Abstract
This review will discuss the formation and potential implications of 7-ketocholesterol (7KCh) in the retina. 7KCh is a proinflammatory oxysterol known to be present in high amounts in oxidized LDL deposits associated with atheromatous plaques. 7KCh is generated in situ in these lipoprotein deposits where it can accumulate and reach very high concentrations. In normal primate retina, 7KCh has been found associated with lipoprotein deposits in the choriocapillaris, Bruch's membrane, and the retinal pigment epithelium (RPE). In photodamaged rats, 7KCh has been found in the neural retina in areas of high mitochondrial content, ganglion cells, photoreceptor inner segments and synapses, and the RPE. Intermediates found by LCMS indicate 7KCh is formed via a free radical-mediated mechanism catalyzed by iron. 7KCh seems to activate several kinase signaling pathways that work via nuclear factor κB and cause the induction of vascular endothelial growth factor, interleukin (IL)-6, and IL-8. There seems to be little evidence of 7KCh metabolism in the retina, although some form of efflux mechanism may be active. The chronic mode of formation and the potent inflammatory properties of 7KCh indicate it may be an “age-related” risk factor in aging diseases such as atherosclerosis, Alzheimer's, and age-related macular degeneration.
Keywords: 7-ketocholesterol, cytokines, oxysterols
In the past year, numerous reviews have been published that extensively discuss the multiple functions of oxysterols and their complex relationships to diseases (1–7). However, the formation and function of oxysterols in the retina has not been well studied. Emerging studies are beginning to reveal how oxysterols are formed and how their actions affect the retina and the retinal pigment epithelium (RPE) (8–10). One particular oxysterol, 7-ketocholesterol (7KCh), has been found more abundantly than other oxysterols in the retina (9, 10). This oxysterol is known to have potent pharmacological properties that lead to inflammation and cell death in a variety of cell types (1–10). This review will focus on what is known about this molecule and the potential implications of its presence in the retina.
The retina is a light-capturing tissue that contains at least 5 cell classes and more than 50 different cell types, each performing unique functions that ultimately provide the visual centers in the brain the information to achieve image formation and visual perception. The retina faces a unique photooxidative environment that generates challenges not encountered by other neurological tissues or organs. Phototransduction requires the retina to have a high metabolic rate (11) and multiple and complex membrane structures (12). The photoreceptor outer segments are enriched in the highly photosensitive docosahexaenoic acid and other easily oxidized polyunsaturated fatty acids (13). Thus, the retina seems to have developed unique mechanisms for lipid uptake and metabolism to adapt to this photooxidative environment. The retina has been shown to uptake circulating LDL (14, 15), providing blood-borne lipids to all the cellular layers of the retina. The retina can also synthesize cholesterol to maintain its dynamic steady-state lipid composition (16). To perform these tasks, the retina expresses the same molecules used in the systemic reverse cholesterol pathway to uptake and transport lipids internally (17). Monkey retina has been found to express apolipoprotein (apo)A1, apoE, apoB, ABCA1, LDL receptor (LDLR), scavenger receptors I and II (SRBI and II), CD36, cholesteryl-ester (CE) transfer protein (CETP), and lethicin-cholesterol acyltransferase (LCAT) (17). The particular location of these molecules in the retina suggests a blood-borne lipoprotein uptake and an intraretinal lipoprotein-based lipid transport process (17).
The retina of humans and other primates contains a specialized structure called the macula, which is highly enriched in cone photoreceptors and ganglion cells (12, 18–20). The macula provides primates with exceptional color vision and visual acuity not enjoyed by other mammals. Human function is highly dependent on a healthy macula and diseases that affect this structure have devastating consequences on the quality of life. There are two general types of diseases affecting the human macula: the juvenile dystrophies (e.g., Best's and Stardgardt's, etc.) and the age-related macular degenerations (AMDs). The juvenile dystrophies usually follow clear genetic patterns of inheritance, whereas AMD is complex, involving multiple types of risk factors. AMD is a complex group of diseases that lead to the degeneration of the macula in individuals older than 60 years of age and is the leading cause of legal blindness in developed nations (19, 20). One of the earliest clinical signs of AMD is the formation of drusen deposits. These deposits generally form between the RPE and Bruch's membrane and are composed of many different proteins and lipid components that originate both from inside and outside of the retina. There are two major forms of AMD: wet or exudative and dry or atrophic. The wet form of the disease is characterized by choroidal neovascularization (CNV). CNV forms when choroidal blood vessels behind the retina grow through Bruch's membrane into the macula, pushing aside the RPE. These bloods vessels tend to be malformed and prone to leakage, causing blood to come in contact with photoreceptors and other retinal cells (19, 20). Unless promptly treated, this causes massive damage to the macular photoreceptors. Although wet AMD composes only 10% of the total cases, it accounts for 90% of the cases that progress to legal blindness. The induction of vascular endothelial growth factor (VEGF) seems to be crucial in the CNV formation (21, 22). Anti-VEGF therapies have proven to be effective at regressing CNV and preserving vision (22). Dry AMD by contrast is much more common (90% of total cases) but usually progresses very slowly and the extent of the vision loss is generally less severe. The most important risk factor in AMD is aging, but genetics and environmental factors also play a very important role in the etiology of this disease (19, 20). The general consensus among vision scientists is that AMD is a disease that initially affects RPE function (19, 20). Chronic inflammation is suspected in being involved in RPE stress and damage (20–22), but the precise mechanisms remain to be elucidated.
In this context, we will discuss how 7KCh and other related oxysterols are formed in the retina and the pharmacological implications of their presence, especially in age-related diseases such as AMD.
WHAT IS 7KCH?
7KCh is an oxidized form of cholesterol commonly found in oxidized LDL (oxLDL) associated with atherosclerotic plaques (1–7, 23–25). 7KCh has been shown to have potent proinflammatory and cell death properties and has been implicated in foam cell formation in macrophages (2, 4, 7, 23–25). These processes result in the formation of the atheromatous plaques (23–25). 7KCh is highly toxic to cultured RPE cells and also has been shown to account for most of the cytotoxicity associated with oxLDL (26). In the oxLDL deposits, 7KCh is also found in the form of oxysteryl esters, covalently bound to oxidized unsaturated fatty acids (27, 28).
CHOLESTEROL OXIDATION IN THE RETINA
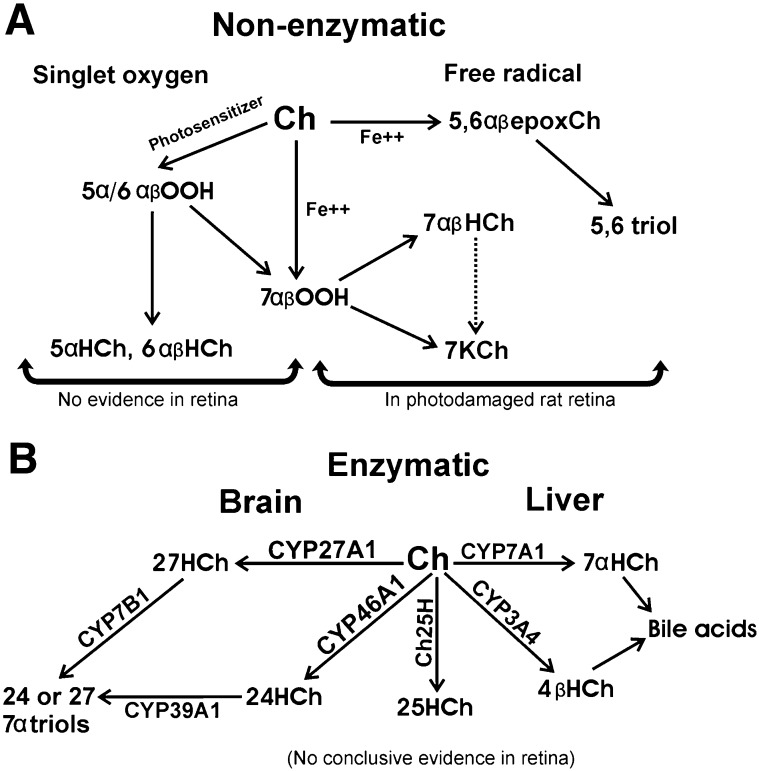
Cholesterol can be oxidized by nonenzymatic and enzymatic pathways (28, 29). The nonenzymatic pathways form mainly B-ring oxysterols like the epimeric 7-hydroxycholesterol (7HCh), 7KCh, and epimeric 5,6-epoxycholesterol (5, 6-epoxCh) (Fig. 1A). This occurs readily in lipoprotein deposits and atherosclerotic plaques. The enzymatic pathways can form both B-ring and side-chain hydroxylated oxysterols depending on the enzyme and the tissue (5, 7, 30–32) (Fig. 1B).
Fig. 1.
Potential cholesterol oxidation pathways in the retina. A: Nonenzymatic mechanisms are singlet oxygen and free radical oxidations. The singlet oxygen mechanism requires a photosensitizer to generate singlet oxygen (O2). In the retina, A2E and ATRA could potentially serve as photosensitizers but are extremely inefficient. The singlet oxygen attack on cholesterol generates three hydroperoxides: 3β-hydroxy-5α-cholest-6-ene-5-hydroperoxide (5α-OOCh), 3β-hydroxycholest-4-ene-6α-hydroperoxide (6α-OOCh), and 3β-hydroxycholest-4-ene-6β-hydroperoxide (6β-OOCh). 5α-OOCh is the main product. The 5α-OOCh, 6α-OOCh, and 6β-OOCh readily convert to the diols 5α-HCh, 6α-HCh, and 6β-HCh. The 5α-OOCh may also partially rearrange to 7α-OOCh, which can then become 7HCh and 7KCh. The free radical-mediated mechanism is essentially the Fenton reaction. This reaction is characterized by the need for a transition metal catalyst (e.g., Fe+2 and Cu+2). Two epimeric cholesterol hydroperoxides and two epimeric cholesterol epoxides are generated during free radical oxidation of Ch: 3β-hydroxycholest-5-ene-7α-hydroperoxide (7α-OOCh) and 3β-hydroxycholest-5-ene-7β-hydroperoxide (7β-OOCh) and the 5,6-epoxide epimers. These rearrange to form 7αHCh, cholest-5-ene-3β,7β-diol (7β-HCh), and 7KCh. A few other minor oxides are also formed. The 7α- and 7β-OOCh are reactive intermediates, whereas the other species are usually end-products, although the epimeric 5,6-epoxides will further oxidize into the epimeric triols. CEs are more sensitive to free radical oxidation than cholesterol. In CEs, both the cholesteryl and the fatty acyl moieties are readily oxidized via this mechanism. In both mechanisms, 7OOCh leads to the formation of 7HCh and 7KCh. At this time we are unaware of any scientific evidence to support the existence of the singlet oxygen mechanism in the retina; only the free radical seems to occur during light damage (10). B: The enzymatic mechanisms for cholesterol oxidation in the retina are speculative. Only CYP27A1 and CYP46A1 are known to be expressed in the retina, although the mRNA for some of the other enzymes can be detected by RT-PCR.
Nonenzymatic oxidation
There are two well-known nonenzymatic mechanisms by which cholesterol may be oxidized to 7KCh and other oxysterols: singlet oxygen and free radical mediated (Fig. 1A).
Singlet oxygen.
The singlet oxygen mechanism is characterized by the formation of the epimeric 6-hydroperoxides (main product), which readily rearrange to form epimeric 7-hydroperoxides (7OOCh) (28). 7OOCh is reactive and will oxidize other molecules and rearrange, becoming epimeric 7HCh and 7KCh (33–35). The singlet oxygen mechanism requires light and a suitable photo-sensitizer to capture the light and oxidize the cholesterol. Hematoporphyrin is a particularly good photo-sensitizer for cholesterol (36) and is often used to synthesize 7-keto derivatives of other oxysterols.
Free radical.
The free radical-mediated mechanism forms a different group of intermediates (27–30) (Fig. 1A). The main intermediates are epimeric 7OOCh, 5, 6-epoxCh, and 7HCh. As mentioned above, 7OOCh will become 7HCh and 7KCh, and 7HCh may also further oxidize to 7KCh, albeit more slowly (24, 30). 7OOCh is very reactive and may also oxidize other molecules. The epimeric 5, 6-epoxCh will also further oxidize to the 5, 6-dihydroxycholesterol (a triol), which also has considerable toxic properties (37). This free radical-mediated mechanism requires a transition metal catalyst. This is known as the Fenton reaction (initially described in 1894 by H.J.H. Fenton). Iron (Fe+2) and/or copper (Cu+2) are particularly effective at catalyzing this reaction (27–30). Both types of reactions (singlet oxygen and free radical) are very effective at oxidizing CEs and can oxidize the cholesteryl as well as the fatty acyl moieties (10, 27, 28, 38).
Enzymatic oxidation
There are a number of enzymes that generate biologically active oxysterols (Fig. 1B). The most important enzymatically generated side-chain oxysterols are 24- hydroxycholesterol (24HCh) and 27-hydroxycholesterol (27HCh), which are formed from cholesterol by the cytochrome P450’s CYP46A1 and CYP27A1, respectively (5). These oxysterols are found more abundantly then other oxysterols in the blood and originate mainly in the brain (5). 27HCh is also found abundantly in atheromatous lesions (38). CYP27A1 may also be involved in cholesterol elimination in macrophages (39, 40). The majority of the enzymatic B-ring hydroxylations of cholesterol take place in the liver by CYP7A1 and CYP3A4, which can oxidize cholesterol to form 7α-hydroxycholesterol (7αHCh) and 4β-hydroxycholesterol, respectively (32, 39). These enzymes oxidize cholesterol for the purpose of synthesizing bile acids and cholesterol elimination. Neither CYP7A1 nor CYP3A4 have been previously reported in the retina. Another enzyme of interest is the cholesterol 25- hydroxylase (41), but its expression has also has not been previously reported in the retina. There are two other 7α-hydroxylases that may also be of interest, although they do not directly hydroxylate cholesterol or 7KCh. These are CYP7B1 and CYP39A1. CYP7B1 preferentially hydroxylates 25- and 27HCh (42), whereas CYP39A1 prefers 24HCh (43). CYP7B1 is broadly expressed in many tissues and has high expression in tissues of neurological origin, but it has not been previously reported in the retina. CYP39A1 is mostly expressed in the liver but has also been reported in the bovine ciliary nonpigmented epithelium of the eye (44). The expression of CYP39A1 in the neural retina has not been previously reported. The potential role of these enzymes in metabolizing 7KCh will be discussed below.
Levels of oxysterols in the retina.
To our knowledge, there are no published studies reporting comprehensive oxysterol levels in the retina. 27HCh has been found in high levels in macrophage foam cells and atherosclerotic plaques (39, 40), but its presence in the retina has not been previously reported. 24HCh was reported in the retina in one study (45), and this will be discussed in more detail below. Our laboratory measured significant levels of 7KCh in monkey retina (9) and rat retina (10) but did not report the levels of the side-chain hydroxylated oxysterols, because they were present only in trace amounts. In monkeys, the levels of 7KCh are between 0.5 and 1.5 pmol/nmol of cholesterol (9). In albino rats (not photodamaged), the levels are between 1 and 4 pmol/nmol of cholesterol (10). The levels of other oxysterols were below our limit of detection or <100 fmol/nmol of cholesterol.
Formation of 7KCh in the retina.
The singlet oxygen- mediated mechanism seemed to be a logical candidate for the formation of 7KCh in the retina. However, careful analyses of retina extracts in monkeys (9) and photodamaged rat retinas (10) found no intermediates to link to this mechanism. The retina seems to lack a suitable photosensitizer to generate 7KCh by this mechanism. Molecules like A2E (46) and all-trans retinoic acid (ATRA) (47), although relatively abundant in the retina, are extremely poor photosensitizers (46–48). In our hands, A2E and ATRA were able to photooxidize cholesterol to 7KCh at <1% the efficiency of hematoporphyrin. A recent study demonstrated that in photodamaged albino rats, 7KCh levels were greatly increased in areas of the retina enriched in mitochondria (10). The unequivocal identification of the oxidized intermediates by LCMS (10) indicates they were produced via a free radical-mediated mechanism (Fig. 1A). This means that iron, which is abundant in the neural retina, was likely involved in catalyzing the oxidation. The source of the iron was not determined, but ferritin and/or cytochrome c are suspected. Ferritin (heavy and light chains) localizes to the mitochondria and is abundant in the retina (49, 50). But most importantly, ferritin is known to release its iron when exposed to bright visible light (51). Ferritin in the presence of light can induce lipid peroxidation in purified photoreceptor outer segments (52) and in LDL (10). The mitochondrial protein cytochrome c is also a potential candidate as a source of iron. Recently, it has been demonstrated that oxidation by hydrogen peroxide can cause cytochrome c to release iron and oxidize cardiolipin via a free radical-mediated mechanism (53). Therefore, the data suggest that light was involved in mediating the oxidation of cholesterol to 7KCh in the retina but not by a singlet oxygen-mediated mechanism. The immunolocalization of 7KCh in the photodamaged rat retina to cell layers rich in mitochondria (RPE, photoreceptor inner segments, and outer plexiform layer and ganglion cells) also supports an iron-catalyzed free radical oxidation.
Cholesterol uptake and reverse cholesterol transport pathway in the retina
How is cholesterol transported in the retina? How does the retina maintain cholesterol homeostasis? These two questions are extremely important to the understanding of 7KCh formation and metabolism. The specific topic of cholesterol transport will be discussed in greater detail in other reviews in this series. Therefore, to avoid redundancy, we will limit our discussion mainly to two studies that identified the reverse cholesterol pathway molecules in the retina (15, 17) and a lipid transport hypothesis based on this and other supporting work.
Cholesterol uptake in the retina.
Considerable evidence suggests that LDL uptake seems to be the main source of lipids in the retina (14, 15, 17, 54–60). Initially, it was observed that lipoproteins were involved in mediating the uptake of docosahexaenoic acid and omega-3 fatty acids (54–56). Later, during photodynamic therapy studies, lipoproteins were found to deliver benzoporphyrin to the RPE (57, 58). In RPE cells, Hayes et al. (59) originally discovered LDL and scavenger receptor activities. Others also later found LDL was taken up by the LDL receptor (14, 15, 60). One of these studies used a blue fluorescent cholesterol analog to visualize and quantify the cholesterol uptake and turnover in the retina (15). Cholestatrienol [cholesta-5, 7, 9(11)-trien-3β-ol] (61) was incorporated into human LDL and injected in the circulation of rats (15). By following the human apoB using a specific antibody and by imaging the retina to localize the cholestatrienol, the uptake of the LDL was visualized and quantified. These results suggested that blood-borne cholesterol turns over in the retina in 6–7 days (15). This is considerably faster than local synthesis, which was reported to be around 17 days (16).
Circulating cholesterol is taken up by the RPE in lipoprotein particles containing a conglomerate of other lipids. The LDL receptor does not seem to be regulated in primary or in RPE-derived cells (14, 60), and this suggests constant lipid uptake by this tissue. This rapid lipid turnover may be an adaptation to photooxidation, because lipids are the most light sensitive of all of the macromolecule classes. Additional evidence for the importance of lipoprotein uptake by the retina has come indirectly from the knockout mice for the LDL (62) and VLDL receptors (63), which both exhibit retinal abnormalities. The LDL receptor knockout mice deposit large amounts of lipids in Bruch's membrane, and this seems to lead to photoreceptor loss and retinal degeneration (62). The VLDL receptor knockouts exhibit extensive neovascularization in the neural retina (63).
Cholesterol transport in the retina.
The uptake of circulating LDL via LDL receptors in the RPE suggests that additional transport mechanisms must be active to maintain lipid homeostasis. These observations lead to a second study that measured and localized the reverse cholesterol pathway proteins in the retina (17). In this study, key proteins in this pathway were localized in monkey retina, leading to a hypothesis for the retinal lipid transport pathway (17). The location of the HDL receptors SRBI and SRBII in the photoreceptors coupled with the presence of apoB, apoA1, LCAT, and CETP in the interphotoreceptor matrix (IPM) suggests a lipoprotein-based outer retina lipid transport or lipid exchange. Assuming that these proteins will generally perform their known function, their presence in those specific locations in the retina provide an insight into the overall lipid transport mechanism.
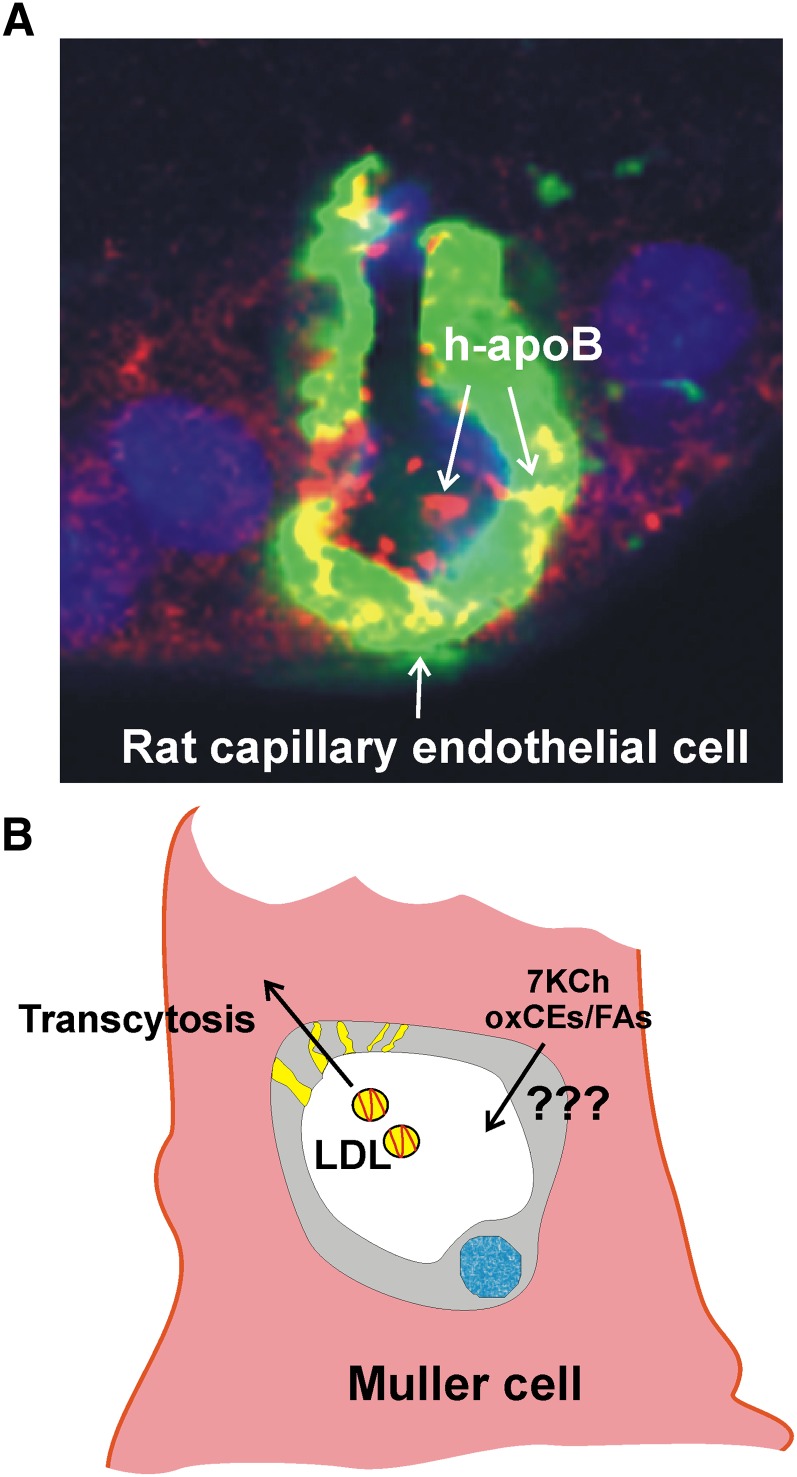
Another important aspect of the retinal lipid transport that has not been well studied is the role of the Müller cells. Unlike the choriocapillary endothelial cells, which are fenestrated and allow direct access of blood components to the RPE, the inner retina capillaries are tightly junctioned and surrounded by Müller cells (18). We know from studies with rhodamine- (14) and cholestatrienol- labeled LDL (15) that the Müller cells participate in the LDL uptake. However, the relative uptake and the rate of lipid exchange from RPE and Müller cells to the rest of the retina are unknown. These studies in rats (14, 15) suggest the presence of some form of transcytosis mechanism through the retinal capillary endothelium cells (Fig. 2). This transcytosis mechanism has been described for vascular endothelium cells (64) but are unknown in the retina.
Fig. 2.
Müller cell LDL uptake. A: Image of a rat capillary endothelial cell surrounded by a Müller cell. The rat was injected with human LDL (14, 15). The capillary endothelial cell was stained with Alexa488-labeled isolectin IB4, and the human apoB was detected with a human-specific anti-apoB antibody and developed with a Cy5-labeled anti-human secondary antibody (red). The nuclei were stained with DAPI (blue). This is an unpublished image from previously published studies (14, 15). B: Cartoon depicting the suspected role of the Müller cell in the LDL uptake and excretion 7KCh and oxCEs. This uptake mechanism is speculative.
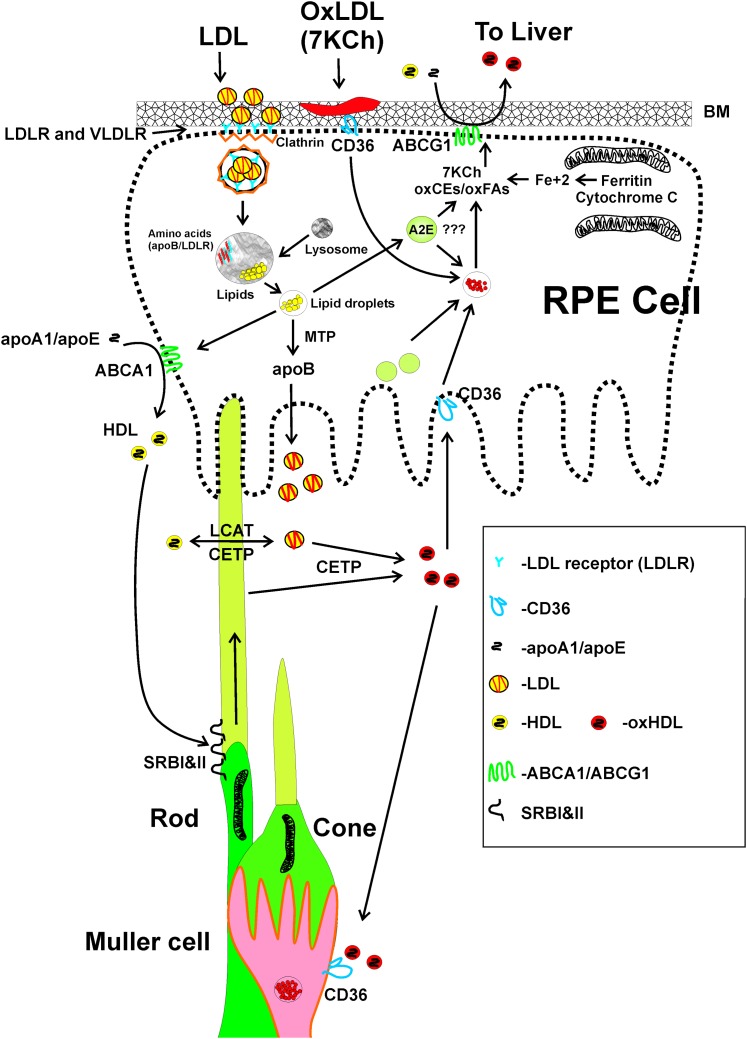
Our overall hypothesis proposes that lipids enter the retina from two locations and two different mechanisms (Fig. 3). In the back of the eye, LDL particles are taken up via LDL receptors located in the basal layer of the RPE. In the neural retina, LDL and VLDL are taken up via VLDL receptors in a transcytosis mechanism (64) through the vascular endothelial cells (Fig. 2). The RPE expresses ABCA1 predominantly on its apical side and secretes apoA1, apoB, LCAT, and CETP into the IPM. HDL-like lipid particles are formed via the ABCA1 transporter and released into the IPM. The LDL-like particles made by the RPE (apoB is also detected in the IPM) transfer lipids to the HDL-like particles with the help of LCAT and CETP. The fresh lipids are then delivered to the photoreceptors and other cells by selective uptake via the SRBI and SRBII scavenger receptors. The LCAT and CETP may also serve to move oxidized lipids out of the membranes into HDL-like lipoprotein particles, which return to the RPE and Müller cells via CD36 receptors and other scavenger receptors. The oxidized lipids are then returned back to circulation via ABCA1/G1 and/or other transporters. This is speculative on our part, because there is no published data demonstrating how oxidized lipids exit the retina.
Fig. 3.
Hypothesis for lipid transport and 7KCh metabolism in the retina. Cholesterol and other fresh unoxidized lipids enter the retina via LDLR and VLDLR in the RPE and capillary endothelial cells/Müller. In the RPE, the clathrin-coated vesicles are formed and fused with lysosomes. In the capillary endothelial cells/Müller, the lipids enter by transcytosis (Fig. 2). The LDLR and apoB are degraded and the lipids are separated into droplets. The RPE then makes fresh LDL particles with endogenous apoB. HDL-like particles may also be formed via ABCA1 (apical side). The lipoprotein particles are then released into the IPM. The HDL-like particles deliver the fresh lipids to the photoreceptors (and other retinal cells) via the SRBI and SRBII HDL receptors. LCAT and CETP in the IPM probably perform similar functions as in the blood, with LCAT forming CEs and CETP transferring them from LDL to HDL. We speculate that LCAT/CETP may also serve to sort oxidized lipids from outer segments and other membranes into HDL-like particles. The oxidized lipid-enriched HDL particles are then internalized by the RPE via CD36 receptors and the lipid reprocessed for excretion and/or exchange with blood lipoproteins via ABCG1 on the basal side of the RPE. The main source of 7KCh is most likely lipoprotein deposits in Bruch's membrane. 7KCh may also be formed at or near the mitochondria from Fe+2 released from ferritin and/or cytochrome c by light exposure. Although A2E is a poor photosensitizer, the large amount of it in the lipofuscin granules could theoretically generate 7KCh if they were to fuse with lipid droplets. Unlike cholesterol, some polyunsaturated fatty acids like docosahexaenoic acid can be photooxidized directly without the need for iron or a photosensitizer and could generate oxidized lipids in the photoreceptor outer segments. We speculate that oxidized lipids are carried by HDL-like particles back to the RPE for processing and excretion back into circulation via ABCG1 (and possibly other transporters) through Bruch's membrane. This is a modification from the cartoon published in Tseretsoodol et al. 2006 (17).
Metabolism of 7KCh
There is no published scientific evidence supporting a detoxification pathway for 7KCh in the retina. To our knowledge, the only cytochrome P450s reported in the retina that are capable of hydroxylating 7KCh are CYP27A1 (39, 65) and CYP46A1 (45). CYP27A1 is a mitochondrial protein that can be readily detected in the retina by immunoblot and has been localized by immunofluorescence (65). Deficiency of CYP27A1 is known to cause a rare autosomal recessive disease called Cerebrotendinous Xanthomatosis (66). This disease is characterized by cataracts as well as a series of neurological and systemic abnormalities (66).These patients also exhibit retinal abnormalities such as optic disc paleness and retinal senescence (67). CYP46A1 has been localized by immunofluorescence in bovine retina (45) and by immunohistochemistry in mouse retina (68). No immunoblot for CYP46A1 in the retina has been published. The immunohistochemistry in the bovine retina (45) was difficult to interpret, but the study in mice (68), using frozen sections, does demonstrate a clear localization of CYP46A1 in retinal ganglion cells as well as cells at the edge of the inner nuclear layer, possibly amacrine cells as well as the RPE. The role of CYP46A1 is unclear, because its levels in the retina are very low. Using quantitative RT-PCR (qRT-PCR), we have determined that the copy number for CYP46A1 mRNA is approximately 6-fold less than CYP27A1 in human and monkey retina RNA extracts. CYP46A1 mRNA is expressed 10-fold less in the retina than in the brain of monkeys (unpublished observations). We also have found discrepancies between the levels of 24HCh reported in bovine retina (45), 3.4 µg/mg cholesterol (3.25 pmol/nmol Ch), and the levels we have observed during the studies in monkey and rat retina (9, 10). Provided care is taken to avoid blood contamination, the levels of 24HCh and 27HCh we have detected are <0.1 pmol/nmol of cholesterol. We cannot explain the reason for this discrepancy, but clearly additional studies are warranted.
Although CYP27A1 and CYP46A1 can readily hydroxylate 7KCh in vitro (69), as mentioned above, the metabolites formed by these enzymes, 24-hydroxy-7-ketocholsterol and 27-hydroxy-7-ketocholesterol, were not detected in monkey retina (9) or in the photodamaged rat retina (10). In the photodamaged rat retina, the levels of 7KCh and 7HCh rose to 6- and 50-fold above controls, respectively. These levels dropped significantly to 2- and 4-fold in 48 h after the cessation of the light treatment (10). However, no side-chain hydroxylated metabolites of 7KCh and 7HCh were detected, although a similar (but not as soluble) triol (5, 6-dihydroxycholesterol) derived from 5, 6-epoxCh was clearly detected (10). The metabolism of 7KCh by CYP27A1 has been previously demonstrated in HepG2 cultured cells (70), and 27-hydroxy-7-ketocholesterol has been detected in very low levels in atheromatous plaques (71). The overall data suggest that metabolism of 7KCh by CYP27A1 (or CYP46A1) is likely a minor process in the retina. However, hydroxylated metabolites of 7KCh have a marked increase in solubility and this decreases their efficiency of extraction and detection. This may be further complicated if these or other cytochrome P450s can cause multiple hydroxylations that enhance efflux by diffusion and avoiding detection. This is another area that needs further investigation.
Another possible pathway for the metabolism of 7KCh is by reduction to 7βHCh by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) as previously suggested (72). This metabolic route, to our knowledge, has not been previously investigated in the retina. In addition, the expression of 11β-HSD1 has also not been previously reported in the retina. We have detected very low levels of 11β-HSD1 expression in human neural retina by qRT-PCR (unpublished observations).
There is one other enzyme that could potentially metabolize 7KCh in the retina, the above-mentioned cholesterol 25-hydroxylase (41). However, we are not aware of any reports demonstrating 7KCh as a substrate and it is detected in very low levels in the retina by qRT-PCR (unpublished observations).
CYP27A1 and CYP46A1 are known to play an important role in maintaining cholesterol homeostasis in the brain (73, 74). However, unlike the retina (15, 16), the brain has a very slow turnover of cholesterol (73, 74). Therefore, CYPs are probably not oxidizing cholesterol for the purpose of maintaining cholesterol homeostasis in the retina. They may play a minor supporting role in this regard, but their main function may be to generate small amounts of side-chain oxysterols for the purpose of gene regulation (40). Liver X receptors (LXRs) are known to be regulated by oxysterols (75, 76) and are abundantly expressed in the retina (unpublished observations). LXRs are also known to regulate key transporters such as ABCA1 and ABCG1 (77–79). Other potential targets for the side-chain oxysterols are the oxysterol binding proteins (OSBPs), which are also abundantly expressed in the retina (80, 81) and are known to mediate numerous and complex cellular responses (4, 10, 82). These OSBPs likely play an important role in mediating the pharmacological responses of oxysterols, but little is known about their function in the retina.
Still another potential mechanism for the metabolism of 7KCh is its removal from the retina into circulation by the RPE and Müller cells via the ATP-binding cassette transporters such as ABCA1 and ABCG1 (77–79) or by some other unknown mechanism. The HDL-mediated lipid efflux from the RPE has been previously reported (83). These transporters have been shown to facilitate the exchange and/or excretion of 7KCh and other oxidized lipids to circulating lipoproteins in macrophages (77–79). It is known that once 7KCh is in circulation, it will be quickly metabolized and cleared by the liver as bile acids (84, 85). Thus, there is no significant toxicity associated with ingested or intravenously delivered 7KCh (84, 85) in animal models. Thus, this efflux process may be extremely important to the retina, because it may help avoid the inflammatory and/or cytotoxicity responses associated with 7KCh accumulation. This lipid efflux mechanism and the potential transporters involved need further investigation.
Sulfotransferases and sulfation of 7KCh.
Another potential detoxification pathway that has been proposed for 7KCh is sulfation (7, 86). One study demonstrated that transfected 293T cells overexpressing SULT2B1b could form 7-ketocholesterol-3-sulfate (7KChS) when incubated with 7KCh (86). Cells overexpressing SULT2B1b showed a slight resistance to 7KCh-medited toxicity (86). However, the scientific evidence to support this pathway in the retina is essentially nonexistent. There is no published evidence to demonstrate expression of SULT2B1b in retina or RPE cells. Our laboratory has failed to detect the formation of 7KChS in cultured RPE cells treated with 7KCh or during the oxysterol analyses in monkey (9) and photodamaged rat retina (10). Moreover, qRT-PCR analyses in monkey and human retina RNA determined that the copy number for SULT2B1b in neural retina is essentially nil (unpublished observations). In addition, to our knowledge, there are no published reports measuring cholesterol sulfate or 7KChS in the retina. However, SULT2B1b is one of the few enzymes known to detoxify 7KCh (86) in vitro. Hence, it may be interesting to investigate the potential of this enzyme to detoxify 7KCh in vivo, especially in tissues like the skin, where it is highly expressed (87). The skin is also susceptible to photooxidation and this could provide information potentially useful to the retina.
Pharmacological properties of 7KCh
There are two major categories of pharmacological properties generally attributed to 7KCh: inflammation and cell death (apoptosis and/or necrosis) (1–7). These two properties seem to be dose related and mechanistically connected. The signaling molecules involved in mediating these processes are not fully understood, and most of what is known has been done in cultured macrophages and vascular endothelial cells (Fig. 4). There are at least three known types of proteins that have been reported to directly bind 7KCh: the caveolins [Cav-1 (88)], the OSBPs [OSBP1 (89) and OSBP2 (81, 90)], and the LXRs (75). Although 7KCh is generally a poor ligand for LXRs (75), we have found that it can significantly induce the expression of ABCA1 and ABCG1 (known to be regulated by LXRs) in cultured RPE cells (unpublished observations). These 7KCh-binding proteins may play unique roles in mediating the pharmacological properties of 7KCh.
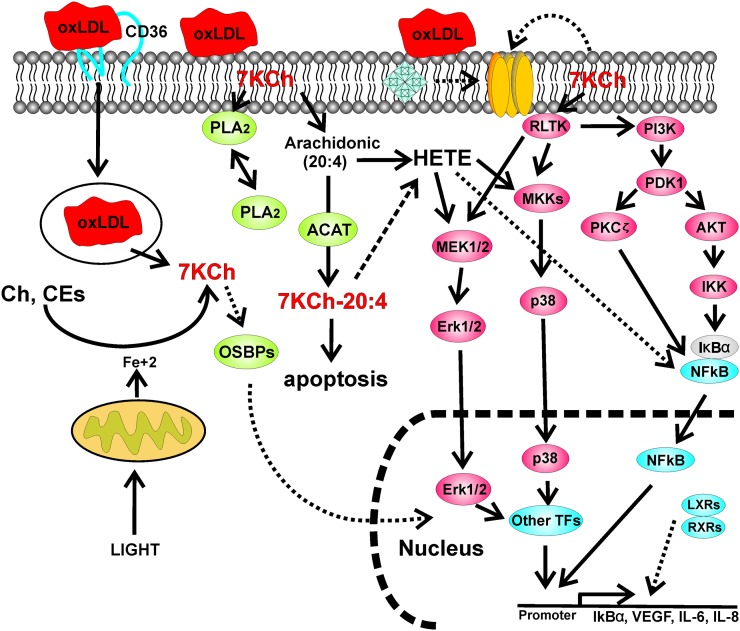
Fig. 4.
Signaling pathways described in macrophages and vascular endothelial cells (mainly). This cartoon depicts some of the major pathways described in the published literature and described in the text. Most of the published literature suggests an NOX4/ROS-mediated activation of NFκB.
Inflammation.
Among the oxidized lipids present in oxLDL, 7KCh seems to be the most potent in initiating inflammatory and cytotoxic responses (9, 21, 91, 92). The inflammatory role of 7KCh has been studied in a variety of cell types, such as monocytes/macrophages (92, 93), astrocytes (94), vascular endothelial cells (95, 96), vascular smooth muscle cells (VSMCs) (97, 98), fibroblasts (99), and retinal cells (8, 9, 100, 101) from different mammals. In culture, 7KCh is able to induce the transcription and release of proinflammatory cytokines such as VEGF (9, 97, 102), interleukin (IL)-1 (95), IL-6 (103), and IL-8 (8, 9, 104–106) in different cell types. The mechanisms for this induction appear to be shared between monocytes/macrophages (102, 105–107), aortic and VSMCs (98, 108), and endothelial cells (95–97). In these cells, 7KCh induces reactive oxygen species (ROS) by a mechanism involving the upregulation of NADPH oxidase 4 (NOX-4) (108). The ROS are then suspected of inducing the proinflammatory pathways by activating nuclear factor κB (NFκB) and the phosphorylation of kinases such as p38 mitogen-activated protein kinase (MAPK), extracellular signal- regulated kinase (ERK), and c-Jun N-terminal protein kinase (109, 110), which lead to the increase in transcription of proinflammatory molecules. The role of ROS in these cells has been supported by studies that used antioxidants to abolish their action (8, 109, 110). Another mechanism described for the proinflammatory role of 7KCh in VSMCs is the translocation of the HuR protein from the nucleus to the cytoplasm (103). HuR is an RNA-binding protein that has been shown to increase the stability of cytokines and inflammation-related mRNAs and thus promotes inflammation (103, 111, 112). Calcium is also suspected of mediating the 7KCh inflammation (110). oxLDL and 7KCh have been shown to cause the translocation of the calcium channel TRPC1 to the caveolin lipid rafts, increasing the intracellular Ca2+ concentration (113). This in turn activates MAP kinases and cytokine formation and release (113). Naito et al. (110), using U937 monocytes, reported that the new calcium channel blocker Azelnidipine was able to reduce the inflammatory response elicited by 7KCh. The observed effects were not due to the blockage of the calcium channel, because Nifedipine, another calcium channel blocker, was ineffective, but to the scavenging properties of Azelnidipine, which were able to reduce the activation of NOX-4. In addition, they reported that NFκB translocates to the nucleus in response to ROS induction and independently of Ca2+ activation. Activation of the Jak/STAT pathway by 7KCh has also been reported in mouse fibroblasts and astrocytes (94, 99). This pathway plays a critical role in the signaling of a wide array of cytokines.
In RPE-derived cells, studies investigating 7KCh-mediated inflammation are few (8, 9, 100, 101) but suggest a different pathway. In cultured RPE cells, as in other cell types, 7KCh is able to induce the formation and release of VEGF and IL-8 (8, 9). However, the pathway by which these cytokines are induced does not seem to involve ROS (8, 9, 100, 101). Porcine primary RPE cells treated with 7KCh demonstrated an increase in ROS as well as a significant IL-8 induction (8). These authors concluded that the ROS was not involved in the NFκB activation, because treatment with vitamin E (an ROS scavenger) did not suppress the production of IL-8. These authors also speculated that the oxysterol-binding LXRs may be involved in the cytokine induction (8). Our laboratory recently reported that the 7KCh-mediated VEGF induction in ARPE19 cells was independent of HIF-1α but partially mediated by LXRs (9). Cholesterol sulfate and 7KChS, which are LXR antagonists (114), were found to attenuate but not eliminate the 7KCh-mediated VEGF induction (9). In ARPE19 cells, the 7KCh-mediated IL-8 induction involved NFκB but was independent of ROS production (9). The lack of involvement of HIF-1α in the VEGF induction (9) also indicates that ROS is not involved in the inflammatory process in RPE-derived cells. ROS formation is known to be required to prevent HIF-1α degradation and sustain the VEGF induction via the HIF pathway (115, 116). 7KCh is capable of directly binding to LXRs (75), which are known to modulate VEGF expression in macrophages (76, 94), although, as mentioned above, 7KCh is not a potent LXR agonist.
A more recent study has shown that 7KCh induces the cytokines VEGF, IL-6, and IL-8 via three different kinase signaling pathways, AKT-PKCζ- NFκB, p38MAPK, and ERK (117). By using a series of specific kinase inhibitors, it was determined that 7KCh inflammation is mediated by NFκB primarily via Akt and PKCζ. The MAPK/ERK pathways do not seem to directly participate in the activation of NFκB. Instead, they seem to activate other downstream transcription factors that work in combination with NFκB to enhance the cytokine induction. Only the complete inhibition of the IκB kinase complex was able to ablate the 7KCh-mediated cytokine induction (117). This study used relatively low concentrations of 7KCh (10–20 µM) to induce inflammation. At this relatively low concentration, no ROS was detected in any of the cell lines tested. The upstream effectors that triggered these inflammatory pathways were not identified. However, it seems unlikely that 7KCh could activate such a broad range of cell surface receptors. Thus, it seems that 7KCh is acting as a nonspecific activator perhaps by forming membrane-disrupting microcrystals as previously demonstrated (118, 119). These types of complex interactions between oxysterols, protein, and membranes have been previously proposed (120) but not fully investigated.
Cell death.
The cell death pathway induced by 7KCh is also complex and not fully understood. Dose seems to be an important factor in the inflammation versus cell death pathways. The doses required for an inflammatory response are in the low micromolar range (5–15 µM) (9, 117), and the doses for cell toxicity are relatively high (25–100 µM) (8, 9, 95–101, 106, 108, 110). The mechanisms by which 7KCh induces cell death may be essentially the same as those that induce inflammation, except more acute and prolonged. Low concentrations of 7KCh induce proinflammatory responses that are usually associated with pro-survival mechanisms that protect the cell. Only when the concentrations of 7KCh are high enough to overwhelm the cell do the death pathways seem to supersede the proinflammatory pathways. This may also explain the ROS formation in some studies but not others. It may be that ROS forms when the 7KCh levels are high enough to cause extensive membrane damage. However, there are no published studies that have specifically addressed this question. As mentioned above, in monocytes and macrophages, 7KCh induces the expression of NOX-4, which in turn increases the intracellular levels of ROS. ROS formation downregulates antiapoptotic genes such as Bcl-2, Bcl-xL, and AKT (121). Simultaneously, ROS upregulates proapoptotic genes like p53, p21, and Bax (121). This has been reported to cause mitochondrial damage and the release of apoptosis-inducing factor (122) and caspases (96, 123), promoting apoptosis. In mouse fibroblasts, the induction of p21 through STAT has also been shown to increase the activation of caspases after treatment with 7KCh (99). In VSMCs, 7KCh causes the release of caspases by increasing the intracellular calcium levels after causing the translocation of the TRPC1 calcium channel to the plasma membrane (113).
Another apoptosis and inflammatory mechanism for 7KCh that has been studied in macrophages is the translocation and activation of phospholipase A2 (PLA2) and arachidonic acid release (124–128). oxLDL and 7KCh have been shown to induce the translocation of PLA2 to the plasma membrane and to cause the formation of cholesteryl- and oxysteryl-esters (124–128). Moreover, in macrophages, 7KCh has been found to not only activate PLA2 but to also become sterified to arachidonic acid by the action of acyl-CoA:cholesterol acyltransferase (127). The 7KCh-arachidonic acid ester is then suspected of being highly toxic and initiating apoptosis via some undetermined pathway (127). Arachidonic acid is known to lead to the formation of 20-hydroxyeicosatetraenoic acid, which can activate NFκB and cytokine release via p38MAPK and ERK pathways (124, 128). This pathway may be the key to understanding the inflammatory properties of 7KCh and needs further investigation.
In RPE-derived culture cells, the mode of cell death does not seem to involve caspase activation. In ARPE19 cells, caspase inhibitors were unable to prevent cell death from 7KCh (123). The mode of cell death seems to resemble that of ECV304 cells (129) in which 7KCh did not activate caspases or cause cytochrome c release or DNA laddering. DNA laddering, which is characteristic of caspase-3 activation, was not observed in ARPE19 cells exposed to menadione, a quinone that generates intracellular ROS (122). By contrast, in the same study, DNA laddering was observed in menadione-treated U937 leukemic monocytes (122). The addition of exogenous cytochrome c and ATP to a cell-free system also resulted in cleavage of caspase-3 and -9 in extracts of U937 but not ARPE19 cells (122). Similarly, when ARPE19 cells were subjected to 7βHCh, an oxysterol structurally related to 7KCh, the cells displayed a caspase-3-independent mode of cell death (130), which resembled the damage caused by endoplasmic reticulum (ER) stressors (131). In ARPE19 cells, 7KCh treatment causes the activation of a number of compensatory cell survival pathway genes, such as Akt, ERK, NFκB, and p38MAPK (117). These are ER stress markers.
ER stress has been identified as a caspase-dependent and -independent pathway for cell death (23, 24). ER stress has been demonstrated to develop an integrated stress response referred to as unfolded protein response, or UPR. This is characterized by disturbances in the redox environment of the ER and the formation of misfolded proteins. This UPR is considered to be protective but can trigger cell death if overstimulated (24, 131). Thus, the UPR may increase the expression of the transcription factor C/EBP homologous protein. This factor is protective in the short term, but with prolonged expression it promotes apoptosis through the increase of ER calcium release. This in turn increases ROS and STAT activation and decreases the compensatory Akt-mediated cell-survival signaling (24).
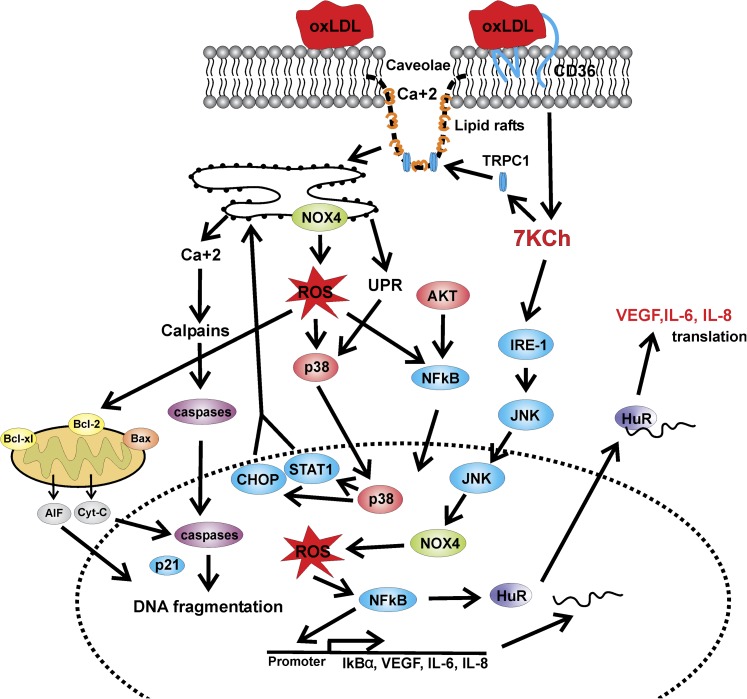
A cartoon summary of the pathways that have been implicated in 7KCh inflammation and toxicity in macrophages and monocytes is depicted in Fig. 4. A cartoon summary of the pathways reported mainly in ARPE19 cells is shown in Fig. 5. Please note that the PLA2 pathway depicted in Fig. 5 has been demonstrated in macrophages only.
Fig. 5.
Signaling pathways described in ARPE19 cells. This cartoon depicts some of the pathways described in ARPE19 cells (9, 117) and photodamaged rat retina (10). The PLA2-acyl-CoA:cholesterol acyltransferase-20-hydroxyeicosatetraenoic acid activation by oxLDL/7KCh has been described only in macrophages in relatively few articles (124–128). However, this pathway may help to explain the activation of the various NFκB-related pathways observed in ARPE19 and other cell types (117).
The main caveat regarding the in vitro work discussed above are the high concentrations of 7KCh needed to initiate inflammation and cytotoxicity in these cultured cells (20–150 µM). In our opinion, there are two main reasons why such high concentrations are warranted: one is technical, and the other physiological in nature.
The technical reasons why high concentrations of 7KCh are needed in these cultured systems are the inefficient methods used to deliver the 7KCh. Most of the in vitro published work used 7KCh dissolved in ethanol or dimethyl sulfoxide. This is a very inefficient method of delivery, because most of the 7KCh will precipitate once added to the culture media. The 7KCh will then adhere to the plastic surfaces of the culture dishes or fall unevenly on the surface of the cells. This results in great variability between experiments. Delivery of 7KCh complexed with hydroxypropyl-β-cyclodextrin (HPBCD) resolves the precipitation problem but is also inefficient. 7KCh will not dissolve in HPBCD in a 1:1 molar ratio, and approximately 15–30 molar excess of HPBCD is needed to sustain 7KCh in solution after dilution in culture media. Unfortunately, HPBCD is also very efficient at removing 7KCh from the plasma membrane (132). This creates a mass-action competition between the membranes and the HPBCD for the 7KCh and increases the effective dose needed to observe the pharmacological effect. HPBCD does not enter the cell, so the 7KCh is delivered to only the plasma membrane. Another method for delivering 7KCh is by complexing it with LDL (26). This delivers the 7KCh internally and may provide different insights into 7KCh efflux, metabolism, and inflammation. The caveat is that this works well only with cells that readily uptake LDL and there is a lag-time while the LDL is processed and the 7KCh released. This increases variability in responses between different cell types.
The physiological reasons why micromolar amounts of 7KCh may be needed to initiate inflammation/cell death are not well understood. We speculate that one potential reason may be that 7KCh works nonspecifically (117). As mentioned above, 7KCh may be needed in concentrations sufficiently high to interact with cell surface receptors. For example, the MAPK/ERK pathways are activated by G-protein coupled receptors that respond to steroid hormones, which, like 7KCh, are keto-sterols. Another possibility is the formation of membrane-disrupting microcrystals (117–120) that in turn nonspecifically activate these receptors or the receptor-linked tyrosine kinases (Fig. 5). Yet another potential explanation is that the activation of the intracellular PLA2, which may be the rate-limiting step in the inflammation cascade, requires micromolar amounts of 7KCh (124–128). At this time, there are no published studies that conclusively demonstrate how 7KCh activates these inflammatory pathways.
This leads to another important question: can 7KCh reach micromolar levels in vivo? 7KCh is not a particularly poisonous substance when taken orally by rodents (84, 85). The toxicity of 7KCh seems to be due to its ability to form and reach very high concentrations in lipoprotein deposits. The levels of 7KCh in atherosclerotic plaques have been measured at >100 µM (38, 39, 71), although most of it is esterified to oxidized forms of different fatty acids, most likely 18:1 (oleic) and 18:2 (linoleic). There is also very convincing evidence demonstrating that Fe+2 and/or Cu+2 can rapidly and thoroughly oxidize the cholesterol and the CEs in LDL deposits to 7KCh, 7HCh, and others mentioned above (27, 28). The published scientific literature to support this is quite extensive and too numerous to cite. Thus, we can safely assume that in any given oxidized lipoprotein deposit, the concentrations of 7KCh or 7KCh esters could easily reach micromolar levels. The more important question is: can 7KCh in these oxLDL deposits reach the plasma membrane of cells at a high enough concentration to induce chronic inflammation? To our knowledge, there is no published literature that has specifically answered this question. However, a recent study by Riazy et al. (133) has shown that oxLDL particles can induce VEGF expression via the AKT-PKCζ-NFκB pathway without lipoprotein uptake. This suggests oxLDL deposits may induce inflammatory responses just by being in contact with the cellular membranes. Cells expressing scavenging receptors like CD36 (134), LOX-1 (135), and SRBI/II (136) as well as other related receptors could potentially enhance the 7KCh levels by internalizing these oxidized lipid deposits, although in this situation, metabolism and efflux may play a role in mitigating the 7KCh accumulation, thus requiring higher doses to induce inflammation. Ultimately, the challenge to investigators is to demonstrate that 7KCh causes these effects in vivo.
7KCh and AMD
Although there is no direct published evidence linking 7KCh to the pathogenesis of AMD, there is considerable circumstantial and indirect evidence that suggest a possible connection. First and foremost is the chronic age-related process by which 7KCh is formed and accumulates (2, 5, 7, 23–25, 38, 89, 91, 120). Most 7KCh is formed by a process of LDL deposition in the vascular system followed by iron and/or copper free-radical mediated oxidation of cholesterol and CEs in the LDL particles (27, 28). This process of deposition and oxidation exists in a form of equilibrium with the macrophages that clean up these deposits (24, 25). As humans age, this process either becomes less efficient and/or is slowly overwhelmed, resulting in more frequent and more highly oxidized deposits that contain increasing levels of 7KCh. The macrophages that encounter these highly oxidized deposits are unable to process them and become intoxicated by the high levels of 7KCh and other oxidized lipids. During this process, 7KCh may elicit inflammatory responses from the macrophages and the surrounding vascular endothelial and smooth muscle cells. The macrophages become foam cells and eventually die, forming large atheromatous plaques that eventually kill the underlying tissues and dislodge, causing cardiac arrest, cerebral infarcts, etc. (7, 24, 25). In the retina, the role of macrophages in this process is less clear, because they are rarely seen in Bruch's membrane where the lipids accumulate. However, the RPE, choroid, and possibly other scavenging cells such as astrocytes are likely to play a role in the inflammatory process.
One question that is often heard is: why is there no strong correlation (epidemiologically) between atherosclerosis and AMD? AMD and atherosclerosis are both complex diseases with multiple genetic and environmental risk factors. Although, in our opinion, both diseases are mechanistically related, the physiological and environmental differences between the retina and the cardiovascular system can statistically disconnect these two diseases in epidemiological studies. In other words, the lack of perfect synchrony in the medical symptoms between these two diseases should not be interpreted as being mechanistically unrelated. The systemic deposition-oxidation process mentioned above also seems to occur in the back of the eye with considerable deposition of oxidized lipoproteins in Bruch's membrane, choriocapillaris, and inner retinal capillaries (9, 14, 15). Lipids are known to accumulate in Bruch's membrane as a consequence of aging, especially around the macular region (137–141). This lipid deposition affects the permeability of Bruch's membrane (138) and if sufficiently oxidized may elicit inflammatory responses in the RPE and the choroidal vascular endothelium (9, 117). Lipoprotein deposits containing 7KCh can be observed even in young monkeys (9). The retina faces additional and unique challenges not experienced by the systemic cardiovascular system. This is basically the processing of visible light and the subsequent lipid oxidation caused by the light exposure and the release of iron (10, 52, 53). This can lead to the formation of 7KCh from sources other than lipoprotein deposits (10, 52, 53). The RPE plays a critical role in the uptake and processing of lipids in and out of the retina (14, 15, 17) (Fig. 3) while serving as the blood-retina barrier (12, 18). The formation of drusen deposits, which is considered an early sign of AMD, is likely caused by loss of function in the RPE. In our opinion, drusen deposits are initially formed by oxidized exit lipids and lipoproteins that are unable to pass through Bruch's membrane either because the membrane is fouled (139) and/or because they are improperly processed by the RPE. The stressed RPE may be unable to properly process the oxidized lipids and “blebs” the material on to Bruch's membrane, bypassing the ABC-type transporters and other eliminatory mechanisms. These drusen deposits are known to contain large quantities of lipoprotein- derived lipids (140, 141) and practically every ocular and circulatory protein (142). Once this material begins to accumulate, it may sequester additional circulating proteins and lipids that cross Bruch's membrane via the fenestrations in the choriocapillaris. These drusen deposits increase the risk of further toxicity and prevent proper exchange of metabolites between the blood and the RPE and/or choriocapillaris. The role of the choroid in this process is unclear. Choroidal cells seem to express similar lipid receptors to the RPE (15, 17). Thus, in addition to providing the structural support and circulatory access to the RPE, choroidal cells may also serve as metabolic assistants to the RPE. At some point, this age-related deposition-oxidation-inflammation process may reach a threshold, then, depending on the particular genetics of the individual (genetic variants) or environmental habits (smoking), the condition may progress from drusen formation to wet or dry AMD.
The scientific evidence for the association of lipids (143) and lipid-related genes (144) with AMD is becoming more convincing. Genetic studies are beginning to support the involvement of lipid-related genes with AMD. OSBP2, which binds 7KCh and is highly expressed in the retina (81), has been genetically linked to AMD (145). A recent genome-wide association study has linked hepatic lipase C, CETP, and ABCA1 to AMD (146). CETP and ABCA1 as well as numerous other reverse cholesterol pathway genes have been previously localized in the retina (17). In our opinion, minor defects in these genes will define the type, age of onset, and/or severity of the AMD in affected individuals.
CONCLUSIONS
AMD is foremost an aging disease that undoubtedly has very significant genetic and environmental risk factors (143–146). The age-related factor(s) in the pathogenesis of AMD will likely be the key to the treatment and/or prevention of this disease. 7KCh is one of potentially many age-related risk factors that may participate in initiating AMD, although conclusive scientific evidence of its involvement remains elusive.
We speculate that chronic inflammation induced by molecules like 7KCh is likely causing three types of effects on cells: loss of function, cell death, and transformation (neoplasia). Activation of NFκB-related inflammatory pathways causes the synthesis and release of cytokines. This pathway also shuts itself down by synthesizing IκB. Therefore, cells chronically exposed to 7KCh will be under a considerable stress cycle that will also stress adjacent cells. The stress cycle caused by the activation/deactivation of the NFκB pathways may result in the loss of certain cellular functions, which could eventually lead to apoptosis. This may not necessarily require an increase in the doses of 7KCh. In some cell types, 7KCh-mediated inflammation may result in the overproduction of certain types of oncogenes, which could result in permanent transformation if suppressor genes are not functioning perfectly. These are processes observed during the chronic nature of aging. Lipid oxidation should be considered as a major influence in the chronic inflammation associated with the aging process.
How may this affect the retina? 7KCh accumulation is likely to affect the choriocapillaris and RPE. Loss of function in the RPE and choriocapillaris could have devastating consequences on the photoreceptors. Induction of VEGF especially in combination with loss of RPE/choroid function may weaken Bruch's membrane (lack of maintenance) and lead to the formation of CNV and wet AMD. The role of the choroid and its interactions with the RPE are also not well-understood but are likely to be involved in any 7KCh-mediated inflammatory process. Whether 7KCh is involved in this process or is part of a more complex group of factors remains to be demonstrated.
The questions for future research regarding the retina are many, but four main areas should be considered regarding 7KCh: 7KCh metabolism/efflux, cholesterol transport and oxidation, 7KCh-interacting proteins, and 7KCh-mediated inflammation. The relationship between metabolism and efflux is very obscure, especially in vivo. The potential role of the cytochrome P450s, especially in protecting the mitochondria from high doses of 7KCh, is particularly interesting. The efflux mechanism and the roles of HDL and ATP-binding cassette transporters are mostly unknown in the retina. The ties between lipid uptake-efflux and the reverse cholesterol pathway need further investigation. Aside from its basic description (17), little is known about this pathway in the retina. However, the association of some of these genes by genome-wide association studies with AMD (146, 147) suggests this may be a very productive area of investigation. There is also remarkably little known about the proteins that bind and interact with 7KCh, especially in relation to the inflammatory pathways. These proteins may be the key to understanding how 7KCh triggers the above-mentioned inflammation. These areas of investigations should also be considered with the overall goal of moving from in vitro systems to in vivo models.
Footnotes
Abbreviations:
- ABCG1
- ATP binding cassette transporter G1
- AMD
- age-related macular degeneration
- apo
- apolipoprotein; CE, cholesteryl-ester
- CETP
- cholesterol-ester transfer protein
- CNV
- choroidal neovascularization
- 5, 6-epoxCh
- 5,6-epoxycholesterol
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- 7HCh
- 7-αβ-hydroxycholesterol
- 24HCh
- 24(S)-hydroxycholesterol
- 25HCh
- 25-hydroxycholesterol
- 27HCh
- 27 or 26-hydroxycholesterol
- HPBCD
- hydroxypropyl-β-cyclodextrin
- 11β-HSD1
- 11β-hydroxysteroid dehydrogenase type 1
- IPM
- interphotoreceptor matrix
- 7KCh
- 7-ketocholesterol
- 7KChS
- ketocholesterol-3-sulfate
- LCAT
- lethicin-cholesterol acyltransferase
- LDLR
- LDL receptor
- IL
- interleukin
- LXR
- liver X receptor
- MAPK
- mitogen-activated protein kinase
- NFκB
- nuclear factor κB
- NOX-4
- NADPH oxidase 4
- 7OOCh
- 7-αβ-hydroperoxides
- OSBP
- oxysterol binding protein
- oxLDL
- oxidized LDL
- PLA2
- phospholipase A2
- RPE
- retinal pigment epithelium
- ROS
- reactive oxygen species
- SRBI and II
- scavenger receptors I and II
- UPR
- unfolded protein response
- 5,6-triol
- 5,6-dihydroxycholesterol
- VEGF
- vascular endothelial growth factor
- VSMC
- vascular smooth muscle cell
REFERENCES
- 1.Lordan S., Mackrill J. J., O'Brien N. M. 2009. Oxysterols and mechanisms of apoptotic signaling: implications in the pathology of degenerative diseases. J. Nutr. Biochem. 20: 321–336. [DOI] [PubMed] [Google Scholar]
- 2.Poli G., Sottero B., Gargiulo S., Leonarduzzi G. 2009. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol. Aspects Med. 30: 180–189. [DOI] [PubMed] [Google Scholar]
- 3.Vejux A., Lizard G. 2009. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Aspects Med. 30: 153–170. [DOI] [PubMed] [Google Scholar]
- 4.Töröcsik D., Szanto A., Nagy L. 2009. Oxysterol signaling links cholesterol metabolism and inflammation via the liver X receptor in macrophages. Mol. Aspects Med. 30: 134–152. [DOI] [PubMed] [Google Scholar]
- 5.Björkhem I., Cedazo-Minguez A., Leoni V., Meaney S. 2009. Oxysterols and neurodegenerative diseases. Mol. Aspects Med. 30: 171–179. [DOI] [PubMed] [Google Scholar]
- 6.Olkkonen V. M., Hynynen R. 2009. Interactions of oxysterols with membranes and proteins. Mol. Aspects Med. 30: 123–133. [DOI] [PubMed] [Google Scholar]
- 7.Brown A. J., Jessup W. 2009. Oxysterols: sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. Mol. Aspects Med. 30: 111–122. [DOI] [PubMed] [Google Scholar]
- 8.Joffre C., Leclere L., Buteau B., Martine L., Cabaret S., Malvitte L., Acar N., Lizard G., Bron A., Creuzot-Garcher C., et al. 2007. Oxysterols induced inflammation and oxidation in primary porcine retinal pigment epithelial cells. Curr. Eye Res. 32: 271–280. [DOI] [PubMed] [Google Scholar]
- 9.Moreira E. F., Larrayoz I. M., Lee J. W., Rodriguez I. R. 2009. 7-Ketocholesterol is present in lipid deposits in the primate retina: potential implication in the induction of VEGF and CNV formation. Invest. Ophthalmol. Vis. Sci. 50: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez I. R., Fliesler S. J. 2009. Photodamage generates 7-keto- and 7-hydroxycholesterol in the rat retina via a free radical-mediated mechanism. Photochem. Photobiol. 85: 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D. Y., Cringle S. J. 2001. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 20: 175–208. [DOI] [PubMed] [Google Scholar]
- 12.Gordon W. C., Bazan N. G. 1997. Cellular organization and biochemistry of the retina. Chapman and Hall, London. [Google Scholar]
- 13.Fliesler S. J., Anderson R. E. 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22: 79–131. [DOI] [PubMed] [Google Scholar]
- 14.Gordiyenko N., Campos M., Lee J. W., Fariss R. N., Sztein J., Rodriguez I. R. 2004. RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 45: 2822–2829. [DOI] [PubMed] [Google Scholar]
- 15.Tserentsoodol N., Sztein J., Campos M., Gordiyenko N. V., Fariss R. N., Lee J. W., Fliesler S. J., Rodriguez I. R. 2006. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 12: 1306–1318. [PubMed] [Google Scholar]
- 16.Fliesler S. J., Florman R., Rapp L. M., Pittler S. J., Keller R. K. 1993. In vivo biosynthesis of cholesterol in the rat retina. FEBS Lett. 335: 234–238. [DOI] [PubMed] [Google Scholar]
- 17.Tserentsoodol N., Gordiyenko N. V., Pascual I., Lee J. W., Fliesler S. J., Rodriguez I. R. 2006. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol. Vis. 12: 1319–1333. [PubMed] [Google Scholar]
- 18.Oyster W. C. 1999. The Human Eye: Structure and Function. Sinauer Associates, Sunderland, MA: 595–699. [Google Scholar]
- 19.Berger J. W., Fine S. L., Maguire M. G. 1999. Age-Related Macular Degeneration. Mosby, Inc; St. Louis, MO. [Google Scholar]
- 20.Chakravarthy U., Evans J., Rosenfeld P. J. 2010. Age related macular degeneration. BMJ. 340: c981. [DOI] [PubMed] [Google Scholar]
- 21.Morohoshi K., Goodwin A. M., Ohbayashi M., Ono S. J. 2009. Autoimmunity in retinal degeneration: autoimmune retinopathy and age-related macular degeneration. J. Autoimmun. 33: 247–254. [DOI] [PubMed] [Google Scholar]
- 22.Ozkiris A. 2010. Anti-VEGF agents for age-related macular degeneration. Expert Opin. Ther. Pat. 20: 103–118. [DOI] [PubMed] [Google Scholar]
- 23.Hakamata H., Miyazaki A., Sakai M., Sakamoto Y. I., Horiuchi S. 1998. Cytotoxic effect of oxidized low density lipoprotein on macrophages. J. Atheroscler. Thromb. 5: 66–75. [DOI] [PubMed] [Google Scholar]
- 24.Seimon T., Tabas I. 2009. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 50(Suppl.): S382–S387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabas I. 2010. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez I. R., Alam S., Lee J. W. 2004. Cytotoxicity of oxidized low-density lipoprotein in cultured RPE cells is dependent on the formation of 7-ketocholesterol. Invest. Ophthalmol. Vis. Sci. 45: 2830–2837. [DOI] [PubMed] [Google Scholar]
- 27.Dzeletovic S., Babiker A., Lund E., Diczfalusy U. 1995. Time course of oxysterol formation during in vitro oxidation of low density lipoprotein. Chem. Phys. Lipids. 78: 119–128. [DOI] [PubMed] [Google Scholar]
- 28.Brown A. J., Dean R. T., Jessup W. 1996. Free and esterified oxysterol: formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J. Lipid Res. 37: 320–335. [PubMed] [Google Scholar]
- 29.Smith L. 1990. Mechanisms of formation of oxysterols: a general survey. Free Radicals, Lipoproteins, and Membrane Lipids. A. Crastes de Paulet, Douste-Blazy L., Paoletti L., Plenum Press, New York: 115–132. [Google Scholar]
- 30.Smith L. L. 1996. Review of progress in sterol oxidations: 1987–1995. Lipids. 31: 453–487. [DOI] [PubMed] [Google Scholar]
- 31.Wielkoszynski T., Gawron K., Strzelczyk J., Bodzek P., Zalewska-Ziob M., Trapp G., Srebniak M., Wiczkowski A. 2006. Cellular toxicity of oxycholesterols. Bioessays. 28: 387–398. [DOI] [PubMed] [Google Scholar]
- 32.Norlin M., Wikvall K. 2007. Enzymes in the conversion of cholesterol into bile acids. Curr. Mol. Med. 7: 199–218. [DOI] [PubMed] [Google Scholar]
- 33.Smith L. L., Teng J. I., Kulig M. J., Hill F. L. 1973. Sterol metabolism XXIII. Cholesterol oxidation by radiation-induced processes. J. Org. Chem. 38: 1763–1765. [DOI] [PubMed] [Google Scholar]
- 34.Girotti A. W. 2001. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem. Photobiol B. 63: 103–113. [DOI] [PubMed] [Google Scholar]
- 35.Korytowski W., Bachowski G. J., Girotti A. W. 1992. Photoperoxidation of cholesterol in homogeneous solution, isolated membranes, and cells: comparison of the 5 alpha- and 6 beta-hydroperoxides as indicators of singlet oxygen intermediacy. Photochem. Photobiol. 56: 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Thomas J. P., Hall R. D., Girotti A. W. 1987. Singlet oxygen intermediacy in the photodynamic action of membrane-bound hematoporphyrin derivative. Cancer Lett. 35: 295–302. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Y. W., Kang J. J., Shih Y. L., Lo Y. L., Wang C. F. 2005. Cholesterol-3-beta, 5-alpha, 6-beta-triol induced genotoxicity through reactive oxygen species formation. Food Chem. Toxicol. 43: 617–622. [DOI] [PubMed] [Google Scholar]
- 38.Van Reyk D. M., Brown A. J., Hult'en L. M., Dean R. T., Jessup W. 2006. Oxysterols in biological systems: sources, metabolism and pathophysiological relevance. Redox Rep. 11: 255–262. [DOI] [PubMed] [Google Scholar]
- 39.Björkhem I., Andersson O., Diczfalusy U., Sevastik B., Xiu R. J., Duan C., Lund E. 1994. Atherosclerosis and sterol 27- hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc. Natl. Acad. Sci. USA. 91: 8592–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luoma P. V. 2008. Cytochrome P450s and gene activation-from pharmacology to cholesterol elimination and regression of atherosclerosis. Eur. J. Pharmacol. 64: 841–850. [DOI] [PubMed] [Google Scholar]
- 41.Lund E. G., Kerr T. A., Sakai J., Li W. P., Russell D. W. 1998. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 273: 34316–34327. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z., Martin K. O., Javitt N. B., Chiang J. Y. 1999. Structure and functions of human oxysterol 7alpha-hydroxylase cDNAs and gene CYP7B1. J. Lipid Res. 40: 2195–2203. [PubMed] [Google Scholar]
- 43.Li-Hawkins J., Lund E. G., Bronsons A. D., Russell D. W. 2000. Expression cloning of an oxysterol 7α-hydroxylase selective for 24-hydroxycholesterol. J. Biol. Chem. 275: 16543–16549. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda H., Ueda M., Ikeda M., Kobayashi H., Honda Y. 2003. Oxysterol 7α-hydroxylase (CYP39A1) in the ciliary non-pigmented epithelium of bovine eye. Lab. Invest. 83: 349–355. [DOI] [PubMed] [Google Scholar]
- 45.Bretillon L., Diczfalusy U., Bjorkhem I., Maire M. A., Martine L., Joffre C., Acar N., Bron A., Creuzot-Garcher C. 2007. Cholesterol-24S-hydroxylase (CYP46A1) is specifically expressed in neurons of the neural retina. Curr. Eye Res. 32: 361–366. [DOI] [PubMed] [Google Scholar]
- 46.Pawlak A., Wrona M., Rozanowska M., Zareba M., Lamb L. E., Roberts J. E., Simon J. D., Sarna T. 2003. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem. Photobiol. 77: 253–258. [DOI] [PubMed] [Google Scholar]
- 47.Harper W. S., Gaillard E. R. 2001. Studies of all-trans-retinal as a photooxidizing agent. Photochem. Photobiol. 73: 71–76. [DOI] [PubMed] [Google Scholar]
- 48.Rozanowska M., Sarna T. 2005. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem. Photobiol. 81: 1305–1330. [DOI] [PubMed] [Google Scholar]
- 49.Yefimova M. G., Jeanny J. C., Guillonneau X., Keller N., Nguyen-Legros J., Sergeant C., Guillou F., Courtois Y. 2000. Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Invest. Ophthalmol. Vis. Sci. 41: 2343–2351. [PubMed] [Google Scholar]
- 50.Hahn P., Dentchev T., Qian Y., Rouault T., Harris Z. L., Dunaief J. L. 2004. Immunolocalization and regulation of iron handling proteins ferritin and ferroportin in the retina. Mol. Vis. 10: 598–607. [PubMed] [Google Scholar]
- 51.Ohishi K., Zhang X. M., Moriwaki S., Hiramitsu T., Matsugo S. 2005. Iron release analyses from ferritin by visible light irradiation. Free Radic. Res. 39: 875–882. [DOI] [PubMed] [Google Scholar]
- 52.Ohishi K., Zhang X. M., Moriwaki S., Hiramitsu T., Matsugo S. 2006. In the presence of ferritin, visible light induces lipid peroxidation of the porcine photoreceptor outer segment. Free Radic. Res. 40: 799–807. [DOI] [PubMed] [Google Scholar]
- 53.Yurkova I., Huster D., Arnhold J. 2009. Free radical fragmentation of cardiolipin by cytochrome c. Chem. Phys. Lipids. 158: 16–21. [DOI] [PubMed] [Google Scholar]
- 54.Bazan N. G., Gordon W. C., Rodriguez de Turco E. B. 1992. Docosahexaenoic acid uptake and metabolism in photoreceptors: retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Adv. Exp. Med. Biol. 318: 295–306. [DOI] [PubMed] [Google Scholar]
- 55.Wang N., Anderson R. E. 1993. Transport of 22:6n-3 in the plasma and uptake into retinal pigment epithelium and retina. Exp. Eye Res. 57: 225–233. [DOI] [PubMed] [Google Scholar]
- 56.Elner V. M. 2002. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans. Am. Ophthalmol. Soc. 100: 301–338. [PMC free article] [PubMed] [Google Scholar]
- 57.Miller J. W., Walsh A. W., Kramer M., Hasan T., Michaud N., Flotte T. J., Haimovici R., Gragoudas E. S. 1995. Photodynamic therapy of experimental choroidal neovascularization using lipoprotein-delivered benzoporphyrin. Arch. Ophthalmol. 113: 810–818. [DOI] [PubMed] [Google Scholar]
- 58.Haimovici R., Kramer M., Miller J. W., Hasan T., Flotte T. J., Schomacker K. T., Gragoudas E. S. 1997. Localization of lipoprotein-delivered benzoporphyrin derivative in the rabbit eye. Curr. Eye Res. 16: 83–90. [DOI] [PubMed] [Google Scholar]
- 59.Hayes K. C., Lindsey S., Stephan Z. F., Brecker D. 1989. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest. Ophthalmol. Vis. Sci. 30: 225–232. [PubMed] [Google Scholar]
- 60.Noske U. M., Schmidt-Erfurth U., Meyer C., Diddens H. 1998. Lipid metabolism in retinal pigment epithelium. Possible significance of lipoprotein receptors. Ophthalmologe. 95: 814–819. [DOI] [PubMed] [Google Scholar]
- 61.Scheidt H. A., Muller P., Herrmann A., Huster D. 2003. The potential of fluorescent and spin-labeled steroid analogs to mimic natural cholesterol. J. Biol. Chem. 278: 45563–45569. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt-Erfurth U., Rudolf M., Funk M., Hofmann-Rummelt C., Franz-Haas N. S., Aherrahrou Z., Schlötzer-Schrehardt U. 2008. Ultrastructural changes in a murine model of graded Bruch membrane lipoidal degeneration and corresponding VEGF164 detection. Invest. Ophthalmol. Vis. Sci. 49: 390–398. [DOI] [PubMed] [Google Scholar]
- 63.Hu W., Jiang A., Liang J., Meng H., Chang B., Gao H., Qiao X. 2008. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest. Ophthalmol. Vis. Sci. 49: 407–415. [DOI] [PubMed] [Google Scholar]
- 64.Sima A. V., Stancu C. S., Simionescu M. 2009. Vascular endothelium in atherosclerosis. Cell Tissue Res. 335: 191–203. [DOI] [PubMed] [Google Scholar]
- 65.Lee J. W., Fuda H., Javitt N. B., Strott C. A., Rodriguez I. R. 2006. Expression and localization of sterol 27-hydroxylase (CYP27A1) in monkey retina. Exp. Eye Res. 83: 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cali J. J., Hsieh C. L., Francke U., Russel D. W. 1991. Mutations in the bile acid biosynthetic enzyme sterol-27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 266: 7779–7783. [PMC free article] [PubMed] [Google Scholar]
- 67.Dotti M. T., Rufa A., Federico A. 2001. Cerebrotendinous xanthomatosis: heterogeneity of clinical phenotype with evidence of previously undescribed ophthalmological findings. J. Inherit. Metab. Dis. 24: 696–706. [DOI] [PubMed] [Google Scholar]
- 68.Ramirez D. M., Andersson S., Russell D. W. 2008. Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J. Comp. Neurol. 507: 1676–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pikuleva I. A. 2008. Cholesterol-metabolizing cytochromes P450: implications for cholesterol lowering. Expert Opin. Drug Metab. Toxicol. 4: 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lyons M. A., Brown A. J. 2001. Metabolism of an oxysterol, 7-ketocholesterol, by sterol 27-hydroxylase in HepG2 cells. Lipids. 36: 701–711. [DOI] [PubMed] [Google Scholar]
- 71.Brown A. J., Watts G. F., Burnett J. R., Dean R. T., Jessup W. 2000. Sterol 27-hydroxylase acts on 7-ketocholesterol in human atherosclerotic lesions and macrophages in culture. J. Biol. Chem. 275: 27627–27633. [DOI] [PubMed] [Google Scholar]
- 72.Jessup W., Brown A. J. 2005. Novel routes for metabolism of 7-ketocholesterol. Rejuvenation Res. 8: 9–12. [DOI] [PubMed] [Google Scholar]
- 73.Bjorkhem I. 2006. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 260: 493–508. [DOI] [PubMed] [Google Scholar]
- 74.Russell D. W., Halford R. W., Ramirez D. M., Shah R., Kotti T. 2009. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu. Rev. Biochem. 78: 1017–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janowski B. A., Grogan M. J., Jones S. A., Wisely G. B., Kliewer S. A., Corey E. J., Mangelsdorf D. J. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA. 96: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baranowski M. 2008. Biological role of liver X receptors. J. Physiol. Pharmacol. 59(Suppl 7): 31–55. [PubMed] [Google Scholar]
- 77.Terasaka N., Wang N., Yvan-Charvet L., Tall A. R. 2007. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA. 104: 15093–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tall A. R., Yvan-Charvet L., Terasaka N., Pagler T., Wang N. 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 7: 365–375. [DOI] [PubMed] [Google Scholar]
- 79.Tall A. R. 2008. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J. Intern. Med. 263: 256–273. [DOI] [PubMed] [Google Scholar]
- 80.Jaworski C. J., Moreira E., Li A., Lee R., Rodriguez I. R. 2001. A family of 12 human genes containing oxysterol-binding domains. Genomics. 78: 185–196. [DOI] [PubMed] [Google Scholar]
- 81.Moreira E. F., Jaworski C., Li A., Rodriguez I. R. 2001. Molecular and biochemical characterization of a novel oxysterol-binding protein (OSBP2) highly expressed in retina. J. Biol. Chem. 276: 18570–18578. [DOI] [PubMed] [Google Scholar]
- 82.Fairn G. D., McMaster C. R. 2008. Emerging roles of the oxysterol-binding protein family in metabolism, transport, and signaling. Cell. Mol. Life Sci. 65: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ishida B. Y., Duncan K. G., Bailey K. R., Kane J. P., Schwartz D. M. 2006. High density lipoprotein mediated lipid efflux from retinal pigment epithelial cells in culture. Br. J. Ophthalmol. 90: 616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lyons M. A., Samman S., Gatto L., Brown A. J. 1999. Rapid hepatic metabolism of 7-ketocholesterol in vivo: implications for dietary oxysterols. J. Lipid Res. 40: 1846–1857. [PubMed] [Google Scholar]
- 85.Lyons M. A., Brown A. J. 2001. 7-Ketocholesterol delivered to mice in chylomicron remnant-like particles is rapidly metabolised, excreted and does not accumulate in aorta. Biochim. Biophys. Acta. 1530: 209–218. [DOI] [PubMed] [Google Scholar]
- 86.Fuda H., Javitt N. B., Mitamura K., Ikegawa S., Strott C. A. 2007. Oxysterols are substrates for cholesterol sulfotransferase. J. Lipid Res. 48: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 87.Falany C. N., He D., Dumas N., Frost A. R., Falany J. L. 2006. Human cytosolic sulfotransferase 2B1: isoform expression, tissue specificity and subcellular localization. J. Steroid Biochem. Mol. Biol. 102: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sleer L. S., Brown A. J., Stanley K. K. 2001. Interaction of caveolin with 7-ketocholesterol. Atherosclerosis. 159: 49–55. [DOI] [PubMed] [Google Scholar]
- 89.Taylor F. R., Kandutsch A. A. 1985. Oxysterol binding protein. Chem. Phys. Lipids. 38: 187–194. [DOI] [PubMed] [Google Scholar]
- 90.Wang C., JeBailey L., Ridgway N. D. 2002. Oxysterol-binding-protein (OSBP)-related protein 4 binds 25-hydroxycholesterol and interacts with vimentin intermediate filaments. Biochem. J. 361: 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lyons M. A., Brown A. J. 1999. 7-Ketocholesterol. Int. J. Biochem. Cell Biol. 31: 369–375. [DOI] [PubMed] [Google Scholar]
- 92.Jessup W., Wilson P., Gaus K., Kritharides L. 2002. Oxidized lipoproteins and macrophages. Vascul. Pharmacol. 38: 239–248. [DOI] [PubMed] [Google Scholar]
- 93.Erridge C., Webb D. J., Spickett C. M. 2007. Toll-like receptor 4 signalling is neither sufficient nor required for oxidised phospholipid mediated induction of interleukin-8 expression. Atherosclerosis. 193: 77–85. [DOI] [PubMed] [Google Scholar]
- 94.Lee C. S., Joe E. H., Jou I. 2006. Oxysterols suppress inducible nitric oxide synthase expression in lipopolysaccharide-stimulated astrocytes through liver X receptor. Neuroreport. 17: 183–187. [DOI] [PubMed] [Google Scholar]
- 95.Lemaire S., Lizard G., Monier S., Miguet C., Gueldry S., Volot F., Gambert P., Neel D. 1998. Different patterns of IL-1beta secretion, adhesion molecule expression and apoptosis induction in human endothelial cells treated with 7alpha-, 7beta-hydroxycholesterol, or 7-ketocholesterol. FEBS Lett. 440: 434–439. [DOI] [PubMed] [Google Scholar]
- 96.Luthra S., Dong J., Gramajo A. L., Chwa M., Kim D. W., Neekhra A., Kuppermann B. D., Kenney M. C. 2008. 7-Ketocholesterol activates caspases-3/7, -8, and -12 in human microvascular endothelial cells in vitro. Microvasc. Res. 75: 343–350. [DOI] [PubMed] [Google Scholar]
- 97.Dulak J., Jozkowicz A., Dichtl W., Alber H., Schwarzacher S. P., Pachinger O., Weidinger F. 2001. Vascular endothelial growth factor synthesis in vascular smooth muscle cells is enhanced by 7-ketocholesterol and lysophosphatidylcholine independently of their effect on nitric oxide generation. Atherosclerosis. 159: 325–332. [DOI] [PubMed] [Google Scholar]
- 98.Lee H. S., Chang J. S., Baek J. A., Chung M. Y., Lee H. C., Rhim B. Y., Sok D. E., Rho M. C., Kim Y. K., Kim K. 2005. TNF-alpha activates death pathway in human aorta smooth muscle cell in the presence of 7-ketocholesterol. Biochem. Biophys. Res. Commun. 333: 1093–1099. [DOI] [PubMed] [Google Scholar]
- 99.Agrawal S., Agarwal M. L., Chatterjee-Kishore M., Stark G. R., Chisolm G. M. 2002. Stat1-dependent, p53-independent expression of p21(waf1) modulates oxysterol-induced apoptosis. Mol. Cell. Biol. 22: 1981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang J. Y., Liu L. Z. 1998. Toxicity of cholesterol oxides on cultured neuroretinal cells. Curr. Eye Res. 17: 95–103. [DOI] [PubMed] [Google Scholar]
- 101.Ong J. M., Aoki A. M., Seigel G. M., Sacerio I., Castellon R., Nesburn A. B., Kenney M. C. 2003. Oxysterol-induced toxicity in R28 and ARPE-19 cells. Neurochem. Res. 28: 883–891. [DOI] [PubMed] [Google Scholar]
- 102.Ramos M. A., Kuzuya M., Esaki T., Miura S., Satake S., Asai T., Kanda S., Hayashi T., Iguchi A. 1998. Induction of macrophage VEGF in response to oxidized LDL and VEGF accumulation in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 18: 1188–1196. [DOI] [PubMed] [Google Scholar]
- 103.Sung S. C., Kim K., Lee K. A., Choi K. H., Kim S. M., Son Y. H., Moon Y. S., Eo S. K., Rhim B. Y. 2009. 7-Ketocholesterol upregulates interleukin-6 via mechanisms that are distinct from those of tumor necrosis factor-alpha, in vascular smooth muscle cells. J. Vasc. Res. 46: 36–44. [DOI] [PubMed] [Google Scholar]
- 104.Terkeltaub R., Banka C. L., Solan J., Santoro D., Brand K., Curtiss L. K. 1994. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler. Thromb. 14: 47–53. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y., Hulten L. M., Wiklund O. 1997. Macrophages isolated from human atherosclerotic plaques produce IL-8, and oxysterols may have a regulatory function for IL-8 production. Arterioscler. Thromb. Vasc. Biol. 17: 317–323. [DOI] [PubMed] [Google Scholar]
- 106.Erridge C., Webb D. J., Spickett C. M. 2007. 25-Hydroxycholesterol, 7beta-hydroxycholesterol and 7-ketocholesterol upregulate interleukin-8 expression independently of Toll-like receptor 1, 2, 4 or 6 signalling in human macrophages. Free Radic. Res. 41: 260–266. [DOI] [PubMed] [Google Scholar]
- 107.Napolitano M., Bravo E. 2005. Lipid metabolism and TNF-alpha secretion in response to dietary sterols in human monocyte derived macrophages. Eur. J. Clin. Invest. 35: 482–490. [DOI] [PubMed] [Google Scholar]
- 108.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J. C., Pouzet C., Samadi M., Elbim C., et al. 2004. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 24: 10703–10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palozza P., Serini S., Verdecchia S., Ameruso M., Trombino S., Picci N., Monego G., Ranelletti F. O. 2007. Redox regulation of 7-ketocholesterol-induced apoptosis by beta-carotene in human macrophages. Free Radic. Biol. Med. 42: 1579–1590. [DOI] [PubMed] [Google Scholar]
- 110.Naito Y., Shimozawa M., Manabe H., Nakabe N., Katada K., Kokura S., Yoshida N., Ichikawa H., Kon T., Yoshikawa T. 2006. Azelnidipine, a new calcium channel blocker, inhibits endothelial inflammatory response by reducing intracellular levels of reactive oxygen species. Eur. J. Pharmacol. 546: 11–18. [DOI] [PubMed] [Google Scholar]
- 111.Choi G. S., Park H. J., Hur G. Y., Choi S. J., Shin S. Y., Ye Y. M., Park H. S. 2009. Vascular endothelial growth factor in allergen-induced nasal inflammation. Clin. Exp. Allergy. 39: 655–661. [DOI] [PubMed] [Google Scholar]
- 112.Krishnamurthy P., Rajasingh J., Lambers E., Qin G., Losordo D. W., Kishore R. 2009. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 104: e9–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ingueneau C., Huynh-Do U., Marcheix B., Athias A., Gambert P., Negre-Salvayre A., Salvayre R., Vindis C. 2009. TRPC1 is regulated by caveolin-1 and is involved in oxidized LDL-induced apoptosis of vascular smooth muscle cells. J. Cell. Mol. Med. 13: 1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song C., Hiipakka R. A., Liao S. 2001. Auto-oxidized cholesterol sulfates are antagonistic ligands of liver X receptors: implications for the development and treatment of atherosclerosis. Steroids. 66: 473–479. [DOI] [PubMed] [Google Scholar]
- 115.Taylor C. T. 2008. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem. J. 409: 19–26. [DOI] [PubMed] [Google Scholar]
- 116.Xia C., Meng Q., Liu L. Z., Rojanasakul Y., Wang X. R., Jiang B. H. 2007. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 67: 10823–10830. [DOI] [PubMed] [Google Scholar]
- 117.Larrayoz I. M., Huang J-D., Lee J-W., Pascual I., Rodriguez I. R. 2010. 7-Ketocholesterol-induced inflammation is mediated by multiple kinase signaling pathways via NFκB but independently of reactive oxygen species formation. Invest. Ophthalmol. Vis. Sci.. Epub ahead of print. June 16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McCourt M. P., Ashraf K., Miller R., Weeks C. M., Li N., Pangborn W., Dorset D. L. 1997. X-ray crystal structure of cytotoxic oxidized cholesterols: 7-ketocholesterol and 25-hydroxycholesterol. J. Lipid Res. 38: 1014–1021. [PubMed] [Google Scholar]
- 119.Phillips J. E., Geng Y. J., Mason R. P. 2001. 7-Ketocholesterol forms crystalline domains in model membranes and murine aortic smooth muscle cells. Atherosclerosis. 159: 125–135. [DOI] [PubMed] [Google Scholar]
- 120.Massey J. B. 2006. Membrane and protein interactions of oxysterols. Curr. Opin. Lipidol. 17: 296–301. [DOI] [PubMed] [Google Scholar]
- 121.Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Berthier A., Bessede G., Corcos L., Gambert P., Neel D., Lizard G. 2005. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 21: 97–114. [DOI] [PubMed] [Google Scholar]
- 122.Zhang C., Baffi J., Cousins S. W., Csaky K. G. 2003. Oxidant-induced cell death in retinal pigment epithelium cells mediated through the release of apoptosis-inducing factor. J. Cell Sci. 116: 1915–1923. [DOI] [PubMed] [Google Scholar]
- 123.Prunet C., Lemaire-Ewing S., Menetrier F., Neel D., Lizard G. 2005. Activation of caspase-3-dependent and -independent pathways during 7-ketocholesterol- and 7beta-hydroxycholesterol-induced cell death: a morphological and biochemical study. J. Biochem. Mol. Toxicol. 19: 311–326. [DOI] [PubMed] [Google Scholar]
- 124.Panini S. R., Yang L., Rusinol A. E., Sinensky M. S., Bonvetre J. V., Leslie C. C. 2001. Arichidonate metabolism and signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. J. Lipid Res. 42: 1678–1686. [PubMed] [Google Scholar]
- 125.Akiba S., Yoneda Y., Ohno S., Nemoto M., Sato T. 2003. Oxidized LDL activates phospholipase A2 to supply fatty acids required for cholesterol esterification. J. Lipid Res. 44: 1676–1685. [DOI] [PubMed] [Google Scholar]
- 126.Akiba S., Ii H., Yoneda Y., Sato T. 2004. Translocation of phospholipase A2 to membranes by oxidized LDL and hydroxyoctadecadienoic acid to contribute to cholesteryl ester formation. Biochim. Biophys. Acta. 1686: 77–84. [DOI] [PubMed] [Google Scholar]
- 127.Freeman N. E., Rusinol A. E., Linton M. R., Hachey D. L., Fazio S., Sinensky M. S., Thewke D. 2005. Acyl-coenzyme A: cholesterol acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis in macrophages. J. Lipid Res. 46: 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ishizuka T., Cheng J., Sing H., Vitto M. D., Manthati V. L., Flack J. R., Laniado-Schwartzman M. 2008. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells. J. Pharmacol. Exp. Ther. 324: 103–110. [DOI] [PubMed] [Google Scholar]
- 129.Ghelli A., Porcelli A. M., Zanna C., Rugolo M. 2002. 7-Ketocholesterol and staurosporine induce opposite changes in intracellular pH, associated with distinct types of cell death in ECV304 cells. Arch. Biochem. Biophys. 402: 208–217. [DOI] [PubMed] [Google Scholar]
- 130.Malvitte L., Montange T., Vejux A., Joffre C., Bron A., Creuzot-Garcher C., Lizard G. 2008. Activation of a caspase-3-independent mode of cell death associated with lysosomal destabilization in cultured human retinal pigment epithelial cells (ARPE-19) exposed to 7beta-hydroxycholesterol. Curr. Eye Res. 33: 769–781. [DOI] [PubMed] [Google Scholar]
- 131.Buytaert E., Callewaert G., Hendrickx N., Scorrano L., Hartmann D., Missiaen L., Vandenheede J. R., Heirman I., Grooten J., Agostinis P. 2006. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 20: 756–758. [DOI] [PubMed] [Google Scholar]
- 132.Kritharides L., Kus M., Brown A. J., Jessup W., Dean R. T. 1996. Hydroxypropyl-beta-cyclodextrin-mediated efflux of 7-ketocholesterol from macrophage foam cells. J. Biol. Chem. 271: 27450–27455. [DOI] [PubMed] [Google Scholar]
- 133.Riazy M., Chen J. H., Steinbrecher U. P. 2009. VEGF secretion by macrophages is stimulated by lipid and protein components of OxLDL via PI3-kinase and PKCzeta activation and is independent of OxLDL uptake. Atherosclerosis. 204: 47–54. [DOI] [PubMed] [Google Scholar]
- 134.Boullier A., Friedman P., Harkewicz R., Hartvigsen K., Green S. R., Almazan F., Dennis E. A., Steinberg D., Witztum J. L., Quehenberger O. 2005. Phosphocholine as a pattern recognition ligand for CD36. J. Lipid Res. 46: 969–976. [DOI] [PubMed] [Google Scholar]
- 135.Ogura S., Kakino A., Sato Y., Fujita Y., Iwamoto S., Otsui K., Yoshimoto R., Sawamura T. 2009. LOX-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ. J. 73: 1993–1999. [DOI] [PubMed] [Google Scholar]
- 136.Rhainds D., Brissette L. 2004. The role of scavenger receptors class B type I (SRBI) in lipid trafficking: defining the rules for lipid traders. Int. J. Biochem. Cell Biol. 36: 39–77. [DOI] [PubMed] [Google Scholar]
- 137.Curcio C. A., Millican C. L., Bailey T., Kruth H. S. 2001. Accumulation of cholesterol with age in human Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 42: 265–274. [PubMed] [Google Scholar]
- 138.Ruberti J. W., Curcio C. A., Millican C. L., Menco B. P., Huang J. D., Johnson M. 2003. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 44: 1753–1759. [DOI] [PubMed] [Google Scholar]
- 139.Rudolf M., Curcio C. A. 2009. Esterified cholesterol is highly localized to bruch's membrane, as revealed by lipid histochemistry in wholemounts of human choroid. J. Histochem. Cytochem. 57: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Malek G., Li C. M., Guidry C., Medeiros N. E., Curcio C. A. 2003. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am. J. Pathol. 162: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Curcio C. A., Presley J. B., Millican C. L., Medeiros N. E. 2005. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp. Eye Res. 80: 761–775. [DOI] [PubMed] [Google Scholar]
- 142.Anderson D. H., Mullins R. F., Hageman G. S., Johnson L. V. 2002. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 134: 411–431. [DOI] [PubMed] [Google Scholar]
- 143.Handa J. T. 2007. New molecular histopathologic insights into the pathogenesis of age-related macular degeneration. Int. Ophthalmol. Clin. 47: 15–50. [DOI] [PubMed] [Google Scholar]
- 144.SanGiovanni J. P., Mehta S., Mehta S. 2009. Variation in lipid-associated genes as they relate to risk of advanced age-related macular degeneration. World Rev. Nutr. Diet. 99: 105–158. [DOI] [PubMed] [Google Scholar]
- 145.Torrini M., Marchese C., Vanzetti M., Marini V., Origone P., Garre C., Mareni C. 2007. Mutation analysis of oxisterol-binding-protein gene in patients with age-related macular degeneration. Genet. Test. 11: 421–426. [DOI] [PubMed] [Google Scholar]
- 146.Chen W., Stambolian D., Edwards A. O., Branhamd K. E., Othmand M., Jakobsdottire J., Tosakulwongc N., Pericak-Vancef M. A., Campochiarog P. A., Klein M. L., et al. 2010. Genetic variants near TIMP3 and HDL-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci USA. 107: 7401–7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Neale B. M., Fagerness J., Reynolds R., Sobrin L., Parker M., Raychaudhuri S, Tan P. L., Oh E. C., Merriam J. E., Souied E., et al. 2010. Genome-wide association study of advanced age- related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc. Natl. Acad. Sci. USA. 107: 7395–7400. [DOI] [PMC free article] [PubMed] [Google Scholar]