Abstract
Electronegative LDL [LDL(–)] is a minor modified LDL subfraction present in blood with inflammatory effects. One of the antiatherogenic properties of HDL is the inhibition of the deleterious effects of in vitro modified LDL. However, the effect of HDL on the inflammatory activity of LDL(–) isolated from plasma is unknown. We aimed to assess the putative protective role of HDL against the cytokine released induced in monocytes by LDL(–). Our results showed that LDL(–) cytokine release was inhibited when LDL(–) was coincubated with HDL and human monocytes and also when LDL(–) was preincubated with HDL and reisolated prior to cell incubation. The addition of apoliprotein (apo)AI instead of HDL reproduced the protective behavior of HDL. HDL preincubated with LDL(–) promoted greater cytokine release than native HDL. Incubation of LDL(–) with HDL decreased the electronegative charge, phospholipase C-like activity, susceptibility to aggregation and nonesterified fatty acid (NEFA) content of LDL(–), whereas these properties increased in HDL. NEFA content in LDL appeared to be related to cytokine production because NEFA-enriched LDL induced cytokine release. HDL, at least in part through apoAI, inhibits phospholipase-C activity and cytokine release in monocytes, thereby counteracting the inflammatory effect of LDL(–). In turn, HDL acquires these properties and becomes inflammatory.
Keywords: cytokines, high density lipoprotein, electronegative low density lipoprotein, monocytes, phospholipase-C activity
HDL plays a central role in many events involved in the development of atherosclerosis, and there is an inverse relationship between plasma HDL-cholesterol levels and cardiovascular risk (1). The atheroprotective role of HDL is often related to its ability to promote reverse cholesterol transport (2). However, HDL presents several other antiatherogenic properties (3) such as inhibition of LDL aggregation (4) and LDL nonenzymatic oxidation, which prevent cellular inflammatory events mediated by oxidized phospholipids (5–7). Furthermore, HDL exerts a protective effect on endothelial cells and monocytes (8–10) because it inhibits cells from producing the inflammatory molecules induced by modified LDL and lipopolysaccharide (LPS).
Some of the protective effects of HDL have been attributed mainly to apolipoprotein (apo)A-I (6–8), the major protein of HDL. However, enzymatic activities associated with HDL, such as paraoxonase (11) and platelet-activating factor acetylhydrolase (PAF-AH) (12), have also been related to its atheroprotective action.
In contrast to atheroprotective HDL, electronegative LDL [LDL(–)] is a minor LDL subfraction in plasma circulation whose proportion is increased in atherosclerosis-associated diseases such as diabetes mellitus or familial hypercholesterolemia (13, 14). We have reported that LDL(–) does not present a different degree of oxidation compared with LDL(+) (15), but other physico-chemical characteristics differ from those in naturaly-occurring LDL. LDL(–) presents higher nonesterified fatty acid (NEFA) content (16), susceptibility to aggregation (15), binding to proteoglycans (PG) (17), PAF-AH (18), phospholipase C (PLC)-like activity (19), and a lower binding to LDL receptor (20) than LDL(+). In addition, LDL(–) exerts inflammatory effects on endothelial cells, such as induction of cytokine release (21), cytotoxicity (22), and apoptosis (23). LDL(–) also exerts an effect on mononuclear leukocytes by inducing several inflammatory molecules such as chemokines or Fas (24, 25).
Because the bioactivity of LDL particles can be regulated by HDL, it is feasible that HDL could exert an anti-inflammatory effect on LDL(–). Therefore, the aim of this study was to evaluate the putative protective role of HDL and apoAI against the proinflammatory activity of LDL(–) evaluated by its capacity to induce cytokine release in monocytes. In addition, because LDL(–) and HDL interaction could lead to biochemical changes in particles, we also assesed their main characteristics after incubation, including lipid and apoprotein composition, electronegative charge, oxidative level, enzymatic activities, aggregation, particle size, and binding to PG. HDL characteristics after incubation with LDL(–) were also evaluated.
METHODS
Lipoprotein isolation
Plasma samples from healthy normolipemic volunteers (total cholesterol < 5.2 mM, triglycerides < 1 mM) were obtained in EDTA-containing Vacutainer tubes. The study was approved by the institutional Ethics Committee and subjects gave their written informed consent. LDL (1.019–1.050 g/mL) and HDL (1.100–1.210 g/mL) were isolated by sequential flotation ultracentrifugation at 4°C. Total LDL was subfractionated into LDL(+) and LDL(–) by preparative anion-exchange chromatography in an ÄKTA-FPLC system (Amersham Pharmacia) (15). The LDL(–) proportion ranged from 4 to 6% of total LDL and the main characteristics of both LDL subfractions were similar to those previously reported (26).
LDL preincubation with NEFA
For some experiments, native LDL was loaded with a mixture of NEFA with a fatty acid proportion similar to that found in human serum as described (16). Briefly, LDL was incubated for 4 h at 37°C in the presence of albumin (45 g/L) with increasing amounts of the NEFA mixture (NEFA concentration: 0.5 mM and 1.5 mM), and LDL was then reisolated by ultracentrifugation and termed NEFA-LDL.
LDL preincubation with HDL and apoAI
LDL(+) and LDL(–) (0.5 g/L apoB) were incubated with HDL and commercial apoAI from human plasma (Sigma) in different proportions of apoB versus apoAI (2:1, 1:1, or 1:2), with 1 = 0.5 g/L, which means that the concentration of apoB was, respectively, double, the same, or half that of apoAI. Incubation was carried out at 37°C for 2 h in phosphate saline buffer with gentle shaking in the presence of butylated hydroxytoluene (20 μM) to avoid oxidation. After incubation, LDL and HDL were reisolated by ultracentrifugation according to their density.
LDL(+), untreated and treated with HDL and apoAI, was used in the experiments as a control. However, data obtained are only shown in Results when considered of interest.
Cytokine release in monocytes by LDL and HDL
Monocytes were isolated from blood of healthy volunteers by density as previously described (24). All participants gave their written informed consent. The general procedure involved incubation of LDL, HDL, and apoAI with monocytes (106 cells) for 20 h in the conditions indicated below. Cell supernatants were then collected to quantify interleukin (IL)6, IL8, IL10 and monocyte chemotactic protein (MCP)1 by ELISA (Bender Medsystems) as described (24). LPS at 0.1 mg/L (Sigma) was used as a positive control of inflammation.
In coincubation experiments, LDL(+) or LDL(–) (150 mg apoB/L) were added to the cells alone or simultaneously with HDL (LDL+HDL, at apoB:apoAI proportions 2:1 and 1:1) or commercial apoAI (LDL+apoAI, at a proportion of 2:1). We also coincubated, at a proportion of 1:1, LDL(+) or LDL(–) with VLDL and LDL(–) with LDL(+). In preincubation experiments, in order to rule out interferences with the presence of HDL in the culture, LDL(+) or LDL(–) were previously incubated with HDL or commercial apoAI, at the same concentrations as in coincubation, and reisolated prior to the addition to monocytes. In preincubation assays, reisolated LDL was termed LDL-HDL and LDL-apoAI, whereas reisolated HDL and apoAI were termed HDL-LDL and apoAI-LDL. Incubation of monocytes with NEFA-LDL was also carried out. In all cases, concentration in the culture was 150 mg apoB/L for LDL and 150 mg apoAI/L for HDL and apoAI.
Lipid and apoprotein composition
Cholesterol, triglyceride, apoB, apoAI (Roche Diagnostic), phospholipids and NEFA (Wako Chemicals) content of LDL, HDL, and apoAI were determined in a Hitachi 917 autoanalyzer. The results were expressed as the percentage of lipoprotein mass and, in the case of NEFA, as μmol NEFA/g apoB for LDL and μmol NEFA/g apoAI for HDL.
Electronegative charge and oxidation tests
The electrophoretic mobility of LDL and HDL by charge was assessed in commercial agarose gels (Biomidi) following the manufacturer's instructions.
Lipid components from samples (200 μg of apoB or apoAI) were extracted by the Bligh and Dyer method (27) and conjugated dienes, as an estimation of lipoperoxidation, were evaluated by normal-phase HPLC using a Beckman Gold System chromatograph as described (28). Conjugated dienes of the samples were measured by calculating the ratio between the phosphatidylcholine (PC) peak areas at 234 nm (oxidized PC) and at 205 nm (maximum absorbance of PC). Peroxide content of lipoproteins (20 μg of apoB for LDL or apoAI for HDL) was quantified by the Auerbach assay (29), based on the reaction with benzoyl leucomethylenblue (TCI), hydroperoxy-octadecadienoic acid (Biomol) was used as standard.
Enzymatic activity measurements
PAF-AH activity of the samples at 0.2 g/L apoB (LDL) and apoAI (HDL) was evaluated by a commercial colorimetric assay based on degradation of 2-thio-PAF (Cayman Chemical), as described (18).
PLC-like activity of LDL(+), LDL(–), HDL, and apoAI in the different incubation conditions was evaluated as previously described (19). Briefly, we used a commercial fluorimetric assay based on enzyme-coupled reactions and a final detection of fluorescent Amplex Red. Lysophosphatidylcholine (LPC) and sphingomyelin (SM) were used as substrates and 30 μg of apoB (LDL) or apoAI (HDL) of the samples were assayed. Fluorescence was monitored for 3 h and activity was calculated by the maximum slope of curve. The PLC-like activity was also assessed in LDL(–) previously treated for 2 h at 37°C with albumin (1 g/L apoB of LDL and 45 g/L albumin).
To corroborate the sphingomyelinase (SMase) activity and avoid possible interferences of the Amplex Red method, the degradation of SM labeled with borondipyrromethene (SM-BODIPY) (Molecular Probes) was also measured. As described, samples (50 μg) were incubated with SM-BODIPY for 3 h at 37°C, followed by lipid extraction and separation by thin layer chromatography (TLC) (19).
Aggregation and PG binding studies
Lipoprotein size and presence of lipoprotein aggregates in the different conditions analyzed were evaluated by nondenaturing polyacrylamide gradient gel electrophoresis (GGE). The basal aggregation level was also evaluated by absorbance at 680 nm (0.5 g/L apoB or apoAI). LDL susceptibility to aggregation was determined by monitoring absorbance at 680 nm after vortexing LDL and HDL (0.2 g/L apoB or apoAI) at increasing times (15).
PG binding of LDL and LDL treated with HDL was assessed by PG affinity chromatography and the LDL-glycosaminoglycan (GAG) precipitation method as widely described (17). Briefly, the PG affinity was performed using a HiTrap column with human arterial PG, and the precipitation method was carried by incubating LDL with GAG and measuring the bound particles, which precipitate in centrifugation.
Statistical analysis
Results are expressed as mean ± SD. A Sigma Stat 2.0 statistical package was used. Differences between groups were tested with Wilcoxon's t-test (for paired data).
RESULTS
Cytokine release induced by LDL(–) and HDL
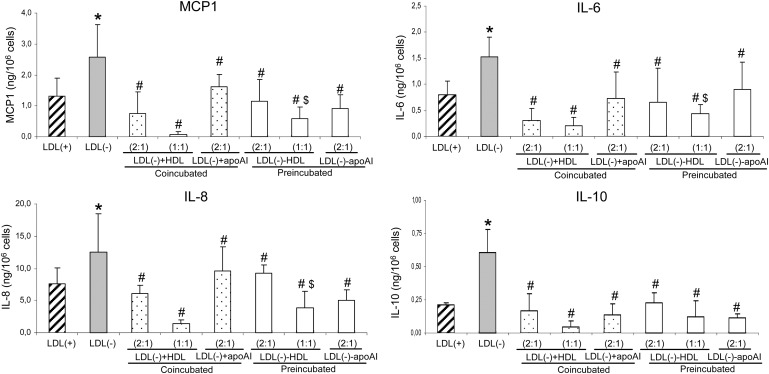
One of the main inflammatory actions of LDL(–) is induction of the release of several cytokines in monocytes. The inhibitory effect promoted by HDL and apoAI on the release of MCP1, IL6, IL8, and IL10 induced by LDL(–) is shown in Fig. 1. This inhibitory action on LDL(–) was found in coincubation with cells [LDL(–)+HDL and LDL(–)+apoAI] as well as in preincubation conditions [LDL(–)-HDL and LDL(–)-apoAI] in which LDL(–) was reisolated prior to the addition to cells. The inhibition promoted by HDL was concentration-dependent and higher than 65% when the same concentration of apoAI and apoB was used. Inhibition in cytokine release by HDL and apoAI was even higher than 50% when half of the amount of apoAI was used except for IL8 in preincubation with HDL and coincubation with apoAI. Coincubation of LDL(–) with VLDL or LDL(+) did not inhibit cytokine release more than 10% (Table 1).
Fig. 1.
Monocyte cytokine release promoted by LDL(+), LDL(–), and LDL(–) treated with HDL and apoAI. The release of MCP1, IL6, IL8, and IL10 expressed as ng/106 cells was evaluated after incubation of monocytes with LDLs. LDL(–) treated with HDL and apoAI in coincubation conditions was named LDL(–)+HDL and LDL(–)+apoAI, respectively, and LDL(–)-HDL and LDL(–)-apoAI in preincubation. The X axis indicates the proportion of apoB:apoAI used in preincubation: 1 = 0.5 g apoB/L of LDL and 0.5 g apoAI/L of HDL. The final amount of LDL and HDL in each well was 150 μg of apoB (LDL) or apoAI (HDL). Release promoted by LDL(+) is shown in striped bars, by LDL(–) in gray bars, by LDL(–) in coincubation conditions in dotted bars, and in preincubation in white bars. Data are the mean ± SD of four experiments. Statistical differences with P = 0.068 are indicated as follows: * LDL(–) vs LDL(+); # LDL(–) treated versus LDL(–) untreated; $ preincubation versus coincubation, at the same lipoprotein concentration.
TABLE 1.
Cytokine release induced by LDL(–) coincubated with LDL(+) and VLDL
| LDL(–) | LDL(–)+LDL(+) | LDL(–)+VLDL | |
|---|---|---|---|
| MCP1 | 1.85 ± 0.44 | 1.69 ± 0.58 | 1.67 ± 0.40 |
| IL6 | 1.30 ± 0.14 | 1.24 ± 0.18 | 1.41 ± 0.45 |
| IL8 | 8.45 ± 3.48 | 7.71 ± 3.07 | 8.67 ± 4.75 |
| IL10 | 0.52 ± 0.16 | 0.48 ± 0.17 | 0.47 ± 0.16 |
The lipoproteins were coincubated, at a proporation of 1:1 (150 mg/L of apoB), with monocytes. Cytokine release is expressed as ng/106cells (n = 3). In LDL(-) incubated with LDL(+) and VLDL, cytokine release induced by the lipoprotein added additionally to LDL(-) was subtracted.
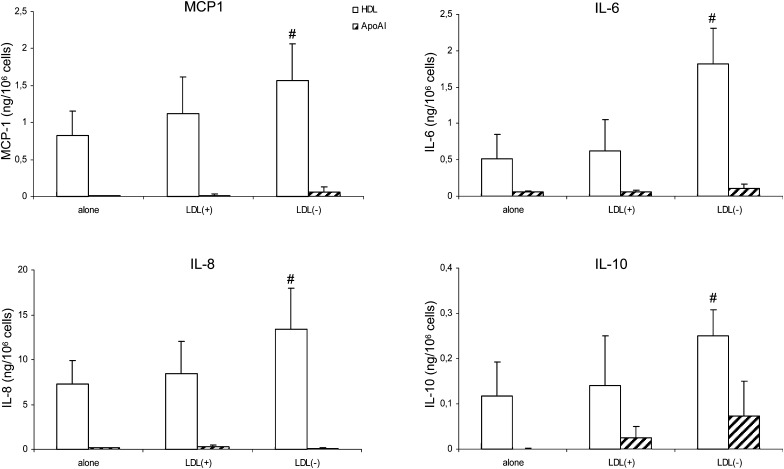
Interestingly, when HDL was preincubated with LDL(–) [HDL-LDL(–)] and then reisolated and added to cells, the cytokine release by monocytes significantly increased compared with native HDL and HDL incubated with LDL(+): approximately 3-fold for IL6 and 2-fold for the other cytokines (Fig. 2). Indeed, HDL-LDL(–) induced production of IL6 and IL8 by monocytes at a similar level to LDL(–) and slightly lower production of MCP1 and IL10. Untreated HDL presented a similar or lower inductor cytokine effect than LDL(+). In contrast, apoAI promoted a lower cytokine release in all cases, even when preincubated with LDL(–). The production of cytokines by apoAI was similar to the basal levels of cells alone, with values lower than 0.1 ng/106 cells.
Fig. 2.
Monocyte cytokine release promoted by HDL and apoAI, in native state and preincubated with LDLs. The release of MCP1, IL6, IL8, and IL10 expressed as ng/106 cells was evaluated after incubation of monocytes with HDL and apoAI. In preincubation, 0.5 g apoB/L of LDL and 0.5 g apoAI/L of HDL were used. The final amount of HDL and apoAI in each well was 150 μg of apoAI. Cytokine release promoted by HDL is shown in white bars and that by apoAI in striped bars. Data are the mean ± SD of four experiments. Statistical differences with P = 0.068 are indicated with # HDL treated versus untreated HDL.
Lipid and apoprotein composition
Some components of LDL(–) could be transferred to HDL or vice versa during preincubation, thereby making LDL(–) lose and HDL gain inflammatory properties. To ascertain which components could be involved, biochemical characterization of the particles was performed after incubation of both lipoproteins and their reisolation.
LDL(+) composition in the different conditions and HDL and apoAI after incubation with LDL(+) did not change (data not shown). The main lipids and apoproteins contained in LDL(–) and HDL also remained unchanged after preincubation (Table 2). ApoAI content in LDL and apoB in HDL were undetectable with the methods used, and consequently lipoproteins were considered free of contamination when reisolated. Regarding apoAI, the particle reisolated after incubation with LDL(–) gained a low proportion of lipids, probably transferred from LDL(–), which were almost undetectable in apoAI alone and incubated with LDL(+).
TABLE 2.
Main components of LDL(-), HDL, and apoAI in native state and after treatements
| LDL(–) |
|||
|---|---|---|---|
| Untreated | After HDL | After apoAI | |
| %CT | 42.2 ± 1.7 | 41.8 ± 2.0 | 42.3 ± 2.5 |
| %TG | 9.4 ± 1.2 | 9.4 ± 1.3 | 9.6 ± 1.1 |
| %PL | 27.1 ± 2.9 | 27.5 ± 3.2 | 27.8 ± 3.5 |
| %ApoB | 21.3 ± 1.1 | 21.3 ± 1.0 | 20.3 ± 1.6 |
| %ApoAI | ND | ND | ND |
| HDL | apoAI | |||
|---|---|---|---|---|
| Untreated | After LDL(–) | Untreated | After LDL(–) | |
| %CT | 18.5 ± 2.2 | 18.4 ± 1.1 | 0.03 ± 0.05 | 1.39 ± 0.59* |
| %TG | 4.6 ± 3.3 | 4.1 ± 0.8 | ND | 1.35 ± 1.59 |
| %PL | 34.8 ± 3.6 | 34.5 ± 3.8 | 0.53 ± 0.61 | 5.84 ± 1.69* |
| %ApoAI | 42.1 ± 4.6 | 43.0 ± 3.6 | 99.4 ± 0.6 | 91.42 ± 1.64* |
| %ApoB | ND | ND | ND | ND |
The amount of each component is expressed as % of mass. The table shows composition of LDL(–) in native state and preincubated with HDL and apoAI in 1:1 proporation (being 1 = 0.5 g apoB/L of LDL and 0.5g apoAI/L of HDL). It also shows composition of HDL and apoAI in native state and preincubated with LDL(–) in 1:1 proportion. CT, cholesterol; TG, triglyceride; PL, phospholipids; ND, nondetectable; n = 9 except for apoAI (n = 4). Significant differences with P = 0.068 are indicated with * versus native state.
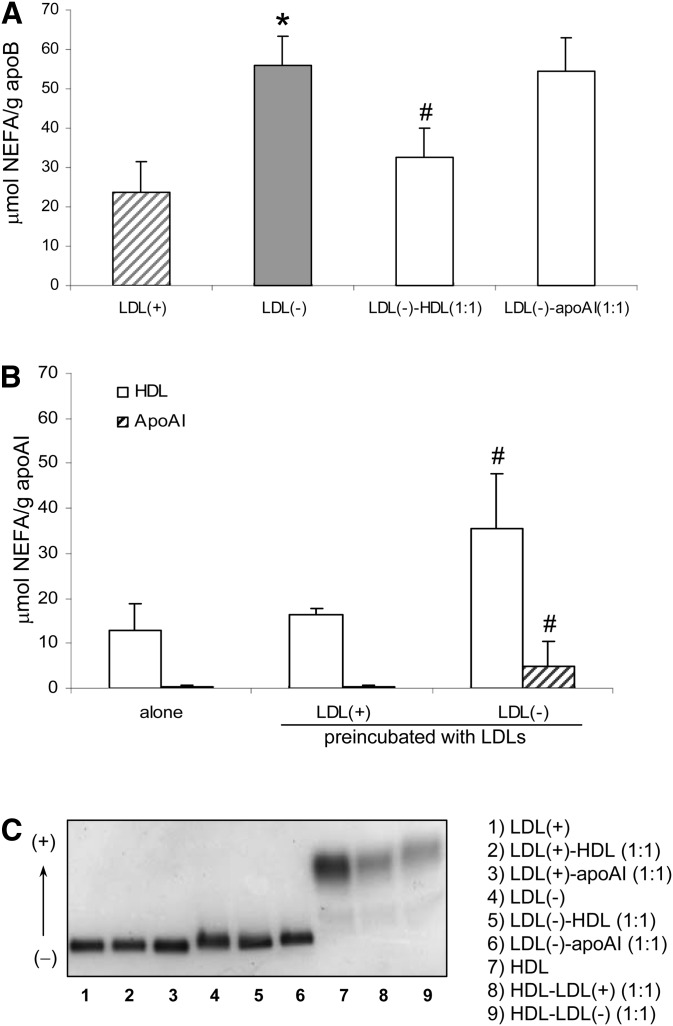
The amount of NEFA, a minor component of LDL which is increased in LDL(–), diminished in this fraction after incubation with HDL (Fig. 3A). In contrast, incubation of LDL(–) with apoAI did not alter its NEFA content. In turn, preincubation of HDL with LDL(–) promoted an increase in NEFA in HDL (Fig. 3B) that concurred with the amount of NEFA removed from LDL(–), around 30 μmols. In apoAI preincubated with LDL(–), a slight increase was found in NEFA content but to a lesser degree than that found in native HDL.
Fig. 3.
NEFA content and electronegative charge of LDL(–), HDL, and apoAI in native state and after treatments. LDLs were preincubated with HDL and apoAI (0.5 g apoB/L of LDL and 0.5 g apoAI/L of HDL). In A, NEFA content expressed as μmol/g apoB is shown in striped bars for LDL(+), gray bars for LDL(–), and white bars for treated LDL(–). In B, NEFA content expressed as μmol/g apoAI is shown in white bars for HDL and in striped bars for apoAI. Data are the mean ± SD of seven experiments except for apoAI in B (n = 4). Statistical differences with P < 0.05 are indicated: * LDL(–) versus LDL(+) and # LDL(–), HDL, and apoAI after preincubation treatment versus native state. The electrophoretic charge of the samples in an agarose gel is shown in .
The electronegativity of the samples followed the same behavior as NEFA because NEFAs possess a high electronegative charge. Figure 3C shows that preincubation of LDL(–) with HDL, but not with apoAI, diminished its mobility in agarose electrophoresis whereas HDL postincubated with LDL(–) increased its mobility.
NEFA effect on cytokine release
To ascertain whether NEFA content was an important factor in inducing cytokine release by monocytes in response to LDL(–), the effect of NEFA-enriched LDL on such release was evaluated. NEFA-LDL induced cytokine release and the effect was dose-related, as observed in Table 3. However, LDL loaded with 1.5 mM NEFA, which reached a NEFA content slightly higher than that of LDL(–), did not promote the same cytokine release as LDL(–) itself.
TABLE 3.
Cytokine release induced in monocytes by NEFA-LDL
| NEFA(0)-LDL | NEFA(0.5)-LDL | NEFA(1.5)-LDL | LDL(-) | |
|---|---|---|---|---|
| NEFA content | 20.4 ± 6.44 | 47.4 ± 6.34* | 70.8 ± 27.4* | 55.92 ± 7.35 |
| MCP1 | 0.96 ± 0.55 | 1.2 ± 0.70* | 1.38 ± 0.61* | 2.58±1.04 |
| IL6 | 0.54 ± 0.13 | 0.69 ± 0.46 | 0.82 ± 0.40* | 1.52 ± 0.38 |
| IL8 | 5.99 ± 1.41 | 6.80 ± 2.44 | 9.34 ± 3.06* | 12.57 ± 5.87 |
| IL10 | 0.18 ± 0.09 | 0.26 ± 0.20 | 0.38 ± 0.31* | 0.61 ± 0.173 |
NEFA concentration in the incubation with LDL is expressed as mM in parentheses. Final NEFA content in LDL is expressed as μmol NEFA/g apoB and cytokine release as ng/106 cells (n = 6). Data of LDL(–), including NEFA content and cytokine release, are extracted from results of Fig. 1 and 3A. Significant differences with P < 0.05 versus NEFA(0)-LDL are indicated with *.
Oxidation levels
Although our previous studies did not show a higher oxidation level in LDL(–) than in LDL(+), we tested whether minor amounts of oxidized lipids could be transferred from LDL(–) to HDL. Peroxide content did not differ either between LDL(+) and LDL(–) or by incubation with HDL in any condition (Table 4), by the Auerbach method and by the degree of oxidation of PC. Oxidized LDL was used as positive control and presented a higher content in lipoperoxides (11.85 ± 3.28 μmol/g apoB).
TABLE 4.
Oxidation levels and PAF-AH activity of LDL(+), LDL(–), and HDL in native state and after treatments
| Ratio 205/234 nm | μmol Peroxides/ g apo (Auerbach) | PAF-AH Activity (μmol/min/mg apo) | |
|---|---|---|---|
| LDL(+) | 62.1 ± 8.8 | 4.20 ± 1.03 | 0.80 ± 0.30 |
| LDL(+)-HDL | 67.6 ± 7.9 | 4.61 ± 1.68 | 1.62 ± 0.24# |
| LDL(–) | 71.1 ± 5.5 | 3.19 ± 1.35 | 3.91 ± 0.69* |
| LDL(–)-HDL | 66.2 ± 10.1 | 3.63 ± 1.63 | 3.62 ± 1.20 |
| HDL | 65.3 ± 1.6 | 4.97 ± 2.67 | 2.34 ± 0.42* |
| HDL-LDL(+) | 65.9 ± 1.8 | 3.92 ± 2.20 | 1.67 ± 0.25# |
| HDL-LDL(–) | 64.5 ± 2.9 | 4.73 ± 2.19 | 2.56 ± 0.83 |
The same concentration of apo B and apoAI was used in preincubation (0.5 g apoB /L of LDL and 0.5 g apoAI/L of HDL) and lipoproteins were then reisolated (n = 4). The assays were performed from the same samples: LDL(+) alone, LDL(+) preincubated with HDL, LDL(–) alone, LDL(–) preincubated with HDL, HDL alone, HDL preincubated with LDL(+) and HDL preincubated with LDL(–). Ratios of the PC peak areas at 234 nm (conjugated dienes) and 205 nm (maximum absorbance of PC) of the samples (200 μg of apoB or apoAI) were evaluated by HPLC. Peroxide content of the samples (20 μg of apoB or apoAI) was quantified by Auerbach assay and expressed as μmol peroxides/g apoB (LDL) or μmol peroxides/g apoAI (HDL). PAF-AH activity of the samples (3 μg of apoB or apoAI) was quantified from the slope of the kinetics of thio-PAF degradation. Significant differences with P = 0.068 are indicated: * untreated LDL(–) and HDL versus LDL(+) and # treated lipoprotein versus respective untreated lipoprotein.
Enzymatic activities
Because LDL(–) possesses increased PAF-AH and PLC-like activity versus LDL(+), we tested whether these enzymatic activities were modified after incubation of LDL(–) with HDL and apoAI.
When LDL(–) was incubated with HDL, its PAF-AH activity did not change (Table 4, right column); in contrast, LDL(+) after incubation with HDL gained PAF-AH activity, which is in parallel with a small but significant decrease in HDL.
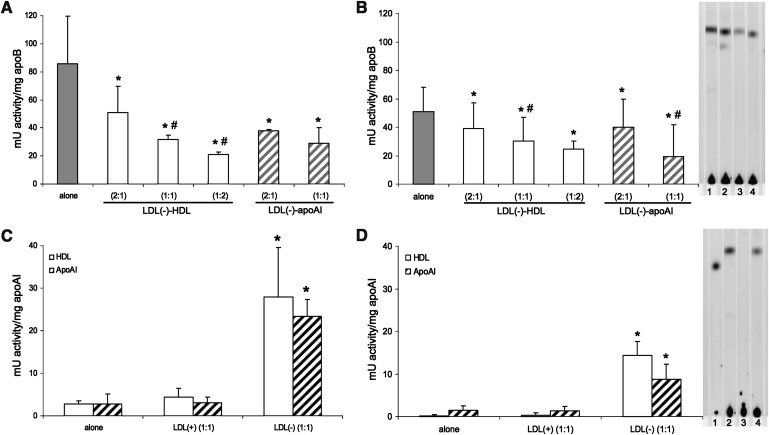
Regarding PLC-like activity, LDL(–) is capable of degrading SM, and particularly LPC, at a high rate whereas LDL(+) did not degrade these substrates (lower than 2 mU activity/mg apoB). LDL(–) depleted in NEFA by incubation with albumin did not lose any PLC-like activity (data not shown), suggesting that the content of NEFA of LDL(–) is not involved in this enzymatic activity. The PLC-like activity of LDL(–) alone and after incubation with HDL and apoAI evaluated by the method of Amplex Red is shown in Fig. 4A (LPC) and 4B (SM). The activity of HDL and apoAI alone and after incubation with LDLs is shown in Fig. 4C (LPC) and 4D (SM).
Fig. 4.
PLC-like activity of LDL(–), HDL and apoAI, in native state and after treatments. The proportion of apoB:apoAI used in preincubation is indicated: 1 = 0.5 g apoB/L of LDL and 0.5 g apoAI/L of HDL. In the assay, 30 μg of apoB (LDL) or apoAI (HDL) were used. The PLC-like activity was calculated in mU activity/mg apoB or apoAI based on the maximal slope of 3 h kinetics. PLC-like activity of LDL(–) in native state and incubated with HDL and apoAI is shown in (substrate: LPC) and B (substrate: SM). Activity of LDL(–) alone is shown in gray bars, preincubated with HDL in white bars, and preincubated with apoAI in striped bars. Activity of HDL and apoAI in native state and incubated with LDL(+) and LDL(–) is shown in (LPC) and D (SM). HDL is represented in white bars and apoAI in striped bars. Data are the mean±SD of four experiments. Significant differences with P = 0.068 are indicated: * versus the respective untreated condition and # versus the lower concentration of treatment. Formation of ceramide-BODIPY by degradation of fluorescent substrate (SM-BODIPY) was evaluated by TLC. In the assay, 50 μg of apoB (LDL) or apoAI (HDL) were used. Representative TLC images are shown in [1: LDL(–), 2: LDL(–)-HDL (2:1), 3: LDL(–)-HDL (1:1), 4: LDL(–)-apoAI (2:1)] and D [1: ceramide-BODIPY, 2: HDL-LDL(–) (1:1), 3: HDL, 4: apoAI-LDL(–) (1:1)].
Taken together, results show how the high PLC-like activity of LDL(–) was reduced when LDL(–) had been incubated previously with HDL and reisolated, and this effect was related to the amount of HDL. ApoAI effect was also evaluated and was found to reproduce the effect promoted by HDL, thereby suggesting that apoAI is involved in the inhibition of the PLC-like effect of HDL. At the same proportion of apoAI and apoB, HDL and apoAI already inhibited around 50% of the activity of LDL(–).
On the other hand, HDL and apoAI reisolated after incubation with LDL(–) gained PLC-like activity. HDL and apoAI alone presented very low activity to hydrolyze LPC and SM, similar to LDL(+); however, this activity increased more than 10-fold when HDL and apoAI were previously incubated with LDL(–).
These data were corroborated by TLC using SM- BODIPY as a substrate; the technique was performed to avoid possible interferences in the Amplex Red method. TLC images are incorporated in Figs. 4B and 4D, where it can be observed that the presence of ceramide, the product of SMase activity, was lower when LDL(–) had been preincubated with HDL and apoAI. In contrast, there was no ceramide production with HDL, but it did appear after incubation with LDL(–) as well as in apoAI-LDL(–).
Aggregation and PG binding
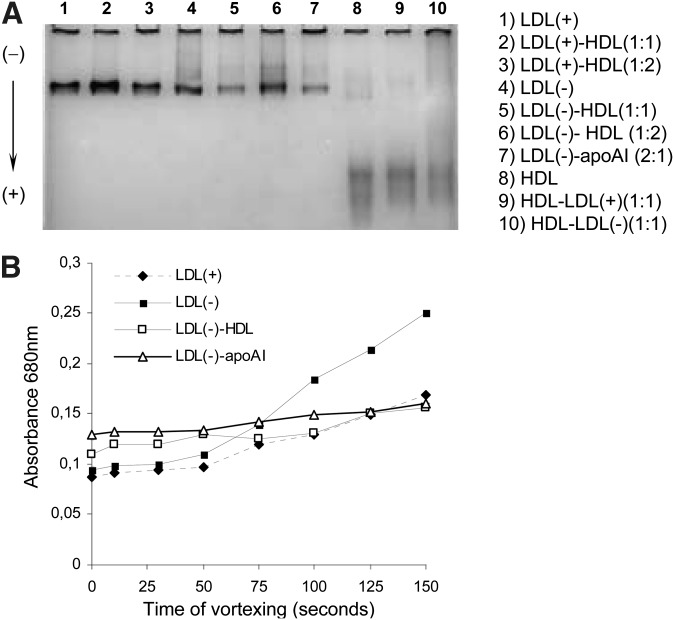
Greater PLC-like activity has been related to an increased aggregation in LDL(–) (19); moreover, LDL(–) is more susceptible to aggregation than LDL(+) (15). After treatment with HDL, changes in LDL(–) size or aggregation level were not found by GGE (Fig. 5A). By measuring the basal absorbance at 680 nm, it was corroborated that it was not modified by treatment with HDL either (differences lower than 4%).
Fig. 5.
Basal aggregation and susceptibility to aggregation of LDL(+), LDL(–), and HDL in native state and after treatments. shows electrophoretic mobility in the experimental conditions in a GGE gel. Particles migrate in GGE gels according to their size and aggregates can be observed. The samples are LDL(+), LDL(–), and HDL in native state and after preincubation. The proportion of apoB:apoAI used in the preincubation is indicated: 1 = 0.5 g/L of apoB for LDL and 0.5 g/L of apoAI for HDL. The concentration used in the gel was 0.5 g/L of apoB or apoAI. shows a representative experiment of susceptibility to aggregation of LDL(+), LDL(–), and LDL(–) preincubated with HDL and apoAI (1:1). Absorbance at 680 nm was measured after increasing times of agitation by vortex.
Fig. 5B shows that susceptibility to aggregation was inhibited when LDL(–) was incubated with HDL and apoAI, thereby reaching values similar to those of LDL(+). Nevertheless, HDL and apoAI were not more aggregable after incubation with LDL(–) because absorbance remained stable (0.05 absorbance units) despite vortexing at increasing times.
In view of previous results in which aggregation level was associated with a higher affinity to PGs (17), we performed assays evaluating this property by PG affinity chromatography and precipitation with GAG methods. Nevertheless, no differences were found by these methods. LDL(–) in the different incubation conditions followed the same profiles in PG affinity chromatograms, and differences by the precipitation method were lower than 5% (data not shown).
DISCUSSION
This study provides new insights into the atheroprotective role of HDL. It has been previously reported that HDL prevents the effect of oxidized LDL and minimally modified LDL (mmLDL) (6, 7); however, our findings show that HDL also neutralizes LDL(–), an inflammatory particle that is isolable from plasma and does not present a higher level of oxidation than native LDL (15). In fact, the properties of LDL(–) differ considerably from those of mmLDL because LDL(–) presents PAF-AH and PLC-like activity and high binding to proteoglycans whereas mmLDL does not. One of the most notable properties of LDL(–) is its capacity to induce the release of cytokines by endothelial and mononuclear cells (24, 26). A major novelty of the current work was that the release of cytokines promoted by LDL(–) in monocytes was counteracted by HDL and even by apoAI alone. Furthermore, PLC-like activity of LDL(–) is also inhibited by HDL. In turn, HDL reisolated after incubation with LDL(–) possessed more PLC-like activity than native HDL and also induced more cytokine release.
Regarding cytokine induction, we found that when HDL and apoAI were added to monocytes together with LDL(–), the cytokine release was significantly lower than that promoted by LDL(–) alone. This inhibitory effect was also observed when LDL(–) was preincubated with HDL or apoAI and isolated prior to its addition to cells. In both cases, LDL(–) effect on cytokine release became similar to that induced by LDL(+). In preincubation experiments, the oxidative level of LDL(–) was not modified by HDL. Hence, in this case, the anti-oxidative role of HDL does not appear to be involved in preventing inflammation as had been suggested previously (6, 7). As LDL(–) presented a decreased cytokine release when reisolated after incubation with HDL and apoAI, it seems that the protective HDL action would not be exerted directly on monocytes or at least not totally. HDL seems to interact with LDL(–) and modify the particle, thereby counteracting its inflammatory effect. As a consequence of this interaction with LDL(–), HDL, but not apoAI, gains the ability to induce cytokine release. These data suggest that LDL(–) could transfer some component to HDL that would be responsible for its inflammatory behavior, although another possibility would be a transfer of some atheroprotective molecule from HDL to LDL. To ascertain which characteristics of LDL(–) could be modified by HDL, several determinations were performed in LDL and HDL reisolated after incubation.
The chemical composition of lipoproteins revealed that only NEFA content changed in LDL as well as in HDL after incubation. We observed a decrease in NEFA content in LDL(–) and an increase in HDL. The amount of NEFA removed from LDL(–) concurs with the quantity of NEFA gained by HDL. Hence, there seems to be a transfer of this component from LDL(–) to HDL. The negative electronegative charge followed the same behavior because NEFA is a major determinant of the electronegative charge of lipoproteins (30). Nevertheless, incubation of LDL(–) with apoAI appears to decrease neither NEFA content nor electronegative charge by the detection methods used. However, apoAI after incubation with LDL(–) gained a certain quantity of lipids, including NEFA, compared with purified apoAI in which these components were almost undetectable. This slight enrichment was probably due to transference from LDL(–), but the amount was too low to be reflected as a loss of the same component in LDL(–) by the methods used. NEFA content in LDL(–) has been described as a significant factor involved in the cytokine release promoted by this particle in endothelial cells (28). In our experiments, HDL decreased the amount of NEFA in LDL(–) and also inhibited its cytokine release by cells. To confirm the involvement of NEFA content in the inflammatory role of LDL(–) on monocytes, experiments loading LDL with a mixture of NEFA were performed, and the NEFA-LDL increased cytokine release in a dose-dependent manner. Nevertheless, LDL enriched with NEFA to a similar content to LDL(–) did not reach the same amount of cytokine release, thereby suggesting that further components of LDL(–) are involved in its inflammatory effect. According to this idea, apoAI, which did not decrease NEFA content in LDL(–), diminished cytokine release promoted by LDL(–). Therefore, other lipids or proteins related to inflammatory action, apart from NEFA, could be transferred from LDL(–) to HDL and apoAI. Because PAF-AH and PLC-like activity are increased in LDL(–) (18, 19), both activities were evaluated in different conditions.
PAF-AH present in HDL has been proposed as involved in its atheroprotective function, mainly by degrading oxidized phospholipids of LDL (12). PAF-AH is easily transferred among lipoproteins in its dissociable form (31). Its involvement in the counteracting action of HDL on LDL(–) is unlikely because LDL(–) is not an oxidized particle (15) and LDL(–) itself presents higher PAF-AH activity than HDL (18). However, PAF-AH could be transferred from LDL(–) to HDL and, because this enzyme yields NEFA and LPC, LDL(–) could decrease its inflammatory action. Nevertheless, current results show that LDL(–) preincubated with HDL did not modify PAF-AH activity whereas LDL(+) had a slight increase in this enzymatic activity after HDL treatment although its inflammatory activity was not modified.
On the other hand, it seems feasible that a protein with PLC-like activity may be transferred from LDL(–) to HDL or apoAI because, after incubation of both lipoproteins, this activity was decreased in LDL(–) and increased in HDL and apoAI. Unfortunately, it is not known which protein is responsible for this PLC-like activity. As apoB has been proposed as a candidate (19, 32), we cannot rule out the possiblity that apoB fragments with this phospholipolytic activity could be transferred from LDL(–) to HDL and apoAI although we did not detect the presence of apoB with the methods used. Another possibility would be the transfer of another nonidentified protein from LDL(–) to HDL or changes in the lipid environment that could modify the conformation or activity of this enzyme. A decrease in NEFA content promoted in LDL(–) after HDL incubation would not be responsible for the loss in PLC-like activity as supported by experiments in which NEFA content was depleted in LDL(–) by incubation with albumin. As it has been reported that incubation of oxidized LDL with albumin removes oxidized lipids from the particle (33), our results suggest that oxidized lipids are not involved in PLC-like activity. Further studies are required to ascertain the protein with PLC-like activity and to determine whether there is transfer of it between LDL(–) and HDL.
Although many aspects of PLC-like activity associated with LDL(–) are unknown, there could be a relationship between phospholipolytic activity and cytokine release. It has been reported that ceramide is an inflammatory mediator (34). Because HDL and apoAI inhibit PLC-like activity of LDL(–), the formation of cell mediators such as ceramide or other products of this activity could be avoided. These products could be generated by hydrolysis of phospholipids from LDL(–) itself or from the cell membrane as reported in the case of other modified LDL (35). Moreover, PLC-like activity would be favored in the incubation with monocytes, because enzymatic activity increases at 37°C, thereby increasing the production of inflammatory cell mediators. This effect would be counteracted in the case of preincubation of LDL(–) with HDL and apoAI.
PLC-like activity is related to the higher degree of aggregation level of LDL(–) (19) and to its increased affinity to PG (17). Therefore, inhibition of phospholipolytic activity by HDL could lead LDL(–) to present lower aggregation and decreased binding to PG. Moreover, NEFA content of lipoproteins is also related to aggregation (36); hence, a decrease in NEFA could also promote a lower aggregation in LDL(–). In fact, LDL(–) is prone to be aggregated in vitro, but we found that HDL and apoAI inhibited its susceptibility to aggregation as described for native LDL in the presence of HDL and apoAI (4). In the case of LDL(–), the decrease in susceptibility to aggregation would possibly not be promoted by inhibition of PLC-like activity but be a consequence of structural or compositional changes such as NEFA decrease in the case of incubation with HDL. In this respect, it has been proposed that apoAI could interact with exposed hydrophobic domains of LDL with perturbed structure and block the intermolecular interactions that cause aggregation (4). However, HDL treatment did not change the basal aggregation level or the affinity of LDL(–) to PG. Hence, HDL prevents susceptibility to aggregation of LDL(–) without diminishing the basal level of aggregation that would be responsible for its increased binding to PG.
Regarding apoAI, an atheroprotective role by inhibiting LDL oxidation and monocyte chemotactic activity has been described (6, 7, 10) and, moreover, it is also able to inhibit LPS-induced inflammation response in cells (37). In our experiments, apoAI seemed to be at least in part responsible for the HDL effect on LDL(–) because it inhibited cytokine release, PLC-like activity, and susceptibility to aggregation. In contrast to apoAI, HDL gains NEFA content and the ability to induce release in parallel with a loss of these properties in LDL(–). This could be related to the observation that HDL can become atherogenic in situations such as acute-phase response and inflammatory states in which HDL loses its protective role and undergoes remarkable structural alterations (9, 38). Lack of cytokine release promoted by apoAI after being treated with LDL(–) could be related to an inability to be recognized by cellular receptors, whereas the whole structure of HDL is, or to its ineffectiveness to gain NEFA, as NEFA develop a role in cytokine induction. In contrast, PLC-like activity of apoAI-LDL(–) would be related to another factor, probably gain of a protein as yet not identified with this enzymatic activity.
In summary, the current study describes a new anti- inflammatory effect of HDL on LDL bioactivity. We found that HDL minimizes the cytokine release induced in monocytes by LDL(–), a nonoxidized LDL subfraction present in plasma. ApoAI seems to be involved at least in part in the counteracting effect of HDL on LDL(–) action. This protective effect of HDL could be mediated by promoting the decrease of NEFA content, PLC-like activity, and susceptibility to aggregation in LDL(–). Nevertheless, further studies should be conducted to delve further into the mechanisms involved in this action.
Acknowledgments
The authors are grateful to Carolyn Newey for editorial assistance.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- BODIPY
- borondipyrromethene
- GAG
- glycosaminoglycan
- GGE
- gradient gel electrophoresis
- IL
- interleukin
- LDL(–)
- electronegative LDL
- LPC
- lysophosphatidylcholine
- LPS
- lipopolysaccharide
- mmLDL
- minimally modified LDL
- NEFA
- nonesterified fatty acid
- PAF-AH
- platelet-activating factor acetylhydrolase
- PC
- phosphatidylcholine
- PG
- proteoglycans
- PLC
- phospholipase C
This work was supported by grants from Fondo de Investigación Sanitaria FIS CP04/110, FIS PI09/160, and FIS PI06/500. S. Benítez is a recipient of the postdoctoral contract FIS CP04/110 and J. L Sánchez of CP06/220. C. Bancells and R. Birkelund are recipients of predoctoral fellowships from the Ministry of Education and Science (MEC) and Generalitat de Catalunya (AGAUR), respectively.
REFERENCES
- 1.Gordon D. J., Rifkind B. M. 1989. High-density lipoprotein-the clinical implications of recent studies. N. Engl. J. Med. 321: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 2.Rader D. J. 2006. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 116: 3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rye K. A., Barter P. J. 2008. Antiinflammatory actions of HDL: a new insight. Arterioscler. Thromb. Vasc. Biol. 28: 1890–1891. [DOI] [PubMed] [Google Scholar]
- 4.Khoo J. C., Miller E., McLoughlin P., Steinberg D. 1990. Prevention of low density lipoprotein aggregation by high density lipoprotein or apolipoprotein A-I. J. Lipid Res. 31: 645–652. [PubMed] [Google Scholar]
- 5.Parthasarathy S., Barnett J., Fong L. G. 1990. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta. 1044: 275–283. [DOI] [PubMed] [Google Scholar]
- 6.Navab M., Hama S. Y., Anantharamaiah G. M., Hassan K., Hough G. P., Watson A. D., Reddy S. T., Sevanian A., Fonarow G. C., Fogelman A. M. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41: 1495–1508. [PubMed] [Google Scholar]
- 7.Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., et al. 2000. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41: 1481–1494. [PubMed] [Google Scholar]
- 8.Murphy A. J., Woollard K. J., Hoang A., Mukhamedova N., Stirzaker R. A., McCormick S. P., Remaley A. T., Sviridov D., Chin-Dusting J. 2008. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 28: 2071–2077. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi L., Gomaraschi M., Franceschini G. 2003. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 23: 1724–1731. [DOI] [PubMed] [Google Scholar]
- 10.Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H., et al. 1991. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88: 2039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackness M. I., Arrol S., Durrington P. N. 1991. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 286: 152–154. [DOI] [PubMed] [Google Scholar]
- 12.Watson A. D., Navab M., Hama S. Y., Sevanian A., Prescott S. M., Stafforini D. M., McIntyre T. M., Du B. N., Fogelman A. M., Berliner J. A. 1995. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J. Clin. Invest. 95: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Quesada J. L., Otal-Entraigas C., Franco M., Jorba O., Gonzalez-Sastre F., Blanco-Vaca F., Ordonez-Llanos J. 1999. Effect of simvastatin treatment on the electronegative low-density lipoprotein present in patients with heterozygous familial hypercholesterolemia. Am. J. Cardiol. 84: 655–659. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Quesada J. L., Perez A., Caixas A., Ordonmez-Llanos J., Carreras G., Payes A., Gonzalez-Sastre F., de Leiva A. 1996. Electronegative low density lipoprotein subform is increased in patients with short-duration IDDM and is closely related to glycaemic control. Diabetologia. 39: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Quesada J. L., Camacho M., Anton R., Benitez S., Vila L., Ordonez-Llanos J. 2003. Electronegative LDL of FH subjects: chemical characterization and induction of chemokine release from human endothelial cells. Atherosclerosis. 166: 261–270. [DOI] [PubMed] [Google Scholar]
- 16.Benitez S., Sanchez-Quesada J. L., Lucero L., Arcelus R., Ribas V., Jorba O., Castellvi A., Alonso E., Blanco-Vaca F., Ordonez-Llanos J. 2002. Changes in low-density lipoprotein electronegativity and oxidizability after aerobic exercise are related to the increase in associated non-esterified fatty acids. Atherosclerosis. 160: 223–232. [DOI] [PubMed] [Google Scholar]
- 17.Bancells C., Benitez S., Jauhiainen M., Ordonez-Llanos J., Kovanen P. T., Villegas S., Sanchez-Quesada J. L., Oorni K. 2009. High binding affinity of electronegative LDL to human aortic proteoglycans depends on its aggregation level. J. Lipid Res. 50: 446–455. [DOI] [PubMed] [Google Scholar]
- 18.Benitez S., Sanchez-Quesada J. L., Ribas V., Jorba O., Blanco-Vaca F., Gonzalez-Sastre F., Ordonez-Llanos J. 2003. Platelet-activating factor acetylhydrolase is mainly associated with electronegative low-density lipoprotein subfraction. Circulation. 108: 92–96. [DOI] [PubMed] [Google Scholar]
- 19.Bancells C., Benitez S., Villegas S., Jorba O., Ordonez-Llanos J., Sanchez-Quesada J. L. 2008. Novel phospholipolytic activities associated with electronegative low-density lipoprotein are involved in increased self-aggregation. Biochemistry. 47: 8186–8194. [DOI] [PubMed] [Google Scholar]
- 20.Benitez S., Villegas V., Bancells C., Jorba O., Gonzalez-Sastre F., Ordonez-Llanos J., Sanchez-Quesada J. L. 2004. Impaired binding affinity of electronegative low-density lipoprotein (LDL) to the LDL receptor is related to nonesterified fatty acids and lysophosphatidylcholine content. Biochemistry. 43: 15863–15872. [DOI] [PubMed] [Google Scholar]
- 21.Benitez S., Camacho M., Bancells C., Vila L., Sanchez-Quesada J. L., Ordonez-Llanos J. 2006. Wide proinflammatory effect of electronegative low-density lipoprotein on human endothelial cells assayed by a protein array. Biochim Biophys Acta. 1761: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 22.Hodis H. N., Kramsch D. M., Avogaro P., Bittolo-Bon G., Cazzolato G., Hwang J., Peterson H., Sevanian A. 1994. Biochemical and cytotoxic characteristics of an in vivo circulating oxidized low density lipoprotein (LDL-). J. Lipid Res. 35: 669–677. [PubMed] [Google Scholar]
- 23.Chen C. H., Jiang T., Yang J. H., Jiang W., Lu J., Marathe G. K., Pownall H. J., Ballantyne C. M., McIntyre T. M., Henry P. D., et al. 2003. Low-density lipoprotein in hypercholesterolemic human plasma induces vascular endothelial cell apoptosis by inhibiting fibroblast growth factor 2 transcription. Circulation. 107: 2102–2108. [DOI] [PubMed] [Google Scholar]
- 24.Benitez S., Bancells C., Ordonez-Llanos J., Sanchez-Quesada J. L. 2007. Pro-inflammatory action of LDL(–) on mononuclear cells is counteracted by increased IL10 production. Biochim. Biophys. Acta. 1771: 613–622. [DOI] [PubMed] [Google Scholar]
- 25.Bancells C., Sánchez-Quesada J. L., Birkelund R., Ordóñez-Llanos J., Benitez S. 2010. Electronegative LDL induces Fas and modifies gene expression in mononuclear cells. Front. Biosci.(Elite Ed.) 2: 78–86. [DOI] [PubMed] [Google Scholar]
- 26.De Castellarnau C., Sanchez-Quesada J. L., Benitez S., Rosa R., Caveda L., Vila L., Ordonez-Llanos J. 2000. Electronegative LDL from normolipemic subjects induces IL-8 and monocyte chemotactic protein secretion by human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20: 2281–2287. [DOI] [PubMed] [Google Scholar]
- 27.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 28.Benitez S., Camacho M., Arcelus R., Vila L., Bancells C., Ordonez-Llanos J., Sanchez-Quesada J. L. 2004. Increased lysophosphatidylcholine and non-esterified fatty acid content in LDL induces chemokine release in endothelial cells. Relationship with electronegative LDL. Atherosclerosis. 177: 299–305. [DOI] [PubMed] [Google Scholar]
- 29.Auerbach B. J., Kiely J. S., Cornicelli J. A. 1992. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal. Biochem. 201: 375–380. [DOI] [PubMed] [Google Scholar]
- 30.Gaubatz J. W., Gillard B. K., Massey J. B., Hoogeveen R. C., Huang M., Lloyd E. E., Raya J. L., Yang C. Y., Pownall H. J. 2007. Dynamics of dense electronegative low density lipoproteins and their preferential association with lipoprotein phospholipase A(2). J. Lipid Res. 48: 348–357. [DOI] [PubMed] [Google Scholar]
- 31.Stafforini D. M., McIntyre T. M., Zimmerman G. A., Prescott S. M. 1997. Platelet-activating factor acetylhydrolases. J. Biol. Chem. 272: 17895–17898. [DOI] [PubMed] [Google Scholar]
- 32.Holopainen J. M., Medina O. P., Metso A. J., Kinnunen P. K. 2000. Sphingomyelinase activity associated with human plasma low density lipoprotein. J. Biol. Chem. 275: 16484–16489. [DOI] [PubMed] [Google Scholar]
- 33.Deigner H. P., Friedrich E., Sinn H., Dresel H. A. 1992. Scavenging of lipid peroxidation products from oxidizing LDL by albumin alters the plasma half-life of a fraction of oxidized LDL particles. Free Radic. Res. Commun. 16: 239–246. [DOI] [PubMed] [Google Scholar]
- 34.Laulederkind S. J., Bielawska A., Raghow R., Hannun Y. A., Ballou L. R. 1995. Ceramide induces interleukin 6 gene expression in human fibroblasts. J. Exp. Med. 182: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinscherf R., Claus R., Deigner H. P., Nauen O., Gehrke C., Hermetter A., Russwurm S., Daniel V., Hack V., Metz J. 1997. Modified low density lipoprotein delivers substrate for ceramide formation and stimulates the sphingomyelin-ceramide pathway in human macrophages. FEBS Lett. 405: 55–59. [DOI] [PubMed] [Google Scholar]
- 36.Hakala J. K., Oorni K., Ala-Korpela M., Kovanen P. T. 1999. Lipolytic modification of LDL by phospholipase A2 induces particle aggregation in the absence and fusion in the presence of heparin. Arterioscler. Thromb. Vasc. Biol. 19: 1276–1283. [DOI] [PubMed] [Google Scholar]
- 37.Gupta H., Dai L., Datta G., Garber D. W., Grenett H., Li Y., Mishra V., Palgunachari M. N., Handattu S., Gianturco S. H., et al. 2005. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circ. Res. 97: 236–243. [DOI] [PubMed] [Google Scholar]
- 38.Kovanen P. T., Pentikainen M. O. 2003. Circulating lipoproteins as proinflammatory and anti-inflammatory particles in atherogenesis. Curr. Opin. Lipidol. 14: 411–419. [DOI] [PubMed] [Google Scholar]