Abstract
Asbestos fibers cause chronic inflammation that may be critical to the development of malignant mesothelioma (MM). Two human MM cell lines (Hmeso, PPM Mill) were used in a SCID mouse xenograft model to assess time-dependent patterns of inflammation and tumor formation. After intraperitoneal (IP) injection of MM cells, mice were euthanized at 7, 14, and 30 days, and peritoneal lavage fluid (PLF) was examined for immune cell profiles and human and mouse cytokines. Increases in human MM-derived IL-6, IL-8, bFGF, and VEGF were observed in mice at 7 days postinjection of either MM line, and a striking neutrophilia was observed at all time points. Free-floating tumor spheroids developed in mice at 14 days, and both spheroids and adherent MM tumor masses occurred in all mice at 30 days. Results suggest that inflammation and cytokine production precede and may be critical to the development of MMs.
Keywords: cytokines, chemokines, asbestos, inflammation, mesothelioma
Introduction
Exposure to asbestos fibers, particularly to amphibole types of asbestos that initiate oxidative stress in vitro and in vivo (reviewed in Ref. 1), is associated with the development of several lung diseases including pulmonary or pleural fibrosis (a nonmalignant disease associated generally with workplace exposures to asbestos), lung cancer, and malignant mesotheliomas (MM) (reviewed in Ref. 2). There is currently no cure for asbestos-related lung/pleural diseases, and treatment options are relatively ineffective. Patients with MM, a devastating tumor arising from the mesothelial cells lining the pleural, peritoneal and pericardial cavity, are often not diagnosed until advanced stages of the disease when curative options are limited. Contributing factors such as the absence of biomarkers and different pathologic subtypes increase the difficulty of treatment, and as a result, individuals with MM generally exhibit an average survival time of 6–8 months from initial diagnosis.3,4 No single-modality MM therapy including chemotherapy, radiation therapy, immunotherapy or surgery has reliably demonstrated superiority to supportive care (reviewed in Refs. 5, 6).
Chronic inflammation has been linked to the initiation and progression of numerous cancers, including lung tumors and MMs which, in approximately 80% of cases examined, are associated with asbestos exposures.7 A number of studies in animal models and human patients have demonstrated that inhalation or injection of asbestos fibers results in a chronic inflammatory response characterized primarily by recruitment of macrophages and neutrophils and production of chemokines and cytokines in the lung (reviewed in Refs. 8, 9) and pleura.10–12 Recent studies in our laboratory have focused on whether exposure of human MM cells to asbestos leads to autocrine production of cytokines, as well as the importance of transcription factors in cytokine production.13,14 In studies here, we hypothesized that inflammation, a known source of generation of reactive oxygen and nitrogen species, and cytokine production by human MM cells, are early features of tumor development in a mouse xenograft model of peritoneal MM after injection of two well-characterized human MM cell lines. We demonstrate an early and sustained neutrophilia accompanied by early detection of a number of cytokines linked to inflammation, cell proliferation and angiogenesis by human MMs in peritoneal lavage fluid (PLF). Such changes, which precede the development of tumors, may be linked causally to MM formation and should be further examined as biomarkers and/or inflammatory events preceding the diagnosis of MMs.
Methods
Human pleural MM cell lines and reagents
Two pleural MM cell lines were used comparatively in our studies. Hmeso cells, originally designated H-MESO-1, were initially isolated by Reale and colleagues15 and supplied by Joseph R. Testa. The PPM Mill line, isolated after surgical resection, was established and provided by Harvey I. Pass. Lines were confirmed as mesothelial in origin using an antibody to calretinin and verified for lack of mycoplasma contamination using a polymerase chain reaction (PCR) assay. In vitro, Hmeso is characterized as epithelioid and PPM Mill is characterized as fibrosarcomatoid. All cell cultures were incubated at 37° C in 5% CO2 and grown to approximately 80–90% confluency in complete medium consisting of DMEM/F12 50/50 (Mediatech, Inc., Herndon, VA), and 10% fetal bovine serum (FBS) (Mediatech), 0.1 µg/mL hydrocortisone (Sigma, St. Louis, MO), 2.5 µg/mL insulin, 2.5 µg/mL transferrin, 2.5 ng/mL sodium selenite (Sigma), and penicillin-streptomycin (50 U/mL penicillin G, 50 µg/mL streptomycin sulfate) (GIBCO, Carlsbad, CA).
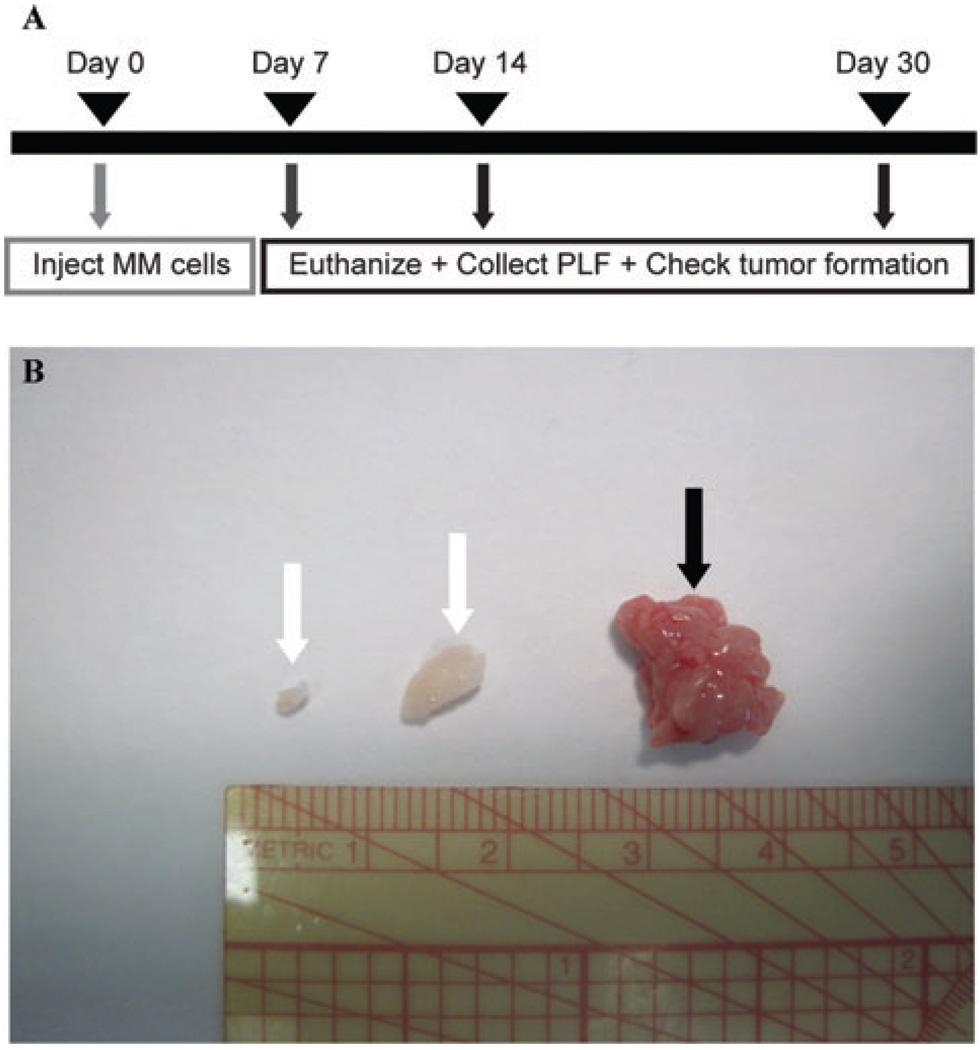
Generation of an intraperitoneal (IP) xenograft model of MM in severe combined immunodeficient (SCID) mice
Hmeso or PPM Mill cells (5 × 106 cells in 50 µL 0.9% NaCl, pH 7.4) were injected into the lower left quadrant of the peritoneal cavity of 6 week-old male Fox Chase SCID mice (n = 3 mice/group/time point). These mice have a genetic autosomal recessive mutation that disrupts the differentiation of both B and T lymphocyte progenitor cells, rendering these cell types nonfunctional. However, these same characteristics make the mice ideal for implantation of foreign tumors and tissues. At 7, 14, and 30 days post-MM cell injection, mice were euthanized by IP injection of sodium pentobarbital. PLF was collected and animals were closely examined for the presence of tumor spheroids or mesenteric masses (Fig. 1B). Those tumors classified as spheroids were small, free-floating nodules, and those classified as mesenteric were larger and attached to the peritoneal mesentery. The total number of spheroid or mesenteric tumors per mouse was determined, along with individual tumor volume and weight (data not shown). Tumors were characterized as MMs using previously described criteria.16,17
Figure 1.
(A) Schematic describing the IP xenograft model used in the studies described here. Briefly, 5 × 106 MM cells were injected IP into SCID mice in 50 µL 0.9% NaCl (pH 7.4). At 7, 14, and 30 days post MM injection, mice were euthanized, their PLF collected for Bio-Plex assays and cytospins, and their peritoneal cavity checked for the presence of spheroid and/or mesenteric tumors. (B) Gross representation of spheroid tumors (white arrows) and a mesenteric tumor (black arrow). Note that spheroids generally possessed smaller tumor volumes and weights than mesenteric tumors.
Collection and preparation of PLF
Following euthanization, the peritoneal cavity of each mouse was instilled with 5 mL of cold sterile PBS using an 18-gauge needle. Leaving the needle in place, the abdomen was gently massaged and PLF was aspirated back into the syringe and placed on ice. PLF was centrifuged at 1000 rpm for 5 min at 4° C, the supernatant removed, and the cell pellet resuspended in 500 µL of 5% bovine serum albumin (BSA) in PBS. The volume required to cytospin 50,000 cells was calculated after determination of total cell counts using an Advia® 120 Hematology System(Siemens Healthcare Diagnostics, Deerfield, IL), placed into cytospin cartridges overlaid on Superfrost® Plus glass slides (Fisher Scientific, Kalamazoo, MI), and spun at 600 rpm for 10 min using a Shandon Cytospin 2 centrifuge (Thermo Scientific, Waltham, MA). Cytospin slides were air dried, fixed for 5 min in 100% methanol, air dried again, and stained using a Hema 3® Stat Pack (Fisher Scientific, Kalamazoo, MI) as per the manufacturer’s recommendations. Slides were cover slipped, and differential cell counts were ascertained by counting 500 cells per slide.
Bio-Plex analysis of cytokine concentrations in PLF
To quantify cytokine levels in PLF isolated from mice, a multiplex suspension protein array was performed using the Bio-Plex protein array system with a human cytokine 27-plex panel or a mouse cytokine 23-plex panel (Bio-Rad, Hercules, CA). This method of analysis is based on Luminex technology and simultaneously measures the following proteins: Interleukin-1α (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, basic fibroblast growth factor (bFGF), eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), IFN-inducible protein 10 (IP-10), keratinocyte chemoattractant (KC), monocyte chemoattractant protein-1 (MCP-1; MCAF), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, platelet-derived growth factor-BB (PDGF-BB), regulated on activation normal T-cell expressed and secreted (RANTES), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF). IL-1ra, IL-7, IL-8, IL-15, bFGF, IP-10, PDGF-BB, and VEGF are unique to the human panel and IL-1α, IL-3, IL-12 (p40), and KC are unique to the mouse panel. Briefly, anticytokine antibody-conjugated beads were added to individual wells of a 96-well filter plate and adhered using vacuum filtration. After washing, 50 µL of prediluted standards (range = 32,000–1.95 pg/mL) or PLF (n = 3/group) was added, and the filter plate shaken at 300 rpm for 30 min at room temperature. Thereafter, the filter plate was washed, and 25 µL of prediluted multiplex detection antibody was added for 30 min. After washing, 50 µL of prediluted streptavidin-conjugated phycoerythrin was added for 10 min followed by an additional wash and the addition of 120 µL of Bio-Plex assay buffer to each well. The filter plate was analyzed using the Bio-Plex protein array system, and concentrations of each cytokine were determined using Bio-Plex Manager Version 3.0 software. Data are expressed as pg of cytokine/mL of PLF. Only differences in cytokine amounts greater than or equal to twofold are presented in Tables 1 and 2.
Table 1.
Characterization of a SCID mouse IP xenograft model using a human epithelioid MM line (Hmeso)
| Days | Differential cell counts in PLFa,b |
Cytokines in PLF Tumor vs. control |
MMc presence (% of mice) | ||
|---|---|---|---|---|---|
| Tumor vs. controld,e | Mouse | Human | Control | Tumor | |
| 7 | Lf: twofold higher | <IL-9 | >IL-6 | Spheroid: 0% | Spheroid: 0% |
| E: threefold higher | <IL-12 (p70) | >IL-8 | Mesenteric: 0% | Mesenteric: 0% | |
| B: sevenfold higher | >G-CSF | >bFGF | |||
| >KC | >VEGF | ||||
| >RANTES | |||||
| 14 | L: threefold higher | <IL-1α | >IL-7 | Spheroid: 0% | Spheroid: 100% |
| N: sixfold higher | >IL-9 | >IL-8 | Mesenteric: 0% | Mesenteric: 0% | |
| E: 11-fold higher | >KC | >IL-9 | |||
| B: twofold higher | <MCP-1 | >G-CSF | |||
| >MCAF | |||||
| >PDGF-BB | |||||
| 30 | M: twofold higher | <IL-1β | >IL-6 | Spheroid: 0% | Spheroid: 100% |
| L: fourfold higher | >IL-3 | >IL-7 | Mesenteric: 0% | Mesenteric: 100% | |
| N: 52-fold higher | >IL-9 | >IL-8 | |||
| >IL-12 (p40) | >IL-9 | ||||
| >IL-13 | >IL-12 (p70) | ||||
| >IL-17 | >G-CSF | ||||
| >G-CSF | >MCAF | ||||
| >KC | >PDGF-BB | ||||
| <MCP-1 | >VEGF | ||||
Fold-changes in percentages of cells.
Peritoneal lavage fluid (PLF).
Malignant mesothelioma (MM).
Control mice injected IP with 50 µL sterile 0.9% NaCl (pH 7.4).
Tumor-bearing mice injected IP with 5 × 106 Hmeso MM cells in 50 µL sterile 0.9% NaCl (pH 7.4); n = 3 mice/group/time point.
Macrophages (M); Lymphocytes (L); Neutrophils (N); Eosinophils (E); and Basophils (B).
Table 2.
Characterization of a SCID mouse IP xenograft model using a human fibrosarcomatoid MM line (PPM Mill)
| Days | Differential cell counts in PLFa,b |
Cytokines in PLF Tumor vs. control |
MMc presence (% of mice) | |||
|---|---|---|---|---|---|---|
| Tumor vs. controld,e | Mouse | Human | Control | Tumor | ||
| 7 | Lf: threefold higher | >IL-1α | >IL-6 | <Eotaxin | Spheroid: 0% | Spheroid: 0% |
| N: 29-fold higher | >IL-3 | >IL-8 | >MCAF | Mesenteric: 0% | Mesenteric: 0% | |
| >IL-10 | <IL-9 | <TNF-α | ||||
| >G-CSF | <IL-10 | >VEGF | ||||
| >KC | <IL-17 | |||||
| >MCAF | >IFN-γ | |||||
| >TNF-α | >bFGF | |||||
| 14 | N: 81-fold higher | >IL-2 | >IL-6 | Spheroid: 0% | Spheroid: 33% | |
| <IL-3 | >IL-8 | Mesenteric: 0% | Mesenteric: 0% | |||
| >G-CSF | >MCAF | |||||
| >KC | ||||||
| >MCAF | ||||||
| >RANTES | ||||||
| 30 | N: 89-fold higher | >IL-2 | >IL-1β | >VEGF | Spheroid: 0% | Spheroid: 100% |
| E: threefold higher | >IL-3 | >IL-1ra | >IP-10 | Mesenteric: 0% | Mesenteric: 100% | |
| B: fourfold higher | >IL-6 | >IL-6 | >IFN-γ | |||
| >IL-9 | >IL-7 | >MCAF | ||||
| >IL-10 | >IL-8 | >bFGF | ||||
| >IFN-γ | <IL-10 | |||||
| >G-CSF | >IL-12 (p70) | |||||
| >KC | >RANTES | |||||
| >TNF-α | <PDGF-BB | |||||
Fold-changes in percentages of cells.
Peritoneal lavage fluid (PLF).
Malignant mesothelioma (MM).
Control mice injected IP with 50 µL sterile 0.9% NaCl (pH 7.4).
Tumor-bearing mice injected IP with 5 × 106 PPM Mill MM cells in 50 µL sterile 0.9% NaCl (pH 7.4); n = 3 mice/group/time point.
Macrophages (M); Lymphocytes (L); Neutrophils (N); Eosinophils (E); and Basophils (B).
Results
Influx of inflammatory cells precedes development of gross intraperitoneal MMs
Figure 1(A) shows the design of the protocol for time course studies to determine the advent of inflammation in relationship to the development of gross tumors in the mouse xenograft model of MM. Although no tumors were observed upon autopsies of mice at 1 week, free-floating spheroids (white arrows) and mesenteric tumors (black arrows) that often invaded other organs in the peritoneal cavities of SCID mice were observed at 14 and 30 days, respectively, post injection of 5 × 106 human MM cells (Fig. 1B). Although the PPM Mill line was fibrosarcomatoid in morphology as compared to the more epithelioid Hmeso line in vitro, both cell lines formed epithelioid or biphasic MMs (the types of MM most frequently observed in human patients) in vivo as determined by histopathology. At 30 days, all mice exhibited spheroid MMs and mesenteric masses upon autopsy, but no gross evidence of peritoneal distension due to accumulation of ascites fluid.
Mice injected with saline in the absence of MM cells maintained consistent PLF cellular profiles at all time points. In general, approximately 81–88% of cells counted were sloughed mesothelial cells, 10–16% were peritoneal macrophages, and <4% were lymphocytes or granulocytes. Differential cell counts of PLF from Hmeso-injected mice revealed twofold, sixfold, and 52-fold higher concentrations of neutrophils at 7, 14, and 30 days, respectively (Table 1). Progressive and striking increases in neutrophils (up to 89-fold increases in comparison to saline-injected mice) were seen at 7, 14, and 30 days after injection of PPM Mill cells (Table 2). Less dramatic increases in eosinophils (three- to 11-fold higher than in saline-injected mice) and basophils (2- to 7-fold higher) were also noted in response to injection of both MM lines. Lymphocyte increases of 3- to 4-fold were noted at 7 days in PPM Mill-injected mice and at 14 and 30 days in Hmeso-injected mice. In addition to these immune cell types, macrophages, normal mesothelial cells, and MM cells were observed in cytospin preparations.
Mouse and human cytokines are released into PLF following IP injection of human MM cells into SCID mice
A unique aspect of our work was the use of both mouse 23-plex and human 27-plex cytokine panels in the Bio-Plex assay system to differentiate between cytokine production by human tumor cells and by the host SCID mouse. After injection of either MM cell line, levels of several human cytokines including IL-6, IL-8, bFGF, and VEGF, were increased in PLF at 7 days (Tables 1 and 2). Increases in the inflammatory cytokine IL-8 was observed at all time points, and elevations in IL-6 levels also were seen with the exception of the 14 day time point in Hmeso-injected mice. Another chemokine increased at almost all time points except for day 7 in Hmeso-injected mice was human MCAF, a monocyte chemoattractant protein. Common increases in the human inflammatory cytokines IL-7 and IL-12 (p70) were also observed at 30 days in both Hmeso-injected and PPM Mill-injected mice. In contrast, levels of human IL-1β, a cytokine activated by the NALP3 inflammasome,9 IL-1ra, RANTES, IP-10, and IFN-γ were selectively increased at 30 days in PLF from PPM Mill-injected mice. Most importantly, a number of known growth factors for MMs including human bFGF, VEGF, G-CSF, and PDGF-BB were detected at 7 days and at more than 1 time point by each MM cell line.
Cytokine profiling also showed that increases in a number of mouse cytokines including KC, a known neutrophil attractant, were increased at all time points in both cell lines. In addition, increases in mouse G-CSF were noted at all time points in mice injected with the PPM Mill line and at 7 and 30 days in mice injected with the Hmeso MM line.
Discussion
We show here that inflammation, as most strikingly demonstrated by increased percentages of neutrophils in PLF and levels of a number of inflammatory cytokines produced by human MMs and SCID mice tumor cell recipients, precedes the development of tumors in an IP mouse xenograft model of MM. Data also suggest that inflammation is in part dictated by the production of inflammatory cytokines that are produced by human MM cells and are essential to tumor growth and development. The fact that these proteins can be measured in PLF is an exciting indication that identification and production of MM growth factors, especially those that are not produced by host mouse cells, may be used as biomarkers of tumor development or as indicators of patient responses to chemotherapeutic regimens or surgical resection of MMs.
In support of our data showing that critical human MM growth factors (bFGF, VEGF, G-CSF, and PDGF-BB) are produced and secreted into PLF prior to the development of tumors, others have reported that human MM cells exhibit overexpression of PDGF receptors and proteins (A and B chains) in vitro that may be linked to growth stimulation and proteoglycan synthesis.18–20 In addition, the proangiogenic factor, VEGF, a potent mitogen for vascular endothelium, is an autocrine growth factor for human MM lines in vitro and is expressed in MM biopsies and in pleural effusions of MM patients.21 These studies also show an inverse correlation between serum VEGF levels and MM patient survival. Both VEGF and bFGF (FGF-2), also a potent proangiogenic factor, have been detected to a greater extent in medium from human MM lines as compared to nonneoplastic mesothelium in vitro as determined by ELISA assays and immunohistochemistry (IHC).22,23 In both studies, a relationship between high serum bFGF levels and reduced patient survival occurred in contrast to no significant correlations between patient prognosis and immunoexpression of other cytokines including VEGF, FGF-1, and TGF-β. These studies also suggest that combined levels of critical cytokines might be more significant predictors of tumor microvascular density and survival outcomes. In addition, expression of G-CSF, another human cytokine observed in PLF in our studies, has been reported in serum and in MMs using ELISA and IHC, respectively.24 We have also documented increased levels of G-CSF and VEGF following Bio-Plex analyses of medium from a nontransformed human peritoneal mesothelial line (LP9/TERT-1) 24 h after exposure to crocidolite asbestos, suggesting that autocrine production of these proliferative and angiogenic cytokines is a rapid response to asbestos-induced injury.13,14
A number of interleukins, most commonly IL-6, IL-8, and IL-12 (p70) were produced by human MM cells and detected in PLF in our models. IL-6 has been shown to be constitutively produced by both primary peritoneal mesothelial cells and MM cells in vitro.25,26 IL-6 also induces the expression of VEGF in MM cells in vitro through the JAK2/STAT3 pathway27 and was found to be produced in an IP orthotopic xenograft model of MM in which murine MM cells were injected into BALB/c or CBA/CAH mice.28 Additionally, IL-6 has been detected in the serum and pleural effusions of patients with MM,29 and has been associated with accelerated release of neutrophils from the bone marrow.30 This may have directly contributed to the neutrophilia observed in our model. Given that IL-6 is a potent growth factor for malignant cells of B-lymphocyte lineage, it has been suggested that this cytokine may similarly affect the proliferation of MM cells.31 IL-8 is also known to activate and be chemotactic for neutrophils,32 and is an integral angiogenic factor for capillary development in vivo.33 In addition, IL-8 is produced by mesothelial cells34 and has been established as an autocrine growth factor for MM.35 In a nude mouse model of pleural MM, the use of an IL-8 antibody to lower pleural fluid IL-8 levels was directly associated with a decreased rate of tumor growth.36 Finally, IL-12 is capable of inducing tumor-specific immune responses and antiangiogenic factors and has been used to prevent subcutaneous MM tumor growth in mice.37,38 The increase of IL-12 observed in our model is therefore somewhat counterintuitive, but may represent a mechanism to regulate tumor growth.
In conclusion, we demonstrate that IP injection of human MM cells into SCID mice results in the generation of a number of cytokines related to inflammation, cell proliferation, and angiogenesis by human MMs, and that these changes precede tumor formation. The fact that MM cells can secrete these factors in an autocrine fashion, and that several of these same cytokines are produced in response to asbestos fibers, suggest that chronic inflammation may be perpetuated by mesothelial cells through common mechanisms over the long latency period of MM.
Acknowledgments
This work is supported by National Cancer Institute Grant P01CA114047 (A.S., J.R.T., H.I.P., M.C., and B.T.M.), National Cancer Institute Grant R01CA106567 (M.C. and B.T.M.), National Institute of Environmental Health Sciences Training Grant T32ES007122 (J.M.H.), and a Lake Champlain Cancer Research Organization J. Walter Juckett Postdoctoral Fellowship (J.M.H.).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Shukla A, et al. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003;34:1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 2.Mossman BT, et al. Asbestos: scientific developments and implications for public policy. Science. 1990;247:294–301. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- 3.Mossman BT, Gee JB. Asbestos-related diseases. N. Engl. J. Med. 1989;320:1721–1730. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BW, Lake RA. Advances in malignant mesothelioma. N. Engl. J. Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 5.Pass HI. Mediastinal staging 2005: pictures, scopes, and scalpels. Semin. Oncol. 2005;32:269–278. doi: 10.1053/j.seminoncol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.O’Byrne K, Rusch V. Malignant Pleural Mesothelioma. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 7.Krishnamoorthy S, Honn KV. Inflammation and disease progression. Cancer Metastasis Rev. 2006;25:481–491. doi: 10.1007/s10555-006-9016-0. [DOI] [PubMed] [Google Scholar]
- 8.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998;157:1666–1680. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 9.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamson IY, Prieditis H, Young L. Lung mesothelial cell and fibroblast responses to pleural and alveolar macrophage supernatants and to lavage fluids from crocidolite-exposed rats. Am. J. Respir. Cell Mol Biol. 1997;16:650–656. doi: 10.1165/ajrcmb.16.6.9191466. [DOI] [PubMed] [Google Scholar]
- 11.Choe N, et al. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ. Health Perspect. 1997;105 Suppl 5:1257–1260. doi: 10.1289/ehp.97105s51257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill GD, et al. Soluble ICAM-1, MCP-1, and MIP-2 protein secretion by rat pleural mesothelial cells following exposure to amosite asbestos. Exp. Lung Res. 2003;29:277–290. doi: 10.1080/01902140303788. [DOI] [PubMed] [Google Scholar]
- 13.Hillegass JM, et al. Utilization of gene profiling and proteomics to determine mineral pathogenicity in a human mesothelial cell line (LP9/TERT-1) J. Toxicol. Environ. Health A. 2010;73:423–436. doi: 10.1080/15287390903486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla A, et al. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am. J. Respir. CellMol. Biol. 2009;41:114–123. doi: 10.1165/rcmb.2008-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reale FR, et al. Characterization of a human malignant mesothelioma cell line (H-MESO-1): a biphasic solid and ascitic tumor model. Cancer Res. 1987;47:3199–3205. [PubMed] [Google Scholar]
- 16.Martarelli D, et al. Characterization of human malignant mesothelioma cell lines orthotopically implanted in the pleural cavity of immunodeficient mice for their ability to grow and form metastasis. BMC Cancer. 2006;6:130. doi: 10.1186/1471-2407-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rdzanek M, et al. Spindle cell tumors of the pleura: differential diagnosis. Semin. Diagn. Pathol. 2006;23:44–55. doi: 10.1053/j.semdp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Gerwin BI, et al. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987;47:6180–6184. [PubMed] [Google Scholar]
- 19.Syrokou A, et al. Proteoglycans in human malignant mesothelioma. Stimulation of their synthesis induced by epidermal, insulin and platelet-derived growth factors involves receptors with tyrosine kinase activity. Biochimie. 1999;81:733–744. doi: 10.1016/s0300-9084(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 20.Versnel MA, et al. Expression of c-sis (PDGF B-chain) and PDGF A-chain genes in ten human malignant mesothelioma cell lines derived from primary and metastatic tumors. Oncogene. 1988;2:601–605. [PubMed] [Google Scholar]
- 21.Strizzi L, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J. Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 22.Kumar-Singh S, et al. Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and -2, and TGF beta expression. J. Pathol. 1999;189:72–78. doi: 10.1002/(SICI)1096-9896(199909)189:1<72::AID-PATH401>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Strizzi L, et al. Basic fibroblast growth factor in mesothelioma pleural effusions: correlation with patient survival and angiogenesis. Int. J. Oncol. 2001;18:1093–1098. doi: 10.3892/ijo.18.5.1093. [DOI] [PubMed] [Google Scholar]
- 24.Kasuga I, et al. Malignant pleural mesothelioma produces functional granulocyte-colony stimulating factor. Chest. 2001;119:981–983. doi: 10.1378/chest.119.3.981. [DOI] [PubMed] [Google Scholar]
- 25.Lanfrancone L, et al. Human peritoneal mesothelial cells produce many cytokines (granulocyte colony-stimulating factor [CSF], granulocyte-monocyte-CSF, macrophage-CSF, interleukin-1 [IL-1], and IL-6) and are activated and stimulated to grow by IL-1. Blood. 1992;80:2835–2842. [PubMed] [Google Scholar]
- 26.Schmitter D, et al. Hematopoietic growth factors secreted by seven human pleural mesothelioma cell lines: interleukin-6 production as a common feature. Int. J. Cancer. 1992;51:296–301. doi: 10.1002/ijc.2910510220. [DOI] [PubMed] [Google Scholar]
- 27.Adachi Y, et al. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int. J. Cancer. 2006;119:1303–1311. doi: 10.1002/ijc.22006. [DOI] [PubMed] [Google Scholar]
- 28.Bielefeldt-Ohmann H, et al. Patho- and immunobiology of malignant mesothelioma: characterisation of tumour infiltrating leucocytes and cytokine production in a murine model. Cancer Immunol. Immunother. 1994;39:347–359. doi: 10.1007/BF01534421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano T, et al. Interleukin 6 and its relationship to clinical parameters in patients with malignant pleural mesothelioma. Br. J. Cancer. 1998;77:907–912. doi: 10.1038/bjc.1998.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Eeden SF, et al. Systemic response to ambient particulate matter: relevance to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005;2:61–67. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 31.Monti G, et al. Intrapleural production of interleukin 6 during mesothelioma and its modulation by gamma-interferon treatment. Cancer Res. 1994;54:4419–4423. [PubMed] [Google Scholar]
- 32.Strieter RM, et al. The immunopathology of chemotactic cytokines: the role of interleukin-8 and monocyte chemoattractant protein-1. J. Lab. Clin. Med. 1994;123:183–197. [PubMed] [Google Scholar]
- 33.Strieter RM, et al. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J. Leukoc. Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 34.Antony VB, et al. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am. J. Respir. Cell Mol Biol. 1995;12:581–588. doi: 10.1165/ajrcmb.12.6.7766422. [DOI] [PubMed] [Google Scholar]
- 35.Galffy G, et al. Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res. 1999;59:367–371. [PubMed] [Google Scholar]
- 36.Galffy G, et al. Inhibition of interleukin-8 reduces human malignant pleural mesothelioma propagation in nude mouse model. Oncol Res. 1999;11:187–194. [PubMed] [Google Scholar]
- 37.Caminschi I, et al. Cytokine gene therapy of mesothelioma. Immune and antitumor effects of transfected interleukin-12. Am. J. Respir. Cell Mol Biol. 1999;21:347–356. doi: 10.1165/ajrcmb.21.3.3575. [DOI] [PubMed] [Google Scholar]
- 38.Caminschi I, et al. Interleukin-12 induces an effective antitumor response in malignant mesothelioma. Am. J. Respir. Cell Mol Biol. 1998;19:738–746. doi: 10.1165/ajrcmb.19.5.3257m. [DOI] [PubMed] [Google Scholar]