Abstract
Background
Estrogen-plus-progestin therapy increases the risk of coronary heart disease (CHD) in postmenopausal women. However, this increased risk might be limited to the first years of use and to women who start therapy late in menopause.
Objective
To estimate the effect of continuous estrogen-plus-progestin therapy on CHD risk over time and stratified by years since menopause, i.e., to estimate an adherence-adjusted effect.
Design
The Women's Health Initiative randomized, double-blinded, placebo-controlled trial.
Setting
40 US clinical centers.
Patients
16,608 postmenopausal women with an intact uterus at baseline in 1993-1998
Intervention
Conjugated equine estrogens, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d or placebo.
Measurements
Adherence-adjusted hazard ratios (HRs) estimated via inverse probability weighting and CHD-free survival curves.
Results
Compared with no use of hormone therapy, the HR (95% confidence interval [CI]) for continuous use of estrogen-plus-progestin was 2.36 (1.55-3.62) for the first 2 years and 1.69 (0.98-2.89) for the first 8 years. For women within 10 years after menopause, the HRs (95% CI) were 1.29 (0.52-3.18) for the first 2 years and 0.64 (0.21-1.99) for the first 8 years, and the CHD-free survival curves for continuous use and no use of estrogen-plus-progestin crossed at about 6 (95% CI: 2-10) years.
Limitations
The analysis may have not fully adjusted for joint determinants of adherence and CHD risk. Sample sizes for some subgroup analyses were small.
Conclusions
There was no suggestion of a decreased risk of CHD from estrogen-plus-progestin within the first 2 years after randomization, including women who initiated therapy within 10 years after menopause, and a cardioprotective effect became apparent only after 6 years of use.
INTRODUCTION
Postmenopausal women who take estrogen-plus-progestin hormone therapy have a greater risk of coronary heart disease (CHD) during the first few years after starting hormone therapy (1-3). Based on both experimental and observational findings, it has been argued that this effect of estrogen-plus-progestin therapy on CHD risk varies by time since menopause (4, 5). Under this “timing hypothesis”, it is unclear whether an increased early risk of CHD exists for newly menopausal women and, if so, whether that risk ever disappears.
To address this question, one needs to compare the CHD-free survival curve of newly menopausal women on hormone therapy with the curve of newly menopausal women not on hormone therapy. In these curves, CHD-free survival is on the vertical axis and time since starting hormone therapy or placebo is on the horizontal axis. If newly menopausal women do indeed have an increased early risk during the first several years of follow-up, the curve for those who take hormone therapy will be lower than the curve for those who do not take hormone therapy. If the increased risk disappears after several years, the curves will converge or cross (i.e., their relative position will reverse). The duration of the increased risk can be measured as the time from starting hormone therapy or placebo until the time when the curves converge or cross.
In the Nurses' Health Study, this crossover time was estimated at approximately 3 years after estrogen-plus-progestin therapy was started in women who initiated therapy within 10 years after menopause, while for women who initiated therapy more than 10 years after menopause the CHD-free survival curve for those who took hormone therapy was always lower than the curve for those who did not take hormone therapy – the curves never crossed (3). However, these estimates are imprecise and perhaps confounded because the Nurses' Health Study was an observational study.
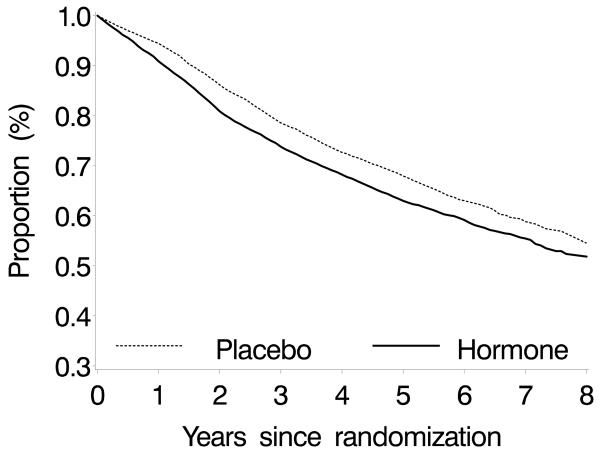
Here we estimate the effect of estrogen-plus-progestin hormone therapy on CHD risk in postmenopausal women with data from the Women's Health Initiative (WHI), a large randomized, double-blinded, placebo-controlled trial. In this study adherence to the assigned treatment decreased substantially with time (Figure 1): approximately 40% of women stopped taking at least 80% of their assigned treatment by the sixth year (1, 6). A standard intention-to-treat approach, which does not adjust for incomplete adherence, might yield a misleading estimate of the crossover time because incomplete adherence may affect the shape of the CHD-free survival curves. Our analyses adjusted for incomplete adherence to the assigned treatment.
Figure 1.
Proportion of women who took at least 80% of the study pills by treatment arm, the Women's Health Initiative estrogen-plus-progestin randomized trial
METHODS
Study design
The WHI estrogen-plus-progestin trial is a double-blinded, placebo-controlled, and multi-centered primary prevention trial in which 16,608 postmenopausal women aged 50-79 years with an intact uterus at baseline were randomized to either a daily hormone regime of 0.625mg conjugated equine estrogens plus 2.5mg medroxyprogesterone acetate (N=8,506) or matching placebo (N=8,102) between 1993 and 1998 (6). A detailed description of the trial has been published elsewhere (6, 7). The limited access dataset we used (obtained from the National Heart, Lung, and Blood Institute [NHLBI]) includes follow-up information updated through July 7, 2002, for an average follow-up of 5.6 years.
Study investigators collected data on demographics; medical, reproductive, and family history; hormone use; dietary intake; and physical examinations at baseline and during the follow-up period. They recorded safety and adherence data 6 weeks after randomization and at semi-annual interviews and annual clinical visits, when they also updated health-related information. The dataset indicates when study participants discontinued assigned treatment and when they initiated hormone therapy outside the study. It also contains estimates of the proportion of assigned pills taken, which were determined by weighing returned bottles, and self-reported frequency of use for assigned pills. CHD was defined as acute myocardial infarction requiring overnight hospitalization, silent myocardial infarction identified through serial electrocardiograms, or death due to CHD. This analysis was approved by the institutional review board at the Harvard School of Public Health, as well as the Publications and Presentations Committee of the WHI and NHLBI.
Statistical analysis
As a preliminary step, we conducted an intention-to-treat analysis to confirm that our results were similar to those previously published by WHI investigators (1, 6, 8). See the appendix for details.
We then adjusted our analyses for adherence to assigned therapy to estimate the CHD risk for continuous hormone use versus no hormone use. The adjustments used inverse probability weighting (9-11), as described in the appendix. Informally, we first estimated each woman's probability of taking her assigned treatment based on her measured prognostic factors, and then gave more weight to observations from women with low estimated probabilities than to those with high estimated probabilities. The goal was to approximate data from a study in which adherence is independent of the prognostic factors. This approach also allowed us to appropriately accommodate the variations in adherence over time and the effect of prior treatment use on subsequent adherence.
We estimated a woman's probability of taking her assigned treatment using a two-stage modeling procedure described in the appendix. To obtain unbiased adherence-adjusted effects, the method requires that all joint determinants of hormone use and CHD risk are included in the models (11). The models included sociodemographic, lifestyle, dietary, and medical factors (see appendix Table 2 for a list of these variables); the number of years since randomization; and the proportion of study pills taken during the previous year. Models that considered additional variables did not materially change the results (not shown). To improve statistical efficiency, the weights were stabilized (9-11). We did not use inverse probability weighting to adjust for selection bias (9-11) because only 3.3% of women were lost to follow-up.
Appendix Table 2.
Variables included in weight models, the Women's Health Initiative estrogen-plus-progestin randomized trial
| No. | Variables | Categories | Baseline | Time-varying |
|---|---|---|---|---|
| 1 | Randomization status in the diet modification trial |
Yes/No | Yes | No |
| 2 | Age | Five-year category | Yes | No |
| 3 | Region | Northeast, South, Midwest, West | Yes | No |
| 4 | Ethnicity | White, African-American, Hispanic, other | Yes | No |
| 5 | Education | High school or less, some college or associate degree, college and above |
Yes | No |
| 6 | Marital status | Never married, divorced or separated, widowed, presently married or married-like relationship |
Yes | No |
| 7 | Cigarette smoking | Never, past, current | Yes | Yes |
| 8 | Physical activity | Quartiles of metabolic equivalent units/week | Yes | Yes |
| 9 | Alcohol intake | None, past, <1 drink/month, <1 drink/week, 1-7 drinks/week, >7 drinks/week |
Yes | Yes |
| 10 | Body mass index | <25, 25-29, 30-34, 35-39, ≥40 kg/m2 | Yes | Yes |
| 11 | Family history of premature myocardial infarction * |
Yes/No | Yes | No |
| 12 | Family history of fracture | Yes/No | Yes | No |
| 13 | Family history of cancer | Yes/No | Yes | No |
| 14 | Personal history of coronary heart disease | Yes/No | Yes | No |
| 15 | Personal history of other cardiovascular diseases |
Yes/No | Yes | Yes |
| 16 | Personal history of diabetes | Yes/No | Yes | Yes |
| 17 | Personal history of high blood pressure | Yes/No | Yes | Yes |
| 18 | Personal history of high cholesterol | Yes/No | Yes | Yes |
| 19 | Personal history of cancer | Yes/No | Yes | Yes |
| 20 | Personal history of osteoporosis or fracture | Yes/No | Yes | Yes |
| 21 | Personal history of other comorbidities | Number of comorbidities | Yes | Yes |
| 22 | General health | Excellent, very good, good, poor/fair | Yes | Yes |
| 23 | Body pain | None, very mild, mild, moderate/severe | Yes | Yes |
| 24 | Physical functioning construct | Quartiles | Yes | Yes |
| 25 | Prior hormone use (duration) | None, <5 years, 5-9 years, ≥10 years | Yes | No |
| 26 | Use of aspirin | Yes/No | Yes | Yes |
| 27 | Use of statins | Yes/No | Yes | Yes |
| 28 | Use of oral contraceptives ever | Yes/No | Yes | No |
| 29 | Use of multivitamin | Yes/No | Yes | Yes |
| 30 | Use of vitamin E | Yes/No | Yes | Yes |
| 31 | Fruit intake | Quintiles of daily intake | Yes | No |
| 32 | Vegetable intake | Quintiles of daily intake | Yes | No |
| 33 | Number of screening/diagnostic procedures | 0, 1, 2, 3, 4, 5, ≥6 | Yes | Yes |
| 34 | Years since menopause | <10, 10-19, ≥20 years | Yes | No |
| 35 | Menopausal symptoms | Quartiles of summary score of menopausal symptoms ‡ | Yes | Yes |
| 36 | Changes in breasts† | Yes/No | No | Yes |
| 37 | Breast tenderness | None, mild, moderate/severe | No | Yes |
| 38 | Vaginal bleeding | none, spotting, light, moderate, severe | No | Yes |
<55 years old in the father or <65 years old in the mother
Including new lumps, nipple discharge, and skin changes
Including hot flushes, night sweats, vaginal or genital irritation or itching, vaginal or genital dryness, vaginal or genital discharge, headaches or migraines, joint pain or stiffness, general aches or pains, lower back pain, neck pain, bloating or gas, swelling of hands or feet, mood swings, and difficulty concentrating (25)
We then fitted a weighted pooled logistic model to estimate the average hazard ratio of CHD for continuous use versus no use of hormone therapy. To do so, we used a time-varying variable for cumulative use of hormone therapy, calculated as the sum of the annual proportion of pills taken since baseline. The effect of continuous use versus no use can be thought of as an adherence-adjusted effect: the effect we would have observed had the women been fully adherent to their assigned therapy. We used a robust variance estimator (12) to calculate conservative 95% confidence intervals (CIs) for the average hazard ratio for the first 2 and 8 years of continuous hormone use.
We fitted a separate weighted pooled logistic model that included an interaction (product) term between cumulative use and months since randomization to allow for time-varying hazard ratios, and we used a Wald test of the product term to test whether the hazard ratio varied during follow-up. The probabilities estimated from this model were used to construct standardized adherence-adjusted CHD-free survival curves (3). The 95% CI for the crossover time was estimated by 200 bootstrap samples with replacement. We used a log-rank test to determine whether the CHD-free survival curves were different. We repeated our analysis among women without prior CHD or a family history of myocardial infarction.
For women with missing data for weights of returned bottles (28% of the total person-time), we estimated the proportion of pills taken using their self-reported frequency of use (none, <1, 1-2, 3-4, 5-6, 7 days/week). When data on self-report also were missing (72% of the person-time in women with missing data for weights of returned bottles), we randomly assigned a number for pills taken that year using a uniform distribution. We also randomly assigned a number for pills taken by the 1,445 women who took hormones outside the study (562 in the hormone arm and 883 in the placebo arm). We repeated our analyses using different assumptions for missing data, for example, that women took all their pills, half their pills, none of their pills, same as previous year, or using a multiple imputation approach; we also used a different distribution and transformation, i.e., the gamma distribution instead of the uniform distribution, and arcsin-root transformation instead of log transformation. Our results were similar under all these assumptions (see appendix).
Comparison with previously published Nurses' Health Study estimates
A recent re-analysis of the observational Nurses' Health Study estimated both the intention-to-treat effect and the adherence-adjusted effect using inverse probability weighting (3). The authors found that the intention-to-treat effect estimates in the observational Nurses' Health Study were similar to the intention-to-treat effect estimates in the WHI randomized trial. They concluded that discrepancies between randomized and observational studies of hormone therapy were likely explained by the different analytic approaches, which contrasted with previous, highly publicized conclusions by others that these discrepancies were likely explained by unmeasured confounding (13).
However, the authors of the re-analysis of the Nurses' Health Study could not compare their adherence-adjusted effect estimates with adherence-adjusted effect estimates from the WHI randomized trial because no such estimates were available. This paper provides those estimates. Therefore, for comparison purposes, our tables include the adherence-adjusted effect estimates from the WHI randomized trial (first reported in this paper) and from the Nurses' Health Study (previously reported). Both sets of estimates are based on U.S. postmenopausal women with similar eligibility criteria who received the same formulation of hormone therapy. Since a Wald test showed little evidence of heterogeneity between our adherence-adjusted estimates and those from the Nurses' Health Study (3), the log hazard ratios from the two studies were weighted by the inverse of their variances to obtain a pooled estimate.
All analyses were performed with statistical software SAS (SAS Institute Inc., Cary NC).
Role of the funding source
The WHI was conducted and supported by the NHLBI in collaboration with the WHI Study Investigators. This study was partially funded by National Institutes of Health (NIH) grant R01 HL080644-01. The funding source had no role in the design, conduct, or report of this study.
RESULTS
During the follow-up period, 188 CHD cases (80 in the first 2 years) occurred in the 8,506 women assigned to hormone therapy, compared to 147 cases (51 in the first 2 years) in the 8,102 women assigned to placebo. Compared with no use of hormone therapy, the estimated hazard ratio of CHD for continuous use of estrogen-plus-progestin was 2.36 (95% CI: 1.55-3.62) for the first 2 years and 1.69 (95% CI: 0.98-2.89) for the first 8 years.
Among women within 10 years of menopause at randomization, 2,782 were randomized to receive hormone, while 2,712 were randomized to receive placebo. Thirty one (14 in the first 2 years) CHD cases were observed among those in the hormone arm, compared to 34 (12 in the first 2 years) in the placebo arm. When the adherence-adjusted analysis was restricted to women within 10 years of menopause, the estimated hazard ratios were 1.29 (95% CI: 0.52-3.18) for the first 2 years and 0.64 (95% CI: 0.21-1.99) for the first 8 years; the p-value for variation of the hazard ratio over follow-up was 0.038.
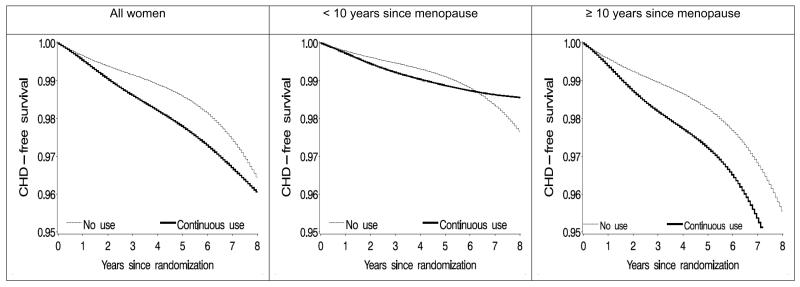
Figure 2 shows the CHD-free survival curves for continuous use of estrogen-plus-progestin and for no use of hormone therapy during the first 8 years after randomization. The p-value for differences between these two survival curves was 0.057 for all women, 0.44 for women within 10 years of menopause, and 0.011 for women more than 10 years after menopause. In women within 10 years of menopause, the curves crossed at year 6 (95% CI: 2-10). The crossover time ranged between 5 and 7 years for various dose-response models and assumptions regarding missing doses. The curves (not shown) did not cross during the first 8 years in women aged less than 60 years or 60 years or older.
Figure 2.
Estimated proportion of women free of coronary heart disease (CHD) under continuous adherence to the assigned treatment, the Women's Health Initiative estrogen-plus-progestin randomized trial
Restriction of the analysis to women without prior CHD before randomization or without a family history of premature myocardial infarction did not materially affect the results.
DISCUSSION
Our analysis of the WHI randomized trial found no suggestion of a reduced risk of CHD during the first 2 years of estrogen-plus-progestin therapy in subgroups of women defined by years since menopause (Table 1). A cardioprotective effect of estrogen-plus-progestin among women within 10 years of menopause was only apparent after approximately 6 years of use.
Table 1.
Hazard ratios (95% confidence intervals) of coronary heart disease for continuous use of estrogen-plus-progestin therapy versus no hormone therapy use by years since baseline, the Women's Health Initiative (WHI) randomized trial and the Nurses' Health Study (NHS)
| Follow-up period | ||||||
|---|---|---|---|---|---|---|
| Overall | ≤2 years | >2 years | ||||
| WHI* | NHS† | WHI‡ | NHS† | WHI§ | NHS† | |
| All women | 1.69 (0.98-2.89) | 1.30 (0.76-2.21) | 2.36 (1.55-3.62) | 1.71 (1.03-2.83) | 1.33 (0.88-2.01) | 1.07 (0.44-2.63) |
| p-value for heterogeneity ∥ | 0.50 | 0.34 | 0.67 | |||
| Years since menopause | ||||||
| < 10 | 0.64 (0.21-1.99) | 0.68 (0.24-1.91) | 1.29 (0.52-3.18) | 1.28 (0.43-3.86) | 0.63 (0.27-1.52) | 0.20 (0.03-1.54) |
| p-value for heterogeneity ∥ | 0.94 | 0.99 | 0.31 | |||
| ≥ 10 | 2.22 (1.20-4.11) | 1.57 (0.86-2.85) | 2.82 (1.73-4.60) | 1.97 (1.11-3.47) | 1.64 (1.01-2.65) | 1.37 (0.54-3.45) |
| p-value for heterogeneity ∥ | 0.43 | 0.35 | 0.74 | |||
| Age at baseline | ||||||
| 50-59 | 1.47 (0.57-3.77) | 0.91 (0.49-1.69) | 2.69 (1.14-6.36) | 1.80 (0.83-3.87) | 1.22 (0.59-2.56) | 0.54 (0.20-1.49) |
| p-value for heterogeneity ∥ | 0.40 | 0.49 | 0.20 | |||
| ≥ 60 | 1.76 (0.97-3.19) | 1.92 (0.90-4.10) | 2.30 (1.43-3.72) | 1.69 (0.87-3.32) | 1.36 (0.86-2.15) | 2.10 (0.68-6.50) |
| p-value for heterogeneity ∥ | 0.86 | 0.47 | 0.49 | |||
Eight-year cumulative use
Women age 50 or older (from Appendix Table 3 of reference 3)
Two-year cumulative use
Six-year cumulative use
Wald test for heterogeneity of the hazard ratios from two studies
These findings are consistent with those of a recent re-analysis of the observational Nurses' Health Study (3). Table 1 compares the randomized and observational estimates of the effect of continuous use of estrogen-plus-progestin. These adherence-adjusted estimates need to be compared after stratification by follow-up period and by time since menopause because women in the WHI and in the re-analysis of the Nurses' Health Study (after excluding women <50 years old) differ with respect to level of nonadherence (42% in hormone arm and 38% in placebo arm in the WHI vs. 61% for hormone initiators and 19% for non-initiators in the Nurses' Health Study), average length of follow-up (5.6 vs. 9.3 years), age distribution (67% vs. 61% aged 60 years or older) and proportion of women within 10 years of menopause (33% vs. 40%). When taken together, the findings from the WHI and the Nurses' Health Study suggest a 29% increase in CHD risk during the first 2 years of use in women within 10 years of menopause (Table 2). This result does not attain traditional statistical significance. Though we need to be cautious when drawing conclusions, it is important to note that our pooled WHI and Nurses' Health Study estimates are, and will probably be for a long time, the best available evidence on this topic. Randomized trial and observational data from the WHI have been previously combined (8, 14), but the WHI observational data contributed few events during the first 2 years after initiation of hormone therapy. One of the strengths of our pooled analysis is the large number of early events when the adverse effect of estrogen-plus-progestin use on CHD is most strikingly manifested.
Table 2.
Pooled hazard ratios (95% confidence intervals) of coronary heart disease for continuous use of estrogen-plus-progestin therapy versus no hormone therapy use, the Women's Health Initiative randomized trial and the Nurses' Health Study
| Follow-up period | |||
|---|---|---|---|
| Overall | ≤2 years | >2 years | |
| All women | 1.48 (1.02-2.16) | 2.06 (1.49-2.86) | 1.28 (0.88-1.86) |
| Years since menopause | |||
| < 10 | 0.66 (0.31-1.42) | 1.29 (0.64-2.59) | 0.53 (0.23-1.18) |
| ≥ 10 | 1.86 (1.21-2.85) | 2.42 (1.67-3.50) | 1.58 (1.03-2.42) |
| p-value for heterogeneity * | 0.021 | 0.12 | 0.018 |
| Age at baseline | |||
| 50-59 | 1.05 (0.63-1.76) | 2.15 (1.21-3.81) | 0.92 (0.51-1.67) |
| ≥ 60 | 1.82 (1.14-2.91) | 2.07 (1.40-3.07) | 1.45 (0.95-2.21) |
| p-value for heterogeneity * | 0.12 | 0.92 | 0.22 |
Wald test for heterogeneity of the stratum-specific hazard ratios
The CHD-free survival curves for continuous use versus no use (Figure 2) showed no indication of a protective effect of estrogen-plus-progestin therapy during the first 6 years of use among women within 10 years of menopause. In the Nurses' Health Study, there was no evidence for a protective effect during the first 3 years of use (3). This difference in the estimated crossover time may be due to random variability (the estimates from both studies are based on relatively few cases), different patient characteristics, failure to include all the joint determinants of hormone use and CHD in either or both of the studies, a misspecified dose-response model in our analysis, or a shorter average time since menopause in the observational study compared with the randomized trial.
This paper does not address the complex clinical and public health issues related to hormone therapy, including risk-benefit considerations. Rather, we focus on the effect of one common formulation of estrogen-plus-progestin therapy (conjugated equine estrogens, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d) on CHD. The evidence summarized above suggests that cardioprotection from this therapy does not occur during the first 3-6 years of use in women within 10 years of menopause. Since most newly menopausal women use this therapy for a short period (2, 15), an expected reduction of CHD risk should not be a consideration for initiation or continuation of hormone therapy in this group.
Though most of the current guidelines do not recommend the use of postmenopausal estrogen-plus-progestin for the prevention of CHD (16-18), a recent report of the International Menopause Society ruled out an early harm of hormone therapy on CHD in newly postmenopausal women (19). This conclusion was based in part on previously published data from the WHI estrogen-only trial (20, 21), which may not be relevant to estrogen-plus-progestin therapy, and from 2 small trials (22) which, when combined, totaled 1 CHD case. Because of the uncertainty surrounding the estimates and the low baseline risk of CHD in younger women, our findings are consistent with current guidelines that recommend short-term use of postmenopausal hormone only for relief of vasomotor symptoms (16-18).
The WHI findings reported here provide some support for the debated timing hypothesis (4, 23), which argues that the effect of postmenopausal hormone therapy varies by the stage of coronary atherosclerosis (4, 5). According to this hypothesis, estrogen may reduce the risk of CHD (through, for example, its effects on lipid profile or endothelial function) among younger women who do not yet have advanced atherosclerotic plaque in their coronary arteries, but trigger CHD (through, for example, its effects on coagulation and inflammatory factors) in the presence of advanced lesions. If the timing hypothesis were true, however, one would also expect a lower relative risk of CHD for hormone users compared with non users in women aged 50-59 years. We did not find a decreased risk in this age group, however. One could try to explain this finding by arguing that time from menopause is a better indicator of stage of coronary atherosclerosis than age, or that the addition of progestin modified the effects of estrogen. Further research on the role of age versus time since menopause is warranted, especially since the Nurses' Health Study found a suggestion of lower relative risk in women under age 60.
In summary, the available evidence suggests that estrogen-plus-progestin therapy does not reduce the CHD risk during the first 3-6 years of use in women who initiated therapy close to menopause. Because the typical duration of use of hormone therapy is short, most women contemplating estrogen-plus-progestin therapy for the relief of menopausal symptoms should not expect protection against CHD.
Appendix Table 3.
Hazard ratios (95% confidence intervals) of coronary heart disease for continuous use of estrogen-plus-progestin therapy versus no hormone therapy use from different imputation methods for missing proportion of hormone use in a given follow-up year, the Women's Health Initiative randomized trial
| Follow-up period | |||
|---|---|---|---|
| Overall (8-year cumulative use) |
≤2 years (2-year cumulative use) |
>2 years (6-year cumulative use) |
|
| All women | |||
| Zero | 1.78 (1.01, 3.15) | 2.29 (1.49, 3.53) | 1.39 (0.89, 2.16) |
| Half | 1.62 (0.94, 2.80) | 2.34 (1.53, 3.58) | 1.29 (0.85, 1.95) |
| One | 1.60 (0.92, 2.79) | 2.28 (1.50, 3.46) | 1.28 (0.84, 1.96) |
| Same as previous year | 1.58 (0.92, 2.72) | 2.33 (1.53, 3.55) | 1.27 (0.84, 1.92) |
| Multiple imputation | 1.43 (0.85, 2.42) | 2.22 (1.46, 3.38) | 1.17 (0.78, 1.76) |
| Years since menopause | |||
| < 10 | |||
| Zero | 0.74 (0.24, 2.30) | 1.22 (0.50, 3.00) | 0.71 (0.30, 1.70) |
| Half | 0.69 (0.22, 2.17) | 1.28 (0.51, 3.18) | 0.67 (0.28, 1.62) |
| One | 0.65 (0.21, 2.01) | 1.26 (0.50, 3.17) | 0.65 (0.28, 1.53) |
| Same as previous year | 0.66 (0.21, 2.04) | 1.34 (0.53, 3.36) | 0.65 (0.27, 1.56) |
| Multiple imputation | 0.56 (0.18, 1.71) | 1.12 (0.46, 2.71) | 0.57 (0.24, 1.36) |
| ≥ 10 | |||
| Zero | 2.19 (1.11, 4.32) | 2.73 (1.66, 4.50) | 1.61 (0.94, 2.77) |
| Half | 2.10 (1.13, 3.88) | 2.79 (1.71, 4.56) | 1.56 (0.96, 2.53) |
| One | 2.11 (1.12, 3.97) | 2.78 (1.72, 4.49) | 1.59 (0.97, 2.61) |
| Same as previous year | 2.00 (1.08, 3.71) | 2.72 (1.67, 4.43) | 1.51 (0.93, 2.45) |
| Multiple imputation | 1.88 (1.04, 3.41) | 2.78 (1.73, 4.49) | 1.47 (0.91, 2.36) |
| Age at baseline (years) | |||
| 50-59 | |||
| Zero | 1.57 (0.60, 4.09) | 2.57 (1.10, 6.04) | 1.29 (0.61, 2.72) |
| Half | 1.46 (0.56, 3.83) | 2.69 (1.13, 6.39) | 1.22 (0.57, 2.59) |
| One | 1.48 (0.58, 3.76) | 2.57 (1.07, 6.15) | 1.23 (0.60, 2.54) |
| Same as previous year | 1.40 (0.55, 3.60) | 2.85 (1.20, 6.78) | 1.19 (0.57, 2.49) |
| Multiple imputation | 1.20 (0.38, 3.79) | 2.23 (0.87, 5.69) | 1.04 (0.43, 2.55) |
| ≥ 60 | |||
| Zero | 1.85 (0.97, 3.52) | 2.24 (1.37, 3.65) | 1.42 (0.86, 2.34) |
| Half | 1.67 (0.92, 3.05) | 2.27 (1.40, 3.66) | 1.31 (0.83, 2.08) |
| One | 1.64 (0.89, 3.02) | 2.22 (1.39, 3.55) | 1.30 (0.82, 2.07) |
| Same as previous year | 1.64 (0.90, 2.97) | 2.23 (1.39, 3.60) | 1.29 (0.82, 2.05) |
| Multiple imputation | 1.50 (0.87, 2.58) | 2.23 (1.40, 3.55) | 1.21 (0.80, 1.84) |
Appendix Table 4.
Hazard ratios (95% confidence intervals) of coronary heart disease for continuous use of estrogen-plus-progestin therapy versus no hormone therapy use with a different distribution and transformation of hormone use, the Women's Health Initiative randomized trial
| Follow-up period | |||
|---|---|---|---|
| Overall (8-year cumulative use) |
≤2 years (2-year cumulative use) |
>2 years (6-year cumulative use) |
|
| All women | |||
| Gamma distribution | 1.66 (0.96, 2.89) | 2.33 (1.53, 3.56) | 1.31 (0.86, 2.00) |
| Arcsin-root transformation | 1.91 (1.05, 3.48) | 2.66 (1.67, 4.23) | 1.44 (0.91, 2.27) |
| Years since menopause | |||
| < 10 | |||
| Gamma distribution | 0.69 (0.22, 2.16) | 1.20 (0.49, 2.97) | 0.67 (0.28, 1.61) |
| Arcsin-root transformation | 0.70 (0.22, 2.28) | 1.40 (0.52, 3.75) | 0.67 (0.27, 1.66) |
| ≥ 10 | |||
| Gamma distribution | 2.16 (1.14, 4.08) | 2.84 (1.75, 4.61) | 1.60 (0.97, 2.63) |
| Arcsin-root transformation | 2.55 (1.28, 5.11) | 3.26 (1.90, 5.61) | 1.80 (1.04, 3.10) |
| Age at baseline (years) | |||
| 50-59 | |||
| Gamma distribution | 1.48 (0.58, 3.80) | 2.50 (1.06, 5.93) | 1.23 (0.59, 2.55) |
| Arcsin-root transformation | 1.76 (0.62, 4.95) | 3.09 (1.19, 8.02) | 1.39 (0.62, 3.12) |
| ≥ 60 | |||
| Gamma distribution | 1.72 (0.93, 3.18) | 2.30 (1.43, 3.70) | 1.34 (0.83, 2.15) |
| Arcsin-root transformation | 1.96 (1.01, 3.81) | 2.57 (1.52, 4.34) | 1.46 (0.88, 2.42) |
ACKNOWLEDGMENTS
The authors would like to acknowledge the dedication of investigators and staff at the WHI Clinical Centers, the WHI Clinical Coordinating Center, and the National Heart, Lung, and Blood Institute Program Office. The complete list of WHI centers and investigators is available at http://www.whiscience.org/publications/WHI_investigators_longlist.pdf. The authors would like to thank Alvaro Alonso, MD, PhD, University of Minnesota, for his analytic support, and Garnet Anderson, PhD, Fred Hutchinson Cancer Research Center, for her expert advice.
FINANCIAL SUPPORT: This study was partially funded by NIH grant R01 HL080644-01
APPENDIX
Intention-to-treat analyses
The published WHI estimates (1, 6, 8) were obtained from a Cox proportional hazards model stratified by age, CHD at baseline, and randomization status in a parallel diet modification trial, and adjusted for the previous history of coronary-artery bypass grafting or percutaneous transluminal coronary angioplasty. We approximated this model through a pooled logistic regression model (24) that included the covariates listed above and months since randomization (modeled by cubic splines). We repeated the analysis stratified by years since menopause (<10, ≥10 years) and age group (<60, ≥60 years old) at baseline.
We also estimated standardized (to the distribution of the baseline covariates) CHD-free survival curves for the hormone and placebo arm from a separate model that included product (“interaction”) terms between treatment arm and months since randomization to allow for time-varying hazard ratios. We tested for heterogeneity of the hazard ratio by time since randomization in women within 10 years of menopause (Wald test for the product term between treatment arm and month). For comparison with the estimated curves, we also constructed unadjusted Kaplan-Meier CHD-free survival curves.
We confirmed that our intention-to-treat hazard ratio estimates coincided, almost exactly, with those previously published by WHI investigators (Appendix Table 1) (1). Compared with women assigned to placebo, women assigned to estrogen-plus-progestin had a 23% increase in CHD incidence during the entire follow-up, and a 55% increase during the first 2 years. The corresponding numbers for women within 10 years of menopause at baseline were -11% and 17%, respectively. Note that the effect within the first 2 years of use among these newly menopausal women, which might be more clinically relevant in current clinical practice, has not been previously reported.
Appendix Table 1.
Intention-to-treat estimates of coronary heart disease, the Women's Health Initiative estrogen-plus-progestin randomized trial *
| Follow-up period | |||||||
|---|---|---|---|---|---|---|---|
| Overall | ≤2 years | >2 years | |||||
| N (E+P/placebo) |
CHD events (E+P/placebo) |
HR (95% CI) |
CHD events (E+P/placebo) |
HR (95% CI) |
CHD events (E+P/placebo) |
HR (95% CI) |
|
| All women | 8,506/8,102 | 188/147 | 1.23 (0.99-1.52) | 80/51 | 1.55 (1.09-2.20) | 108/96 | 1.07 (0.81-1.40) |
| Years since menopause † | |||||||
| < 10 | 2,782/2,712 | 31/34 | 0.89 (0.55-1.46) | 14/12 | 1.17 (0.54-2.52) | 17/22 | 0.74 (0.39-1.40) |
| ≥ 10 | 4,897/4,797 | 137/95 | 1.44 (1.10-1.87) | 62/36 | 1.74 (1.15-2.63) | 75/59 | 1.27 (0.90-1.79) |
| p-value for heterogeneity § | 0.092 | 0.37 | 0.144 | ||||
| Age at baseline | |||||||
| 50-59 | 2,839/2,683 | 37/27 | 1.30 (0.79-2.15) | 16/10 | 1.60 (0.73-3.55) | 21/17 | 1.14 (0.60-2.16) |
| ≥ 60 | 5,667/5,419 | 151/120 | 1.21 (0.95-1.54) | 64/41 | 1.54 (1.04-2.29) | 87/79 | 1.05 (0.77-1.42) |
| p-value for heterogeneity § | 0.80 | 0.93 | 0.82 | ||||
E+P: estrogen-plus-progestin therapy; CHD: coronary heart disease; HR: hazard ratio; CI: confidence interval
1,420 women (827 in E+P group and 593 in placebo group) had missing years since menopause
Wald test for heterogeneity of the stratum-specific hazard ratios
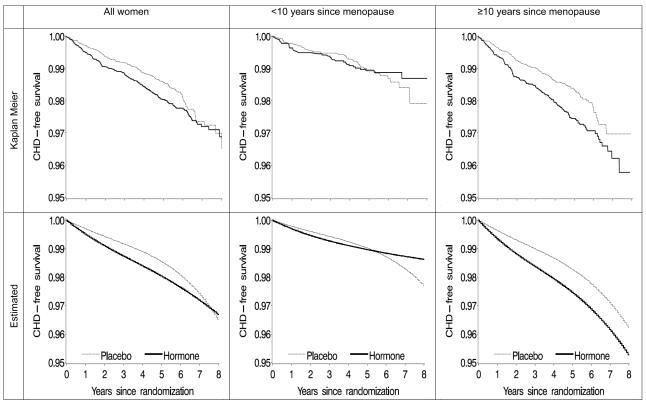
The Appendix Figure shows unadjusted and standardized CHD-free survival curves for all women, women within 10 years of menopause, and women more than 10 years after menopause. Unadjusted and standardized curves were similar, which suggests that our model for the standardized curves was adequately specified. The survival curves crossed at about 5 (95% CI: 0.5-10) years after randomization for women within 10 years of menopause, but did not cross for women who were at least 10 years past their menopause. The p-value from a log-rank test for heterogeneity of the survival curves between hormone and placebo arm was 0.058 for all women, 0.64 for women within 10 years of menopause, and 0.007 for women more than 10 years after menopause. In women within 10 years of menopause, the p-value for heterogeneity of the hazard ratio by time since randomization was 0.060. Overall, the intention-to-treat survival curves were qualitatively similar to the adherence-adjusted survival curves for continuous use versus no use (Figure 2).
Appendix Figure.
Proportion of women free of coronary heart disease (CHD)--intention-to-treat analysis, the Women's Health Initiative estrogen-plus-progestin randomized trial
The curves for women under and above age 60 (not shown) crossed at about 8 and 7 years, respectively. In women aged 50-59 years, 16% were more than 10 years from menopause and 10% had an unknown time of menopause. Restriction of the analysis to women without prior CHD before randomization or without a family history of premature myocardial infarction did not materially affect the results.
Adherence-adjusted analyses
The inverse probability weights were estimated by fitting, separately for each arm, (1) a logistic regression model to estimate each participant's probability of receiving hormone therapy during each follow-up year, and (2) a linear regression model to estimate each participant's density of receiving their actual proportion of pills taken (log transformed) among those with non-zero use during that year (9, 25). A participant contributed as many observations to the models as years she was in the study, i.e., from baseline to the occurrence of CHD, death, or end of study, whichever occurred first.
Both models included years since randomization (linear and quadratic), proportion of study pills taken during the previous year (linear and quadratic), as well as sociodemographic, lifestyle, dietary, and medical factors measured at baseline and, for time-varying covariates, at the most recent visit (Appendix Table 2). To improve statistical efficiency, the weights were stabilized (9-11) by adding to their numerator the estimated density of received treatment history conditional on the baseline covariates included in the intention-to-treat model plus body mass index, cigarette smoking, diabetes, high blood pressure, high cholesterol, physical activity, alcohol intake, family history of premature myocardial infarction, years since menopause, and previous hormone use. Including the additional baseline variables region, ethnicity, education, physical functioning, use of statins, aspirin, oral contraceptives and multivitamin, and fruit and vegetable intake yielded similar results. The mean of the estimated stabilized inverse probability weights for adherence adjustment was 1.00 (standard deviation: 0.30).
We then fit a pooled logistic model identical to the one used in our intention-to-treat analysis, except that (i) the treatment arm indicator was replaced by a time-varying variable for cumulative use of hormone therapy, calculated as the sum of the annual proportion of pills taken since baseline, (ii) included the additional baseline variables used to estimate the numerator of the weights, and (iii) each individual contribution was weighted by the estimated time-varying inverse probability weights. We added product terms between cumulative use and indicators for age (<60, ≥60 years old), time since menopause (<10, ≥10 years), and period of follow-up (≤2 and >2 years) to obtain the corresponding stratum-specific estimates of the hazard ratio. Unlike a previous analysis of the Nurses' Health Study (3), our analysis requires the specification of such dose-response function because we could not censor women at the first occurrence of noncompliance (the available information does not allow us to establish the temporal sequence of noncompliance and CHD). Models that included a quadratic term of cumulative use, or that replaced cumulative use by cumulative use on the logarithmic scale yielded similar estimates (not shown).
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00000611
REPRODUCIBLE RESEARCH STATEMENTS - Protocol: Available at http://www.whiscience.org/about/design.php; Statistical code: Available to interested readers by contacting Dr. Toh (darrentoh@post.harvard.edu); Data: The limited access dataset is available through application at the National Heart, Lung, and Blood Institute data repository
REFERENCES
- 1.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349(6):523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 2.Hernán MA, Robins JM, García Rodríguez LA. Discussion of Statistical issues arising in the Women's Health Initiative by Prentice RL, Pettinger M, Andreson GL. Biometrics. 2005;61(4):922–930. doi: 10.1111/j.0006-341X.2005.454_1.x. [DOI] [PubMed] [Google Scholar]
- 3.Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–79. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manson JE, Bassuk SS. Invited commentary: hormone therapy and risk of coronary heart disease why renew the focus on the early years of menopause? Am J Epidemiol. 2007;166(5):511–7. doi: 10.1093/aje/kwm213. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356(25):2639–41. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 6.The Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 7.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Prentice RL, Langer R, Stefanick ML, Howard BV, Pettinger M, Anderson G, et al. Combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women's Health Initiative clinical trial. Am J Epidemiol. 2005;162(5):404–14. doi: 10.1093/aje/kwi223. [DOI] [PubMed] [Google Scholar]
- 9.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Toh S, Hernán MA. Causal inference from longitudinal studies with baseline randomization. 2008;4(1) doi: 10.2202/1557-4679.1117. Article 22 (Available at: http://www.bepress.com/ijb/vol4/iss1/22) [DOI] [PMC free article] [PubMed]
- 12.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 13.Hernán MA, Robins JM. Authors' response, part I: Observational studies analyzed like randomized experiments--Best of both worlds. Epidemiology. 2008;19(6):789–792. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connelly MT, Richardson M, Platt R. Prevalence and duration of postmenopausal hormone replacement therapy use in a managed care organization, 1990-1995. J Gen Intern Med. 2000;15(8):542–50. doi: 10.1046/j.1525-1497.2000.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ACOG Task Force on Hormone Therapy Hormone therapy. Obstet Gynecol. 2004;104(4 Suppl):1S–131S. doi: 10.1097/01.AOG.0000138807.32767.32. [DOI] [PubMed] [Google Scholar]
- 17.Hormone therapy for the prevention of chronic conditions in postmenopausal women: recommendations from the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(10):855–60. [PubMed] [Google Scholar]
- 18.Estrogen and progestogen use in peri- and postmenopausal women: March 2007 position statement of The North American Menopause Society. Menopause. 2007;14(2):168–82. doi: 10.1097/gme.0b013e31803167ab. [DOI] [PubMed] [Google Scholar]
- 19.Pines A, Sturdee DW, Birkhauser MH, de Villiers T, Naftolin F, Gompel A, et al. HRT in the early menopause: scientific evidence and common perceptions. Climacteric. 2008;11(4):267–72. doi: 10.1080/13697130802226866. [DOI] [PubMed] [Google Scholar]
- 20.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 21.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356(25):2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 22.Lobo RA. Evaluation of cardiovascular event rates with hormone therapy in healthy, early postmenopausal women: results from 2 large clinical trials. Arch Intern Med. 2004;164(5):482–4. doi: 10.1001/archinte.164.5.482. [DOI] [PubMed] [Google Scholar]
- 23.Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. Am J Epidemiol. 2007;166(5):506–10. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- 24.Thompson WA., Jr On the treatment of grouped observations in life studies. Biometrics. 1977;33(3):463–70. [PubMed] [Google Scholar]
- 25.Cotter D, Zhang Y, Thamer M, Kaufman J, Hernan MA. The effect of epoetin dose on hematocrit. Kidney Int. 2008;73(3):347–53. doi: 10.1038/sj.ki.5002688. [DOI] [PubMed] [Google Scholar]
- 26.Barnabei VM, Cochrane BB, Aragaki AK, Nygaard I, Williams RS, McGovern PG, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women's Health Initiative. Obstet Gynecol. 2005;105(5 Pt 1):1063–73. doi: 10.1097/01.AOG.0000158120.47542.18. [DOI] [PubMed] [Google Scholar]