Abstract
Macrophages constitute a major part of the cell response to wear particles produced at articulating and non-articulating interfaces of joint replacements. This foreign body reaction can result in periprosthetic osteolysis and implant loosening. We demonstrate that ultra high molecular weight polyethylene (UHMWPE) particles induce systemic trafficking of macrophages by non-invasive in vivo imaging and immunohistochemistry. The distal femora of nude mice were injected with 60 mg/ml UHMWPE suspension, or saline alone. Reporter RAW264.7 macrophages which stably expressed the bioluminescent reporter gene and the fluorescence reporter gene were injected intravenously. Bioluminescence imaging was performed using an in vivo imaging system immediately after macrophage injection, and at 2-day intervals. Compared to the non-operated contralateral femora, at day 4, 6, and 8, the bioluminescent signal of femora containing UHMWPE suspension increased 1.30 ± 0.09, 2.36 ± 0.92, and 10.32 ± 7.61 fold, respectively. The values at same time points for saline injected control group were 1.08 ± 0.07, 1.14 ± 0.27, and 1.14 ± 0.35 fold, respectively. The relative bioluminescence of the UHMWPE group was higher at all post-injection days and significantly greater than the saline group at day 8 (p < 0.05). Histological analysis confirmed the presence of reporter macrophages within the medullary canal of mice with implanted UHMWPE particles. The presence of UHMWPE particles induced enhanced bone remodeling activity. Clinically relevant UHMWPE particles stimulated the systemic recruitment of macrophages during an early time course using the murine femoral implant model. Interference with systemic macrophage trafficking may potentially mitigate UHMWPE particle-induced periprosthetic osteolysis.
Keywords: macrophage trafficking, polyethylene particles, bioluminescence, non-invasive in vivo imaging
INTRODUCTION
Excessive production of wear particles from joint replacements is associated with periprosthetic osteolysis, which can lead to implant loosening.1–5 Phagocytic cells engulf wear debris and become activated, releasing proinflammatory cytokines, chemokines, degradative enzymes, and other substances which stimulate osteoclasts to undermine the prosthetic bone bed.6–12 One of the key cells in the foreign body and chronic inflammatory response to wear particles is the macrophage.13–15 Cells of the monocyte/macrophage lineage differentiate and maturate into phagocytic macrophages, foreign body giant cells and osteoclast precursors. In communication with stromal cells and other cell types, the phagocytic cells are primarily responsible for the cascade of events culminating in periprosthetic osteolysis.
Ultra high molecular weight polyethylene (UHMWPE) is one of the most commonly used materials for total joint arthroplasty. It is believed that wear debris of UHMWPE generated at the articulating surfaces enters the periprosthetic tissue and activates macrophages. Previously we showed that implanted polymethylmethacrylate (PMMA) bone cement particles induce systemic migration and concentration of macrophages using a murine femoral implant model.16 PMMA particles are locally produced and generally not widely disseminated, whereas UHMWPE particles from the bearing surfaces are more widely dispersed.17 In the present study, we demonstrate that clinically relevant UHMWPE particles stimulate an intense systemic recruitment of macrophages during an early time course using the murine femoral implant model.
MATERIALS AND METHODS
Animals and Cells
Twelve-week old adult male nude mice (Charles River Laboratories, Inc., MA) were housed and fed in our institution’s animal facility. The murine macrophage cell line RAW264.7 was transfected with the lentiviral vector to express the bioluminescent optical reporter gene, firefly luciferase (fluc), and a fluorescence reporter gene, green fluorescent protein (gfp).18
Ultra high molecular weight polyethylene particles
Conventional (non-highly cross-linked) UHMWPE particles from mechanical testing simulator studies of metal-on-conventional polyethylene bearings (a kind gift from Dr. Timothy Wright of the Hospital for Special Surgery, New York) were isolated by ultracentrifugation.19 The size of the UHMWPE particles was found to be 1.0 ± 0.1 μm (mean ± SE) in length using a Scanning Electronic Microscopy (Hitachi S-3400N). The particles tested negative for endotoxin using a Limulus Amebocyte Lysate kit (BioWhittaker, MD) and were suspended in sterile saline at a concentration of 60 mg/ml (based on the volume and density of the UHMWPE particles isolated, 60 mg/ml equals around 1.2 × 1011 particles/ml).
Surgical procedure
Our university’s guidelines for the care and use of laboratory animals were strictly followed. Thirty nude mice were injected UHMWPE particles (60 mg/ml) in their left femoral medullary cavities; another 13 nude mice served as saline-injected controls. All the operations were done under isoflurane anesthesia (2–3% in 100% oxygen) using mask inhalation. We used the transpatellar tendon approach for distal femoral medullary cavity injection.16,20 Briefly, the patellar tendon was exposed through a 5 mm lateral skin incision, and then the lateral aspect of the femoral shaft was exposed by another 5 mm incision over the distal quadriceps. The intramedullary injection (10 μl) of UHMWPE particle suspension or saline was performed through the patellar tendon into the inter-condylar region of the femur with a 5 mm insertion of the needle guided by the femoral shaft. After injection, the quadriceps-patellar complex and the skin were repaired by suture. Buprenorphine (0.1 mg/kg, Ben Venue Laboratories, Bedford, OH) was given subcutaneously immediately after surgery and 4 hours later post-operatively for pain control.
Bioluminescence imaging
Before performing the macrophage injections, we waited at least 7 days post-operation, until wound healing was complete. Then macrophages (5 × 105 cells) suspended in 0.1 ml Hanks’ balanced salt solution (Invitrogen, Carlsbad, CA) were injected intravenously through the lateral tail vein of mice. Luciferase substrate D-luciferin (3 mg/mouse, Biosynth International) was administrated intraperitoneally. Five minutes later, bioluminescence images were taken of the entire mouse with an in vivo imaging system (IVIS) employing a cooled charge-coupled device camera (Caliper LifeSciences, MA) in the Stanford Small Animal Imaging Facility. Prone and lateral images were obtained from each animal at each time point to better determine the origin of photon emission. Animals were imaged at 2-day intervals post-macrophage injection for 10 days. Bioluminescence images were quantified by drawing uniformly sized regions of interest (ROIs) over the implantation position throughout the whole experiment, and the data were collected as to photon/second/cm2/steradian (p/s/cm2/sr).
Histology and immunohistology
All the femora were collected from experimental and control groups after completion of the imaging experiments. Femora from 3 animals in both the UHMWPE particle and saline groups were randomly chosen for histological study. Frozen sections were collected from the distal to the middle of each femur. The sections collected from the diaphyses were used for immunostaining. Mouse anti-GFP monoclonal antibody (Chemicon International, CA) was used to detect exogenous macrophages tagged with GFP. Rat anti mouse CD68 (AbD Serotec, NC) was used to detect macrophages. Anti-vitronectin receptor αVβ3 antibody (Chemicon International, CA) and anti-osteocalcin antibody (LifeSpan Biosciences, WA) were used to identify osteoclasts and osteoblasts respectively. The secondary antibody used was biotin-conjugated goat anti-mouse/rat IgG (Invitrogen, CA). Alkaline phosphotase (AP)-conjugated streptavidin (Invitrogen, CA) was used to bind with biotinylated secondary antibodies. Briefly, cold acetone fixed frozen sections (6 μm thick) were blocked by levamisole (200 mM in PBS, Sigma) for 24 hours followed with treatment of Ultra V Block (Lab Vision, CA) for 5 minutes at room temperature (RT). Primary antibodies were incubated with sections at RT for 3 hours; then the sections were incubated with secondary antibodies for 1 hour at RT; AP-streptavidin were incubated for 15 minutes at RT. Buffer washing was performed after each step. Nuclear fast red (Vector Laboratories, CA) was used for counter staining.
Statistical Methods
The ratio of the bioluminescence within the ROI of the operated divided by the non-operated femora was calculated for saline-infused (control) and particle-infused (treatment) animals. The non-parametric Mann-Whitney U test was used for statistical analyses between groups and the signed rank test was used to compare right and left limbs in the same animals.
RESULTS
Imaging and bioluminescent signals of nude mice in UHMWPE and control group
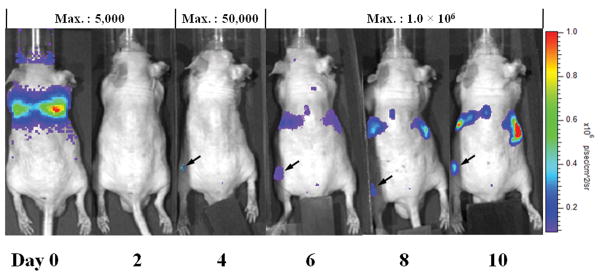
The pulmonary bioluminescent signal immediately after injection indicated the success of intravenous injection of reporter macrophages. In a typical experimental animal, a strong signal was seen in the lungs on day 0 (Figure 1). From day 4 onward, bioluminescent signals were detected in the operated left femur which received UHMWPE particles (Figure 1). With subsequent distribution of the tagged macrophages throughout the body, signals could also be seen in the vicinity of the liver and kidney (Figure 1) after day 6. For the saline group, there was no difference of the bioluminescence signals for injected and non-injected femora (data not shown).
Fig. 1. In vivo imaging of UHMWPE particles injected in the left femur of a nude mouse from day 0 to day 10 post-injection of Fluc labeled macrophages.
The animal is lying in the prone position. The bioluminescence from implanted femur can be detected since day 4 (arrowed). The Maximum reading of the scale bar for different days of imaging are listed on the top of the graph. The unit of the signal is p/s/cm2/sr as shown in the right of the figure.
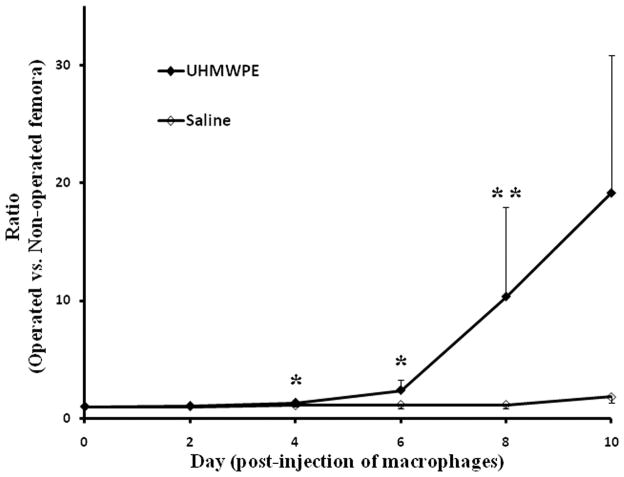
In Figure 2, the ratios of bioluminescent signals from operated divided by the non-operated contralateral femora of the UHMWPE particle and saline groups are shown. At day 0 and day 2 post-injection of macrophages, there were few differences between the operated and non-operated femora in both groups. From day 4 onwards, there were higher bioluminescent signals detected from femora receiving UHMWPE particles compared to those receiving saline alone. The ratios of the bioluminescence of UHMWPE particle injected femora versus non-operated femora in the experimental group were 1.30 ± 0.09, 2.36 ± 0.92, and 10.32 ± 7.61 at day 4, 6, and 8, whereas the values for the control group were 1.08 ± 0.07, 1.14 ± 0.27, and 1.14 ± 0.35, respectively. The increased bioluminescent signals of the UHMWPE group were significantly higher than those from the saline group at day 8 (p < 0.05) and a trend was seen at day 4, and day 6 (p < 0.1).
Fig. 2. The ratio of bioluminescence of 60 mg/ml (w/v) UHMWPE particle suspension femora, and saline injected femora versus bioluminescence of the corresponding non-operated contralateral femur from day 0 to 10 post-injection of macrophages.
The Y-axis is a normalized ratio of bioluminescence (unit: p/s/cm2/sr) from the operated femur versus non-operated femur in each animal. The values represent: mean ± SE. Number of animals in UHMWPE and saline injected groups are 30 and 13, respectively. Non-parametric Mann-Whitney test: *: p < 0.1; **: p < 0.05.
Immunohistochemistry
After completion of all of the imaging experiments, animals were euthanized and femora were collected for immunohistochemistry analysis. As shown in Figure 3, both the macrophage marker CD68 and the reporter gene GFP from RAW264.7 macrophage cells could be detected in both the UHMWPE particle injected (Figure 3A and 3B) and non-operated contralateral femora (Figure 3E and F) of the UHMWPE group. However, in the non-operated femora, there was much less GFP signal compared to the operated side implanted with particles (Figure 3B and 3F). For the osteoblast marker osteocalcin (Figure 3C and 3G) and the osteoclast marker αVβ3 (Figure 3D and 3H), there was high expression in the trabecular bone in the sections from the UHMWPE particle injected group compared to the contralateral non-injected femora. However, in the saline group, both injected and non-injected contralateral femora showed little evidence of macrophage, osteoclast or osteoblast staining (Figure 3I – 3L). We used a high concentration of levamisole (200 mM in PBS) to block endogenous AP activity for the 24 hour incubation period during immunostaining. However, if this step is omitted during processing of the sections, strong AP activity could be observed within the periosteum and the bone marrow cavity in sections from the UHMWPE particle injected femora, but not in the sections from control group (data not shown).
Fig. 3. Immunohistology of frozen section of UHMWPE particle group and control group.
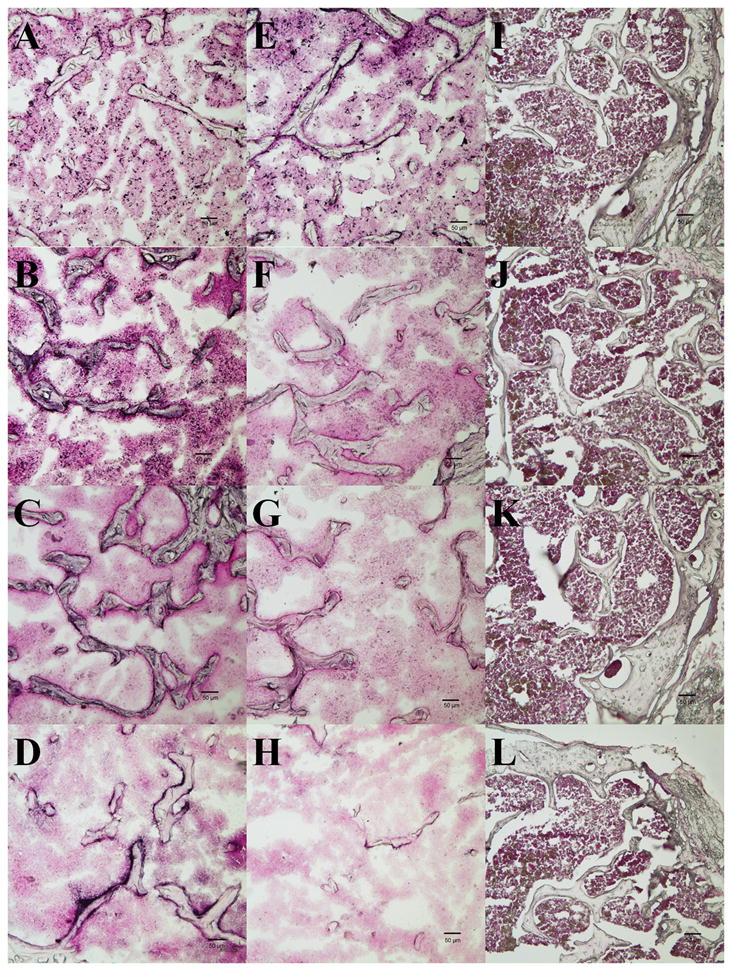
The first column (A–D) contains sections from UHMWPE particle injected femora; the 2nd column (E–H) contains sections from non-operated femora of UHMWPE particle groups; the 3rd column (I–L) contains sections from the saline injected femora. Sections in 1st row are stained with anti-CD68 antibody, 2nd – 4th rows are stained with anti-GFP, anti-osteocalcin, and anti-αVβ3 antibody, respectively. AP and its substrate BCIP/NBT were used for chromogenic reaction; Nuclear fast red was used for counter staining. Arrows in the panels C and D pointed the positive staining of anti-osteocalcin and anti-αVβ3. The scale bar length is 50 μm.
DISCUSSION
Wear particle-induced periprosthetic osteolysis and implant loosening are the major causes of failure of joint replacements. UHMWPE wear debris play an important role in these processes. The UHMWPE particles in this study have an average length of 1.0 ± 0.1 μm based on SEM measurements, which is a size capable of being phagocytosed and stimulating an inflammatory reaction.21, 22 The volume of the particle suspension injected was 10 μl, containing about 1.2 × 109 particles for placement into the marrow cavity.
In this study, we used in vivo imaging and immunohistochemistry to observe trafficking of intravenously injected mouse macrophages (RAW264.7), in which fluc and gfp reporter genes were expressed, to the UHMWPE particles implanted in the femora of nude mice. To avoid the immediate inflammatory phase associated with surgical trauma, intravenous injection of macrophages was performed 7 to 10 days post particle injection. In addition, since the reporter RAW264.7 cells used in this experiment are an immortal macrophage cell line, the imaging was performed no more than 14 days post-macrophage infusion to minimize the potential longer-term adverse effects of systemic growth of the RAW264.7 cells. In Figure 1, the bioluminescence signals in the liver and kidney were seen as early as day 6 and became stronger thereafter. The background signal due to continued growth of RAW264.7 cells in the body would increase with time. The immortal characteristics of the RAW264.7 cell line would induce not only unwanted signal, but also cachexia of the animals. The bodyweight of animals usually starts to fall around day 6 and drops abruptly (more than 10%) between day 12 and day 14 post-injection with the current cell dosage used. In order to collect data from animals in a healthy state, we accumulated data from day 0 to day 10 post-macrophage injection in this study.
As shown in Figure 1, at day 0, the strongest signals were located in the lungs, as this signified successful intravenous injection of reporter cells. At day 2, the majority of injected cells are then widely distributed throughout the body and the signal from these diffusely located cells was not strong enough to be detected. By day 4, bioluminescent signal at the UHMWPE particle implanted site was evident and became stronger subsequently. In contrast to the bioluminescent signals from the operated femur with particles, the bioluminescent signals from the contralateral limb were minimal. For the control group, the saline-injected femora had very low levels of bioluminescent signal, similar to the non-operated contralateral femora.
From the bioluminescent signal detected from the UHMWPE particle implanted femora, we concluded that reporter macrophages migrated to the region of inflammation induced by the polymer particles. Based on previous studies23–27, tail vein injected reporter cells will travel to the lung via the inferior vena cava and pulmonary artery initially; in our studies, these cells could be detected as early as 5 minutes post-injection. Approximately 2 hours post-injection, cells migrate systemically to the liver, spleen, kidney, and to the marrow of long bones.23–27 In rodents, the number of distributed reporter cells to the long bones is much lower than to viscera such as liver, lung, kidney, and spleen; the concentration of cells localized in bone was around 50 times lower than those in liver and lung, 10 times lower than in the kidney, and 2 times lower than in spleen, respectively.23–27 In this study, the signals from the liver and kidney area reflected the normal distribution rate of cells in rodents. The comparatively strong signal from the distal femora in which particles were implanted indicated that the UHMWPE particles induced robust systemic migration of infused macrophages to the location of the particles.
We used the ratio (bioluminescent signals from operated divided by the non-operated contralateral femora of each animal) to minimize individual difference amongst animals. The increased bioluminescent signals of the UHMWPE group were significantly higher than those from the saline group at day 8 (p < 0.05) and a trend was seen at day 4, and day 6 (p < 0.1).
We used immunohistochemistry to demonstrate the migration of the reporter macrophages to the UHMWPE particle implanted distal femora. The macrophage marker CD68 (staining all macrophages), and the GFP protein marker expressed by reporter RAW264.7 cells only were used to detect all the macrophages versus exogenous macrophages injected from a remote site, respectively. The existence of GFP immunostaining confirmed that reporter macrophages in the circulation had trafficked to the site of implanted UHMWPE particles. In contrast, there were few CD68 or GFP positive staining cells in the saline-injected femora or contralateral controls. In addition, the majority of macrophages in the UHMWPE group stained for both CD68 and GFP. This suggested that the majority of the macrophages in the UHMWPE treated femora were systemically derived cells, rather than of local origin.
It is also noted that there were comparatively lower levels of anti-CD68 and anti-GFP immunoreactions in the sections from contralateral non-operated femora in UHMWPE group, but more than in either limb of the control group. This suggests that the systemic signaling induced by the implanted UHMWPE particles in the left femora was associated with macrophage migration and activation in the non-operated contralateral limbs. But, maybe due to the lag of the inflammation signal transferring, most macrophages in the non-operated limbs were endogenous GFP-free macrophages. The migration of macrophages induced by systemic signaling to a non-operated limb has also been observed in other inflammatory animal models and human conditions.28–31
We also qualitatively investigated markers of bone remodeling in the UHMWPE and saline implanted animals. We observed increased osteocalcin staining on the trabecular bone within the bone marrow in the UHMWPE particle containing femora), but much less for the non-operated femora on the contralateral side, and in the saline injected femora. Positive staining for the osteoclast marker αVβ3 was noted around the trabecular bone in the UHMWPE particle-containing femora but staining was rare in the non-operated UHMWPE femora and was not found in the femora of the saline group. Thus, UHMWPE particle implantation stimulated increased local osteoblast and osteoclast activity, which has been reported by others previously.13, 32–34 In addition to the osteoblast and osteoclast specific marker immunostaining, we noted strong alkaline phosphatase activity in the periosteum and marrow of the UHMWPE containing femora without the levamisole treatment (to block AP activity), but not in the control group or the contralateral femora of the UHMWPE group. This implied that UHMWPE particle implantation stimulated bone remodeling, with regards to both osteoblast and osteoclast activities.
CONCLUSIONS
Macrophages are one of the major cells that participate in the foreign body and chronic inflammatory reaction to wear debris. The foreign body and chronic inflammatory reaction to wear debris is primarily responsible for periprosthetic osteolysis. In this study, we employed the techniques of non-invasive in vivo bioluminescence imaging and immunohistochemistry to demonstrate the systemic macrophages recruitment to UHMWPE particles implanted in murine femora. Understanding the mechanisms of macrophage trafficking and homing to areas of wear particle generation may suggest potential strategies and targets for mitigation of periprosthetic osteolysis. Given the fact that other downstream approaches to minimize osteolysis have had limited success, it seems logical to understand and neutralize the initial processes of cellular recruitment and activation. This could potentially increase the longevity of joint replacements.
Acknowledgments
We gratefully acknowledge Dr. Gobalakrishnan Sundaresan (Gambhir lab, Stanford University, CA) who supplied the reporter macrophage cell line and Dr. T. Wright (Hospital of Special Surgery, NY) for UHMWPE particles. This study was supported by R01 AR055650 from the National Institute of Health, and the Ellenburg Chair in Surgery at Stanford University.
References
- 1.Maloney WJ, Smith RL. Periprosthetic osteolysis in total hip arthroplasty: the role of particulate wear debris. J Bone Joint Surg Am. 1995;77:1448–1461. [PubMed] [Google Scholar]
- 2.Bauer TW. Particles and Periimplant Bone Resorption. Clin Orthop Relat Res. 2002;405:138–143. doi: 10.1097/00003086-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Wang ML, Sharkey PF, Tuan RS. Particle bioreactivity and wear-mediated osteolysis. J Arthroplasty. 2004;19(8):1028–1038. doi: 10.1016/j.arth.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Archibeck MJ, Jacobs JJ, Roebuck KA, Glant TT. The basic science of periprosthetic osteolysis. Instr Course Lect. 2001;50:185–195. [PubMed] [Google Scholar]
- 5.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 6.Glant TT, Jacobs JJ, Molnár G, Shanbhag AS, Valyon M, Galante JO. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993;8(9):1071–1079. doi: 10.1002/jbmr.5650080907. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima Y, Sun DH, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Induction of matrix metalloproteinase expression in human macrophages by orthopaedic particulate debris in vitro. J Bone Joint Surg Br. 1998;80:694–700. doi: 10.1302/0301-620x.80b4.8374. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima Y, Sun DH, Trindade MC, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81:603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-α mediates orthopedic implant osteolysis. Am J Pathol. 1999;154(1):203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinov P, Leithner A, Radl R, Bodo K, Khoschsorur GA, Schauenstein K, Windhager R. Role of free radicals in aseptic loosening of hip arthroplasty. J Orthop Res. 2006;24(1):55–62. doi: 10.1002/jor.20013. [DOI] [PubMed] [Google Scholar]
- 11.Talmo CT, Shanbhag AS, Rubash HE. Nonsurgical management of osteolysis: challenges and opportunities. Clin Orthop Relat Res. 2006;453:254–264. doi: 10.1097/01.blo.0000246531.59876.a8. [DOI] [PubMed] [Google Scholar]
- 12.Drees P, Eckardt A, Gay RE, Gay S, Huber LC. Mechanisms of disease: Molecular insights into aseptic loosening of orthopedic implants. Nat Clin Pract Rheumatol. 2007;3(3):165–171. doi: 10.1038/ncprheum0428. [DOI] [PubMed] [Google Scholar]
- 13.Athanasou NA, Quinn J, Bulstrode CJK. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J Bone Joint Surg Br. 1992;74B:57–62. doi: 10.1302/0301-620X.74B1.1732267. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz SM, Purdon MA. Mediator interactions in macrophage/particulate bone resorption. J Biomed Mater Res. 1995;29:477–484. doi: 10.1002/jbm.820290407. [DOI] [PubMed] [Google Scholar]
- 15.Miyanishi K, Trindade MC, Ma T, Goodman SB, Schurman DJ, Smith RL. Periprosthetic osteolysis: induction of vascular endothelial growth factor from human monocyte/macrophages by orthopaedic biomaterial particles. J Bone Miner Res. 2003;18(9):1573–1583. doi: 10.1359/jbmr.2003.18.9.1573. [DOI] [PubMed] [Google Scholar]
- 16.Ren P-G, Lee S-W, Biswal S, Goodman SB. Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials. 2008;29(36):4760–4765. doi: 10.1016/j.biomaterials.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg. 2000;82:457–477. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 18.De A, Zhou X-M, Gambhir SS. Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice. Molecular Therapy. 2003;7(5):681–691. doi: 10.1016/s1525-0016(03)00070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell P, Ma S, Yeom B, McKellop H, Schmalzried TP, Amstutz HC. Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res. 1995;29(1):127–131. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 20.Zilber S, Epstein NJ, Lee S-W, Larsen M, Ma T, Smith RL, Biswal S, Goodman SB. Mouse femoral intramedullary injection model: technique and micro CT scan validation. J Biomed Mater Res B. 2008;84(1):286–290. doi: 10.1002/jbm.b.30872. [DOI] [PubMed] [Google Scholar]
- 21.Schmalzried TP, Jasty M, Harris TP. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed] [Google Scholar]
- 22.Green TR, Fisher J, Matthews JB, Stone MH, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res (Appl Biomater) 2000;53:490–497. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Lotze MT, Line BR, Mathisend J, Rostnberg SA. The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implication for the adoptive immunotherapy of tumors. J Immunol. 1980;125:1487–1493. [PubMed] [Google Scholar]
- 24.Maghazachi AA, Herbermarn B, Vujanovinc L, Hiseodt JC. In vivo distribution and tissue localization of highly purified rat lymphokine-activated killer (LAK) cells. Cell Immunol. 1988;115:179–194. doi: 10.1016/0008-8749(88)90172-4. [DOI] [PubMed] [Google Scholar]
- 25.Felgar E, Hiserodt JC. In vivo migration and tissue localization of highly purified lymphokine-activated killer cells (ALAK cells) in tumor-bearing rats. Cell Immunol. 1990;129:288–298. doi: 10.1016/0008-8749(90)90205-6. [DOI] [PubMed] [Google Scholar]
- 26.Kuppen PJ, Marinelli A, Camps JA, Pauwels EK, van de Velde CJ, Fleuren GJ, Eggermont AM. Biodistribution of lymphokine-activated killer (LAK) cells in Wag rats after hepatic-artery or jugular-vein infusion. Int J Cancer. 1992;52(2):266–270. doi: 10.1002/ijc.2910520219. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Dennisa JE, Muzicb RF, Lundberga M, Caplan AI. The Dynamic in vivo Distribution of Bone Marrow-Derived Mesenchymal Stem Cells after Infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 28.Kelly S, Dunham JP, Donaldson LF. Sensory nerves have altered function contralateral to a monoarthritis and may contribute to the symmetrical spread of inflammation. Eur J Neurosci. 2007;26(4):935–942. doi: 10.1111/j.1460-9568.2007.05737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decaris E, Guingamp C, Chat M, Philippe L, Grillasca JP, Abid A, Minn A, Gillet P, Netter P, Terlain B. Evidence for neurogenic transmission inducing degenerative cartilage damage distant from local inflammation. Arthritis Rheum. 1999;42:1951–1960. doi: 10.1002/1529-0131(199909)42:9<1951::AID-ANR22>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson LF, Seckl JR, McQueen DS. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J Neurosci Meth. 1993;49:5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26(2):165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 32.Quinn J, Neale S, Fujikawa Y, McGee J, Athanasou NA. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int. 1998;62:527–531. doi: 10.1007/s002239900473. [DOI] [PubMed] [Google Scholar]
- 33.Quinn J, Joyner C, Triffitt JT, Athanasou NA. Polymethylmethacrylate-induced inflammatory macrophages resorb bone. J Bone Joint Surg Br. 1992;74B:652–658. doi: 10.1302/0301-620X.74B5.1527108. [DOI] [PubMed] [Google Scholar]
- 34.Sabokbar A, Pandey R, Quinn JMW, Athanasou NA. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch Orthop Trauma Surg. 1998;117:136–140. doi: 10.1007/s004020050213. [DOI] [PubMed] [Google Scholar]