Abstract
Oxidative stress is a core abnormality responsible for disease progression in nonalcoholic fatty liver disease (NAFLD). However, the pathways that contribute to oxidative damage in vivo are poorly understood. Our aims were to define the circulating profile of lipid oxidation products in NAFLD patients, the source of these products, and assess whether their circulating levels reflect histological changes in the liver. The levels of multiple structurally specific oxidized fatty acids, including individual hydroxy-eicosatetraenoic acids (HETE), hydroxy-octadecadenoic acids (HODE), and oxo-octadecadenoic acids (oxoODE), were measured by mass spectrometry in plasma at time of liver biopsy in an initial cohort of 73 and a validation cohort of 49 consecutive patients. Of the markers monitored, 9- and 13-HODEs and 9- and 13-oxoODEs, products of free radical-mediated oxidation of linoleic acid (LA), were significantly elevated in patients with nonalcoholic steatohepatitis (NASH), compared with patients with steatosis. A strong correlation was revealed between these oxidation products and liver histopathology (inflammation, fibrosis, and steatosis). Further analyses of HODEs showed equivalent R and S chiral distribution. A risk score for NASH (oxNASH) was developed in the initial clinical cohort and shown to have high diagnostic accuracy for NASH versus steatosis in the independent validation cohort. Subjects with elevated oxNASH levels (top tertile) were 9.7-fold (P < 0.0001) more likely to have NASH than those with low levels (bottom tertile). Collectively, these findings support a key role for free radical-mediated linoleic acid oxidation in human NASH and define a risk score, oxNASH, for noninvasive detection of the presence of NASH.
Keywords: oxidized fatty acids, mass spectrometry, chiral mass spectrometry
Nonalcoholic fatty liver disease (NAFLD) is currently the most common form of chronic liver disease affecting both adults and children, and it is strongly associated with obesity and insulin resistance (1, 2). One in three adults and one in ten children or adolescents in the United States have hepatic steatosis, a stage within the spectrum of NAFLD that is characterized by triglyceride accumulation in liver cells and follows a benign, nonprogressive clinical course (3, 4). Nonalcoholic steatohepatitis (NASH) is defined as lipid accumulation with evidence of cellular damage, inflammation, and different degrees of scarring or fibrosis (5). NASH is a serious condition as approximately 25% of these patients progress to cirrhosis and its feared complications of portal hypertension, liver failure, and hepatocellular carcinoma (6–8).
At present, the available noninvasive markers for NAFLD include a set of clinical signs and symptoms, nonspecific laboratory and radiological imaging tests, and combinations of clinical and blood test results (9). Although several of these markers are generally useful for the diagnostic evaluation of a patient with suspected NAFLD, they lack specificity and sensitivity to distinguish NAFLD from NASH and to determine the presence and stage of fibrosis (10). This represents a key clinical problem because patients with NASH and fibrosis are those who need closer monitoring and follow-up, and they are the potential targets for therapeutic intervention when specific treatments for this condition become available. To date, liver biopsy, an invasive procedure, remains the gold standard for diagnosis of NAFLD and NASH (10). Therefore, there is a great need for development of noninvasive methods that can reliably identify patients with NASH and stage the magnitude of fibrosis present.
Oxidative stress (OS) is recognized as mechanism contributing to hepatocyte injury and disease progression in NAFLD (11–15). However, identification of structurally specific oxidation products whose levels discriminate between NAFLD and NASH remains unknown. Identification of noninvasive markers that reflect the magnitude of hepatic oxidative stress is an attractive target for development of a disease biomarker. While experimental and human studies have demonstrated enhanced OS and lipid peroxidation products in circulation and the liver of different animal models and patients with NASH (16–18), the studies have been limited due to the lack of sensitive and structurally specific markers and poor understanding of the relative importance of different oxidation pathways in the context of NAFLD and NASH. In the present study, by employing a highly sensitive liquid chromatography-mass spectrometric approach in a well-characterized group of patients, we are able to define the circulating profile of bioactive lipid peroxidation products characteristic of patients with NASH and the role of free radical-mediated processes in the generation of these products. We also show the surprising finding that circulating levels of only a subset of structurally specific oxidized fatty acids (oxFA) serve as markers of hepatic inflammation, steatosis, and fibrosis in NASH patients.
METHODS
Patient characteristics and sample collection
The study was approved by the Cleveland Clinic Institutional Review Board, and all patients gave written informed consent prior to participation. Our cohort consisted of an initial group of 73 consecutive patients and a subsequent validation group of 49 consecutive patients in whom fasting blood was drawn the morning of scheduled elective liver biopsy at the Cleveland Clinic. The inclusion/exclusion criteria are detailed below (see “Clinical Diagnosis”). Extensive demographic, clinical, and laboratory data were collected from each patient. Whole blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes. Blood was immediately placed on ice or in a refrigerator, and samples centrifuged at 3500 rpm for 10 min at 4°C within 2 h of collection. Plasma was then immediately stored under conditions to minimize artificial oxidation (i.e., with an antioxidant cocktail under inert atmosphere) (19). Briefly, plasma aliquots were placed into cryotubes with screw caps and o-rings. Antioxidant cocktail (from 100 × stocks) was added, consisting of butylated hydroxytoluene (500 μM final) and diethylenetriamine pentaacetic acid (2 mM final). Head space in the cryotube was purged with argon, and then sealed tubes were snap-frozen in liquid nitrogen for storage at –80°C until analysis. Liver biopsy tissue was collected and sent to the Department of Anatomic Pathology for histopathological analysis (see “Liver biopsy”).
Clinical diagnosis
Information regarding demographics, medical history, and medications were obtained by patient interview and confirmed by chart review. Race information was based on self-reporting, and the information used for analyses was prespecified prior to the study. The clinical outcome data were verified by source documentation. Subjects included in the study were between the age of 18 and 70 years inclusive, had less than 20 g/day of alcohol consumption (for males) and less than 10 g/day (for females), and were referred by the treating physician for a baseline liver biopsy in the context of diagnostic evaluation for suspected NAFLD. Currently there are no established guidelines of when to perform a liver biopsy in either adult or pediatric patients with suspected NAFLD. Thus, the decision to perform a baseline liver biopsy in our patient population was made on an individual basis by the treating gastroenterologist independent of the present study. In the most cases, the decision for biopsy indication was based on the presence of persistently abnormal liver enzymes [mainly serum alanine transaminase (ALT)] in a patient with suspected NAFLD. Patients were excluded from the study if other liver diseases were detected, including viral, drug related, autoimmune, and metabolic/genetic liver disease. These other liver diseases were ruled out in all cases based on standard clinical studies, imaging and/or liver biopsy features, or laboratory studies, including viral hepatitis panel, ceruloplasmin, α-1-antitrypsin, autoantibodies, and metabolic/inborn error panel.
Liver biopsy
Liver histology was assessed by an experienced liver pathologist blinded to patient clinical and laboratory data. Biopsy length and the average number of portal tracts were recorded for each patient. The diagnosis of NASH was established based on Brunt's Criteria (20). The NAFLD activity scoring system developed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NASH Clinical Research Network (NASH CRN) was also calculated for each patient (21). According to this scoring system, the degree of steatosis, liver injury, and inflammatory activity are measured using a 0 to 8 scale (steatosis, 0–3; lobular inflammation, 0–3; and ballooning, 0–2). The NAFLD activity score (NAS) is the unweighted sum of the steatosis, lobular inflammation, and hepatocellular ballooning score. The degree of fibrosis was measured using a 6-point scale (1a, b = zone 3 perisinusoidal fibrosis; 1c = portal fibrosis only; 2 = zone 3 and portal/periportal fibrosis; 3 = bridging fibrosis; and 4 = cirrhosis). Severity of fibrosis was defined as: stage 1 = no fibrosis or mild fibrosis; stage 2 = moderate fibrosis; and stage 3 or 4 = severe fibrosis. The quality of liver biopsies was rated as follows: optimal-quality biopsy = greater than 2.5 cm length, more than 10 portal tracts, and no fragmentation; good-quality biopsy = greater than 1.5 cm length, more than 6 portal tracts, and no fragmentation; inadequate biopsy = less than 1.5 cm length, fewer than 6 portal tracts, and fragmentation. Only optimal and good quality samples were included in the study.
Lipid extraction from human plasma
Lipid extractions and protein hydrolyses were performed using disposable threaded borosilicate glass test tubes with PTFE lined caps. Before use, all glassware tubes, caps, and pipette tips were washed with nitric acid to remove trace transition metals, extensively rinsed with Chelex-treated water containing 1 μM diethylenetriamine pentaacetic acid (DTPA; pH 7.0 in H2O), and then rinsed with pure Chelex-treated water. Plastic tips were further rinsed in methanol and air-dried prior to use. Test tubes were baked at 500°C overnight to remove residual potential organics. All plasma samples for analyses contained anti-oxidant cocktail [DTPA (2 mM final) and butylated hydroxytoluene (500 μM final)] with head space overlaid with argon. Samples were thawed in ice/water bath immediately prior to sample handling for LC/MS/MS analysis. Fatty acids and oxidized fatty acids in plasma were extracted as previously described (22). Briefly, plasma (50 μl), internal standard [synthetic 15(S)-HETE-d8] and potassium hydroxide were added to the glass test tubes, overlaid with argon, and sealed. Lipids were hydrolyzed at 60°C under argon atmosphere for 2 h and then the released fatty acids were extracted into the hexane layer twice by liquid/liquid extraction. With each extraction, argon was used to purge the head space of the tube prior to sealing and vortexing/centrifugation. The combined hexane layers were dried under nitrogen gas and then resuspended in 200 μl 85% methanol/water (v/v).
Liquid chromatography online electrospray ionization tandem mass spectrometry
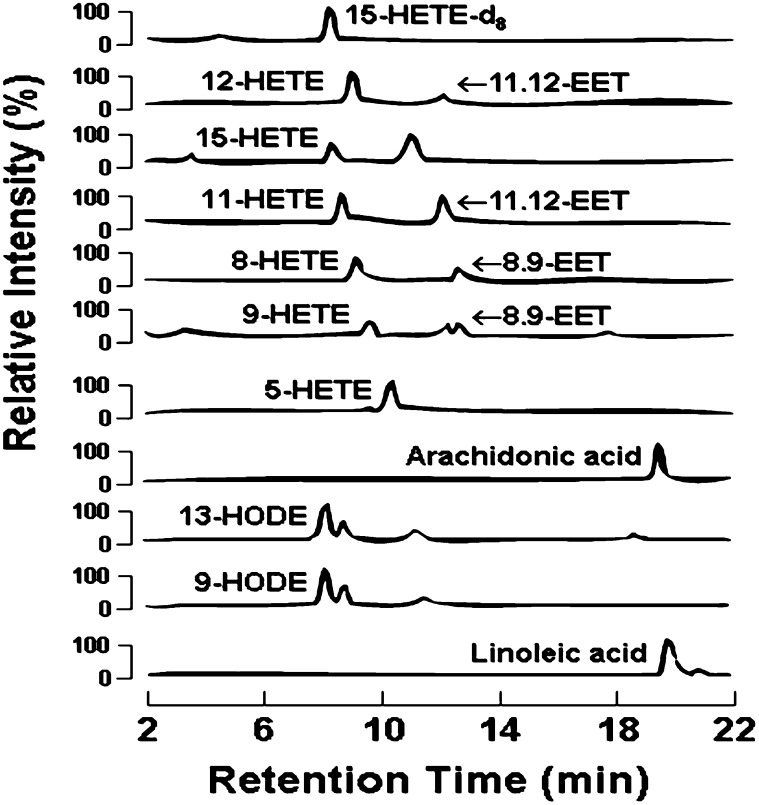
Levels of multiple fatty acid oxidation products (free plus esterified) in plasma were quantified using liquid chromatography online electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) (Fig. 1) (22). Briefly, lipid extract was injected onto an HPLC (Waters 2690 Separations Module, Franklin, MA), and the oxidized fatty acids and their precursors were separated through a C18 column (Phenomenex ODS (2), 2 × 150 mm, 5 μm, Rancho Palos Verdes, CA) using a gradient starting from 85% methanol containing 0.2% acetic acid over 10 min and then to 100% methanol containing 0.2% acetic acid over 2 min, followed by 100% methanol containing 0.2% acetic for 15 min. The oxidized fatty acids and their precursors were quantified on a triple quadrupole mass spectrometer (Quattro Ultima, Micromass, Manchester, UK) using ESI in negative ion mode and multiple reaction monitoring (MRM) using characteristic parent → daughter ion transitions for the specific molecular species monitored (22). The lipid peroxidation products analyzed included structurally specific species of hydroxy-eicosatetraenoic acids (HETE 5, 8, 9, 11, 12, and 15), hydroxy-octadecadienoic acids (HODE 9 and 13), oxo-octadecadienoic acids (HODE 9 and 13) and their precursor's arachidonic acid and linoleic acid (AA). The sample preparation and the quantitation of oxidized fatty acids by LC/ESI/MS/MS were performed by an investigator who was blinded to the liver histology and other clinical data. 15-HETE-d8 (Cayman Chemicals, Ann Arbor, MI) was used as internal standard for calibration of oxidized fatty acids in plasma, as previously described (22). To further assess the role of free radical versus stereoselective (enzymatic) processes in formation of the lipid peroxidation molecular species, isomers of 13-HODE, 13(S)-HODE and 13(R)-HODE were separated by liquid chromatography on a chiral phase to quantify stereo specificity. Lipid extract was injected onto and separated through a Chiral column (Chiralpak IA, 4.6 × 250 mm, Chiral Tech, West Chester, PA) on a HPLC (Beckman 126, Palatine, IL) using mobile phase hexane/2-propanol (90/10,v/v) at a flow rate of 0.5 ml/min. Based on the difference of their retention time versus authentic synthetic chiral standards, the HPLC fractions containing 13(S)-HODE and 13(R)-HODE were individually collected, dried under nitrogen gas flow, and reconstituted in 50% methanol. The quantities of 13(S)-HODE and 13(R)-HODE were then determined by LC/ESI/MS/MS.
Fig. 1.
Detection and quantification of oxFA profile by ESI/LC/MS/MS. Individual isomers of HETEs, EETs, HODEs, and oxoODEs formed by oxidation of arachidonic acid and linoleic acid are quantified with one single injection. Lipid extracts are resolved by HPLC and monitored online by ESI/LC/MS/MS as detailed under “Methods.” Abbreviations: EET, epoxy eicosatetraenoic acid; HETE: hydroxy eicosatetraenoic acid; HODE: hydroxy octadecadenoic acid; oxFA, oxidized fatty acid; oxoODE, oxo-octadecadenoic acid; 15-HETE-d8, internal standard.
In control studies, both the influence of sample processing time and assay methodology were evaluated to ensure no artificial formation of oxidation products occurred under the conditions employed. Linoleate-d4 and arachidonate-d8 (Cayman Chemicals, Ann Arbor, MI) were added to human plasma (100 μM each final) that had either been isolated immediately following blood draw or from whole blood (drawn into a purple top EDTA plasma tube) that was kept on ice for 4 h prior to plasma isolation. Plasma samples were then treated with the typical antioxidant cocktail of BHT and DTPA, and then analyzed under typical conditions as outlined above. During LC/MS/MS analyses parent → daughter transitions were monitored for the endogenous fatty acids and their oxidation products, as well as the isotopomers that would be formed from the deuterated parent fatty acids if artificial oxidation occurred. After completion of the plasma analyses using the above assay methods, only trace levels (or none for many species) of the deuterated HODEs, oxoODEs, and HETEs were detected, with calculated production of all monitored, structurally specific, oxidized fatty acids being less than 5% of the endogenous levels detected. Moreover, comparison of the analyses from plasma isolated immediately upon blood draw, versus delay on ice prior to plasma isolation, showed similar results (within ±5%). These data confirm that the sample handling prior to plasma isolation and the assay methodology used did not significantly artificially produce the specific lipid oxidation products monitored.
Statistical analysis
Clinical diagnosis, histopathological diagnosis, and laboratory and mass spectrometry assays were performed by investigators blinded to sample identity other than study barcode. Continuous variables are presented as median (25th and 75th percentiles) and categorical variables as numbers and percentages. Kruskal-Wallis tests for continuous and ordinal factors and Pearson's chi-square for categorical factors were used to assess differences between the three patient groups. Ad-hoc pairwise comparisons were done using Pearson's Chi-square for categorical factors and the Stee-Dwass procedure for continuous factors; a significance criterion of 0.017 was used for these. Spearman Rank correlation coefficients (rho) were calculated to test the correlation between the different oxidized fatty acid levels with liver histology features (inflammation, degree of steatosis, and degree of fibrosis) and the HOMA index for metabolic function. A multivariable logistic regression model for prediction of NASH in the initial cohort was constructed by performing automated stepwise variable selection on 1,000 bootstrap samples; the four most frequently included variables were incorporated in the final model. The multivariable logistic regression model (z) obtained from the original cohort was defined as follows: z = −10.051 + 0.0463 × Age(years) + 0.147 × BMI(kg/m2) + 0.0293 × AST(IU/l) + 2.658 × HODE-13:LA ratio(mmol/mol). This value was then converted into a probability distribution with a value between 0 to 100 and called “oxNASH” by the following formula: oxNASH = 100 × exp(z)/[1 + exp(z)]. Using this formula, oxNASH was then calculated for an independent group of patients to validate the predictive ability of the score. The area under the receiver operating characteristic (ROC) curves for aspartate transaminase (AST), ALT, and the final model were estimated and compared using Delong's method (23). Subgroup analyses were carried out to compare 13-HODE, 9-HODE, and 9-oxoODE levels according to the presence or absence of metabolic factors (diabetes mellitus, hypertension, and obesity) in patients with histopathological diagnosis of NASH. A P < 0.05 was considered statistically significant. SAS version 9.2 software (SAS Institute, Cary, NC) and R version 2.4.1 software (R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
RESULTS
Systemic levels of a select subset of oxFA are markedly increased in plasma of patients with NASH
We initially quantified the oxFA profile using LC/MS/MS in a well-characterized group of patients with suspected NAFLD. The main clinical and serological features of the study participants, stratified according to their liver biopsy results, are summarized in Table 1. Of the initial 73 subjects (initial cohort), 37 had NASH (51%); 23 had hepatic steatosis (32%); and 13 (17%), who had either no or minimal steatosis (less than 5%) and no or minimal inflammation, were classified as “controls” (i.e., normal liver biopsy). Participants were of similar age and predominantly Caucasian. Subjects with NASH had greater prevalence of history of diabetes mellitus, hyperlipidemia, and hypertension, but this did not reach statistical significance. The level of high density lipoprotein cholesterol (HDL) was lower in participants with NASH, while triglyceride levels were significantly higher in the NASH group. Patients with NASH had significantly higher body mass index (BMI) and the Homeostatic Model Assessment (HOMA) index, which is a sensitive measure of insulin resistance (24).
TABLE 1.
Demographic, clinical, and histological characteristics of the patients
| Factor | Normal Biopsy (N = 13) | Steatosis (N = 23) | NASH (N = 37) | P |
|---|---|---|---|---|
| Demographic | ||||
| Female | 7 (53.9) | 11 (47.8) | 21 (56.8) | 0.8 |
| Caucasian | 10 (76.9) | 18 (78.3) | 33 (89.2) | 0.42 |
| Age | 45 (42, 53) | 47 (38, 59) | 53 (44, 58) | 0.26 |
| Clinical | ||||
| BMI | 29.7 (26.8, 32) | 30.6 (27, 33.4) | 32 (30.7, 34.5) | 0.039 |
| AST | 43 (34, 56) | 50 (36, 60) | 63 (46, 84)a | 0.019 |
| ALT | 59 (44, 71) | 65 (42, 90) | 82 (42, 112) | 0.56 |
| HOMA | 1 (0.4, 1.6) | 1.4 (0.8, 2.7) | 5.5 (3, 12.9)a,b | <0.001 |
| Diabetesc | 3 (25) | 5 (21.7) | 14 (38.9) | 0.34 |
| Hyperlipidemiad | 7 (58.3) | 10 (43.5) | 20 (55.6) | 0.59 |
| Hypertensione | 3 (25) | 8 (36.4) | 18 (51.4) | 0.22 |
| Platelet | 269 (231.5, 304.5) | 247 (207, 312) | 225 (199, 262) | 0.076 |
| Cholesterol | 160 (158, 211) | 211 (169, 239) | 192 (162, 236) | 0.39 |
| Triglycerides | 86 (69, 94) | 160 (107, 210)a | 192 (139, 241)a | 0.003 |
| HDL | 62 (50, 70) | 56 (47, 63) | 44 (40, 50)a,b | 0.003 |
| LDL | 77.6 (75.2, 128) | 119.0 (94.4, 135.6) | 107.0 (94.4, 153.4) | 0.54 |
| Histologic | ||||
| Fibrosis | <0.001 | |||
| 0 | 13 (100) | 20 (87) | 2 (5.4)a,b | |
| 1 | 0 (0.0) | 3 (13.0) | 11 (29.7) | |
| 2 | 0 (0.0) | 0 (0.0) | 6 (16.2) | |
| 3 | 0 (0.0) | 0 (0.0) | 12 (32.4) | |
| 4 | 0 (0.0) | 0 (0.0) | 6 (16.2) | |
| Inflammation | – | |||
| None/minimal | 13 (100) | 19 (82.6) | 0 (0.0) | |
| Mild | 0 (0.0) | 4 (17.4) | 16 (44.4) | |
| Moderate | 0 (0.0) | 0 (0.0) | 19 (52.8) | |
| Severe | 0 (0.0) | 0 (0.0) | 1 (2.8) | |
| Steatosis | – | |||
| None/minimal | 13 (100) | 0 (0.0) | 0 (0.0) | |
| 5–33% | 1 (7.7) | 13 (56.5) | 4 (11.1) | |
| 34–66% | 0 (0.0) | 10 (43.5) | 20 (55.6) | |
| >66% | 0 (0.0) | 0 (0.0) | 12 (33.3) | |
| Ballooning | – | |||
| None | 13 (100) | 21 (91.3) | 1 (2.8) | |
| Few | 0 (0.0) | 2 (8.7) | 16 (44.4) | |
| Many | 0 (0.0) | 0 (0.0) | 19 (52.8) | |
| NAS | 0.0 (0.0, 0.0) | 2 (1, 2) | 5 (5, 6) | – |
Values presented as mean (± SD) or median (25th–75th quartile). Pairwise comparisons were done using Pearson's chi-square for categorical factors and the Stee-Dwass procedure for continuous factors. Significance criterion is 0.017. ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; HOMA index: Homeostatic Model Assessment index; NAS, NAFLD activity score. Boldface type indicates statistically significant. (See Statistical methods for details.)
Significantly different from normal biopsy group.
Significantly different from hepatic steatosis group.
Diabetes mellitus: history of diabetes, fasting plasma glucose ≥126 mg/dl and/or on hypoglycemic agents.
Hyperlipidemia: history of high plasma cholesterol, total cholesterol ≥200 mg/dl, and/or on hypolipidemic agents.
Hypertension: history of hypertension, systolic and diastolic blood pressure ≥ 120/80 mm Hg, and/or on anti-hypertensive agents.
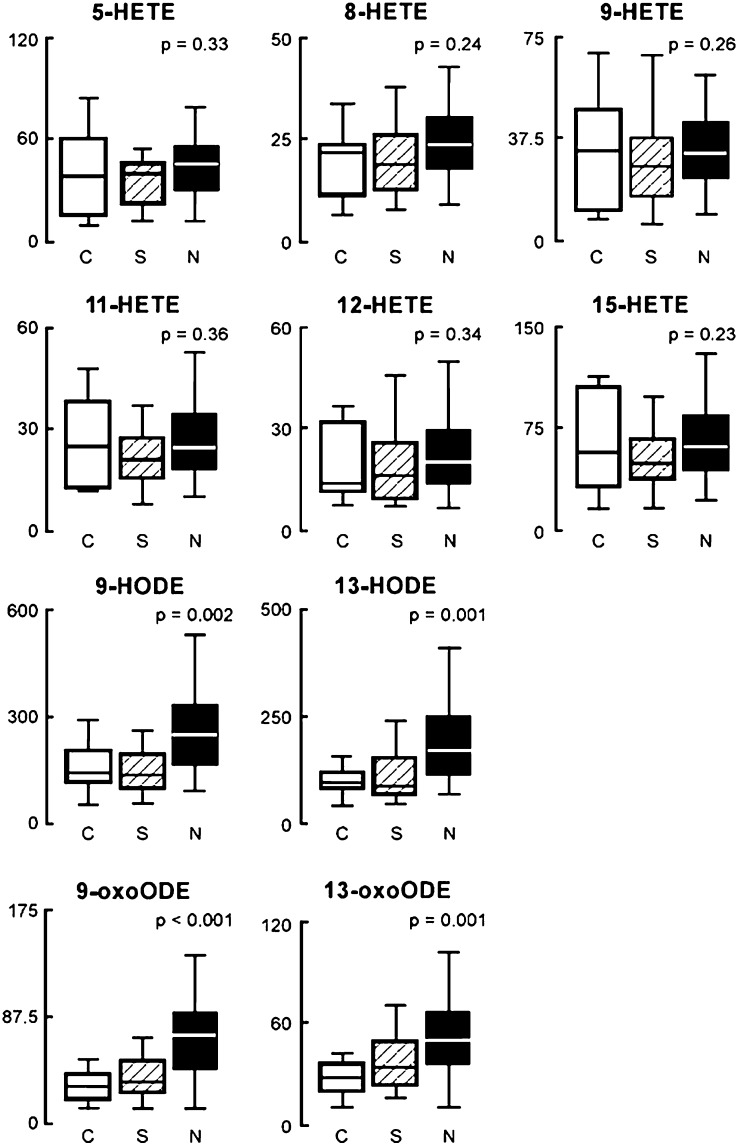
Plasma levels of multiple, structurally specified oxFA were quantified in our study participants, and the levels were analyzed for their relationship with histopathological changes. Remarkably, of the markers monitored, only 9-HODE, 13-HODE, 9-oxoODE, and 13-oxoODE, products of free radical-mediated oxidation of linoleic acid, were significantly elevated in patients with NASH compared with patients with hepatic steatosis and normal liver biopsy (Fig. 2). Patients with simple steatosis had slightly higher indices of oxidative stress compared with patients with the normal liver biopsy, but the difference in oxFA levels between the two groups failed to reach statistical significance for most analytes monitored (Fig. 2). The data expressed as the ratio of oxFA product to specific precursor were consistent with these findings and showed that patients with NASH had the highest oxlipid/precursor ratio for 9-HODE, 13-HODE, 9-oxoODE, and 13-oxoODE compared with patients with hepatic steatosis and normal liver biopsy (Table 2). The lipid oxidation product/precursor ratio was similar in the hepatic steatosis and normal liver biopsy groups for most of the fatty acid oxidation products monitored.
Fig. 2.
OxFA levels are markedly increased in the blood of patients with NASH. The box-whisker plot is represented with the lower boundary of the box indicating the 25th percentile, the line within the box indicating the median value, and the upper boundary of the box indicating the 75th percentile. The whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box. P values represent differences among groups. Abbreviations: HETE: hydroxy-eicosatetraenoic acid; HODE: hydroxy-octadecadienoic acid; NASH, nonalcoholic steatohepatitis; oxFA, oxidized fatty acid; oxoODE: oxo-octadecadienoic acid.
TABLE 2.
Plasma levels of oxidized fatty acid:precursor ratios in patients with suspected NAFLD
| Lipid:Precursor Ratioa | Normal Biopsy | Steatosis | NASH | P |
|---|---|---|---|---|
| mmol/mol of plasma | N = 13 | N = 20 | N = 37 | |
| 12-HETE | 0.14 (0.09, 0.33) | 0.24 (0.11, 0.32) | 0.25 (0.16, 0.39) | 0.17 |
| 15-HETE | 0.54 (0.22, 1.09) | 0.57 (0.30, 0.99) | 0.86 (0.48, 1.09) | 0.3 |
| 11-HETE | 0.22 (0.09, 0.40) | 0.25 (0.14, 0.32) | 0.27 (0.19, 0.41) | 0.31 |
| 8-HETE | 0.24 (0.07, 0.27) | 0.24 (0.12, 0.30) | 0.25 (0.18, 0.35) | 0.21 |
| 9-HETE | 0.33 (0.06, 0.54) | 0.30 (0.14, 0.43) | 0.37 (0.28, 0.60) | 0.16 |
| 5-HETE | 0.37 (0.11, 0.64) | 0.45 (0.24, 0.68) | 0.51 (0.34, 0.72) | 0.21 |
| 13-HODE | 0.29 (0.22, 0.47) | 0.31 (0.21, 0.57) | 0.51 (0.35, 0.77) | 0.01 |
| 9-HODE | 0.43 (0.30, 0.74) | 0.44 (0.28, 0.89) | 0.72 (0.46, 1.08) | 0.02 |
| 9-oxoODE | 0.10 (0.08, 0.16) | 0.13 (0.09, 0.19) | 0.24 (0.16, 0.32) | 0.002 |
| 13-oxoODE | 0.09 (0.07, 0.12) | 0.11 (0.08, 0.14) | 0.16 (0.10, 0.22) | 0.01 |
Median (25th–75th percentile) reported for all parameters. HETE, hydroxy-eicosatetraenoic acid; HODE, hydroxy-octadecadienoic acid; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; oxoODE, oxo-octadecadienoic acid. Boldface type indicates statistically significant. (See Statistical methods for details.)
Ratio of HETEs, HODEs, and oxoODEs to their parent compound arachidonic acid and linoleic acid, respectively.
OxFA levels correlate with liver histopathology independent of other metabolic factors
A strong positive correlation was revealed between systemic levels of specific oxidation products and liver histology that included inflammation, degree of steatosis, and stage of fibrosis (Table 3). The Spearman Rank correlation (r) between 13-HODE with inflammation was 0.42 (P < 0.001). A similar positive correlation was observed between 13-HODE and both degree of steatosis and stage of fibrosis (r = 0.36, P < 0.01; and r = 0.36, P = 0.01, respectively). We also compared circulating oxFA levels in the subgroup of NASH patients according to the presence or absence of diabetes mellitus, hypertension, and obesity. Of the 37 patients (initial cohort) with NASH, 14 (38.9%) had diabetes, 18 (51.4%) hypertension, and 29 (78.4%) were obese. Levels of 13-HODE, 9-HODE, and 9-oxoODE were not found to be significantly associated with either factor (supplementary Table I).
TABLE 3.
Oxidized fatty acids and disease severity
| Lipids | Inflammation | Steatosis | Fibrosis | NAS |
|---|---|---|---|---|
| Spearman's correlation coefficients (rho) | ||||
| 12-HETE | 0.096 | 0.131 | 0.121 | 0.137 |
| 15-HETE | 0.136 | 0.127 | 0.118 | 0.16 |
| 11-HETE | 0.085 | 0.082 | 0.081 | 0.119 |
| 8-HETE | 0.126 | 0.109 | 0.11 | 0.137 |
| 9-HETE | 0.098 | 0.186 | 0.111 | 0.185 |
| 5-HETE | 0.106 | 0.125 | 0.073 | 0.17 |
| AA | −0.169 | −0.211 | −0.18 | −0.214 |
| 13-HODE | 0.415c | 0.355b | 0.359b | 0.418c |
| 9-HODE | 0.387c | 0.338b | 0.299a | 0.399c |
| 9-oxoODE | 0.438c | 0.440c | 0.300a | 0.499c |
| 13-oxoODE | 0.362b | 0.396c | 0.247a | 0.425c |
| LA | 0.095 | 0.031 | 0.114 | 0.059 |
AA, arachidonic acid; HETE: hydroxy-eicosatetraenoic acid; HODE: hydroxy-octadecadienoic acid; LA, linoleic acid; NAS, NAFLD activity score; oxoODE: oxo-octadecadienoic acid.
P < 0.05.
P < 0.01.
P < 0.001.
OxFA level profile for NASH diagnosis
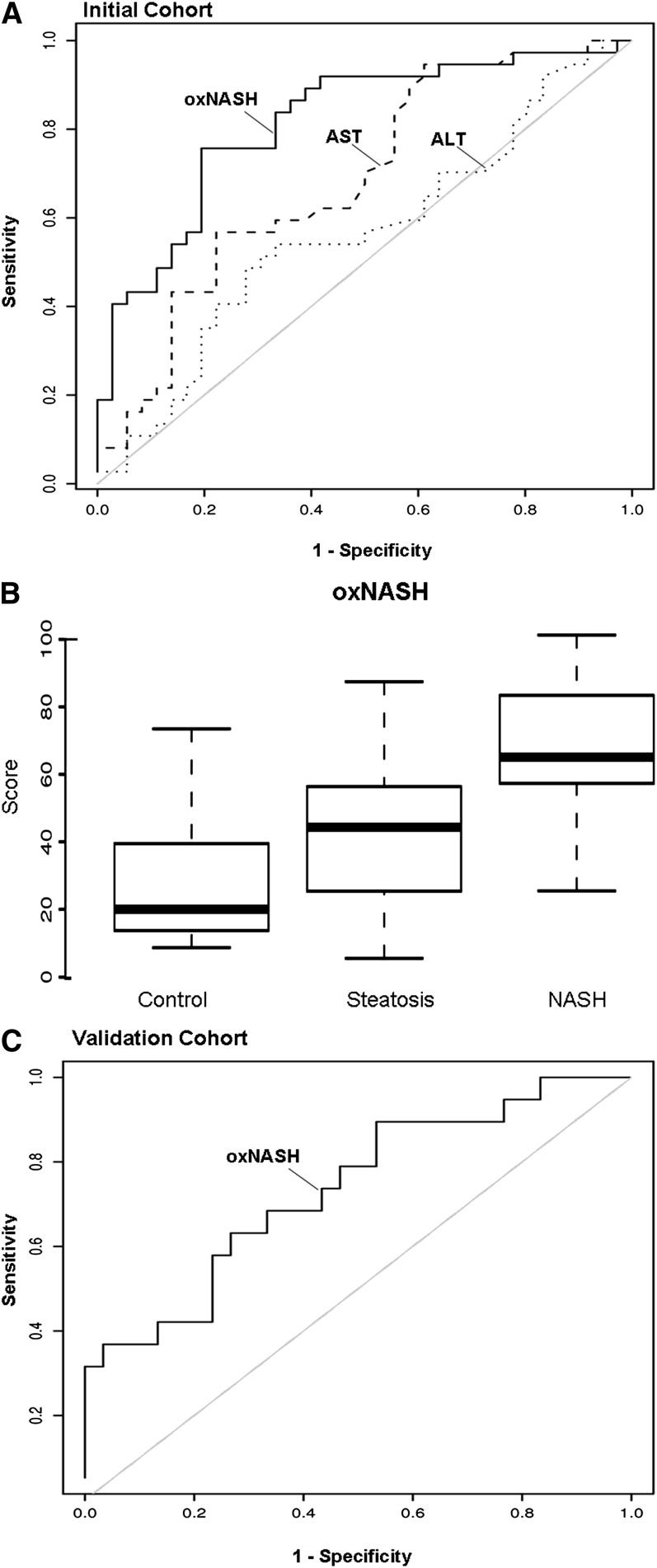
To ascertain whether plasma oxFA levels independently predicted the presence of NASH, we conducted multivariable logistic regression analysis. A risk score for the histopathologic diagnosis of NASH called “oxNASH” was generated by multivariable modeling that showed the best prediction for NASH diagnosis (see “Statistical Methods”). The oxNASH was calculated from the ratio of 13-HODE to LA, age, BMI, and AST (Fig. 3). In this prediction model, a 0.5 mmol/mol increase in 13-HODE/LA ratio was associated with a 3.8-fold increase in the likelihood of having NASH [OR: 3.8 (95% CI: 1.4-10.6; P = 0.01)] (Table 4). The addition of other factors, including gender, race, history of diabetes, history of hypertension, HDL, Tg/HDL ratio, history of hyperlipidemia, and HOMA did not have a confounding effect on the model. Within the initial cohort, the area under the curve (AUC) for oxNASH was estimated to be 0.83 (95% CI: 0.73, 0.93) and was found to be significantly higher than the AUC of either serum ALT 0.56 (95% CI: 0.43, 0.70) or serum AST 0.69 (95% CI: 0.56, 0.81) (P < 0.01) (Fig. 3A). Fig. 3B illustrates how the distribution of levels of oxNASH was significantly elevated in patients with NASH compared with patients with either hepatic steatosis (P < 0.01) or normal (P < 0.01) liver biopsy (Fig. 3B). Using the AUC curve, two cutoff values were selected to minimize the rate of false positive and negative results. A low cutoff point for oxNASH of 55 was able to exclude the presence of NASH with a sensitivity of 81%, and a high cutoff value for oxNASH of 73 was able to detect the presence of NASH with 97% specificity.
Fig. 3.
Circulating oxFA levels as predictors of NASH in patients with suspected NAFLD. A scoring system (oxNASH) that included 13-HODE/LA ratio, age, BMI, and AST showed best prediction of NASH diagnosis. (A) The AUC curve on the initial training cohort (n = 73) for oxNASH was estimated to be 0.83 (95% CI: 0.73, 0.93) and was found to be significantly higher than the AUCs of both that of serum AST 0.69 (95% CI: 0.56, 0.81) and serum ALT 0.56 (95% CI: 0.43, 0.70) (P < 0.01). (B) The box-whisker plot for oxNASH in the three groups of patients is represented with the lower boundary of the box indicating the 25th percentile, the line within the box indicating the median value, and the upper boundary of the box indicating the 75th percentile. The whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box. (C) The AUC in the independent validation group (n = 49) was estimated to be 0.74 (95% CI: 0.6, 0.88). Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; AUC, area under the curve; BMI, body mass index; HODE, hydroxy-octadecadenoic acid; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; oxFA, oxidized fatty acid.
TABLE 4.
Multivariable modeling of oxidized fatty acids in prediction of NASH
| Factor | Odds Ratio (95% CI) | P |
|---|---|---|
| Age (5-year increase) | 1.3 (0.97, 1.6) | 0.083 |
| BMI (1 kg/m increase) | 1.2 (1.02, 1.3) | 0.025 |
| AST (5 IU/l increase) | 1.2 (1.03, 1.3) | 0.01 |
| 13-HODE/LA (0.5 mmol/mol increase) | 3.8 (1.4, 10.6) | 0.011 |
AST, aspartate transaminase; BMI, body mass index; HODE: hydroxy-octadecadienoic acid; LA, linoleic acid; NASH, nonalcoholic steatohepatitis.
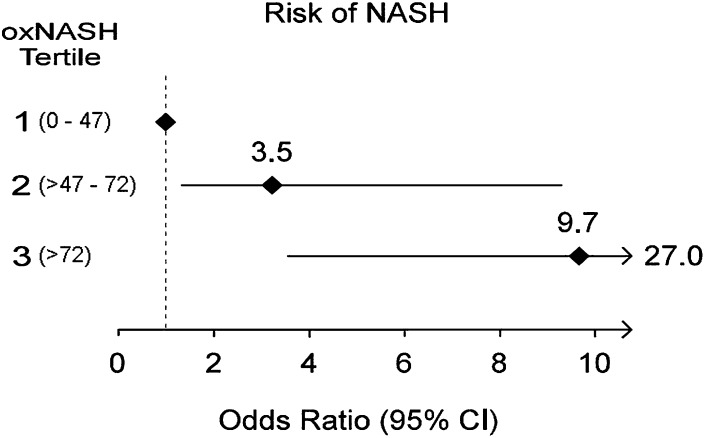
We next examined the diagnostic accuracy of the oxNASH prediction model in an independent validation cohort of 49 consecutive patients. Within the validation cohort, 19 (39%) had NASH, 22 (45%) had hepatic steatosis, and 8 were classified as normal biopsies (16%). The AUC for oxNASH in the validation cohort for prediction of histopathologic diagnosis of NASH was 0.74 (95% CI: 0.6, 0.88) (Fig. 3C). By applying the low cutoff point in the validation set, the diagnosis of NASH on liver biopsy was able to be excluded with a sensitivity of 84%. While applying the high cutoff, the presence of NASH was able to be established with a specificity of 63%. The risk of NASH diagnosis for subjects with oxNASH levels in the second or third tertile versus the lowest tertile was 3.5-fold (P < 0.001) and 9.7-fold (P < 0.001) higher odds, respectively (Fig. 4).
Fig. 4.
Risk of having NASH on liver biopsy based on oxNASH levels. Forest plot illustrating the odds ratio and 95 confidence intervals for risk of histopathologic diagnosis of NASH based upon oxNASH tertiles. Numbers in parentheses represent oxNASH tertile ranges. For each comparison, the first tertile served as the reference group. Abbreviation: NASH, nonalcoholic steatohepatitis.
Free radical-mediated processes are key mediators of lipid oxidation in NASH
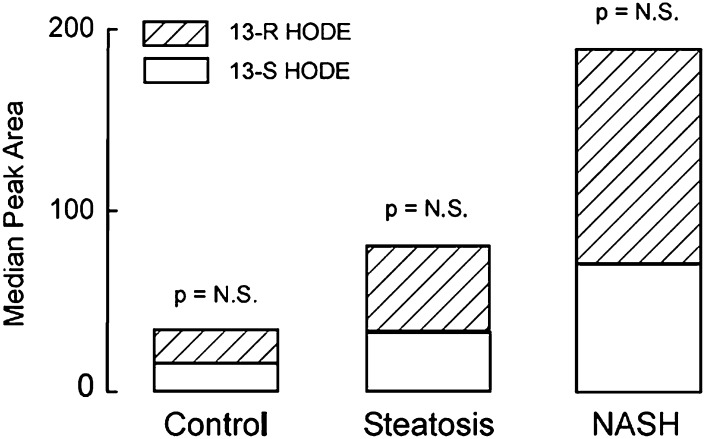
The initial findings showing significantly higher levels of 9-HODE and 13-HODE in patients with NASH suggested that free radical-mediated oxidation processes are involved in the generation of lipid oxidation products in these patients. To further assess the role of free radical versus stereoselective (enzymatic) processes in the formation of lipid oxidation products in patients with NAFLD, chiral phase separation of individual stereoisomers coupled with mass spectrometry was used to identify and quantify the structural isomers and the chiral distribution of specific oxidized lipid species. A significant increase in peak areas of both 13(S)-HODE and 13(R)-HODE were observed in patients with NASH compared with patients with simple steatosis and compared with patients with normal liver biopsy. Furthermore, the peak area of 13(S)-HODE was similar to that of 13(R)-HODE with a P value of 0.1 (Fig. 5). Taken together, these observations strongly suggest that in the context of NASH, free radical-mediated processes are mainly responsible for generation of lipid oxidation products (particularly 13-HODE).
Fig. 5.
Free radical-mediated processes are key mediators of lipid oxidation in NAFLD. Specific oxidized lipid species were separated by liquid chromatography on a chiral phase to identify and quantify the structural isomers and their chiral distribution. A significant increase in peak area of both 13-S HODE and 13-R HODE were observed in patients with NASH compared with patients with hepatic steatosis and patients with normal liver biopsy. The peak area of 13-S HODE was similar to that of 13-R HODE in the three groups of patients. Abbreviations: HODE, hydroxy-octadecadenoic acid; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
DISCUSSION
The principal findings of this study relate to the identification of specific fatty acid oxidation products as potential novel, systemic, noninvasive (plasma) markers to identify NASH. The results demonstrate that specific oxFA products are markedly increased in the blood of patients with NASH and are mainly the result of free radical-mediated processes. The findings also show that the levels of these oxidation products correlate with severity of liver disease, independent of other metabolic factors. Finally, a risk score termed oxNASH was generated using the initial cohort and validated in an independent validation cohort for the diagnosis of NASH. While multiple laboratory values (e.g., triglcyerides, HDL, HOMA, ALT, and AST) are found to be elevated in NASH, discrimination between simple steatosis versus NASH was optimized by including the 13-HODE/LA ratio, age, BMI, and AST in oxNASH. This simple risk score may be clinically useful in establishing the presence and severity of disease.
Obesity and type 2 diabetes have reached epidemic proportions in most of the Western world, and both conditions are strongly associated with NAFLD (25). NAFLD encompasses a wide spectrum of conditions associated with over-accumulation of lipids in the liver, ranging from simple fatty liver (hepatic steatosis), in which there is evidence for fat accumulation without signs of liver cell injury or inflammation, to nonalcoholic steatohepatitis (NASH), characterized by the accumulation of fat in the liver along with evidence of liver cell damage, inflammation, and different degrees of scarring or fibrosis (2). While most patients with steatosis tend to have a benign, nonprogressive clinical course, a significant proportion of those with NASH show progressive liver disease with significant associated risk of developing cirrhosis and its complications (portal hypertension, liver failure, and hepatocellular carcinoma) (6–8). In light of the dramatic increase in the prevalence of NASH together with the significant research effort aimed at developing novel therapies targeted to patients with NASH, the development of noninvasive, simple, reproducible, and reliable biomarkers are greatly needed. They would not only help in the diagnosis of NASH but would also be useful for assessment of treatment response and prognosis. Oxidative stress and formation of bioactive lipid peroxidation products are associated with liver damage and disease progression to NASH (26, 27), yet precise molecular species have not yet been identified. Several oxidation pathways may play a role in the overproduction of lipid peroxidation products in NASH, including enzymatic and nonenzymatic free radical-mediated processes. Each of these pathways may generate different oxidation products that potentially could be quantified. Based on the importance of oxidative stress in the pathogenesis of NASH, several groups have attempted to elucidate whether measurement of systemic markers of OS may be used as biomarkers (16–18, 28). However, three key questions have remained unanswered: Which specific oxFA are enriched in the livers during NASH development? What are the primary pathways involved in their generation? And, as ROS react rapidly in the environment they are produced, does measuring these markers in blood reflect what is happening in the liver? By using a highly sensitive and specific tandem mass spectrometry approach, we defined for the first time the profile of oxFA in patients with NAFLD. Indeed, our results demonstrate that a specific subset of oxFA are markedly increased in blood from patients with NASH and that their levels correlate with histopathological changes found in the liver independent of other metabolic factors, such as presence of diabetes or hypertension. Because of the large amount of blood flow to the liver, this organ is the likely source of much of the oxFA monitored in blood.
The current study has several strengths and limitations. The mass spectrometry-based techniques employed served as a powerful tool to identify relevant oxidation pathways operative in NASH. They also allowed for definition of novel mechanism-based markers for this disease. Another strength is the clinical relevance of the patient population studied—those undergoing elective liver biopsy for diagnostic evaluation for suspected NAFLD. The subjects examined included a large group of consecutive, well-characterized patients who represented the whole spectrum of normality and disease, thus minimizing a potential selection bias that typically occurs in clinical studies on NAFLD in which only patients with clearcut NASH or hepatic steatosis are included. One potential limitation of our study is the lack of a control group of apparently healthy subjects with no known liver disease. Such a group, however, does not by definition have elevated liver function tests and would be of little clinical relevance. Furthermore, recruitment of a healthy control group for a study such as this is extremely difficult, as performing a liver biopsy without clinical indication is ethically questionable. Another potential limitation of our study is the intentional exclusion of patients with chronic hepatitis C or alcohol liver disease (i.e., lack of a disease control group). Whether oxNASH would allow distinguishing NASH patients from those with other chronic liver diseases remains to be established and requires future investigation.
There is currently a great need to develop tests that can distinguish NASH from hepatic steatosis and assess the severity of liver damage in patients (10, 29). Our data suggest oxNASH or specific individual linoleic oxidation products may serve as such a test. By multivariable modeling, we generated the scoring system oxNASH that predicted the presence of NASH with high accuracy. A combination of 13-HODE/LA ratio, age, BMI, and AST resulted in an AUC of 0.83 in the training set and 0.74 in an independent validation group. The performance of oxNASH is significantly better than the AUC for serum liver enzymes, currently the most commonly used test to identify the presence of NASH and to select patients for liver biopsy (9). These observations suggest that the oxNASH risk score has the potential to become a useful instrument in clinical practice. Future, larger studies are clearly warranted. The clinical utility of oxNASH in monitoring interval changes with links to outcomes is needed.
One of the more intriguing findings of the present study is that direct examination of the distribution of circulating oxFA in patients suggests that free radical-mediated processes are the predominant oxidation pathway involved in generation of lipid peroxidation products during NASH. Surprisingly, NASH subjects showed specific elevations only in a subset of linoleic acid oxidation products, including marked increased levels of 13-HODE, 9-HODE, 9-oxoODE, and 13-oxoODE. A role for free radical processes in production of these species is supported both by the regiospecificity of the products formed and the chirality of the oxFA. While linoleic acid may serve as a substrate for lipid peroxidation by enzymes such as lipoxygenases and cyclooxygenases, the regiospecificity for oxidation preferentially produces products with oxidation sites alternative to the 9- and 13- positions. In contrast, free radical-driven oxidation shows significantly less regiospecificity and produces the 9- and 13-HODEs and oxoODEs monitored. A free radical origin for the species of interest is further supported by the analyses of the stereospecificity of the oxFA as it showed a symmetric (50:50) R and S chiral distribution. Such a distribution is typical of free radical-mediated lipid oxidation (19). The precise mechanism(s) of free radical generation in NASH, however, will require further studies. It may reflect generation of a diffusible radical intermediate that initiates lipid peroxidation, or alternatively, it could represent the importance of free radical processes in the propagation phase of lipid peroxidation, with initiation involving a stereoselective enzymatic source. Moreover, a chiral HODE, for example, can be also be further oxidized to its corresponding keto form, and then reduced again to a racemic compound. Thus, the precise oxidative pathway(s) participating in NASH and elevation of the linoleate lipid oxidation species within the circulation of NASH subjects awaits further examination.
In summary, the current studies uncover the profile of oxFA in human NAFLD and identify species that provide strong discriminatory prognostic utility in the diagnosis of NASH versus steatosis or normal liver biopsy. The results support a model in which NASH development is accompanied by generation of a subset of oxFAs, mainly by free radical-mediated processes that are then released into the bloodstream. OxNASH, a model risk score we developed that may serve as a signature for NASH risk, is based upon specific circulating markers of liver damage, including a subset of those derived from free radical-mediated oxidation of linoleic acid. The present data have significant implications for both development of NASH biomarkers and potential novel targets for therapeutic intervention.
Supplementary Material
Footnotes
Abbreviations:
- AA
- linoleic acid
- ALT
- alanine transaminase
- AST
- aspartate transaminase
- BMI
- body mass index
- HETE
- hydroxy-eicosatetraenoic acid
- HODE
- hydroxy-octadecadenoic acid
- NAFLD
- nonalcoholic fatty liver disease
- NAS
- NAFLD activity score
- NASH
- nonalcoholic steatohepatitis
- OR
- odds ratio
- OS
- oxidative stress
- oxFA
- oxidized fatty acid
- oxoODE
- oxo-octadecadenoic acid.
This work was supported by National Institutes of Health Grants DK-076852, AA-017748, and P01 HL-087018-020001. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. S. L. Hazen and A. E. Feldstein are named as co-inventors on pending patents filed by the Cleveland Clinic that refer to the use of biomarkers in fatty liver disorders.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table.
REFERENCES
- 1.Wieckowska A., Feldstein A. E. 2005. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr. Opin. Pediatr. 17: 636–641. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. 2002. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 3.Browning J. D., Szczepaniak L. S., Dobbins R., Nuremberg P., Horton J. D., Cohen J. C., Grundy S. M., Hobbs H. H. 2004. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 40: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer J. B., Deutsch R., Kahen T., Lavine J. E., Stanley C., Behling C. 2006. Prevalence of fatty liver in children and adolescents. Pediatrics. 118: 1388–1393. [DOI] [PubMed] [Google Scholar]
- 5.Brunt E. M., Neuschwander-Tetri B. A., Oliver D., Wehmeier K. R., Bacon B. R. 2004. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum. Pathol. 35: 1070–1082. [DOI] [PubMed] [Google Scholar]
- 6.Adams L. A., Lymp J. F., St Sauver J., Sanderson S. O., Lindor K. D., Feldstein A., Angulo P. 2005. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 129: 113–121. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni C. A., Younossi Z. M., Gramlich T., Boparai N., Liu Y. C., McCullough A. J. 1999. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 116: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M., Franzen L. E., Mathiesen U. L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. 2006. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 44: 865–873. [DOI] [PubMed] [Google Scholar]
- 9.Wieckowska A., McCullough A. J., Feldstein A. E. 2007. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 46: 582–589. [DOI] [PubMed] [Google Scholar]
- 10.Wieckowska A., Feldstein A. E. 2008. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin. Liver Dis. 28: 386–395. [DOI] [PubMed] [Google Scholar]
- 11.Day C. P. 2002. Pathogenesis of steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 16: 663–678. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira C. P., da Costa Gayotto L. C., Tatai C., Della Bina B. I., Janiszewski M., Lima E. S., Abdalla D. S., Lopasso F. P., Laurindo F. R., Laudanna A. A. 2002. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J. Cell. Mol. Med. 6: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roskams T., Yang S. Q., Koteish A., Durnez A., DeVos R., Huang X., Achten R., Verslype C., Diehl A. M. 2003. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am. J. Pathol. 163: 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Videla L. A., Rodrigo R., Araya J., Poniachik J. 2004. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic. Biol. Med. 37: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 15.Gao D., Wei C., Chen L., Huang J., Yang S., Diehl A. M. 2004. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 287: G1070–G1077. [DOI] [PubMed] [Google Scholar]
- 16.Bonnefont-Rousselot D., Ratziu V., Giral P., Charlotte F., Beucler I., Poynard T. 2006. Blood oxidative stress markers are unreliable markers of hepatic steatosis. Aliment. Pharmacol. Ther. 23: 91–98. [DOI] [PubMed] [Google Scholar]
- 17.Chalasani N., Deeg M. A., Crabb D. W. 2004. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 99: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 18.Horoz M., Bolukbas C., Bolukbas F. F., Sabuncu T., Aslan M., Sarifakiogullari S., Gunaydin N., Erel O. 2005. Measurement of the total antioxidant response using a novel automated method in subjects with nonalcoholic steatohepatitis. BMC Gastroenterol. 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R., Shen Z., Nauseef W. M., Hazen S. L. 2002. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 99: 1802–1810. [PubMed] [Google Scholar]
- 20.Brunt E. M., Janney C. G., Di Bisceglie A. M., Neuschwander-Tetri B. A., Bacon B. R. 1999. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 94: 2467–2474. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner D. E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., Ferrell L. D., Liu Y. C., Torbenson M. S., Unalp-Arida A., et al. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 22.Shishehbor M. H., Zhang R., Medina H., Brennan M. L., Brennan D. M., Ellis S. G., Topol E. J., Hazen S. L. 2006. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic. Biol. Med. 41: 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLong E. R., DeLong D. M., Clarke-Pearson D. L. 1988. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 44: 837–845. [PubMed] [Google Scholar]
- 24.Bonora E., Formentini G., Calcaterra F., Lombardi S., Marini F., Zenari L., Saggiani F., Poli M., Perbellini S., Raffaelli A., et al. 2002. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 25: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 25.Clark J. M., Brancati F. L., Diehl A. M. 2002. Nonalcoholic fatty liver disease. Gastroenterology. 122: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke H., Gores G. J., Cederbaum A. I., Hinson J. A., Pessayre D., Lemasters J. J. 2002. Mechanisms of hepatotoxicity. Toxicol. Sci. 65: 166–176. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi H., Gores G. J. 2003. Mechanisms of liver injury: an overview. Curr. Mol. Med. 3: 483–490. [DOI] [PubMed] [Google Scholar]
- 28.Solga S. F., Alkhuraishe A., Cope K., Tabesh A., Clark J. M., Torbenson M., Schwartz P., Magnuson T., Diehl A. M., Risby T. H. 2006. Breath biomarkers and non-alcoholic fatty liver disease: preliminary observations. Biomarkers. 11: 174–183. [DOI] [PubMed] [Google Scholar]
- 29.Wieckowska A., Zein N. N., Yerian L. M., Lopez A. R., McCullough A. J., Feldstein A. E. 2006. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 44: 27–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.