Abstract
Men with castration-resistant prostate cancer (PCa) frequently develop metastasis in bone. The reason for this association is unclear. We have previously shown that cadherin-11 (also known as OB-cadherin), a homophilic cell adhesion molecule that mediates osteoblast adhesion, plays a role in the metastasis of PCa to bone. Here, we report that androgen deprivation therapy upregulates cadherin-11 expression in PCa. In human PCa specimens, immunohistochemical staining showed that 22 of 26 (85%) primary PCa tumors from men with castration-resistant PCa expressed cadherin-11. In contrast, only 7 of 50 (14%) androgen-dependent PCa tumors expressed cadherin-11. In the MDA-PCa-2b xenograft animal model, cadherin-11 was expressed in the recurrent tumors following castration. In the PCa cell lines, there is an inverse correlation between expression of cadherin-11 and androgen receptor (AR), and cadherin-11 is expressed in very low levels or not expressed in AR positive cell lines, including LNCaP, C4-2B4, and VCaP cells. We showed that AR likely regulates cadherin-11 expression in PCa through an indirect mechanism. Although re-expression of AR in the AR-negative PC3 cells led to the inhibition of cadherin-11 expression, depletion of androgen from the culture medium or down regulation of AR by RNA interference in the C4-2B4 cells or VCaP cells only produce a modest increase of cadherin-11 expression. Promoter analysis indicated that cadherin-11 promoter does not contain a typical AR binding element, and AR elicits a modest inhibition of cadherin-11 promoter activity, suggesting that AR does not regulate cadherin-11 expression directly. Together, these results suggest that androgen deprivation upregulates cadherin-11 expression in prostate cancer and this may contribute to the metastasis of PCa to bone. Our study suggests that therapeutic strategies that block cadherin-11 expression or function may be considered when applying androgen ablation therapy.
Keywords: PCa, androgen receptor, cadherin-11, bone metastasis, osteoblast
Introduction
Prostate cancer (PCa) is the most common cancer in man. While improved efforts at diagnosis have resulted in early diagnosis and early treatments, a portion of patients relapses after local therapy [1]. Because the proliferation of PCa cells is regulated and stimulated by engagement of the androgen receptor (AR) by androgens, androgen ablation therapy is the mainstay of treatment for advanced PCa [1]. However, ultimately all patients on androgen deprivation therapy invariably become resistant to hormonal manipulation [2].
Castration-resistant progression is frequently associated with the development of metastasis in bone, a lethal progression of the disease that accounts for the majority of disease-related mortality. The mechanism linking the castration-resistant progression to metastasis in bone is not known. One of the key events associated with the tropism of PCa for bone likely involves cell-cell binding between the cancer and bone marrow cells. As PCa progresses, cancer cells may undergo epithelial to mesenchymal transition, resulting in the up-regulation of adhesion molecules that mediate interactions with other cell types.
In our previous studies, we found that osteoblast cadherin (cadherin-11, OB-cadherin) plays a role in the metastasis of PCa cells to bone. We have previously shown that the homophilic cell adhesion molecule osteoblast (OB)-cadherin is expressed in PCa cell lines derived from bone metastasis and in metastatic lesions particularly in bone [3]. In addition, we found that expression of cadherin-11 in PCa cells increases the motility and invasiveness of PCa cells (manuscript submitted for publication). Furthermore, in an intracardiac injection model, downregulation of cadherin-11 in PC3-mm2 cells significantly decreased their ability to colonize in bone in vivo [3]. These observations suggest that aberrant expression of cadherin-11 in PCa cells may contribute to bone homing by facilitating adhesion between PCa cells and osteoblasts. Because metastases in bone frequently occur following castration-resistant progression, we hypothesize that androgen/AR pathways may be involved in the expression of cadherin-11 in PCa cells.
In this study, we examined the effect of androgen/AR on cadherin-11 expression. We found that androgen depletion is one of the mechanisms that lead to the upregulation of cadherin-11 in PCa.
Methods
Cell Cultures and Androgen Treatment
Human PCa cell line PC3-mm2 was provided by Dr. I. J. Fildler (M.D. Anderson Cancer Center). PC3 and LNCaP cell lines were purchased from American Type Culture Collection (ATCC; Manassas, CA). C4-2B4 is a LNCaP subline generated from multiple passages of LNCaP in mice [4] and was kindly provided by Dr. Robert Sikes (University of Delaware). All four cell lines were maintained in RPMI 1640 supplemented with 10% FBS. VCaP cell line (ATCC; Manassas, CA) [5] was maintained in DMEM with 10% FBS. For androgen treatment, cells were cultured in phenol red free medium supplemented with 5% charcoal-stripped FBS (cFBS) (Invitrogen) or 5% FBS, and treated with DHT or R1881 for 3 days. For bicalutamide (Casodex) treatment, cells were cultured in phenol red free medium supplemented with 5% charcoal-stripped FBS (cFBS) (Invitrogen) plus 1nM DHT, and treated with various concentrations of bicalutamide for 3 days.
Androgen-Independent Human Prostate Cancer Specimens
Formalin-fixed, paraffin-embedded tissue samples of localized PCa from men who had undergone salvage prostatectomy subsequent to androgen deprivation therapy were selected from the PCa tissue bank (supported by a SPORE award to The University of Texas M.D. Anderson Cancer Center).
Androgen Ablation in Mice Bearing Subcutaneous Tumors
MDA-PCa-2b cells (4 × 106) were injected subcutaneously into 6- to 8-week-old male nude mice. Tumor development was monitored and surgical castration was performed when tumor volume reached about 500 mm3. Mice were killed and tumors collected before castration (2 tumors), at 1 week after castration (3 tumors), at 2 weeks after castration (3 tumors), and at the 15th week after castration (2 tumors). All tumors were fixed in formalin and embedded in paraffin for immunostaining. Immunostaining was performed as described previously [3].
Reverse Transcription PCR and quantitative PCR Analysis
Total RNA was extracted from cells using TRIZOL (Invitrogen), according to the manufacturer’s instructions, followed by DNase I treatment and purified through the RNeasy mini kit (Invitrogen). Reverse transcription PCR (RT-PCR) was done with the following primers; human cadherin-11 forward, 5’-ACCCTCACCATCAAAGTCTG-3. and reverse, 5’-TCAGGGTCACAAACAATACT; human androgen receptor, forward, 5’-CGGGGACATGCGTTTGGAGA-3’, reverse, 5’-AGTTGCGGAAGCCAGGCAAG-3’; glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), forward, 5’-TGATGACATCAAGAAGGTGGTGAAG-3’ and reverse, 5’-TCCTTGGAGGCCATGTGGGCCAT. Expression levels of cadherin-11 or androgen receptor were quantified using quantitative real-time PCR and normalized by beta-actin expression level. The specific primers and probes for quantitative real-time PCR were purchased from Applied Biosystems. Real-time PCR were performed with Taqman gene expression master mix using ABI prism 7500 sequence detection system (Applied Biosystems).
Western Blotting
Cells were washed with PBS and lysed in lysis buffer (2%NP-40, 20mM Tris, pH, 7.4, 5mM MgCl2 and 5mM EDTA) before being processed for Western blotting. Membranes were probed with a monoclonal anti-human cadherin-11 (Invitrogen), rabbit polyclonal anti-human androgen receptor (Upstate), or monoclonal anti-alpha-tublin (Santz Cruz, CA) antibody. Proteins were detected using super signal chemiluminenscence kit (Pierce).
Generation of PC3-mm2-AR Cells
The human AR cDNA in the pcDNA 3.1-AR vector was subcloned into plasmid pBMN-I-GFP (a gift from Dr. Gary Nolan, Stanford University) through NotI/BamHI sites to generate pBMN-AR-I-GFP. The pBMN-AR-I-GFP plasmid was used to generate retrovirus [6] and to infect PC3-mm2 cells. The transduced PC3-mm2 cells were selected based on GFP positivity by fluorescence activated cell sorting.
shRNA Transfection
Androgen receptor specific shRNA and control non-target shRNA (mission shRNA, Sigma) were electroporated into C4-2B4 cells using the Amaxa Nucleofector II. After transfection, cells were grown for additional 4 days and total RNA were prepared for further analyses.
Cadherin-11 ELISA
ELISA assay was performed by coating anti-cadherin-11 monoclonal antibody 2C7 (4 µg/ml in PBS) in 96-well dish overnight. Cell lysate prepared from L-cadherin11 cells [6] was used as standards. Samples and standards were incubated in the wells for 2 hours. The captured cadherin-11 was then incubated with the biotinylated anti-cadherin-11 antibody, which was generated by biotinylation of Cad11 MoAb (Invitrogen) with sulfo-NHS-LC-Biotin (Pierce). After binding with Streptavidin –HRP (R&D), the color was developed using color reagent A and B (R&D).
Cloning of the Human Cadherin-11 Promoter
The promoter region of the human cadherin-11 gene was cloned by PCR of genomic DNA prepared from PC-3 cells. Primers specific to the upstream 2-kb region of exon 1, forward primer 5′-CCACGATCTGCTCTCTGGCGCTCTACAGGA-3′ and reverse primer 5′-GGGGCCCTTGAGGGTGGACGCAACCTCCGAGCC-3′, were used and the expected size of the PCR amplicon is 2 kb. The 2 kb fragment was subcloned into pGL3 plasmid (Promega) through NheI/BglII sites (pGL-2kb). Plasmid containing 1 kb promoter fragment was generated from pGL-2kb by removing the 1 kb upstream promoter sequence by Kpn1 restriction enzyme digestion followed with self-ligation (pGL-1kb). The plasmids containing 0.5, 0.4, 0.3, 0.2, 0.1, 0.05 kb promoter fragments were generated by PCR using the pGL-2kb as template. All of the promoter fragments were subcloned into pGL3.
Promoter activity measurement
All transfection assays were performed using Fugene 6 Transfection Reagent (Roche) and used 24-well plate. The cadherin-11 promoter constructs (50 ng/well), the AR plasmid (100 ng/well), and the Renilla reporter (5 pg/well) were co-transfected into 293 cells while the cadherin-11 promoter constructs and the Renilla reporter were co-transfected into PC3-mm2-AR cells. The transfected cells were grown in 5% cFBS with or without 10 nM DHT for 48 hrs and the promoter activity were determined using the Dual Luciferase Reporter Assay (Promega). The luciferase activity was measured at 20/20 Luminometer (Turner Biosystem) and expressed as a ratio of Firefly luciferase activity to Renilla luciferase activity.
Results
Cadherin-11 Expression in Recurrent Tumors After Androgen-Deprivation Therapy
To study whether androgen deprivation therapy affects cadherin-11 expression, prostate tumors were obtained from patients undergoing salvage prostectomy due to local recurrence after androgen deprivation therapy. We found that 22 of the 26 specimens showed increased cadherin-11 expression by immunohistochemical analysis (Figure 1). These observations are in contrast to the primary prostate specimens from patients without androgen deprivation therapy, in which most of the samples did not express cadherin-11 (Figure 1) and only 7 of the 50 specimens showed focal cadherin-11 expression [3]. Statistical analysis using chi-square test indicated a p value of less than 0.00001 between castration-resistant and androgen-dependent tumors. Because recurrent tumors are poorly differentiated, thus may have higher Gleason scores than the primary tumors, it is possible that cadherin-11 expression in recurrent tumors may correlate with higher Gleason scores. We used the same criteria to score the androgen-dependent and castration-resistant prostate tumors. We found that there is no correlation between cadherin-11 expression and Gleason score in either androgen-dependent samples (13–17%) (p=1.0, Chi-square test) or recurrent tumors (75–100%) (p=1.0, Chi-square test) (Table 1). These observations suggest that androgen deprivation therapy upregulates cadherin-11 expression in prostate cancer.
Figure 1.

Cadherin-11 expression in human prostate tumors from patients with androgen-dependent and androgen-independent disease status. The immunohistochemical staining patterns of three representative tumors from each group are shown. The majority of androgen-independent tumors stained positive (brown) with anti-cadherin-11 antibody while androgen-dependent prostate tumors do not. Original magnification, ×400.
Table 1.
Cadherin-11 expression in primary and recurrent prostate cancer with different Gleason score.
| Gleason Score | Primary Tumors (#cadherin-11 positive/# samples) |
Recurrent Tumors (#cadherin-11 positive/# samples) |
|---|---|---|
| 6 | 17% (2/12) | 100% (1/1) |
| 7 | 14% (1/7) | 100% (3/3) |
| 8 | 13% (1/8) | 80% (4/5) |
| 9 | 13% (2/15) | 75% (9/12) |
| 10 | 13% (1/8) | 100% (5/5) |
Effect of Castration on Cadherin-11 Expression in MDA-PCa-2b Tumors
We then determined the effect of castration on cadherin-11 expression in a xenograft tumor model. The androgen receptor positive MDA-PCa-2b cells were injected into male SCID mice and tumors were collected from mice before castration (pre-castration), one week post-castration, two weeks post-castration, and fifteen weeks post-castration (recurrent tumors). We examined the expression of cadherin-11 in these tumors by immunohistochemistry. As shown in Figure 2, cadherin-11 expression was not detectable before castration (0/2) or at one-week post-castration (0/3). At two-weeks post-castration, one of the three tumors expressed cadherin-11 (1/3). The recurrent tumors at 15 weeks post-castration were all positive with cadherin-11 (2/2). These observations suggest that cadherin-11 expression is upregulated in the castration-induced recurrent tumors.
Figure 2.

Expression of cadherin-11 in MDA-PCa-2b tumors before and after castration. Tumors collected before castration, one week post-castration, two weeks post-castration, and 15 weeks post-castration were immunostained with a goat anti-cadherin-11 polyclonal antibody as described previously [3]. Tumors generated by injecting PC3 cells subcutaneously into nude mice was used as positive controls. Original magnification, ×400.
Cadherin-11 is mainly expressed in AR-negative PCa cell lines
Next, we examined cadherin-11 expression and AR status in several established PCa cell lines. Using gene-specific RT-PCR, we found that steady-state levels of cadherin-11 transcripts were detected in the AR-negative PC-3 cells but not in the AR-positive cell lines such as VCaP, LNCaP, C4-2B4, and MDA-PCa-2b (Figure 3a). Using antibodies against cadherin-11 or AR in western blotting, we found that the expression of cadherin-11 and AR protein in these cell lines correlated with the presence of their respective RNA messages (Figure 3b). These data suggest that there is an inverse relationship between AR status and cadherin-11 expression in PCa cell lines.
Figure 3.

Expression of cadherin-11 and AR in PCa cells. (a) The levels of cadherin-11 and AR transcripts were examined by semi-quantitative RT-PCR. GAPDH was used as a loading control. (b) The protein levels of cadherin-11 and AR in the PCa cell lines as indicated were examined by Western blotting. Tubulin was used as a loading control.
Re-expression of AR in PC-3 cells decreased cadherin-11 expression
To examine whether AR activity is involved in the regulation of cadherin-11 expression in PCa cells, we examined the expression of cadherin-11 in PC3-AR(A2) cell line [7], a PC3 cell line in which AR expression has been introduced via the stable transfection of a wild type AR cDNA expression plasmid. We compared the effect of AR activity on cadherin-11 expression by treating PC3-AR(A2) cells with different doses of dihydrotestosterone (DHT) or R1881 in medium containing 5% charcoal-stripped fetal bovine serum (cFBS). Analyses by semi-quantitative RT-PCR (Figure 4a) or TaqMan quantitative real-time PCR (Q-PCR) (Figure 4b) showed that DHT or R1881 treatments led to a decrease (around 65%) in cadherin-11 transcript levels (Figure 4a and b). A dose-dependence was observed between 0 to 1 nM DHT and increase in the concentrations of DHT or R1881 from 1 nM to 10 nM did not further enhance the inhibition (Figure 4b). Analysis by Western blotting showed an approximate 50% reduction of cadherin-11 protein in androgen-treated PC3-AR(A2) cells (Figure 4c). These results confirmed that activated AR could indeed negatively affect cadherin-11 expression in PCa cells. Given that the decrease in transcript levels correlated with the decrease in the protein levels, our data suggest that AR activity regulates cadherin-11 expression primarily at the transcriptional level.
Figure 4.
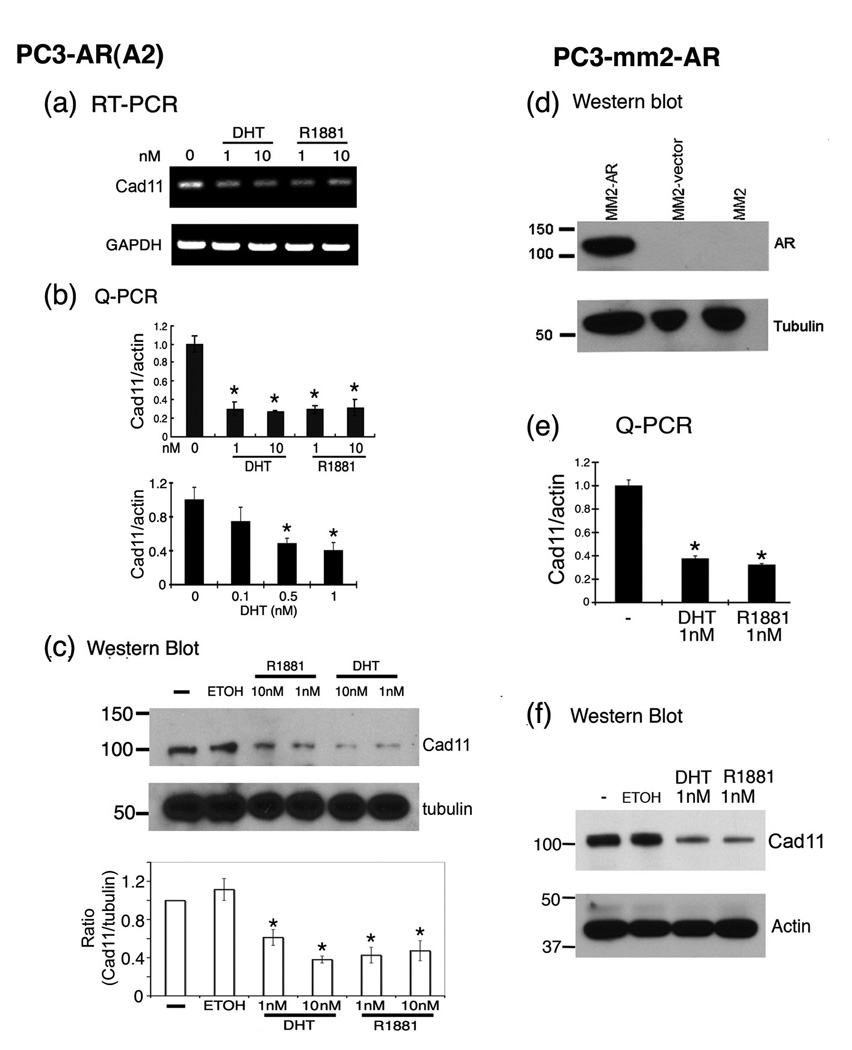
Suppression of cadherin-11 expression by re-expressing AR in PC3 and PC3-mm2 cells. (a) PC3-AR(A2) cells grown in charcoal-stripped fetal bovine serum or treated with DHT or R1881 at the concentrations as indicated. RNAs were prepared and cadherin-11 transcripts were detected by semi-quantitative RT-PCR. (b) Quantification of the levels of cadherin-11 transcripts by real-time PCR. (c) Western blotting of cadherin-11 from the PC3-AR(A2) cells treated with androgens. (d) Western blotting for androgen receptor (AR) expression in PC3-mm2 cells transduced with AR retroviral vector. PC3-mm2 cells or PC3-mm2 cells transduced with control retroviral vector were used as a control. (e) The levels of cadherin-11 transcripts were determined by real-time PCR after PC3-mm2-AR cells were treated with 1 nM of DHT or R1881 for 48 hrs. (f) The levels of cadherin-11 proteins in PC3-mm2-AR cells were determined by Western blot. *, p < 0.01.
To eliminate the possibility that the effect of AR on cadherin-11 expression in PC3-AR(A2) cells is due to clonal effect, we generated pooled AR-expressing PC3-mm2 cells. PC3-mm2 is a highly metastatic variant of the PC3 cell line [8]. We used a bicistronic retroviral expression system to introduce wild type AR together with GFP into PC3-mm2 cells and the AR-expressing PC3-mm2 cells were selected by FACS sorting. The expression of AR in these cells was confirmed by Western blot using anti-AR antibody (Figure 4d). Similar to those with PC3-AR(A2) cells, we found that treatment of PC3-mm2-AR cells with 1 nM of DHT or R1881 led to the inhibition of cadherin-11 expression in both the RNA transcript (Figure 4e) and protein levels (Figure 4f). Addition of androgen to PC3 cells, which do not express AR, does not suppress cadherin-11 expression in PC-3 cells (data not shown), suggesting that suppression of cadherin-11 expression requires AR. Thus, our data suggest that androgen stimulation of the AR pathway suppresses cadherin-11 expression in PC3-AR(A2) and PC3-mm2 cells.
Effect of androgen depletion or knockdown of AR on cadherin-11 expression
Next, we examined whether depleting androgens from the culture medium has an effect on cadherin-11 expression in AR-positive C4-2B4 or VCaP cells, which express AR (Figure 5a). Both C4-2B4 and VCaP cells expressed very low to undetectable levels of cadherin-11. When VCaP cells were grown in phenol red-free medium supplemented with cFBS instead of FBS, we detected a small but significant increase in cadherin-11 message by Q-PCR (Figure 5a). Addition of DHT (1 nM) led to a partial inhibition of cadherin-11 expression. Because depletion of organic compounds in FBS by activated charcoal also removes other factors, e.g. estrogens, this observation suggests that other factors in the FBS may also play a role in modulating cadherin-11 expression. Western blot did not detect the increase of cadherin-11 in protein level (data not shown), likely due to the small increase in cadherin-11 message. However, a more sensitive ELISA did detect a small increase (4.4±1.0 fold) in cadherin-11 protein levels in cFBS treated VCaP cells compared to that in FBS treated cells (Figure 5a). Addition of DHT to cFBS inhibited the expression of cadherin-11 protein (Figure 5a). A similar result was obtained when C4-2B4 cells were grown on cFBS compared to that in FBS (2.3±0.5 fold), and the addition of DHT to cFBS suppressed cadherin-11 protein expression (Figure 5a). Because the AR antagonist bicalutamide (Casodex) is commonly used in the treatment of prostate cancer, we also examined the effect of bicalutamide [9] on cadherin-11 expression. C4-2B4 or VCaP cells were treated with various doses of bicalutamide and cadherin-11 expression was assessed by ELISA. We found that bicalutamide treatments led to an upregulation of cadherin-11 expression in these PCa cells (Fig. 5b).
Figure 5.

Induction of cadherin-11 expression by inactivation of AR activity. (a) VCaP cells were cultured in 10% fetal bovine serum (FBS), charcoal-stripped FBS (cFBS), or cFBS supplemented with 1nM DHT for 72 h, and cadherin-11 transcripts were detected by real-time PCR. The protein levels of cadherin-11 were determined by ELISA. (b) C4-2B4 or VCaP cells were cultured in cFBS supplemented with 1nM DHT plus various concentrations of bicalutamide as indicated for 72 h. The protein levels of cadherin-11 were determined by ELISA. (c) Semi-quantitative RT-PCR and real-time PCR was used for detection of AR and cadherin-11 transcript in the control shRNA or the AR-specific shRNA treated C4-2B4 cells. All experiments were repeated three times with similar results. *, p < 0.05.
We further used RNA interference to transiently knockdown AR expression in C4-2B4 cells. AR-specific shRNA expression plasmid in a lentiviral vector was used to deliver the shRNA. Cells transfected with non-specific shRNA were used as controls. Using semi-quantitative RT-PCR and Q-PCR analyses, we found that the cadherin-11 message was very low in the C4-2B4 cells and its level was increased (5 fold) after knocking down AR levels in C4-2B4 cells in culture (Figure 5c). Together, these results suggest that inactivation of AR activity, by removal of the ligand or its receptor or by treatment with an AR antagonist, causes a modest but significant increase in cadherin-11 expression in AR expressing PCa cells.
Cloning and characterization of human cadherin-11 promoter
The promoter region of cadherin-11 has not been reported. Upon analysis of the genomic structure of human cadherin-11, we found that cadherin-11 contains 13 exons with the first ATG located in exon 3 and the termination codon in exon 13 (Figure 6a). Interestingly, the first two exons, which contain the 5’ untranslated region, are about 117 kb upstream of exon 3. The sizes for introns 1 and 2 are unusually large, at 68 kb and 49 kb, respectively (Figure 6a). We cloned a 2-kb human cadherin-11 promoter fragment located upstream of exon 1 by PCR of the genomic DNA from PC-3 cells (Figure 6b). Deletion mutants of the 2-kb promoter, i.e. 1 kb, 0.5 kb, 0.4 kb, 0.3 kb, 0.2 kb, 0.1 kb and 0.05 kb were also generated (Figure 6b). Transient transfection experiments showed that the minimal promoter for cadherin-11 is present between 0.2 kb and 0.1 kb region (Figure 6c), which contains several E-box transcriptional binding sites; however, a bona fide androgen receptor response element (ARE) was not detected in this region by DNA sequence analysis. Co-transfection of AR with cadherin-11 promoter constructs into 293 cells led to a small, but significant, inhibition of cadherin-11 promoter activity in the presence of DHT (Figure 6c). Similarly, treatment of PC3-mm2-AR cells with DHT suppressed the transfected cadherin-11 promoter activity (Figure 6d). The small decrease in cadherin-11 promoter activity by DHT treatment suggests that AR does not suppress cadherin-11 expression directly through this 2 kb promoter. Our data suggest that AR may modulate other molecules to affect cadherin-11 expression at the transcriptional level.
Figure 6.
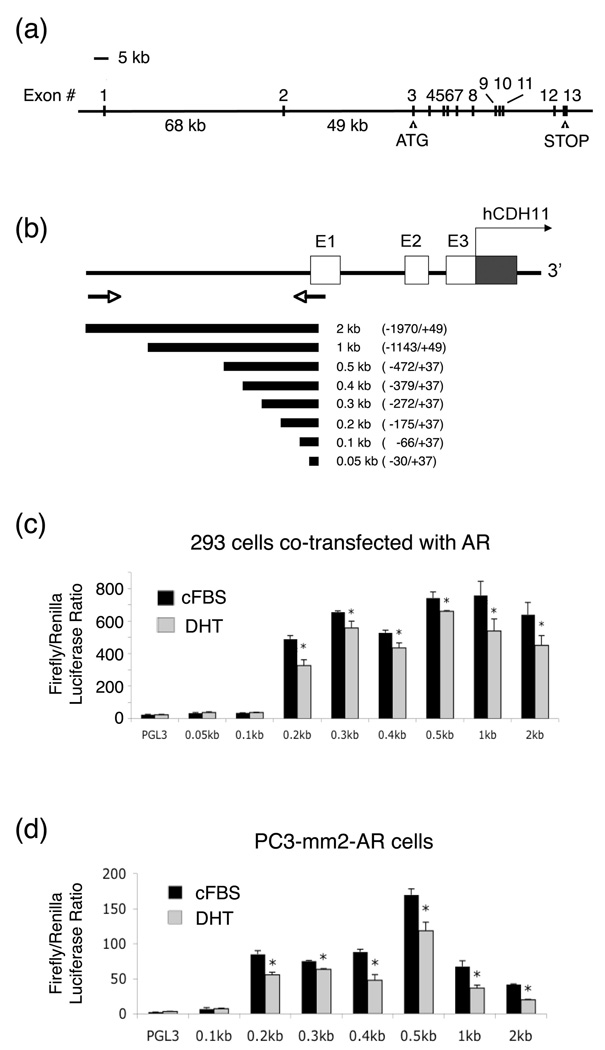
Promoter activity of human cadherin-11. (a) Genomic organization of the human cadherin-11 gene. (b) Cloning of a 2-kb fragment of the cadherin-11 promoter from PC-3 DNA. (Note: the predicted sizes of introns and exons are not drawn to scale.) (c) Transcriptional activity of cadherin-11 promoters co-transfected with AR in 293 cells. Cells were grown in charcoal-stripped FBS (cFBS) in the absence or presence of 10 nM DHT. (d) Cadherin-11 promoter activity in PC3-mm2-AR cells grown in the absence or presence of 10 nM DHT. These experiments were done twice and the luciferase assays were carried out in triplicates. *, p < 0.01.
Discussion
We showed that androgens suppress cadherin-11 expression in PCa cells. First, cadherin-11 was expressed in a majority of recurrent tumors (86%) from patients who failed androgen ablation therapy while only 14% of androgen-dependent prostate tumors express cadherin-11. Second, in the AR positive MDA-PCa-2b tumors implanted in nude mice, cadherin-11 expression was induced in these tumors following castration. Third, cadherin-11 was mainly expressed in the AR-negative PC-3 cell line and re-expression of AR in PC-3 cells resulted in a decrease in cadherin-11 expression. Fourth, cell culture analyses show that the regulation of cadherin-11 by AR activity is mediated at the transcription level. In support of these observations, studies by Best et al. [10] comparing the gene expression profiles of androgen-independent primary prostate tumor biopsies with that from primary androgen-dependent tumors found that cadherin-11 is up-regulated in androgen-independent tumors. We thus suggest that the reduction or lost of AR activity due to clinical intervention or cancer progression may lift the repression on cadherin-11 gene. Because we have previously shown that cadherin-11 plays a role in the metastasis of PCa to bone, upregulation of cadherin-11 expression in PCa cells could enhance their dissemination to bone. Our study thus provides a mechanism linking the castration-resistant progression to the proclivity of PCa to metastasize to bone. These findings raise the possibility that therapeutic strategies involving blocking cadherin-11 expression or function may reduce bone metastasis in patients undergoing androgen ablation therapy.
Our studies suggest that androgen deprivation therapy is one of the causes that lead to cadherin-11 expression in PCa cells. The mechanism by which AR suppresses cadherin-11 expression is not known. Our studies showed that AR may modulate cadherin-11 gene expression through its downstream regulator(s). First, cadherin-11 was not detected in tumors at 7 days post-castration, but it was expressed in some tumors at 14 days post-castration (Fig. 2). Because a decrease in PSA usually immediately follows castration [11], the time course of cadherin-11 expression suggests that AR regulates cadherin-11 transcription through an indirect manner. Second, we have cloned a 2-kb human cadherin-11 promoter fragment located upstream of exon 1 and identified the minimal promoter for cadherin-11 to be between the 0.2 kb and 0.1 kb region (Fig. 6). DNA sequence analysis indicated that a bona fide androgen receptor response element (ARE) [12] is not present in the 2 kb cadherin-11 promoter region. Consistently, we found that the cloned cadherin-11 promoter was active in both AR negative (PC-3) and AR positive (LNCaP) PCa cells (data not shown). The pathways that lead to suppression of cadherin-11 by AR await further investigation.
Our observations also raise an interesting question concerning whether the induction of cadherin-11 expression is due to the reduction of AR or the depletion of androgens. In the castration study using MDA-PCa-2b tumor, we found that AR was expressed in the MDA-PCa-2b tumors before castration. The AR levels were significantly decreased in 1-week and 2-week post-castration tumors, likely due to AR degradation in the absence of androgen. However, AR was re-expressed in the recurrent tumors (data not shown). Although AR protein levels increased following long time castration, the transcriptional activity of AR in the presence of a low concentration of androgens might not be sufficient to suppress cadherin-11 expression. Based on our tissue culture and prostate tumor xenograft studies, it seems that androgen depletion followed by the reduction of AR transcriptional activity is critical for cadherin-11 expression.
Prostate cancers are known to exhibit high cellular heterogeneity, which is also reflected in the PCa cell lines generated. We have previously examined cadherin-11 expression in several PCa cell lines, including the brain metastasis-derived, AR-negative DU145 cells. We found that DU145 has no detectable cadherin-11 [3]. DU145 is one exception among the nine PCa cell lines we have examined so far. Because cadherin-11 expression is regulated indirectly by AR activity, it is possible that the lack of expression of cadherin-11 in DU145 cells is due to other factors in the pathway.
Upregulation of cadherin-11 was also observed in invasive breast cancer [13], which has a high incidence of bone metastasis. As observed in PCa [3], cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone [14]. However, whether cadherin-11 expression in breast cancer cells is regulated by hormonal status is not known.
Our study raises important issues in the treatment strategies for advanced PCa. Androgen deprivation therapy is the mainstay for the treatment of advanced PCa [1]. However, our studies showed that depletion of androgen leads to upregulation of cadherin-11, which may increase the metastasis of PCa to bone. Thus, therapeutic strategies involving blocking cadherin-11 expression or its function should be considered to reduce the likelihood of bone metastasis during androgen ablation therapy. In this regard, we have recently developed a method to express a dimerized form of the extracellular domain of cadherin-11 as an Fc fusion protein (OB-CAD-Fc), which could be used to inhibit the binding between PCa cells and osteoblasts via competition for binding [6]. Alternatively, anti-cadherin-11 antibodies that interfere with the interactions between PCa and osteoblasts may be considered. Whether these blocking strategies have sufficient avidity to inhibit cadherin-11-mediated PCa bone metastasis awaits further investigation.
Acknowledgments
This work was supported in part by National Institutes of Health grants CA111479, P50-CA140388, U.S. Department of Defense grant PC093132, and an award from the Prostate Cancer Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest.
References
- 1.Ye XC, Choueiri M, Tu SM, Lin SH. Biology and clinical management of prostate cancer bone metastasis. Front Biosci. 2007;12:3273–3286. doi: 10.2741/2311. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum E, Carducci MA. Pharmacotherapy of hormone refractory prostate cancer: new developments and challenges. Expert Opin Pharmacother. 2003;4:875–887. doi: 10.1517/14656566.4.6.875. [DOI] [PubMed] [Google Scholar]
- 3.Chu K, Cheng C-J, Ye X, Lee Y-C, Zurita AJ, Chen D-T, et al. Cadherin-11 Promotes the Metastasis of Prostate Cancer Cells to Bone. Mol Cancer Res. 2008;6:1259–1267. doi: 10.1158/1541-7786.MCR-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R, et al. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15:163–168. [PubMed] [Google Scholar]
- 6.Lira CB, Chu K, Lee YC, Hu MC, Lin S-H. Expression of the extracellular domain of OB-cadherin as an Fc fusion protein using bicistronic retroviral expression vector. Protein Expr Purif. 2008;61:220–226. doi: 10.1016/j.pep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 8.Pettaway CA, Pathak S, Greene G, Ramirez E, Wilson MR, Killion JJ, et al. Selection of highly metastatic variants of different human prostatic carcinomas using orthotopic implantation in nude mice. Clin Cancer Res. 1996;2:1627–1636. [PubMed] [Google Scholar]
- 9.Furr BJ, Tucker H. The preclinical development of bicalutamide: pharmacodynamics and mechanism of action. Urology. 1996;47(1A Suppl):13–25. doi: 10.1016/s0090-4295(96)80003-3. [DOI] [PubMed] [Google Scholar]
- 10.Best CJ, Gillespie JW, Yi Y, Chandramouli GV, Perlmutter MA, Gathright Y, et al. Molecular alterations in primary prostate cancer after androgen ablation therapy. Clin Cancer Res. 2005;11:6823–6834. doi: 10.1158/1078-0432.CCR-05-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng CJ, Ye XC, Vakar-Lopez F, Kim J, Tu SM, Chen DT, et al. Bone Microenvironment and Androgen Status Modulate Subcellular Localization of ErbB3 in Prostate Cancer Cells. Mol Cancer Res. 2007;5:675–684. doi: 10.1158/1541-7786.MCR-06-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–952. [PubMed] [Google Scholar]
- 14.Tamura D, Hiraga T, Myoui A, Yoshikawa H, Yoneda T. Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int J Oncol. 2008;33:17–24. [PubMed] [Google Scholar]


