Abstract
Lipoprotein(a) [Lp(a)] has enhanced atherothrombotic properties. The ability of Lp(a) levels to predict adverse cardiovascular outcomes in patients undergoing coronary angiography has not been examined. The relationship between serum Lp(a) levels and both the extent of angiographic disease and 3-year incidence of major adverse cardiovascular events (MACE: death, myocardial infarction, stroke, and coronary revascularization) was investigated in 2,769 patients who underwent coronary angiography [median Lp(a) 16.4 mg/dl, elevated levels (≥30 mg/dl) in 38%]. An elevated Lp(a) was associated with a 2.3-fold [95% confidence interval (CI), 1.7–3.2, P < 0.001] greater likelihood of having a significant angiographic stenosis and 1.5-fold (95 CI, 1.3–1.7, P < 0.001) greater chance of three-vessel disease. Lp(a)≥30 mg/dl was associated with a greater rate of MACE (41.8 vs. 35.8%, P = 0.005), primarily due to a greater need for coronary revascularization (30.9 vs. 26.0%, P = 0.02). A relationship between Lp(a) levels and cardiovascular outcome was observed in patients with an LDL cholesterol (LDL-C) ≥70-100 mg/dl (P = 0.049) and >100 mg/dl (P = 0.02), but not <70 mg/dl (P = 0.77). Polymorphisms of Lp(a) were also associated with both plasma Lp(a) levels and coronary stenosis, but not a greater rate of MACE. Lp(a) levels correlate with the extent of obstructive disease and predict the need for coronary revascularization in subjects with suboptimal LDL-C control. This supports the need to intensify lipid management in patients with elevated Lp(a) levels.
Keywords: atherosclerosis, risk factor, coronary angiography
For more than a century, evidence has continued to accumulate supporting the pivotal role of cholesterol in the pathogenesis of atherosclerotic cardiovascular disease (CVD). The finding of a curvilinear relationship between systemic levels of LDL cholesterol (LDL-C) and prospective cardiovascular risk in population studies (1) and unequivocal clinical benefit of therapies that lower LDL-C (2) have prompted its central role in the approach to risk prediction and preventive strategies. However, the finding that many patients with established CVD have normal LDL-C levels and no identifiable risk factor suggests that additional factors are likely to play a contributory role in promoting cardiovascular risk.
A number of additional lipid risk factors have been proposed to be independently associated with CVD. Lipoprotein (a) [Lp(a)] contains apolipoprotein (apo)B-100, linked by a disulfide bond to apo(a). Data supports the idea that Lp(a) possesses potent atherogenic and thrombogenic properties (3–6). This is likely to be derived not only from the presence of apoB-100, but also due to the presence of a kringle structure with remarkable homology to plasminogen, which may alter hemostatic activity. Although epidemiologic studies have reported variable findings with regard to the relationship between Lp(a) levels and cardiovascular risk (7–18), it was recently reported that genetic polymorphisms associated with elevated Lp(a) levels are associated with an excessive rate of myocardial infarction (19–22). This implicates a direct role for Lp(a) in acute thrombotic/ischemic events. In addition, accumulating evidence suggests a relationship between apo(a) isoform size and both Lp(a) levels and cardiovascular risk (23).
The relationship between Lp(a) and cardiovascular events requires ongoing exploration in different patient cohorts in order to elucidate its potential role as a marker of clinical risk and target for therapeutic manipulation. In particular, its role in higher risk patients, regardless of their level of LDL-C or concomitant use of high-potency statin therapy, has not been defined. The objective of the current analysis was to investigate the clinical and genetic relationship between baseline levels of Lp(a) and both the extent of angiographic disease and the subsequent incidence of cardiovascular events in a cohort of patients who presented for a clinically-indicated coronary angiogram and with a contemporary treatment regimen.
METHODS
Study population
Patients were enrolled in the GeneBank study, a repository of clinical, biochemical, and angiographic data from consenting subjects undergoing elective diagnostic cardiac evaluation, including coronary angiography, at the Cleveland Clinic. All participants gave written informed consent and the Institutional Review Board of the Cleveland Clinic approved the study protocol. The current analysis evaluated sequentially enrolled subjects without biochemical evidence of myocardial necrosis (troponin I [cTnI] <0.03 ng/ml), and who did not undergo coronary revascularization or experience an ischemic clinical event (death, MI, or stroke) within 30 days of the index cardiac catheterization.
Biochemical determination
Specimens were stored at –80°C prior to analysis. Lp(a) was measured in serum samples by an automated latex enhanced immunoassay (QUANTIA Lp(a) assay and standard) using the Abbott Architect ci8200 platform (Abbott Laboratories, Abbott Park, IL). For this assay, a concentration up to 30 mg/dl was considered within the normal range, with an inter- and intra-assay coefficient of variation of 3.5% at 13 mg/dl, limit of detection of 0.4 mg/dl, and precision less than 5%. This assay is not influenced by apo(a) isoform size. Cardiac troponin I was also measured using a high sensitivity assay on the Abbott Architect platform, as previously described (24). LDL-C was calculated by using the Friedewald equation. An estimate of creatinine clearance (CrCl) was calculated using the Cockcroft-Gault equation.
Endpoint determination
Adjudicated outcomes were ascertained over the ensuing 3 years for all subjects following enrollment. Coronary artery disease (CAD) was defined as any clinical history of myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass surgery prior to enrollment, or angiographic evidence of CAD (≥50% stenosis) in one or more major coronary arteries at index coronary angiography examination at time of enrollment. A MACE was defined as death (all-cause, from the Social Security Disease Index), nonfatal MI, nonfatal stroke or coronary revascularization [PCI or coronary artery bypass grafting [CABG)] following enrollment. Revascularization procedures recorded during the follow-up period included atherectomy, balloon angioplasty, CABG, PCI, or stent. For subjects with multiple events, only the first event was considered for this analysis.
Genetic analyses
As part of ongoing genome-wide association studies (GWASs) in GeneBank, 3,031 samples were genotyped using the Affymetrix 6.0 GeneChip. In addition to single nucleotide polymorphisms (SNPs) spanning the Lp(a) locus on chromosome 6, five SNPs previously reported to be associated with plasma Lp(a) levels and/or CAD phenotypes (21, 25) were also present on the 6.0 chip and selected for the present analysis. All variants were tested for Hardy-Weinberg equilibrium using a χ2 test prior to analysis. Haplotypes of the four previously reported SNPs (rs2048327, rs3127599, rs7767084, rs10755578) were estimated using an expectation-maximization algorithm to generate maximum likelihood estimates of haplotype frequencies, which assigns the probability that each individual possesses a particular haplotype pair. Association analyses of these haplotypes were only carried out in those individuals whose haplotypes could be determined with greater than 80% probability. Quantitative association tests were carried out in 2,376 subjects for whom plasma Lp(a) levels were available using multiple linear regression under an additive genetic model with adjustment for age and gender. Due to non normality, Lp(a) values were natural log transformed prior to analysis. To test for association with CAD, we used logistic regression under an additive genetic model with adjustment for age and gender and reported the results as odds ratios (ORs) with 95% confidence intervals (CIs). Cox proportional hazard models were used to calculate the risk for experiencing a MACE, with adjustment for traditional cardiac risk factors.
Statistical analysis
Patients were stratified according to baseline Lp(a) levels < or ≥30 mg/dl for all analyses. Continuous data was summarized by mean ± SD across level of Lp(a) and compared using the Student's t-test if data was normally distributed. Nonnormally distributed continuous data was summarized as median (interquartile range) and compared using the Wilcoxon rank-sum test. Categorical data was presented as percentage and compared using the χ2 test. Kaplan-Meier methods were used to estimate time to 3-year endpoints. Cox proportional hazards models were used to calculate hazard ratios and 95% CIs in determining the risk of 3-year MACE and revascularization events for patients with an elevated Lp(a). ORs from a logistic regression analysis were generated to summarize the association of high Lp(a) and both the rate of maximum angiographic stenosis ≥50% and presence of three-vessel disease. To determine if the risk with an elevated Lp(a) differs across LDL-cholesterol levels, 3-year MACE and revascularization rates were also investigated in patients, stratified according to baseline LDL-C of <70, ≥70–100, ≥100 mg/dl. All analyses were performed using SAS version 9.1 (Cary, NC). P values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
The median (interquartile range) Lp(a) level in the entire cohort was 16.4 (6.8, 60.5) mg/dl. Whereas 37.9% of patients had a Lp(a) ≥30 mg/dl, the upper quartile of patients had a Lp(a) at baseline in excess of 60 mg/dl. A high rate of significant obstructive disease was present at the time of the baseline angiogram. A stenosis >50% was observed in at least one coronary artery on angiography in 91.8% of patients. Significant three-vessel CAD was demonstrated in 42.5%. MACE occurred in 36.6% of patients (25.6% coronary revascularization, 10.5% death, 5.2% myocardial infarction, 1.1% stroke) during a 3-year follow-up.
Clinical characteristics, medication use, and biochemical parameters in patients stratified according to their level of Lp(a) are summarized in Table 1. Patients with a Lp(a) ≥30 mg/dl were younger (63.0 ± 10.7 vs. 63.9 ± 10.9 years, P = 0.04), less likely to be Caucasian (96.2 vs. 98.4%, P < 0.001) and diabetic (25.8 vs. 29.7%, P = 0.03), more likely to have a history of hyperlipidemia (90.8 vs. 87.9%, P = 0.02), and had lower body mass index (29.2 ± 5.8 vs. 29.9 ± 5.9 kg/m2, P = 0.001) . An elevated Lp(a) was associated with a greater history of CAD (96.6 vs. 91.7%, P < 0.001) and peripheral arterial disease (30.2 vs. 25.0%, P = 0.003), with a higher rate of previous MI (53.7 vs. 49.2%, P = 0.03) and CABG (44.8 vs. 37.0%, P < 0.001). The presence of an elevated Lp(a) was accompanied by greater LDL-C (101.0 ± 31.8 vs. 94.9 ± 31.0 mg/dl, P < 0.001) and HDL cholesterol levels (34.8 ± 9.9 vs. 33.6 ± 9.8, P = 0.001) and lower triglycerides (119 vs. 123 mg/dl, P = 0.03). As a result, patients with an elevated Lp(a) were more likely to be treated with a statin (72.3 vs. 63.1%, P < 0.001).
TABLE 1.
Clinical characteristics, medication use and risk factor control in patients stratified according to baseline Lp(a) level
| Parameter | Lp(a) <30 (n = 1720) | Lp(a) ≥30 (n = 1049) | P |
|---|---|---|---|
| Age (years) | 63.9 ± 10.9 | 63.0 ± 10.7 | 0.04 |
| Male (%) | 71.1 | 68.6 | 0.17 |
| Body Mass Index (kg/m2) | 29.9 ± 5.9 | 29.2 ± 5.8 | 0.001 |
| Caucasian (%) | 98.4 | 96.2 | <0.001 |
| Diabetes (%) | 29.7 | 25.8 | 0.03 |
| Hypertension (%) | 87.0 | 85.9 | 0.42 |
| Hyperlipidemia (%) | 87.9 | 90.8 | 0.02 |
| History of CAD (%) | 91.7 | 96.6 | <0.001 |
| History of PAD (%) | 25.0 | 30.2 | 0.003 |
| Previous MI (%) | 49.2 | 53.7 | 0.03 |
| Previous CABG (%) | 37.0 | 44.8 | <0.001 |
| Previous PCI (%) | 37.0 | 39.5 | 0.19 |
| Previous stroke (%) | 6.7 | 7.7 | 0.35 |
| Aspirin use (%) | 78.3 | 77.4 | 0.06 |
| Beta-blocker use (%) | 66.6 | 68.3 | 0.36 |
| Statin use (%) | 63.1 | 72.3 | <0.001 |
| ACE inhibitor use (%) | 52.1 | 54.1 | 0.32 |
| Systolic blood pressure (mmHg) | 134.0 ± 21.2 | 133.1 ± 22.1 | 0.28 |
| Diastolic blood pressure (mmHg) | 74.1 ± 12.7 | 74.3 ± 12.5 | 0.76 |
| LDL cholesterol (mg/dl) | 94.9 ± 31.0 | 101.0 ± 31.8 | <0.001 |
| HDL cholesterol (mg/dl) | 33.6 ± 9.8 | 34.8 ± 9.9 | 0.001 |
| Triglycerides (mg/dl)a | 123.0 (87.0, 179.0) | 119.0 (84.0, 166.0) | 0.03 |
| CRP (mg/L)a | 2.5 (1.1, 5.9) | 2.4 (1.0, 5.9) | 0.19 |
| Creatinine clearance (ml/min/1.73m2) | 95.2 ± 39.9 | 92.6 ± 39.3 | 0.13 |
Results expressed mean ± SD for continuous variables or percentage for categorical variables. ACE, angiotensin converting enzyme; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CRP, c-reactive protein; MI, myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention.
median (interquartile range).
Lp(a) and angiographic disease
The prevalence of angiographic CAD in patients stratified according to Lp(a) levels is summarized in Table 2. Patients with an elevated Lp(a) had a greater rate of single (95.3 vs. 89.7%, P < 0.001) or three-vessel (48.2 vs. 39.0%, P < 0.001) disease. The presence of an elevated Lp(a) was associated with a 2.3-fold (95% CI, 1.7, 3.2, P < 0.001) greater likelihood of having a stenosis >50% in at least one coronary artery and a 1.5-fold (95% CI, 1.3, 1.7, P < 0.001) greater likelihood of having three-vessel disease.
TABLE 2.
Angiographic measures of patients stratified according to baseline Lp(a) level
| Parameter | Lp(a) <30 | Lp(a) ≥30 | P |
|---|---|---|---|
| Any stenosis >50% (%) | 89.7 | 95.3 | <0.001 |
| 3-vessel disease (%) | 39.0 | 48.2 | <0.001 |
| Left ventricular ejection fraction (%) | 51.7 ± 12.7 | 51.6 ± 12.3 | 0.54 |
| Odds ratio for stenosis >50% | 1.0 | 2.3 (1.7, 3.2) | <0.001 |
| Odds ratio for 3-vessel disease | 1.0 | 1.5 (1.3, 1.7) | <0.001 |
Lp(a) and cardiovascular events
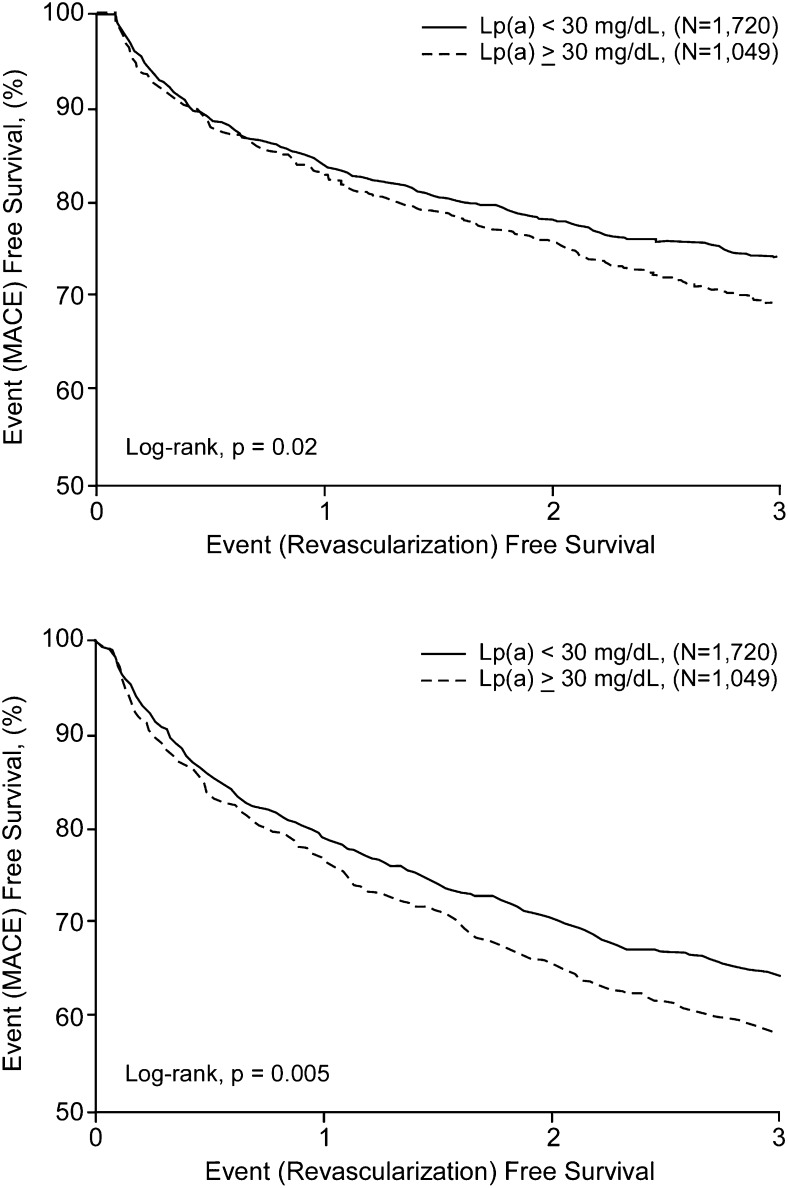
The incidence of cardiovascular events during 3 years of follow-up in patients stratified according to baseline Lp(a) levels is summarized in Table 3 and Fig. 1. The presence of an elevated Lp(a) was associated with a greater rate of MACE (41.8 vs. 35.8%, P = 0.005), which was primarily due to an excessive requirement for coronary revascularization (30.9 vs. 26.0%, P = 0.02). In contrast, the rate of death (12.2 vs. 10.5%, P = 0.20), MI (6.0 vs. 5.7%, P = 0.84), and stroke (1.0 vs. 1.3%, P = 0.64) did not significantly differ between patients with low or high levels of Lp(a). Overall, having Lp(a) ≥30 mg/dl was associated with a 1.2-fold (95% CI 1.1, 1.4, P = 0.005) greater likelihood of MACE and a 1.2-fold (95% CI 1.0, 1.4, P = 0.02) greater likelihood of coronary revascularization.
TABLE 3.
Cardiovascular event rates in patients stratified according to baseline Lp(a) level
| Parameter | Lp(a) <30 | Lp(a) ≥30 | P |
|---|---|---|---|
| MACE (%) | 35.8 | 41.8 | 0.005 |
| Coronary revascularization (%) | 26.0 | 30.9 | 0.02 |
| Death (%) | 10.5 | 12.2 | 0.20 |
| Myocardial infarction (%) | 5.7 | 6.0 | 0.84 |
| Stroke (%) | 1.3 | 1.0 | 0.64 |
| Hazard ratio for MACE | 1.0 | 1.20 (1.06, 1.37) | 0.005 |
| Hazard ratio for coronary revascularization | 1.0 | 1.20 (1.02, 1.40) | 0.02 |
MACE, major adverse cardiovascular events; death, myocardial infarction, stroke, coronary revascularization.
Fig. 1.
Cox proportional hazard plots for frequency of major adverse cardiovascular events (MACE: death, myocardial infarction, stroke, coronary revascularization; A) and coronary revascularization (B) in subjects stratified according to Lp(a) <30 mg/dl (solid line) or ≥30 mg/dl (broken line).
Lp(a), lipid levels, and cardiovascular risk
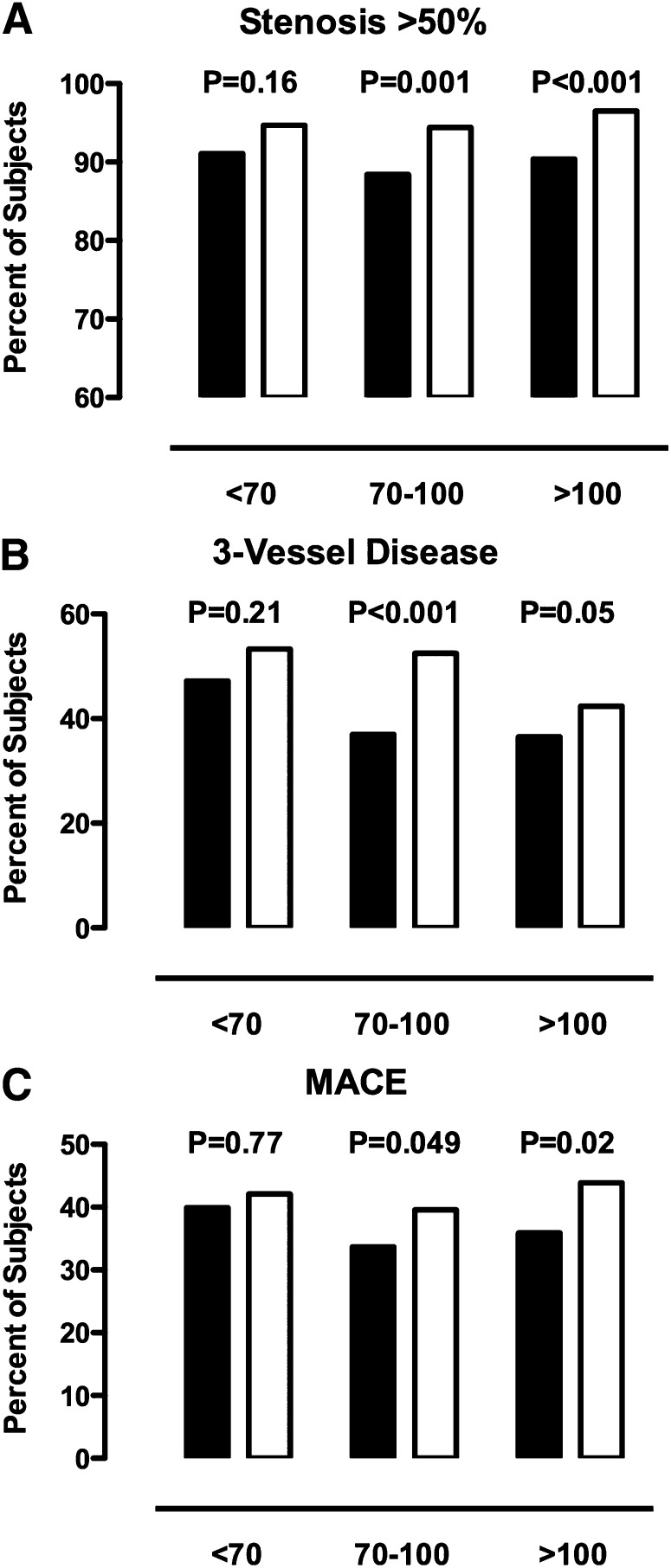
The relationship between the rate of significant obstructive disease on angiography and cardiovascular event rates in patients with an elevated Lp(a), stratified according to baseline levels of LDL cholesterol, is summarized in Fig. 2. An elevated Lp(a) was associated with a greater rate of significant obstructive disease, three-vessel disease, or major adverse cardiovascular events with an elevated Lp(a) in groups of patients with an LDL-C between 70 and 100 mg/dl and >100 mg/dl. In contrast, patients with very well-controlled LDL-C levels (<70 mg/dl) demonstrated no greater risk of angiographic disease or of an adverse cardiovascular outcome in the setting of an elevated Lp(a). When LDL-C levels were adjusted for Lp(a) levels, we continued to observe a greater rate of MACE in subjects with higher LDL-C levels. (data not shown). An elevated Lp(a) was associated with a greater rate of MACE in subjects with either HDL cholesterol less (42.6 vs. 36.7%, P = 0.01) or greater (39.4 vs. 32.7%, P = 0.16) than 40 mg/dl, although these results failed to meet statistical significance in the latter group of patients.
Fig. 2.
Percentage of subjects with any stenosis >50% (A), 3-vessel disease (B) and major adverse cardiovascular events (MACE: death, myocardial infarction, stroke, coronary revascularization; C) stratified according to Lp(a) <30 mg/dl (solid bars) or ≥30 mg/dl (open bars) and levels of LDL-C.
Genetic polymorphisms, Lp(a), and cardiovascular risk
Several recent studies have reported the association of SNPs at the LPA locus with both plasma Lp(a) levels and CAD-related phenotypes (21, 25, 26). In particular, Tregouet et al. (21) reported that two haplotypes comprised of four SNPs (rs2048327, rs3127599, rs7767084, rs10755578) spanning a large region encompassing the SLC22A3-LPAL2-LPA gene cluster on chromosome 6 were associated with CAD. In a subsequent study, a single intronic LPA SNP was also reported to be associated with both plasma Lp(a) levels and CAD-related phenotypes (25). As part of ongoing genetic studies in GeneBank, we used genotyping data generated from the Affymetrix 6.0 GeneChip in 3,031 GeneBank subjects to first replicate the association of the five previously reported SNPs and haplotypes at the LPA locus with plasma Lp(a) levels. As shown in Table 4, four of these reported SNPs and the two four-SNP haplotypes were significantly associated with plasma Lp(a) levels in GeneBank subjects, replicating previous observations. We also observed association of several additional SNPs present on the 6.0 GeneChip located within the LPA locus with plasma Lp(a) levels, two of which (rs9457925 and rs2292334) exhibited highly significant p-values (Table 4).
TABLE 4.
Association of SNPs and haplotypes at the LPA locus with plasma Lp(a) levels in GeneBank
| Lp(a) (mg/dl) |
||||
|---|---|---|---|---|
| Number of alleles | 0 | 1 | 2 | *P |
| rs2048327a | 24 ± 30 | 41 ± 42 | 55 ± 48 | 6.2 × 10−37 |
| rs3127599a | 32 ± 40 | 39 ± 39 | 45 ± 41 | 1.7 × 10−10 |
| rs7767084a | 37 ± 40 | 35 ± 41 | 28 ± 33 | 0.13 |
| rs10755578a | 32 ± 39 | 37 ± 40 | 41 ± 40 | 9.3 × 10−10 |
| CTTGb | 30 ± 37 | 50 ± 42 | 64 ± 45 | 1.7 × 10−38 |
| CCTCb | 33 ± 37 | 91 ± 54 | 69 ± 41 | 1.4 × 10−26 |
| rs6919346c | 39 ± 41 | 30 ± 36 | 18 ± 27 | 3.7 × 10−19 |
| rs9457925d | 33 ± 37 | 95 ± 50 | 114 ± 47 | 2.0 × 10−32 |
| rs2292334d | 24 ± 30 | 41 ± 42 | 55 ± 48 | 9.3 × 10−38 |
Data for Lp(a) are shown as mean ± SD and all analyses were adjusted for age and gender.
Derived from analyses using natural log transformed values.
Reported by Tregouet et al. (21).
Risk haplotypes of rs2048327, rs3127599, rs7767084, and rs10755578 reported by Tregouet et al. (21).
Reported by Ober et al. (25).
Part of ongoing GWAS currently being carried out at the Cleveland Clinic using the Affymetrix 6.0 GeneChip.
Based on these results, we next determined whether these LPA variants were also associated with CAD and future risk of MACE. Using logistic regression analysis, two previously reported SNPs (rs2048327 and rs10755578) and one of the SNPs present on the 6.0 GeneChip (rs2292334) were significantly associated with risk of CAD (Table 5). One of the previously reported haplotypes (CTTG) also yielded a similar trend, although this association did not achieve statistical significance (Table 5). Importantly, the effects of these variants/haplotypes on increasing the risk of CAD are consistent with their effects on increasing plasma Lp(a) levels. We next carried out two stratified analyses with CAD. In the first sub-analysis, we only included those subjects with LDL-C levels above 100 mg/dl and observed that the magnitude of the associations was attenuated (Table 5), perhaps as a result of decreased sample size. In the second sub-analysis, we defined affected subjects as only those with angiographic evidence for severe CAD, defined as the presence of three or more major epicardial vessels with >50% stenosis. The association of rs2048327 and rs2292334 with severe CAD remained significant with P-values of 0.02 and 0.02, respectively. In addition, the CTTG and CCTC haplotypes also demonstrated significant (P = 0.02) and suggestive association (P = 0.07) with severe CAD, respectively. Using Cox proportional hazard models, we did not observe association of the LPA SNPs/haplotypes with increased prospective risk of MACE over 3 years of follow-up (data not shown). Taken together, these results confirm previously reported associations and further support the biochemical and genetic relationship between LPA variants, plasma Lp(a) levels, and increased risk of CAD (Table 5).
TABLE 5.
Association of SNPs and haplotypes with CAD in GeneBank
| All Subjects (n = 3031) |
Subjects with LDL > 100 mg/dl (n = 1212) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of alleles | 0 | 1 | 2 | P | 0 | 1 | 2 | P |
| rs2048327a | 1 | 1.2 (0.9–1.6) | 1.5 (1.0–2.2) | 0.04 | 1 | 1.2 (0.8–1.8) | 1.7 (0.9–3.0) | 0.09 |
| rs3127599a | 1 | 1.2 (0.9–1.6) | 1.1 (0.7–1.6) | 0.32 | 1 | 1.5 (1.0–2.2) | 0.9 (0.5–1.7) | 0.44 |
| rs7767084a | 1 | 1.3 (0.9–1.7) | 0.8 (0.4–1.5) | 0.40 | 1 | 1.2 (0.8–1.9) | 0.8 (0.2–2.8) | 0.50 |
| rs10755578a | 1 | 1.3 (0.9–1.6) | 1.4 (1.0–2.0) | 0.04 | 1 | 1.1 (0.7–1.6) | 1.4 (0.0–2.4) | 0.19 |
| CTTGb | 1 | 1.1 (0.9–1.5) | 2.2 (0.9–5.2) | 0.09 | 1 | 1.2 (0.8–1.8) | 1.2 (0.4–3.4) | 0.46 |
| CCTCb | 1 | 1.3 (0.7–2.7) | *NA | 0.39 | 1 | 1.9 (0.6–5.8) | *NA | 0.23 |
| rs6919346c | 1 | 1.0 (0.8–1.4) | 0.5 (0.2–0.9) | 0.32 | 1 | 0.9 (0.6–1.4) | 0.4 (0.1–1.0) | 0.15 |
| rs9457925d | 1 | 2.2 (0.9–5.2) | *NA | 0.07 | 1 | 2.6 (0.8–9.0) | *NA | 0.12 |
| rs2292334d | 1 | 1.2 (1.0–1.6) | 1.5 (1.0–2.2) | 0.03 | 1 | 1.2 (0.8–1.7) | 1.7 (1.0–3.2) | 0.07 |
Data are shown as OR (95% CI) with adjustment for age and gender.
ORs were not calculated for homozygous carriers of these variants since there were only three subjects carrying two alleles.
Reported by Tregouet et al. (2009).
Risk haplotypes of rs2048327, rs3127599, rs7767084, and rs10755578 reported by Tregouet et al. (2009).
Reported by Ober et al. (2009).
Part of ongoing GWAS currently being carried out at the Cleveland Clinic using the Affymetrix 6.0 GeneChip.
DISCUSSION
In the current analysis, Lp(a) levels were investigated in stable patients presenting for elective coronary angiography. Elevated Lp(a) levels were associated with the presence of more extensive obstructive disease on angiography and subsequent need for coronary revascularization during 3-year follow-up. The ability of Lp(a) to predict an adverse cardiovascular outcome was observed in patients with concomitant LDL-cholesterol levels greater than 70 mg/dl. Importantly, the attributable cardiovascular risk associated with Lp(a) is dramatically attenuated among subjects in whom LDL cholesterol has been reduced to below the more aggressive goal of <70 mg/dl. These findings suggest that the potential atherothrombotic impact of Lp(a) may be influenced by circulating LDL levels. Taken together, these results support the role of Lp(a) as a marker of cardiovascular risk in patients and the use of intensive lipid management in subjects with elevated Lp(a).
Accumulating evidence has suggested that Lp(a) plays an important role in promoting CVD. The association between the atherogenic apoB100 and kringle structure with potential to impair endogenous fibrinolytic activity suggests that Lp(a) plays an important role in the propagation of atherosclerosis and thrombus formation in the setting of plaque rupture (3–6). This is supported by early observations that many patients with premature CAD and no major risk factors are found to have an elevated Lp(a) (27). These reports were subsequently confirmed by the finding of a relationship between Lp(a) and cardiovascular risk in a number of, but not all, population cohorts (7–18). Variability in findings are likely to reflect differences in concomitant risk factors and their range of Lp(a) levels. Recent reports that genetic polymorphisms resulting in elevated Lp(a) levels predict MI further supports Lp(a)’s role in ischemic CVD (19–21), an observation that we replicate in our study as well. The current findings extrapolate this relationship to a cohort of patients with a high prevalence of atherosclerotic disease and with a high rate of use of established medical therapies. It is important to note, however, that the current analysis is unable to determine whether Lp(a) plays a direct role in disease pathogenesis in these patients or simply acts as a marker of elevated cardiovascular risk.
Although Lp(a) should likely receive increased attention in risk prediction algorithms, the therapeutic options are limited. Whereas niacin and hormone replacement therapy have each been reported to have a favorable impact on Lp(a) levels, the majority of lipid-modifying strategies have little influence on Lp(a). As a result, there is an ongoing need to identify novel effective therapeutic agents to lower Lp(a), such as thyromimetic agents (28), with the objective of reducing cardiovascular risk. An elevated Lp(a) or genetic variant may identify a patient who is more likely to benefit from use of other preventive therapeutic approaches (19). Given the limited therapeutic options available, one approach is to lower LDL cholesterol. However, little has been reported on Lp(a) attributable risks stratified by LDL-C levels. The current findings support the notion that a relationship between elevated Lp(a) levels and increased adverse cardiovascular outcomes may be attenuated with aggressive LDL-C reduction, because enhanced risk is not observed in the current patient cohort where LDL cholesterol is less than 70 mg/dl. The present findings thus suggest that Lp(a) is an important marker of cardiovascular risk that identifies patients who are likely to derive more clinical benefit from intense lipid lowering with reduction of LDL cholesterol to < 70 mg/dl. The current observations provide further support for the concept that an elevated Lp(a) increases the risk associated with the presence of traditional risk factors such as LDL cholesterol (29–32). Ultimately, the impact of altering the degree of LDL-C lowering on the basis of an elevated Lp(a) remains to be investigated in a prospective randomized clinical trial. Moreover, the relative merit of combination therapy with statin and niacin compared with statin monotherapy on cardiovascular event rates remains under investigation in ongoing clinical trials. It is important to note, however, that these studies did not select patients on the basis of an elevated Lp(a) level and given that niacin has additional properties beyond its ability to lower Lp(a), it cannot be simply inferred that any clinical benefit can be attributed to changes in that lipid parameter alone.
A number of caveats should be noted with regard to the present analysis. The Lp(a) threshold that was selected for the current analysis reflects one that is typically used in clinical practice. It remains to be determined whether this will ultimately represent the most optimal for stratification of risk on the basis of Lp(a). The current analysis was also performed in patients with a high prevalence of established atherosclerotic CVD. It remains to be determined whether Lp(a) levels predict the extent of obstructive disease and need for coronary revascularization in asymptomatic apparently healthy subjects. However, it should be noted that in studies of apparently healthy men/women, Lp(a) was identified as a predictor of prospective risk for development of CVD and mortality (33). It is also important to consider that use of niacin, an agent that has been demonstrated to both lower Lp(a) levels and in clinical trials to reduce cardiovascular event rates, was not recorded. Another potential caveat of the present study is that patients were predominantly Caucasian. Given that there may be differences in the potential atherogenicity of Lp(a) in different ethnic groups, it is uncertain to what extent these findings can be extrapolated to other groups. Although Lp(a) levels were only determined at baseline, several groups have reported very little fluctuation in levels in an individual over a period of decades, due to the fact they are predominantly influenced by genetic rather than environmental factors. Accordingly, it is likely that Lp(a) levels remained relatively stable during the duration of patient follow-up. It is possible that additional clinical factors may have influenced the relationship between Lp(a) and cardiovascular risk. In addition, apo(a) isoform size may also be relevant, although these data were not available in the current analysis. Furthermore, it is important to note that much of the previous controversy in the Lp(a) field was likely to reflect the use of suboptimal immunologically based assays, with poor reproducibility, methodological issues with sample storage and handling, and investigations of relatively low risk cohorts. The more recent trend toward consistent findings of a relationship between Lp(a) and outcome may reflect the use of new generation assays.
In conclusion, the current findings provide further evidence that Lp(a) predicts the extent of angiographic disease and subsequent incidence of cardiovascular events in patients presenting for elective cardiac evaluation. Further, the attributable risk associated with elevated Lp(a) appears to be substantially attenuated among subjects in whom aggressive LDL-C lowering has occurred to levels <70 mg/dl. The present findings further support the association between Lp(a) and atherosclerotic CVD. The lack of relationship at low levels of LDL-C suggests that the presence of an elevated Lp(a) identifies a patient who is more likely to benefit from use of intensive lipid lowering strategies.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- CABG
- coronary artery bypass grafting
- CAD
- coronary artery disease
- CI
- confidence interval
- CrCl
- creatinine clearance
- CVD
- cardiovascular disease
- GWAS
- genome-wide association study
- Lp(a)
- lipoprotein(a)
- LDL-C
- LDL cholesterol
- MACE
- major adverse cardiovascular event
- MI
- myocardial infarction
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- SNP
- single nucleotide polymorphism
This study was supported by the National Institutes of Health grants 1P01 HL098055-01, P01 HL087018-020001, 1R01 DK080732-01A1, P50 HL077107-050004, and 1RO1 HL103931-01 and a gift from the Dare Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Stamler J., Wentworth D., Neaton J. D. 1986. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 256: 2823–2828. [PubMed] [Google Scholar]
- 2.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 3.Anuurad E., Boffa M. B., Koschinsky M. L., Berglund L. 2006. Lipoprotein(a): a unique risk factor for cardiovascular disease. Clin. Lab. Med. 26: 751–772. [DOI] [PubMed] [Google Scholar]
- 4.Berglund L., Ramakrishnan R. 2004. Lipoprotein(a): an elusive cardiovascular risk factor. Arterioscler. Thromb. Vasc. Biol. 24: 2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boffa M. B., Marcovina S. M., Koschinsky M. L. 2004. Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: mechanistic insights from animal models. Clin. Biochem. 37: 333–343. [DOI] [PubMed] [Google Scholar]
- 6.Koschinsky M. L. 2005. Lipoprotein(a) and atherosclerosis: new perspectives on the mechanism of action of an enigmatic lipoprotein. Curr. Atheroscler. Rep. 7: 389–395. [DOI] [PubMed] [Google Scholar]
- 7.Bennet A., Di Angelantonio E., Erqou S., Eiriksdottir G., Sigurdsson G., Woodward M., Rumley A., Lowe G. D., Danesh J., Gudnason V. 2008. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch. Intern. Med. 168: 598–608. [DOI] [PubMed] [Google Scholar]
- 8.Craig W. Y., Neveux L. M., Palomaki G. E., Cleveland M. M., Haddow J. E. 1998. Lipoprotein(a) as a risk factor for ischemic heart disease: metaanalysis of prospective studies. Clin. Chem. 44: 2301–2306. [PubMed] [Google Scholar]
- 9.Danesh J., Collins R., Peto R. 2000. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation. 102: 1082–1085. [DOI] [PubMed] [Google Scholar]
- 10.Rifai N., Ma J., Sacks F. M., Ridker P. M., Hernandez W. J., Stampfer M. J., Marcovina S. M. 2004. Apolipoprotein(a) size and lipoprotein(a) concentration and future risk of angina pectoris with evidence of severe coronary atherosclerosis in men: the Physicians’ Health Study. Clin. Chem. 50: 1364–1371. [DOI] [PubMed] [Google Scholar]
- 11.Suk Danik J., Rifai N., Buring J. E., Ridker P. M. 2006. Lipoprotein(a), measured with an assay independent of apolipoprotein(a) isoform size, and risk of future cardiovascular events among initially healthy women. JAMA. 296: 1363–1370. [DOI] [PubMed] [Google Scholar]
- 12.Suk Danik J., Rifai N., Buring J. E., Ridker P. M. 2008. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J. Am. Coll. Cardiol. 52: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolibar I., Thompson S. G., von Eckardstein A., Sandkamp M., Assmann G. 1995. Dose-response relationships of serum lipid measurements with the extent of coronary stenosis. Strong, independent, and comprehensive. ECAT Angina Pectoris Study Group. Arterioscler. Thromb. Vasc. Biol. 15: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 14.Bolibar I., von Eckardstein A., Assmann G., Thompson S. 2000. Short-term prognostic value of lipid measurements in patients with angina pectoris. The ECAT Angina Pectoris Study Group: European Concerted Action on Thrombosis and Disabilities. Thromb. Haemost. 84: 955–960. [PubMed] [Google Scholar]
- 15.Berg K., Dahlen G., Christophersen B., Cook T., Kjekshus J., Pedersen T. 1997. Lp(a) lipoprotein level predicts survival and major coronary events in the Scandinavian Simvastatin Survival Study. Clin. Genet. 52: 254–261. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer E. J., Lamon-Fava S., Jenner J. L., McNamara J. R., Ordovas J. M., Davis C. E., Abolafia J. M., Lippel K., Levy R. I. 1994. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 271: 999–1003. [DOI] [PubMed] [Google Scholar]
- 17.Cremer P., Nagel D., Labrot B., Mann H., Muche R., Elster H., Seidel D. 1994. Lipoprotein Lp(a) as predictor of myocardial infarction in comparison to fibrinogen, LDL cholesterol and other risk factors: results from the prospective Gottingen Risk Incidence and Prevalence Study (GRIPS). Eur. J. Clin. Invest. 24: 444–453. [DOI] [PubMed] [Google Scholar]
- 18.Seed M., Hoppichler F., Reaveley D., McCarthy S., Thompson G. R., Boerwinkle E., Utermann G. 1990. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N. Engl. J. Med. 322: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 19.Chasman D. I., Shiffman D., Zee R. Y., Louie J. Z., Luke M. M., Rowland C. M., Catanese J. J., Buring J. E., Devlin J. J., Ridker P. M. 2009. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 203: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamstrup P. R., Tybjaerg-Hansen A., Steffensen R., Nordestgaard B. G. 2009. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 301: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 21.Tregouet D. A., Konig I. R., Erdmann J., Munteanu A., Braund P. S., Hall A. S., Grosshennig A., Linsel-Nitschke P., Perret C., DeSuremain M., et al. 2009. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 41: 283–285. [DOI] [PubMed] [Google Scholar]
- 22.Clarke R., Peden J. F., Hopewell J. C., Kyriakou T., Goel A., Heath S. C., Parish S., Barlera S., Franzosi M. G., Rust S., et al. 2009. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361: 2518–2528. [DOI] [PubMed] [Google Scholar]
- 23.Erqou S., Thompson A., Di Angelantonio E., Saleheen D., Kaptoge S., Marcovina S., Danesh J. 2010. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J. Am. Coll. Cardiol. 55: 2160–2167. [DOI] [PubMed] [Google Scholar]
- 24.Tang W. H., Wu Y., Nicholls S. J., Brennan D. M., Pepoy M., Mann S., Pratt A., Van Lente F., Hazen S. L. 2010. Subclinical myocardial necrosis and cardiovascular risk in stable patients undergoing elective cardiac evaluation. Arterioscler. Thromb. Vasc. Biol. 30: 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ober C., Nord A. S., Thompson E. E., Pan L., Tan Z., Cusanovich D., Sun Y., Nicolae R., Edelstein C., Schneider D. H., et al. 2009. Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J. Lipid Res. 50: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luke M. M., Kane J. P., Liu D. M., Rowland C. M., Shiffman D., Cassano J., Catanese J. J., Pullinger C. R., Leong D. U., Arellano A. R., et al. 2007. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 27: 2030–2036. [DOI] [PubMed] [Google Scholar]
- 27.Dahlen G., Ericson C., Furberg C., Lundkvist L., Svardsudd K. 1972. Studies on an extra pre-beta lipoprotein fraction. Acta Med. Scand. Suppl. 531: 1–29. [PubMed] [Google Scholar]
- 28.Ladenson P. W., Kristensen J. D., Ridgway E. C., Olsson A. G., Carlsson B., Klein I., Baxter J. D., Angelin B. 2010. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N. Engl. J. Med. 362: 906–916. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong V. W., Cremer P., Eberle E., Manke A., Schulze F., Wieland H., Kreuzer H., Seidel D. 1986. The association between serum Lp(a) concentrations and angiographically assessed coronary atherosclerosis. Dependence on serum LDL levels. Atherosclerosis. 62: 249–257. [DOI] [PubMed] [Google Scholar]
- 30.Cantin B., Gagnon F., Moorjani S., Despres J. P., Lamarche B., Lupien P. J., Dagenais G. R. 1998. Is lipoprotein(a) an independent risk factor for ischemic heart disease in men? The Quebec Cardiovascular Study. J. Am. Coll. Cardiol. 31: 519–525. [DOI] [PubMed] [Google Scholar]
- 31.Maher V. M., Brown B. G., Marcovina S. M., Hillger L. A., Zhao X. Q., Albers J. J. 1995. Effects of lowering elevated LDL cholesterol on the cardiovascular risk of lipoprotein(a). JAMA. 274: 1771–1774. [PubMed] [Google Scholar]
- 32.von Eckardstein A., Schulte H., Cullen P., Assmann G. 2001. Lipoprotein(a) further increases the risk of coronary events in men with high global cardiovascular risk. J. Am. Coll. Cardiol. 37: 434–439. [DOI] [PubMed] [Google Scholar]
- 33.Erqou S., Kaptoge S., Perry P. L., Di Angelantonio E., Thompson A., White I. R., Marcovina S. M., Collins R., Thompson S. G., Danesh J. 2009. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 302: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]