 175/177/179 (C12).
DISCUSSION
Brought on by difficulties encountered during development of a method for quantitative analyses of NAEs in biological tissues, studies were undertaken to investigate the pitfalls that may arise from the use of different brands of SPE columns and organic solvents.
During the method development, a marked difference in recovery of NAEs and 2-AG was observed when changing the brand of silica SPE column. Therefore, a study to compare the recoveries of 2-AG and seven NAEs from commercially available SPE columns, packed with silica stationary phases, was performed. Silica was chosen as packing material, because the initial step in most sample preparations includes solvent extraction of NAE's from tissue using a nonpolar solvent. This means that using a silica packing material will allow for direct application of the tissue solvent extract to be applied to the column without the need for evaporation and redissolving in aqueous solvent (with the solubility problems that may entail), as will be required if using a C18 packing material. It was found that while recoveries from the SPE columns in general were acceptable, there were significant differences between columns from the four vendors. Furthermore, the retention and recoveries were compound dependent, with significant loss of 2-AG during the wash step on all columns and with OEA having the lowest recovery on all the columns (Fig. 2A, B). A similar observation on differences in column performance was made by Hardison et al. (21), who turned toward replacement of silica with C18 packing material as a way to solve problems with poor analyte recovery. The present study describes in detail for the first time that silica-based SPE columns may still be useful provided an appropriate brand of silica is chosen. Furthermore, the addition of acids or bases, e.g., TEA or TFA, to the elution solvent was not investigated in the present study but has previously been reported to have a marked effect on recoveries (19).
A very important and most surprising result, arising from the development and validation of an assay for measuring endocannabinoids and NAEs in biological tissues in our laboratory, was the discovery of PEA and SEA as permanent contaminants of some commercially branded chloroform products. Proof of the presence of these compounds as actual contaminations in the chloroforms, and not just artifacts caused by, e.g., carry-over in the analytical system, was given by the fact that concentrating increasing volumes of solvent lead to corresponding increases in the amount of PEA and SEA found in the samples (Fig. 3A, B). Furthermore, the identities of the contaminations were confirmed based on retention times and MS/MS spectra identical to authentic standards of PEA and SEA. Initially, the presence of these contaminants in the solvents was somewhat confounding. However, a careful review of the literature revealed that NAEs are widely used in the industry as additives in cosmetics, detergent powders, detergent liquids, fabric softeners, corrosion inhibitors, textile auxiliaries, and shampoos to improve foam stabilization and viscosity (28). The contaminations of commercially branded chloroform products could therefore originate from detergents used to clean manufacturing plant facilities or containers and bottles used to store the chloroform products. Along with this, it could be speculated that the contaminations stem from detergents used to clean glassware in the laboratory. However, due to the negative results obtained when analyzing for the contaminants in, e.g., ethylacetate or hexane, this appears not to be the main source. The findings are particularly interesting, because chloroform is used in Folch's method for lipid extraction and in most sample preparation methods prior to MS-based analysis of NAEs, including PEA and SEA (13, 16, 20, 29–31). The concentrations of PEA and SEA in chloroform are relatively small, however, and usually will not contribute significantly to the analytical signal, even when analyzing small amounts of PEA or SEA in samples dissolved directly in this solvent. The problem may arise when using large volumes of chloroform for lipid extraction and sample purification on silica SPE columns. The PEA and SEA contaminations will be concentrated on the silica SPE columns during the sample preparation and are eluted along with the endogenous amounts of PEA and SEA. This issue becomes problematic, because it leads to measurements of artificially high levels of PEA and SEA in biological samples and may be a plausible explanation for some of the discrepancies among published tissue levels of, e.g., PEA and SEA, especially for very small biological samples (e.g., 50–100 mg of brain homogenates), where the contaminations of PEA and SEA in chloroform could contribute significantly to the analytical signal. A general tendency in the majority of published papers is that no information is given on the particular brand of chloroform used in the lipid extraction and sample preparation procedures. The presence of PEA and SEA in some brands of chloroform, as has been presented in this paper, gives rise to difficulties in discerning reliable data from false positive values or artificially high concentrations of these compounds.
Due to the presence of PEA and SEA in the initial chloroform brand used in our lab, which was encountered during development of a sample preparation method using silica SPE columns, a change was made to use Merck LiChrosolv chloroform instead. However, it was observed that the use of Merck LiChrosolv chloroform resulted in the disappearance of OEA from samples. Initial thoughts were centered around the reaction between the amylene used to stabilize the chloroform and the alcohol group on the ethanolamine part of OEA. This was based in part on data found in the literature, reporting on the reactivity of amylene (2-methyl-2-butene) with ethanol (32, 33) and also on the presence of a reaction product with m/z 396, i.e., a mass increase of 70 u, which corresponded to the expected mass difference from the reaction with amylene. However, analysis of the isotope pattern of the reaction product revealed the presence of two chlorine atoms, which did not support the idea of the reaction with amylene (Fig. 5B). To investigate whether the disappearance of OEA was a general problem for chloroforms containing amylene as stabilizer, incubations of OEA in chloroforms of different brands, stabilized with either amylene or EtOH, were performed. These showed that disappearance of OEA was not generally happening in chloroforms containing amylene or EtOH, but was constrained to a single brand (Fig. 4A, B). This further substantiated that amylene was not responsible for the disappearance of OEA.
The mass increase relative to OEA of 70 u in the reaction product corresponded well with the addition of two chlorine atoms to OEA, with the most likely explanation being an addition of chlorine radicals to the unsaturated ω-9 double-bond of OEA, resulting in the possible reaction product 9,10-dichloro-SEA. Further evidence of this came from the isotope pattern of the reaction product, which also corresponded to the presence of two chlorine atoms in the molecule. To support this notion, synthesis of 9,10-dichloro-SEA from OEA was performed by leading chlorine gas through a solution of OEA in dichloromethane in the presence of reduced iron as a catalyst, which is a classic method for chlorination of alkenes. This resulted in a product with similar retention time and m/z value to the observed reaction product OEA in Merck LiChrosolv, as measure by LC-MS.
MS/MS analyses of the reaction products with m/z 396 revealed that the ethanolamine moiety was retained unchanged, as was seen from the presence of the characteristic ions at m/z 44 and 62. Apart from the precursor ion at m/z 396.25, the fragments at m/z 378.3 ([9,10-dichloro-SEA - H2O + H]+), m/z 379.0 ([9,10-dichloro-SEA − OH + H]+), and the minor fragment at m/z 343.3 ([9,10-dichloro-SEA - H2O − Cl + H]+), none of the other fragments were seen to contain chlorine, indicating that the two chlorine atoms are very labile during collision-induced dissociation (Fig. 6B). Further information about the position of the chlorine atoms was difficult to extract from the MS/MS spectra of the reaction product, even through comparison with the MS/MS spectra for OEA. Structural information about fatty acids is notoriously difficult to obtain using soft ionization techniques (ESI) with low-energy collision-induced dissociation, because a number of mechanisms give rise to both charge-remote fragmentation, hydrogen shifts, and possibly charge-mediated fragmentations (34–36). However, some evidence for the saturation of the double bond in the reaction product could be inferred from the fact that in the spectra for OEA few fragment ions were seen past the vinylic C10-11 bond, whereas for the reaction product with m/z 396 more fragments were seen up to C12-13 (34).
To further elucidate the structure of the reaction product, 1H-NMR was performed on OEA and the purified reaction product. Comparison of the NMR spectra showed most notably a shift of two protons (L+M) from δ 5.50 to δ 4.31 in the spectra for the reaction product and a splitting of the signal due to the prochiral properties of the vicinal K and N protons, corresponding to addition of two chlorine atoms to the double bond of OEA (Fig. 7). A minor shift in the value of protons N+K was correspondingly observed from δ 2.12 to δ 1.97, with splitting of the signals for these protons also. The positions of these proton pairs were corroborated by the COSY-spectrum, where a clear coupling between the changed L and M protons and the N and K protons could be identified (refer to supplementary Fig. VA, B). Taken together with MS and MS/MS data, the reaction product was identified as 9,10-dichloro-SEA [9,10-dichloro-N-(2-hydroxyethyl)-octadecanamide].
Fig. 7.
1H-NMR of 9,10-dichloro-SEA.
The source of the chlorine that reacted with OEA may be the chloroform itself, which, upon reaction with UV light and oxygen and/or with metal ions, readily produces free chlorine, phosgene, and hydrochloric acid (37–39). Alternatively, chlorine may stem from the manufacturing of chloroform by chlorination of methane or from produced carbon tetrachloride left over from the manufacturing process that has not been adequately removed. Chlorine, either as Cl2, HCl or as radicals from chloroform or carbon tetrachloride, may react with the unsaturated bond in OEA and possibly also with nonvinylic carbons to yield the corresponding chlorinated products of OEA. The latter were not identified in this study, because attention was focused on the major reaction product observed, 9,10-dichloro-SEA. As mentioned previously, however, a number of minor reaction products were also observed by MS of the mixture of OEA with Merck LiChrosolv chloroform, and it cannot be ruled out that other chlorinated reaction products on nonvinylic carbons could be found among these. Consequently, it should be realized that reactions with chlorine may occur for all unsaturated NAEs, e.g., anandamide and 2-arachidinoylglycerol, two endocannabinoids often measured in mammalian tissues (40, 41), as well as possibly saturated NAEs, provided a susceptible brand of chloroform is used.
The described results emphasize the need for thorough method validation in bioanalysis with regards to analyte recovery during sample preparation, and the potential presence of interfering compounds from the matrix or solvents used (i.e., PEA and SEA in chloroform). Furthermore, some thought should be given to the quality of the solvents used. Specifically, initial and subsequent periodic checks should be performed for the presence of phosgene and chlorine in chloroform, because these may react with unsaturated NAEs, as was demonstrated in this study.
In all circumstances, method revalidation should be performed if changes are made to the brand of SPE column or brand of solvent, and if possible, QC samples should be included in the analyses in order to catch problems at an early point and to improve quantitation.
Supplementary Material
Footnotes
Abbreviations:
- AEA
- N-arachidonoylethanolamine
- 2-AG
- 2-arachidonoylglycerol
- CID
- collision-induced dissociation
- DHEA
- N-docosahexaenoylethanolamine
- EPEA
- N-eicosapentatenoylethanolamine
- EtOH
- ethanol
- ISTD
- internal standard
- LEA
- N-linoleoylethanolamine (18:2-NAE)
- MeCN
- acetonitrile
- MeOH
- methanol
- NAE
- N-acylethanolamine
- OEA
- N-oleoylethanolamine (18:1-NAE)
- PEA
- N-palmitoylethanolamine (16:0-NAE)
- SEA
- N-stearoylethanolamine (18:0-NAE)
- SIM
- selected-ion monitoring
- SPE
- solid phase extraction
This work was supported by the Novonordisk Foundation. This work was carried out as a part of the research program of the UNIK: Food, Fitness & Pharma for Health and Disease (see www.foodfitnesspharma.ku.dk). The UNIK project is supported by the Danish Ministry of Science, Technology and Innovation.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table and five figures.
REFERENCES
- 1.Capasso R., Izzo A. A. 2008. Gastrointestinal regulation of food intake: general aspects and focus on anandamide and oleoylethanolamide. J. Neuroendocrinol. 20(Suppl. 1): 39–46. [DOI] [PubMed] [Google Scholar]
- 2.Fu J., Kim J., Oveisi F., Astarita G., Piomelli D. 2008. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295: R45–R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen H. S., Diep T. A. 2009. N-acylethanolamines, anandamide and food intake. Biochem. Pharmacol. 78: 553–560. [DOI] [PubMed] [Google Scholar]
- 4.Cerrato S., Brazis P., Della Valle M. F., Miolo A., Puigdemont A. 2010. Effects of palmitoylethanolamide on immunologically induced histamine, PGD(2) and TNFalpha release from canine skin mast cells. Vet. Immunol. Immunopathol. 133: 9–15. [DOI] [PubMed] [Google Scholar]
- 5.LoVerme J., Russo R., La R. G., Fu J., Farthing J., Mattace-Raso G., Meli R., Hohmann A., Calignano A., Piomelli D. 2006. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J. Pharmacol. Exp. Ther. 319: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 6.Maccarrone M., Pauselli R., Di R. M., Finazzi-Agro A. 2002. Binding, degradation and apoptotic activity of stearoylethanolamide in rat C6 glioma cells. Biochem. J. 366: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artmann A., Petersen G., Hellgren L. I., Boberg J., Skonberg C., Nellemann C., Hansen S. H., Hansen H. S. 2008. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta. 1781: 200–212. [DOI] [PubMed] [Google Scholar]
- 8.Hansen H. S., Moesgaard B., Hansen H. H., Petersen G. 2000. N-Acylethanolamines and precursor phospholipids: relation to cell injury. Chem. Phys. Lipids. 108: 135–150. [DOI] [PubMed] [Google Scholar]
- 9.Schmid H. H., Schmid P. C., Berdyshev E. V. 2002. Cell signaling by endocannabinoids and their congeners: questions of selectivity and other challenges. Chem. Phys. Lipids. 121: 111–134. [DOI] [PubMed] [Google Scholar]
- 10.Fontana A., Di M., Cadas V. H., Piomelli D. 1995. Analysis of anandamide, an endogenous cannabinoid substance, and of other natural N-acylethanolamines. Prostaglandins Leukot. Essent. Fatty Acids. 53: 301–308. [DOI] [PubMed] [Google Scholar]
- 11.Kasai H. F., Tsubuki M., Takahashi K., Honda T., Ueda H. 2003. Analyses of anandamide and endocannabinoid-like compounds using collision-induced dissociation in fast atom bombardment ionization-mass spectrometry and gas chromatography/chemical ionization-mass spectrometry. Anal. Sci. 19: 1593–1598. [DOI] [PubMed] [Google Scholar]
- 12.Muccioli G. G., Stella N. 2008. An optimized GC-MS method detects nanomolar amounts of anandamide in mouse brain. Anal. Biochem. 373: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid P. C., Schwartz K. D., Smith C. N., Krebsbach R. J., Berdyshev E. V., Schmid H. H. 2000. A sensitive endocannabinoid assay. The simultaneous analysis of N-acylethanolamines and 2-monoacylglycerols. Chem. Phys. Lipids. 104: 185–191. [DOI] [PubMed] [Google Scholar]
- 14.Zoerner A. A., Gutzki F. M., Suchy M. T., Beckmann B., Engeli S., Jordan J., Tsikas D. 2009. Targeted stable-isotope dilution GC-MS/MS analysis of the endocannabinoid anandamide and other fatty acid ethanol amides in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2909–2923. [DOI] [PubMed] [Google Scholar]
- 15.Di Marzo V., Breivogel C. S., Tao Q., Bridgen D. T., Razdan R. K., Zimmer A. M., Zimmer A., Martin B. R. 2000. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J. Neurochem. 75: 2434–2444. [DOI] [PubMed] [Google Scholar]
- 16.Giuffrida A., Rodriguez de Fonseca F., Piomelli D. 2000. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal. Biochem. 280: 87–93. [DOI] [PubMed] [Google Scholar]
- 17.Kingsley P. J., Marnett L. J. 2003. Analysis of endocannabinoids by Ag+ coordination tandem mass spectrometry. Anal. Biochem. 314: 8–15. [DOI] [PubMed] [Google Scholar]
- 18.Koga D., Santa T., Fukushima T., Homma H., Imai K. 1997. Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric determination of anandamide and its analogs in rat brain and peripheral tissues. J. Chromatogr. B Biomed. Sci. Appl. 690: 7–13. [DOI] [PubMed] [Google Scholar]
- 19.Richardson D., Ortori C. A., Chapman V., Kendall D. A., Barrett D. A. 2007. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal. Biochem. 360: 216–226. [DOI] [PubMed] [Google Scholar]
- 20.Folch J., Lees M., Stanley G. H. S. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 21.Hardison S., Weintraub S. T., Giuffrida A. 2006. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins Other Lipid Mediat. 81: 106–112. [DOI] [PubMed] [Google Scholar]
- 22.Degn M., Lambertsen K. L., Petersen G., Meldgaard M., Artmann A., Clausen B. H., Hansen S. H., Finsen B., Hansen H. S., Lund T. M. 2007. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J. Neurochem. 103: 1907–1916. [DOI] [PubMed] [Google Scholar]
- 23.Hansen S. L., Nielsen A. H., Knudsen K. E., Artmann A., Petersen G., Kristiansen U., Hansen S. H., Hansen H. S. 2009. Ketogenic diet is antiepileptogenic in pentylenetetrazole kindled mice and decrease levels of N-acylethanolamines in hippocampus. Neurochem. Int. 54: 199–204. [DOI] [PubMed] [Google Scholar]
- 24.Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258: 1946–1949. [DOI] [PubMed] [Google Scholar]
- 25.Ozalp A., Barroso B. 2009. Simultaneous quantitative analysis of N-acylethanolamides in clinical samples. Anal. Biochem. 395: 68–76. [DOI] [PubMed] [Google Scholar]
- 26.Palandra J., Prusakiewicz J., Ozer J. S., Zhang Y., Heath T. G. 2009. Endogenous ethanolamide analysis in human plasma using HPLC tandem MS with electrospray ionization. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2052–2060. [DOI] [PubMed] [Google Scholar]
- 27.Artmann A. 2008. Endocannabinoids and N-acylethanolamines. PhD Thesis. University of Copenhagen, Faculty of Pharmaceutical Sciences, Department of Pharmacology and Pharmacotherapy, Copenhagen, ISBN 978–87–92199–13–3. [Google Scholar]
- 28.Maag H. 1984. Fatty-acid derivatives: important surfactants for household, cosmetic and industrial purposes. J. Am. Oil Chem. Soc. 61: 259–267. [Google Scholar]
- 29.Beltramo M., de Fonseca F. R., Navarro M., Calignano A., Gorriti M. A., Grammatikopoulos G., Sadile A. G., Giuffrida A., Piomelli D. 2000. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J. Neurosci. 20: 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrendero F., Sepe N., Ramos J. A., Di Marzo V., Fernandez-Ruiz J. J. 1999. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 33: 181–191. [DOI] [PubMed] [Google Scholar]
- 31.Bisogno T., Berrendero F., Ambrosino G., Cebeira M., Ramos J. A., Fernandez-Ruiz J. J., Di Marzo V. 1999. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem. Biophys. Res. Commun. 256: 377–380. [DOI] [PubMed] [Google Scholar]
- 32.Linnekoski J. A., Kiviranta-Paakkonen P., Krause A. O., Rihko-Struckmann L. K. 1999. Simultaneous isomerization and etherification of isoamylenes. Ind. Eng. Chem. Res. 38: 4563–4570. [Google Scholar]
- 33.Rihko L. K., Linnekoski J. A., Krause A. O. I. 1994. Reaction equilibria in the synthesis of 2-methoxy-2-methylbutane and 2-ethoxy-2-methylbutane in the liquid-phase. J. Chem. Eng. Data. 39: 700–704. [Google Scholar]
- 34.Griffiths W. J. 2003. Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids. Mass Spectrom. Rev. 22: 81–152. [DOI] [PubMed] [Google Scholar]
- 35.de Hoffmann E., Stroobant V. 2007. Fragmentation reactions. Mass Spectrometry: Principles and Applications. 3rd edition de Hoffmann E., Stroobant V., John Wiley & Sons Ltd, Chichester, West Sussex, England: 274–303. [Google Scholar]
- 36.de Hoffmann E., Stroobant V. 2007. Analysis of Biomolecules. Mass Spectrometry: Principles and Applications. 3rd edition. de Hoffmann E., Stroobant V., John Wiley & Sons Ltd, Chichester, West Sussex, England: 306–402. [Google Scholar]
- 37.Crummett W. B., Stenger V. A. 1956. Thermal stability of methyl chloroform and carbon tetrachloride. Ind. Eng. Chem. 48: 434–436. [Google Scholar]
- 38.Jensen K. A. 1976. Substitutionsprodukter: halogen- og nitroforbindelser. Grundrids af den organiske kemi. 2nd edition Jensen K. A., editor Polyteknisk Forlag, Lyngby, Denmark: 133–161. [Google Scholar]
- 39.Yu W. H. S. 1970. Photolysis of chloroform in presence of ethane at 25 degrees C. J. Chem. Phys. 52: 2736. [Google Scholar]
- 40.Hansen H. S., Artmann A. 2008. Endocannabinoids and nutrition. J. Neuroendocrinol. 20(Suppl. 1): 94–99. [DOI] [PubMed] [Google Scholar]
- 41.Ligresti A., Petrosino S., Di Marzo V. 2009. From endocannabinoid profiling to ‘endocannabinoid therapeutics’. Curr. Opin. Chem. Biol. 13: 321–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.