Abstract
Posterior parietal cortex (PPC) links primate visual and motor systems and is central to visually guided action. Relating the anatomical connections of PPC to its neurophysiological functions may elucidate the organization of the parietal–frontal network. In owl and squirrel monkeys, long-duration electrical stimulation distinguished several functional zones within the PPC and motor/premotor cortex (M1/PM). Multijoint forelimb movements reminiscent of reach, defense, and grasp behaviors characterized each functional zone. In PPC, functional zones were organized parallel to the lateral sulcus. Thalamocortical connections of PPC and M1/PM zones were investigated with retrograde tracers. After several days of tracer transport, brains were processed, and labeled cells in thalamic nuclei were plotted. All PPC zones received dense inputs from the lateral posterior nucleus and the anterior pulvinar. PPC zones received additional projections from ventral lateral (VL) divisions of motor thalamus, which were also the primary source of input to M1/PM. Projections to PPC from rostral motor thalamus were sparse. Dense projections from ventral posterior (VP) nucleus of somatosensory thalamus distinguished the rostrolateral grasp zone from the other PPC zones. PPC connections with VL and VP provide links to cerebellar nuclei and the somatosensory system, respectively, that may integrate PPC functions with M1/PM.
Keywords: intracortical microstimulation, motor cortex, premotor cortex, pulvinar, ventral lateral thalamus
Introduction
The present paper is part of a series of comparative studies on the connections of functionally defined zones of posterior parietal cortex (PPC) and frontal cortex motor regions in nonhuman primates. The PPC of macaque monkeys in the region of the intraparietal sulcus (IPS) has been subdivided into a number of functionally related areas on the basis of connectional, architectonic, and functional criteria. Frontal motor cortex has long been divided into primary motor (M1), dorsal premotor, ventral premotor, and supplementary motor areas using similar criteria. The functional zones in PPC and frontal cortex motor regions are major components of the “dorsal stream” involved in visually driven motor action (Wise et al. 1997).
Recently, Graziano et al. (2002) extended the understanding of the organization of motor/premotor cortex (M1/PM). Delivering longer than commonly used trains of electrical pulses with microelectrodes in macaque monkeys revealed an organization of M1/PM not apparent in studies based on short bursts of electrical pulses (Asanuma and Rosen 1972; Strick and Peterson 1978; Sessle and Wiesendanger 1982; Gould et al. 1986; Donoghue et al. 1992; Nudo et al. 1992; Stepniewska et al. 1993). In brief, electrical stimulation of different cortical sites evoked complex motor behaviors that resembled reaching, defensive, and grasping behaviors. Each behavioral category was evoked from a discrete zone, and the entire map extended across M1/PM. In a less extensive exploration, long trains of electrical stimulation evoked defensive behavior from the ventral intraparietal area (VIP) of PPC (Cooke et al. 2003). Using similar electrical stimulation mapping parameters, Stepniewska et al. (2005) evoked reaching, defensive, and grasping behaviors from functionally distinct zones of PPC in prosimian primates (galagos). More recent results indicate that such complex movements can also be evoked from different zones of M1/PM in galagos (Stepniewska, Cerkevich, et al. 2009; Stepniewska, Fang, and Kaas 2009). Collectively, results in Old World primates and prosimian primates suggest that PPC and M1/PM have similar functional zones specialized in mediating ethologically relevant behaviors. If this is indeed the case, we may expect PPC and M1/PM to share some connections with other components of the motor subsystems but that the connections also differ in ways that reflect the roles of PPC and M1/PM.
Although the thalamocortical connections of PPC have been extensively studied in macaque monkeys (Jones et al. 1979; Yeterian and Pandya 1985; Schmahmann and Pandya 1990; Cappe et al. 2007), the connections of functional zones in PPC as defined by neurophysiological mapping, and how they overlap or differ across functional zones, are not known. In addition, much less is known in general about the thalamocortical connections of PPC and M1/PM in New World monkeys than in macaque monkeys. Here, we used long trains of electrical stimulation to define functional zones in PPC and regions of M1/PM in New World squirrel and owl monkeys. Anatomical tracers were injected into some of these functional zones to reveal their connections. To some extent, injections were delivered into zones of similar functions in both PPC and M1/PM to facilitate direct comparisons of their thalamocortical connections. Squirrel and owl monkeys were used because the IPS is absent, and M1/PM is rostral to the central sulcus. Thus, most of the functional zones in PPC and M1/PM are accessible on the cortical surface where they can be readily explored with electrical stimulation and localized with facility for tracer injections. The thalamocortical connections revealed by tracer injections are described in the present report. Subsequent reports will describe the electrical stimulation results in detail, as well as patterns of cortical connections. Some of the present results have appeared previously in an abstract format (Gharbawie et al. 2008).
Materials and Methods
Animals
Three squirrel monkeys (Saimiri sciureus) and 5 owl monkeys (Aotus trivirgatus) were studied. Animals from both species were 3–6 years old. Squirrel monkeys weighed 800–900 g, whereas owl monkeys weighed 800–1300 g. All procedures were approved by Vanderbilt University Animal Care and Use Committee and followed the guidelines of the National Institutes of Health guide for the care and use of laboratory animals.
Intracortical Electrical Stimulation
Monkeys were preanesthetized with ketamine hydrochloride (10–30 mg/kg i.m.) and maintained on 2% isoflurane during surgical procedures. Animals were placed in a stereotaxic frame for aseptic surgery. The skull was opened to expose the parietal and frontal lobes of a single hemisphere. The opening extended from the tip of the lateral sulcus to approximately 7 mm rostral to the central sulcus. In its caudal extent, the craniotomy was bound by the midline of the hemisphere and the lateral sulcus. The opening widened rostrally to ensure that the hand–forelimb representations of M1/PM were exposed. The more medial trunk representations of M1/PM were also exposed. The dura was dissected, and the exposed cortex was covered with inert silicon fluid. The surface of the cortex was digitally photographed, and a printout was used to record microelectrode penetrations. Anesthesia was switched at this point to a continuous perfusion of ketamine mixed in physiological saline (20–40 mg/kg/h) delivered through the tail vein.
Intracortical electrical stimulation motor mapping was conducted with the objective of identifying the topography of functional zones in PPC and in M1/PM according to the forelimb movements evoked. A tungsten microelectrode (1-MΩ impedance) was perpendicularly lowered with a micromanipulator into the cortex to depths 1600–1800 μm beneath the surface. Interpenetration distances were 0.5–1.0 mm, varying primarily to avoid vascular branches on the cortex. Stimulation trains consisted of 150 biphasic pulses delivered over 500 ms. The duration of each phase was 0.2 ms at 350 Hz. Similar stimulation parameters have been shown to evoke multijoint movements reminiscent of ethologically relevant behaviors from PPC of macaque monkeys (Cooke et al. 2003) and galagos (Stepniewska et al. 2005). For M1/PM electrical stimulation, current intensity was increased from low levels (20 μA) until a movement was reliably evoked to a maximum of 200 μA, whereas the starting point for PPC was 150 μA to a maximum of 400 μA.
Because the primary objective was to investigate PPC thalamocortical connections, PPC was mapped to identify as many functional zones as possible for tracer injections. In the interest of minimizing duration of anesthesia, M1/PM was less comprehensively mapped. Current thresholds were therefore not consistently determined to identify the borders between motor and premotor cortex. Thus, the 2 areas are referred to as M1/PM. In addition, M1/PM was not explored in its entirety. Nevertheless, several zones were consistently identified in M1/PM and injected in each animal for comparison to the PPC results.
Anatomical Tracer Injection
Once electrical stimulation mapping was complete, 2–4 retrograde tracers were injected into M1/PM and PPC of each animal. A total of 27 injections were delivered into 8 hemispheres of 8 monkeys. Tracers were pressure injected from a 1- or 2-μL Hamilton syringe fitted with a glass pipette beveled to a sharp tip. Tracers included cholera toxin b-subunit (CTB; Molecular Probes, Carlsbad, CA; 10% in distilled water), and the fluorescent tracers Diamidino Yellow (DY; Sigma, 2% in distilled water), Fluoro Ruby (FR; Molecular Probes, 10% in distilled water), and Fast Blue (FB; Polysciences, Warrington, PA; 2% in distilled water). Two depths beneath the surface of the cortex (800 and 400 μm) were targeted at each injection site. Total volume for each injection site was 0.4 μL for DY, FB, and CTB and 0.6 μL for FR.
Histology
Approximately 8 days were allowed for tracer transport. Animals were then injected with a lethal dose of sodium pentobarbital (80 mg/kg) and perfused intracardially with phosphate-buffered saline (PBS; pH 7.4). For fixation, 2% paraformaldehyde in PBS and 2% paraformaldehyde in PBS with 10% sucrose solution were delivered in succession. The brain was removed from the skull. The thalamus and brainstem were separated, blocked, and submerged in 2% paraformaldehyde in PBS with 30% sucrose overnight for additional fixation and cryoprotection. The thalamus was sectioned in the coronal plane at 40 μm and saved in 5 series of adjacent sections. One series was mounted onto glass slides unprocessed for analysis of the distribution of fluorochrome-labeled cells. A second series was reacted for CTB immunohistochemistry, and then labeled cells were visualized with a diaminobenzidine dihydrochloride reaction that was nickel intensified (Veenman et al. 1992). Three successive series were stained for cytochrome oxidase (CO, Wong-Riley 1979), acetylcholinesterase (AChE, Geneser-Jensen and Blackstad 1971), or Nissl substance to identify architectonic subdivisions of thalamus.
Data Analysis
Distributions of labeled cells in the thalamus were plotted with a Leitz microscope (Leica Microsystems, Wetzlar, Germany) connected to an X–Y encoder. An experimenter manually marked the positions of labeled cells on a computer system running Neurolucida software (V. 5.05.4, Chicago, IL). Cells labeled with DY and FB were visualized with fluorescence illumination passed through a 360-nm wavelength filter, whereas a 530- to 560-nm-wavelength filter was used for FR-labeled cells. Cells labeled with CTB were visualized under bright field illumination.
Architectonic borders of thalamic nuclei in sections stained for CO, AChE, and Nissl were traced on paper using a projection microscope. Tracings were digitized with a scanner and architectonic borders were retraced using Adobe Illustrator software (CS2). Plots generated in Neurolucida were aligned to digitized tracings of thalamic nuclei using Adobe Illustrator. Blood vessels and major fiber pathways guided alignment. Symbols marking the location of labeled cells were adjusted for shape and color and digitally merged onto the retraced borders.
Results
Electrical stimulation mapping was conducted to identify functional zones in PPC and M1/PM for tracer injections. Functional zones were identified in PPC and in M1/PM according to constellations of evoked multijoint movements. Thalamocortical connections of M1/PM were primarily with ventral lateral (VL) divisions of motor thalamus. PPC connections were primarily with lateral posterior nucleus (LP) and anterior pulvinar (PA). Additional PPC connections were with VL divisions and ventral posterior (VP) nuclei.
Thalamic Nuclei Architectonic Borders
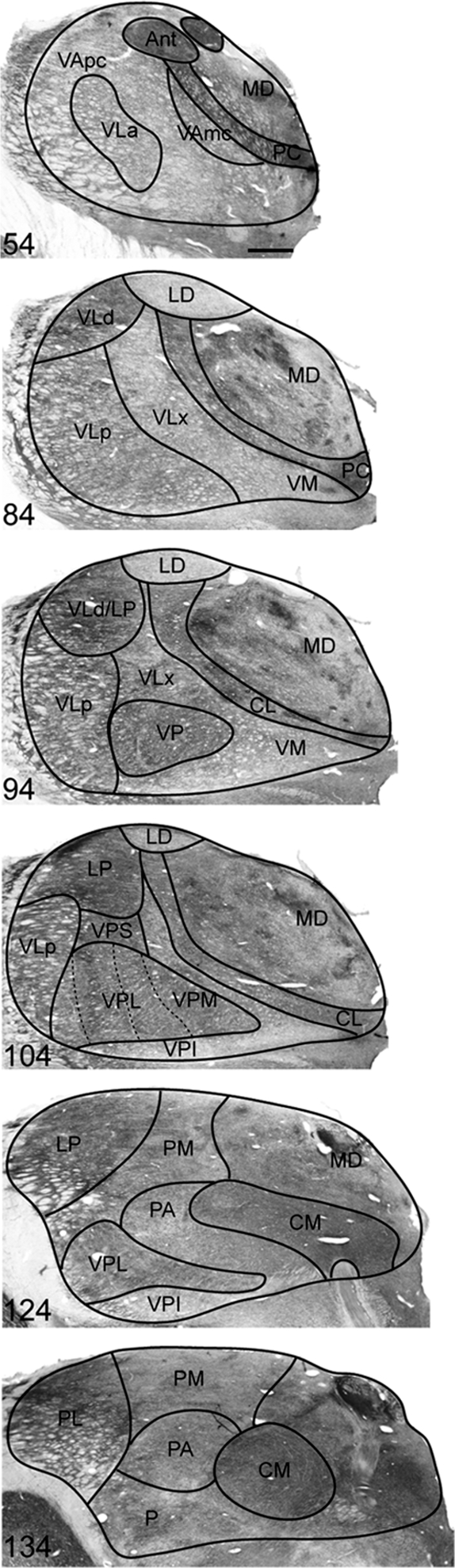
Thalamic nuclei relevant to the present study are shown in sections from an owl monkey (Fig. 1). The same nuclei were identified in the present squirrel monkeys as well as in previous squirrel monkeys (Emmers and Akert 1963) and macaque monkeys (Olszewski 1952; Jones 1985). Although most of the nomenclature in the present study is consistent with this previous use, nuclei of motor thalamus follow those described for owl monkeys (Stepniewska et al. 1994a, 1994b) and the recently revised nomenclature for macaque monkeys (Jones 2007). Accordingly, 4 main divisions of VL thalamus are recognized. Those were most readily identified with AChE staining. The most anterior is VLa, which has been referred to as VLo (Olszewski 1952) or VLa (Jones 1985). The largest division of VL is VLp, which has been referred to as VPLo (Olszewski 1952) or VLp (Jones 1985). The VLx division is medial to VLp and has been referred to as area X (Olszewski 1952) or recognized only as part of VLp (Jones 1985). The most dorsal division is VLd, which has been referred to as VLc (Olszewski 1952) or recognized only as part of VLp (Jones 1985).
Figure 1.
Photomicrographs of coronal sections of owl monkey thalamus cut at 40 μm and stained for AChE. Representative sections were selected to show nuclei pertinent to cell labeling from tracers injections. Organization is from rostral (top) to caudal (bottom). Section numbers are in the bottom left corner of each panel. Scale bar = 1 mm.
The motor thalamus also includes the ventral anterior nucleus (VA), which is mostly rostral to VL. The VA subdivision characterized by large cells is referred to as magnocellular (VAmc), whereas the subdivision characterized by smaller cells is coined parvocellular (VApc). This terminology is consistent with macaque and squirrel monkey nomenclature (Olszewski 1952; Emmers and Akert 1963; Jones 1985). It is generally agreed upon that VA subdivisions and VLa receive basal ganglia projections, whereas the remainder of VL receives cerebellar projections. The present ventral medial nucleus (VM) has been considered a subdivision of the VL complex, VLm (Olszewski 1952), or recognized as separate from VL (Jones 1985).
Additional differences in terminology are with reference to parts of somatosensory thalamus. The lateral division of the VP nucleus, which receives most of the somatosensory input from below the face (via the cuneate and gracilis nuclei), is termed VPL in the present study but has been referred to as VPLc (Olszewski 1952; Jones 1985). The PA is also known as the oral division of pulvinar or pulvinar pars oralis (PO) (Olszewski 1952; Jones 1985).
Tracer Injections
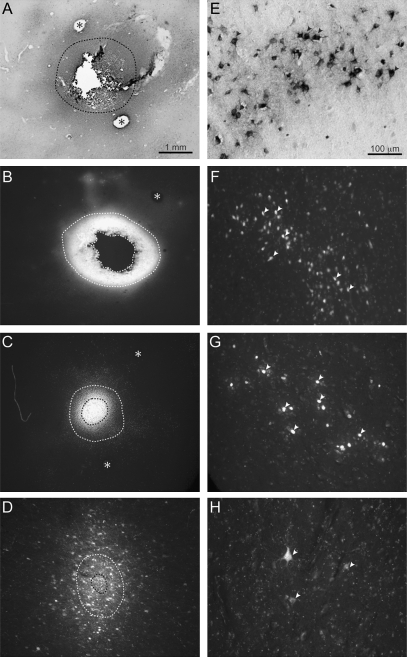
Retrograde tracers were injected into functional zones of PPC and M1/PM and labeled cells in thalamic nuclei. Electrolytic lesions induced at the completion of mapping confirmed in postmortem tissue that tracer injections were confined to target zones (e.g., Fig. 2).
Figure 2.
Photomicrographs of tracer injection sites in the cortex (left panels) and examples of the corresponding cells labeled in the thalamus (right panels). Examples are from squirrel monkey case 08-09 shown in more detail in Figure 3. Sections of cortex were flattened and cut parallel to the surface at 40 μm. Photomicrographs were captured using a light microscope to show (A) CTB or using a fluorescent microscope to show (B) FB, (C) DY, and (D) FR. The inner outline marks the core of each tracer injection, whereas the surrounding outline marks a halo of intense tracer diffusion. Electrolytic lesions—delivered at the end of motor mapping to mark the boundaries of specific zones identified with electrical stimulation—are indicated with asterisks. The medial and lateral borders of the M1/PM grasp zone are marked with asterisks in (A). The caudomedial border of the PPC reach zone is marked in (B). The medial and lateral borders of PPC grasp zone are marked in (C). One-millimeter scale bar applies to all panels showing tracer injections. Cells labeled from corresponding injections to show (E) CTB-labeled cells in VLx, (F) FB-labeled cells in VLd/LP, (G) DY-labeled nuclei in VLx, (H) FR-labeled cells in VLx. Arrows point to examples of clearly labeled cells. Contrast was digitally enhanced for FR- and DY-labeled cells to improve illustration. Photomicrographs of labeled cells were captured from thalamic section 59 in Figure 3. Hundred-micrometer scale bar applies to all panels showing labeled cells.
Forelimb Movements Evoked with Electrical Stimulation
Short trains of microstimulation (18 monophasic pulses in 50 ms) evoked muscle twitches from M1/PM but no apparent movement from PPC. Long trains of electrical stimulation (150 biphasic pulses in 500 ms) evoked multijoint forelimb movements reminiscent of ethologically relevant behaviors from M1/PM and PPC. Body movements were primarily contralateral to electrical stimulation, but bilateral trunk and hindlimb movements were evoked from some sites. Although several classes of movement were evoked with long trains of electrical stimulation from different zones, only those pertinent to tracer injections are described here. Detailed mapping results will be presented in a future report. The reaching and defensive movements described below are similar to those illustrated for galagos (Stepniewska et al. 2005).
Reaching Movements
The shoulder was flexed and the forearm extended. Digits extension and opening were typically concurrent with forearm extension. Forelimb trajectories were consistent for repeated electrical stimulation of a penetration site. Nevertheless, trajectories varied across sites to include end points in upper space, lower space, or level with the animal's horizontal body.
Defensive Movements
Two constellations of movements were characterized as defensive. First, the shoulder extended and the forelimb concurrently abducted while the digits extended. The end point of the open palm was either near the face or lateral to the body presumably for protection. Second, the forelimb was withdrawn directly toward the body by a shoulder retraction and a slight elbow adduction. The utility of the actions was presumed to remove the forelimb from a threatening stimulus. Aggressive face gestures were evoked either independently or in conjunction with defensive forelimb movements. The ear pinna retracted against the neck, the eye blinked, and the upper lip exposed the teeth in a grimace posture.
Grasping Movements
The digits flexed and the wrist concurrently dorsiflexed. In some sites, these movements were accompanied with wrist supination toward the mouth.
M1/PM Organization (Squirrel Monkeys)
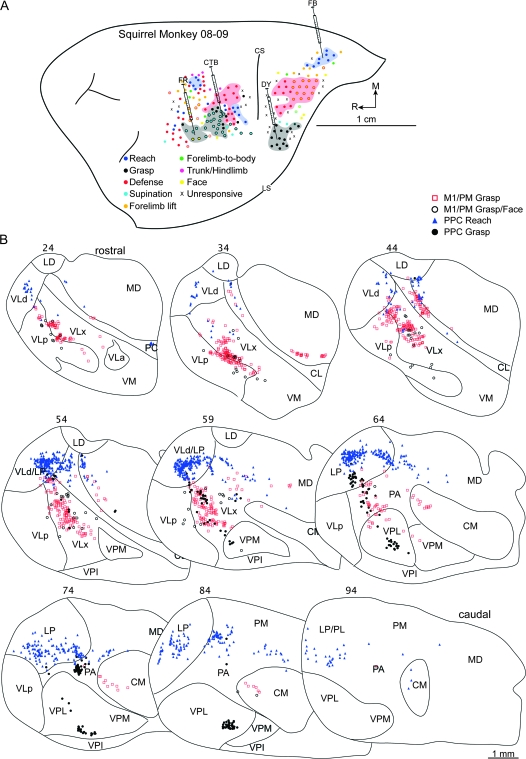
Functional zones were identified with electrical stimulation in 3 squirrel monkeys. Organization of M1/PM varied across cases and zones intermingled within each case. In addition, similar forelimb movements could be evoked from separate zones in M1/PM. Nevertheless, the general topography included a defense zone caudally. A grasp zone bordered it rostrally and included sites of grasp and concurrent wrist supination in its lateral extension (Fig. 3A). A reach zone was rostral and medial to the grasp zone. A zone of concurrent forelimb and face movements was at the approximate rostral extent as the reach zone but lateral. The 2 movement constellations primarily evoked from this concurrent response zone included grasp and mouth open as well as defensive forelimb movements and aggressive face gestures.
Figure 3.
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation (0.2-ms biphasic current trains delivered at 300 Hz for 500 ms) from squirrel monkey case 08-09. Microelectrode penetration sites are color coded to reflect evoked movements. Sites that evoked dual movements are represented in 2 colors derived from the color code in the figure legend. Major functional zones are highlighted. M1/PM was mostly characterized by reach, defense, grasp/wrist supination zones, organized from caudal to rostral. The 3 zones shared borders. Rostral and medial to M1 was another reach zone. At the same rostral extent, but lateral, was another grasp zone that included sites of concurrent wrist supination or mouth opening. In PPC, zones of reach, defense, and grasp were organized in a caudomedial to rostrolateral progression. The defense zone involved forelimb movements, but aggressive face gesturers were evoked from the caudal half of this functional zone. Reach and defense zones were separated by sites that evoked forelimb-lift responses, whereas the defense and grasp zones were separated by sites that evoked forelimb supination, face movements, and unresponsive sites. Four retrograde tracers were injected: CTB into M1/PM grasp zone, FR into M1/PM near sites that evoked concurrent grasping and mouth opening, FB into PPC reach zone, and DY into the PPC grasp zone. Major landmarks include lateral sulcus (LS) and central sulcus (CS). (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case. Sections were cut at 40 μm, and the ascending section numbers are organized from rostral to caudal. Each symbol represents a single cell labeled by 1 of the 4 retrograde tracers. Borders of thalamic nuclei were identified from architectonic analysis in adjacent sections.
M1/PM Thalamocortical Connections (Squirrel Monkeys)
Eight days were allowed for tracer transport except in case 08-03, which was terminated approximately 48 h after tracer injections. Numerous cells were labeled in the thalamus of this monkey however to merit inclusion. Distributions of labeled cells in thalamic nuclei from 5 tracer injections in M1/PM are summarized in Table 1.
Table 1.
Distributions of labeled cells from retrograde tracer injections in M1/PM functional zones in 3 squirrel monkeys and 5 owl monkeys
| Defense |
Grasp |
Reach |
Concurrent |
|||||||||
| Sq | Sq | Owl | Sq | Owl | Sq | Owl | Owl | Owl | Sq | Owl | Owl | |
| 07-118 | 08-03 | 07-103 | 08-09 | 07-85 | 08-03 | 08-41 | 07-85 | 07-77 | 08-09 | 08-45 | 07-103 | |
| FB | FB | FR | CTB | CTB | FR | FB | FR | FR | FR | DY | DY | |
| 1705.00 | 50.00 | 10.00 | 1423.00 | 685.00 | 20.00 | 804.00 | 50.00 | 117.00 | 117.00 | 432.00 | 807.00 | |
| Vapc | 1.11 | — | 30.00 | — | 1.46 | — | 18.16 | 4.00 | — | — | 22.45 | 13.75 |
| Vla | 11.03 | — | 10.00 | — | 6.57 | 10.00 | 22.14 | 4.00 | — | — | 12.73 | 20.20 |
| VLD | — | — | — | 0.77 | — | 30.00 | 2.99 | — | — | — | — | 0.25 |
| VLx | 16.48 | 80.00 | 20.00 | 56.50 | 44.53 | 55.00 | 28.73 | 64.00 | 57.98 | 56.41 | 34.26 | 39.16 |
| VLp | 36.89 | — | 40.00 | 15.04 | 18.69 | — | 10.32 | 2.00 | 28.57 | 36.75 | 0.46 | 13.63 |
| VM | 3.40 | — | — | — | — | — | — | 2.00 | — | 1.71 | — | 1.61 |
| PC | 2.11 | — | — | 0.63 | — | — | 0.62 | 2.00 | 0.84 | — | 6.71 | 2.11 |
| CL | 0.76 | — | — | 9.98 | 0.29 | — | 7.59 | — | 0.84 | — | 11.34 | 6.07 |
| CM | 4.63 | — | — | 4.78 | 10.07 | — | 1.24 | — | — | 2.56 | — | — |
| LD | — | — | — | 0.07 | — | — | 0.62 | — | — | — | — | — |
| MD | 2.35 | — | — | 7.24 | 0.44 | 5.00 | 7.21 | 18.00 | 8.40 | — | 7.64 | 2.60 |
| VPM | 0.65 | 2.00 | — | 0.14 | 0.88 | — | — | 4.00 | — | — | 0.69 | 0.25 |
| VPL | 7.51 | 14.00 | — | 1.48 | 11.24 | — | — | — | 1.68 | 1.71 | 0.46 | — |
| VPS | 5.22 | — | — | — | 1.02 | — | — | — | — | — | — | — |
| VPI | 0.11 | — | — | — | — | — | — | — | — | — | — | — |
| LP | 0.35 | 4.00 | — | 0.56 | — | — | — | — | 1.68 | — | 0.23 | 0.25 |
| PL | — | — | — | 0.35 | — | — | — | — | — | — | — | — |
| PA | 7.33 | — | — | 2.46 | 4.82 | — | 0.37 | — | — | 0.85 | 3.01 | 0.12 |
| PM | — | — | — | — | — | — | — | — | — | — | — | — |
Rows sequentially list M1/PM functional zones, species of monkey investigated (Sq: squirrel monkey and Owl: owl monkey), case number of each monkey, tracer injected, and total number of cells labeled in the thalamus from each injection. Thalamic nuclei are listed in the first column, and successive columns contain the percentage of cells labeled in each thalamic nucleus. The densest concentration of labeled cells for each injection is listed in bold.
Defense Zone
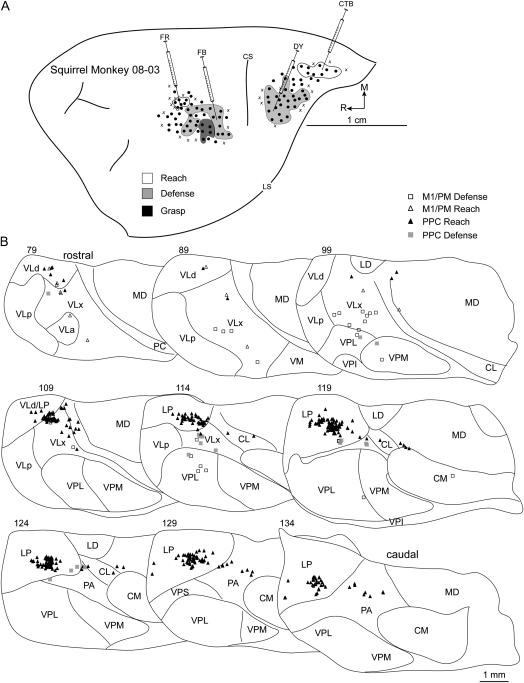
Defense zones were consistently identified in caudal aspects of M1/PM in all squirrel monkeys and were injected with tracers in 2 cases. In case 08-03, FB was injected into the caudal extent of the defense zone (Fig. 4A). The densest concentration (80%) of labeled cells was in VLx (Table 1). A few labeled cells were in VP, and they were primarily concentrated in VPL (Fig. 4B). Even fewer cells were present in LP. In case 07-118, FB was injected near the center of the defense zone (Fig. 5A) at the approximate location of the previous injection. Nearly 65% of labeled cells were in motor thalamus with the densest concentration in VLp (Table 1). Labeled cells were primarily concentrated in VLp in rostral thalamic sections and shifted medially into VLx in more caudal sections (Fig. 5B). Most of the other cells labeled in the motor thalamus were in VLa. The number of cells labeled in intralaminar nuclei was minimal and mostly limited to CM. Only a small number of cells was labeled in MD. The proportion of cells labeled in VP was comparable with the previous injection, albeit with most of the labeled cells distributed between VPL and VPS. Perhaps the most distinguishing feature about the present distribution of labeled cells was the comparatively dense proportion (7%) identified in PA. The caudal location of the injection and distribution of labeled cells outside of motor thalamus suggests that the present injection may have encroached into area 3a.
Figure 4.
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from squirrel monkey case 08-03. Four retrograde tracers were injected: FB into M1/PM defense zone, FR into M1/PM reach zone, CTB into PPC reach zone, and DY into PPC defense zone. (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case. A brief survival period limited tracer transport for at least 3 injections. The adequate number of labeled cells from tracer injection in the PPC reach zone was likely due to rapid CTB transport. Borders of thalamic nuclei were identified from architectonic analysis in adjacent sections.
Figure 5.
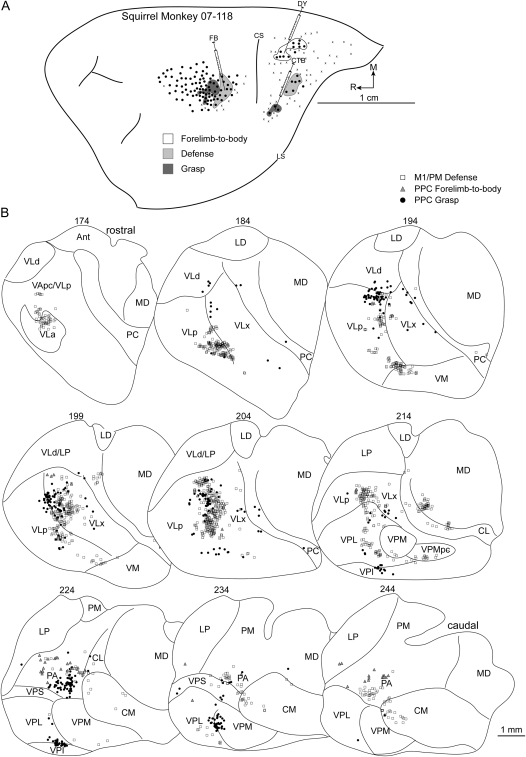
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from squirrel monkey case 07-118. Three retrograde tracers were injected: FB into M1/PM defense zone, DY into PPC forelimb-to-body representation, and CTB into PPC grasp zone. (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case.
Grasp Zone
In case 08-09, CTB was injected in the caudal aspect of the grasp zone, which was located just rostral to the defense zone (Fig. 3A). This injection was therefore rostral relative to the previous defense zone injections. Nearly 72% of the labeled cells were in VLp and VLx, with a higher concentration in VLx (Table 1). Labeled cells were concentrated in the VLp/VLx border in rostral sections of the thalamus but shifted medially into VLx in more caudal sections (Fig. 3B). A small concentration of labeled cells was in intralaminar nuclei (CL and CM) as well as in adjacent MD. A few cells were labeled in VPL and PA.
Reach Zone
In case 08-03, FR was injected near the center of the reach zone (Fig. 4A) and labeled 20 cells with the densest concentration in VLx and the remainder in VLd and VLa (Table 1; Fig. 4B).
Concurrent Response Zone
In case 08-09, FR was injected near electrical stimulation sites that evoked concurrent grasping and mouth opening movements (Fig. 3A). This injection was at the approximate rostral level of the previous injection. Nearly all labeled cells were in motor thalamus (Fig. 3B) with the densest concentration in VLx (56%) and to a lesser extent in VLp (Table 1).
M1/PM Organization (Owl Monkeys)
Functional zones of M1/PM were identified with electrical stimulation in 5 owl monkeys. In most cases, a caudal grasp zone characterized M1/PM (Fig. 6A). The representations rostral to the grasp zone varied among cases and included defense, reach, forelimb-to-body, or forelimb supination zones. As in squirrel monkeys, a reach zone was rostral and medial within M1/PM. Also, as in squirrel monkeys, a zone that evoked concurrent forelimb and face movements was at the same rostral extent as the reach zone but lateral.
Figure 6.
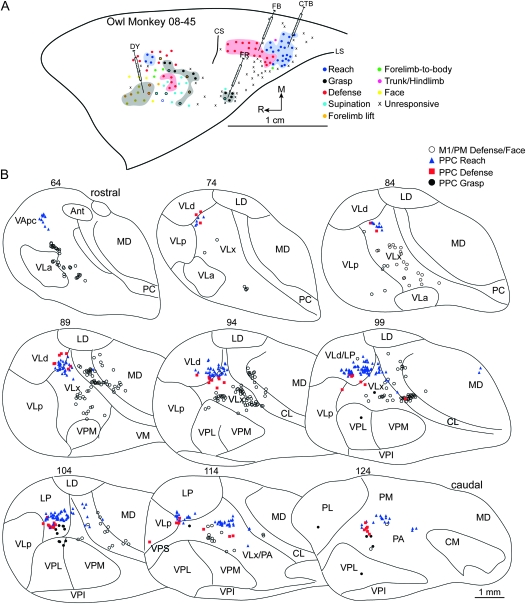
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from owl monkey case 08-45. Microelectrode penetration sites are color coded to reflect evoked movements. Sites that evoked dual movements are represented in 2 colors derived from the color code in the figure legend. Major functional zones are highlighted. M1/PM was primarily characterized by grasp, wrist supination, and defense zones. A reach zone was rostral and medial in M1/PM, whereas concurrent face and forelimb movements were evoked from the same approximate rostral extent but lateral. In PPC, reach, defense, and grasp zones were organized in a caudomedial to rostrolateral progression. Reach and defense zones bordered one another, whereas unresponsive sites separated the defense and grasp zones of PPC. Four retrograde tracers were injected: DY into M1/PM near sites that evoked concurrent defensive forelimb movements and aggressive face gestures, CTB into PPC reach zone, FB into PPC defense zone, FR into PPC grasp zone. (B) Distributions of labeled cells in a series of coronal thalamic sections (40 μm) from the same case. A limited number of cells were labeled from the PPC grasp zone because a small volume of FR was purposely injected to minimize tracer spread beyond this limited target zone.
M1/PM Thalamocortical Connections (Owl Monkeys)
Survival for tracer transport ranged from 7 to 9 days. The distributions of labeled cells in thalamic nuclei from 7 injections in M1/PM are summarized in Table 1. The pattern of connections was similar to squirrel monkeys with cells primarily labeled in VL divisions.
Defense Zone
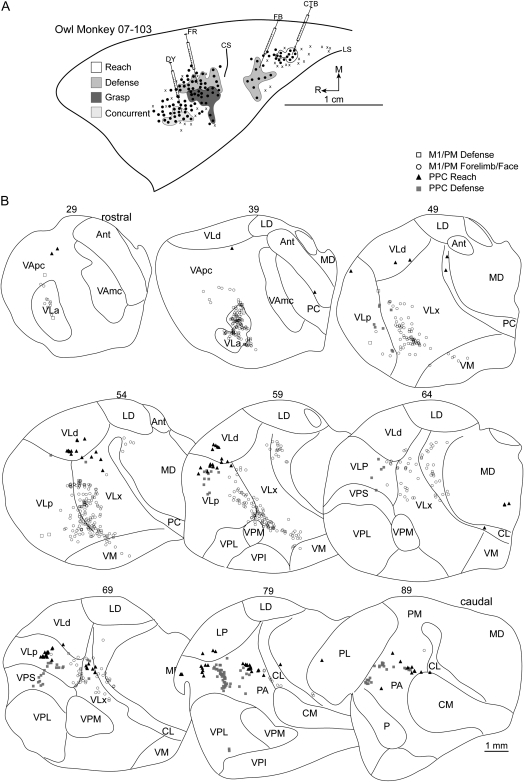
In case 07-103, a large defense zone bordered the grasp zone medially in caudal M1/PM (Fig. 7A). An injection of FR near the center of this zone labeled a limited number of cells (Table 1) but all were in motor thalamus (Fig. 7B). The densest concentration was in VLp followed in decreasing order by VApc, VLx, and VLa.
Figure 7.
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from owl monkey case 07-103. Four retrograde tracers were injected: FR into the M1/PM defense zone, DY into M1/PM near sites that evoked grasping and mouth opening, CTB into PPC reach zone, and FB into PPC defense zone. (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case. A limited number of cells were labeled from the M1/PM defense zone injection likely because of a small injection volume of FR.
Grasp Zone
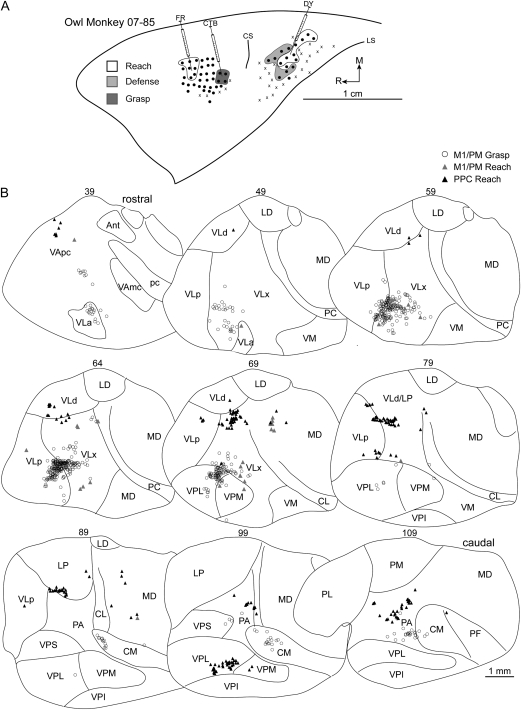
In case 07-85, CTB was injected into the center of the grasp zone in caudal M1/PM (Fig. 8A). More than 71% of labeled cells were in the motor thalamus with the densest concentration in VLx (Table 1). The second densest concentration of labeled cells was in VLp (Fig. 8B). The number of cells labeled in rostral motor thalamus was markedly less. A small proportion of labeled cells were in intralaminar nuclei, primarily in CM. A small proportion of cells was labeled in VP, largely in VPL. A small proportion of labeled cells was also identified in PA.
Figure 8.
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from owl monkey case 07-85. Three retrograde tracers were injected: CTB into M1/PM grasp zone, FR into M1/PM reach zone, DY into the PPC reach zone. (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case. A limited number of cells were labeled from the M1/PM reach zone injection likely due to a small injection volume of FR. Borders of thalamic nuclei were identified from architectonic analysis in adjacent sections.
Reach Zone
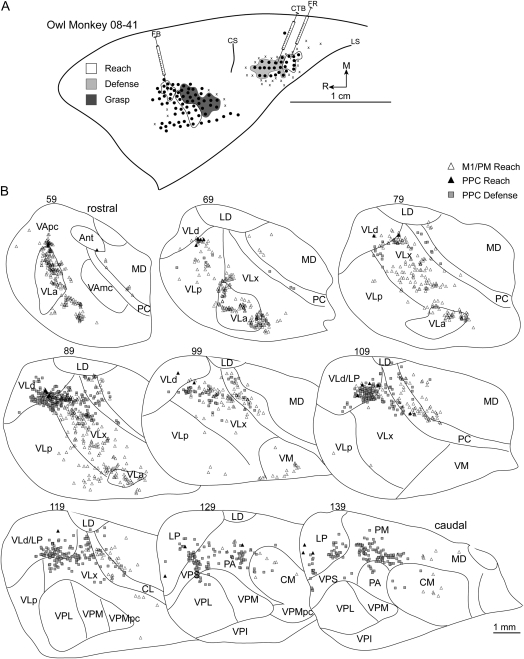
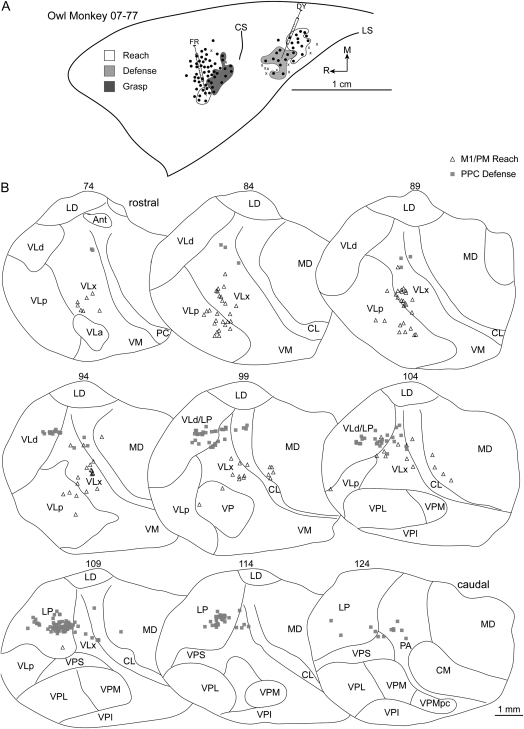
Injections into the reach zone in rostral/medial M1/PM in 3 cases revealed consistent patterns of cell labeling. In case 08-41, FB was injected into the center of the reach zone (Fig. 9A). More than 82% of labeled cells were in motor thalamus with the densest concentration in VLx (Table 1). The next densest concentration of labeled cells was in VLa and VApc (Fig. 9B). The proportion of labeled cells in VLp and VLd was comparatively less. A small proportion of cells was labeled in intralaminar nuclei and was primarily concentrated in CL. A comparable proportion of cells was labeled in adjacent MD. In case 07-85, a small volume of FR was injected near the center of the reach zone (Fig. 8A) at the approximate location of the previous injection. A modest number of cells was labeled (Table 1), and the majority (74%) was in the motor thalamus, with the densest concentration in VLx (Fig. 8B). A few cells were labeled in MD. In case 07-77, FR was injected near the center of the reach zone at a more lateral location than the previous 2 injections (Fig. 10A). Nearly 87% of labeled cells were in motor thalamus (Table 1). The densest concentration was in VLx, and fewer cells were labeled in VLp (Fig. 10B). A few cells were labeled in MD.
Figure 9.
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from owl monkey case 08-41. Three retrograde tracers were injected: FB into M1/PM reach zone, FR into PPC reach zone, and CTB into PPC defense zone. (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case.
Figure 10.
(A) Map of multijoint forelimb movements evoked with intracortical electrical stimulation from owl monkey case 07-77. Two retrograde tracers were injected: FR into M1/PM reach zone and DY into PPC defense zone. (B) Distributions of labeled cells in a series of coronal thalamic sections from the same case.
Concurrent Response Zone
Tracers were injected into the concurrent response zone in rostral/lateral M1/PM in 2 cases. In case 08-45, DY injection was near sites that produced concurrent defensive forelimb movements and face grimace (Fig. 6A). Labeled cells were primarily in motor thalamus (70%) with the densest concentration in VLx (34%). Rostral motor thalamus comprised the next densest concentration of labeled cells (Table 1). The majority of those cells was in VApc, and the remainder was in VLa. A small proportion of cells were identified in intralaminar nuclei PC and CL as well as in adjacent MD. A few cells were labeled in PA. In case 07-103, DY injection (Fig. 7A) was near sites that evoked concurrent grasping and mouth opening and was in the approximate location of the previous injection. The distribution of labeled cells was similar for the 2 injections. Nearly 87% of labeled cells were in motor thalamus (Table 1). The densest concentration was in VLx (39%), whereas only half that proportion was in VLa (Fig. 7B). An equal number of cells were labeled in VLp and VApc. A small proportion of labeled cells was in intralaminar nuclei and was primarily limited to CL.
PPC Organization (Squirrel Monkeys)
Functional zones of PPC were identified with intracortical electrical stimulation in the same squirrel monkeys. The responsive region was medial to the lateral sulcus and caudal to the central sulcus. Functional zones were organized approximately parallel to the lateral sulcus (Fig. 3A). A reach zone was in caudomedial PPC. A defensive zone was lateral and slightly rostral to the reach zone. A grasp zone was lateral to the defense zone. The reach and defense zones were often separated by sites that evoked forelimb lifting as well as forelimb adduction to the body. The defense and grasp zones were separated by sites that evoked wrist supination to the mouth and by unresponsive sites.
PPC Thalamocortical Connections (Squirrel Monkeys)
Distributions of labeled cells from 6 injections in PPC are summarized in Table 2. In general, labeled cells were concentrated in dorsal and posterior thalamic nuclei. Concentrations of labeled cells were densest in LP and PA. Cells were also labeled in the motor thalamus and to a lesser extent in VP.
Table 2.
Distributions of cells labeled from retrograde tracer injections in PPC functional zones in 3 squirrel monkeys and 5 owl monkeys
| Reach |
Defense |
Grasp |
||||||||||||
| Sq | Sq | Owl | Owl | Owl | Owl | Sq | Owl | Owl | Owl | Owl | Sq | Sq | Owl | |
| 08-09 | 08-03 | 08-45 | 07-85 | 08-41 | 07-103 | 08-03 | 08-45 | 07-77 | 07-103 | 08-41 | 08-09 | 07-118 | 08-45 | |
| FB | CTB | CTB | DY | FR | CTB | DY | FB | DY | FB | CTB | DY | CTB | FR | |
| 1743.00 | 424.00 | 425.00 | 414.00 | 56.00 | 171.00 | 19.00 | 74.00 | 252.00 | 188.00 | 994.00 | 344.00 | 642.00 | 27.00 | |
| Vapc | — | — | 4.00 | 2.42 | 3.57 | 2.92 | — | — | — | 0 | 0.30 | — | — | — |
| Vla | — | — | — | — | — | — | — | — | — | — | — | 0.29 | 0.31 | — |
| VLd | 17.27 | 4.72 | 9.41 | 6.28 | 37.50 | 13.45 | — | 14.86 | 16.27 | — | 26.26 | 0.29 | 2.65 | — |
| VLx | 5.22 | 9.19 | 24.24 | 18.12 | 1.79 | 13.45 | 5.26 | 27.03 | 13.49 | 8.51 | 21.23 | 14.82 | 18.38 | 7.41 |
| VLp | 0.34 | — | 4.71 | 6.76 | 7.14 | 25.15 | — | 12.16 | — | 48.94 | 7.55 | 0.29 | 18.85 | — |
| VM | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| PC | 0.69 | 0.24 | 0.24 | 0.24 | — | 2.34 | — | — | 1.19 | — | 0.91 | 0.29 | 2.18 | — |
| CL | 6.94 | 2.59 | 4.47 | 2.17 | — | 1.75 | — | 1.35 | 4.37 | — | 2.52 | 1.74 | 1.09 | — |
| CM | 0.92 | 0.47 | 1.41 | 0.72 | — | 1.17 | — | — | — | — | 1.41 | — | 0.78 | — |
| LD | 10.04 | 1.65 | 0.24 | 5.07 | — | 2.34 | — | — | 0.40 | — | 0.20 | 0.29 | 0.00 | — |
| MD | 10.04 | 1.65 | 0.24 | 5.07 | — | 2.34 | — | — | 0.40 | — | 0.80 | 0.87 | 0.16 | — |
| VPM | — | — | — | 0.48 | — | — | 21.05 | — | — | — | 0.00 | 4.65 | 4.67 | 7.41 |
| VPL | — | — | — | 12.56 | — | — | 10.53 | — | — | 1.06 | 0.20 | 29.94 | 16.36 | 18.52 |
| VPS | — | — | — | 1.69 | — | — | — | — | 0.79 | 4.26 | 1.11 | — | 1.56 | 11.11 |
| VPI | — | — | — | — | — | — | — | — | — | — | — | — | 5.76 | — |
| LP | 45.09 | 64.62 | 37.18 | 20.29 | 35.70 | 4.09 | 15.79 | 35.14 | 59.52 | 7.98 | 14.10 | 13.08 | 0.31 | 22.22 |
| PL | 2.35 | 6.6 | — | — | — | 5.85 | — | — | — | 3.19 | 0.80 | — | — | 3.70 |
| PA | 8.84 | — | 8.94 | 21.50 | 1.79 | 26.90 | 47.37 | 9.46 | 2.38 | 26.06 | 11.97 | 34.43 | 26.95 | 29.63 |
| PM | 1.20 | 9.91 | 5.18 | 1.45 | 10.71 | — | — | — | 1.59 | — | 10.66 | — | — | — |
Rows sequentially list M1/PM functional zones, species of monkey investigated (Sq: squirrel monkey, Owl: owl monkey), case number of each monkey, tracer injected, and total number of cells labeled in the thalamus from each injection. Thalamic nuclei are listed in the first column, and successive columns contain the percentage of cells labeled in each thalamic nucleus. The densest concentration of labeled cells for each injection is listed in bold.
Reach Zone
A reach zone was identified in the most caudomedial aspect of PPC in 2 cases. In case 08-09, FB was injected near the center of the PPC reach zone (Fig. 3A). The densest concentration of labeled cells was in LP (45%). A less dense concentration (23%) was in the motor thalamus, primarily localized in VLd with fewer cells labeled in VLx (Table 2). In some sections, cells labeled in VLx were close to the VLd border (Fig. 3B). Cells labeled in VLd might have been a rostral extension to those labeled in LP. Similarly, cells labeled in PL might have been a caudal extension to those labeled in LP. A small proportion of cells was labeled in CL, but the extent of labeling in the intralaminar nuclei was otherwise negligible. A slightly higher concentration of labeled cells was identified in adjacent MD. The proportion of cells labeled in the pulvinar nuclei (13%) was primarily concentrated in PA. In case 08-03, an injection of CTB in the center of the PPC reach zone (Fig. 4A) confirmed the distribution pattern of cells labeled from the previous injection. Nearly 65% of all labeled cells were in LP (Table 2). Cells labeled in the motor thalamus were primarily in VLx and to a lesser extent in VLd (Fig. 4B). Pulvinar labeling from the present injection was limited to PL and PM.
Forelimb-to-Body Zone
In case 07-118, sites that evoked forelimb adduction to the body were concentrated in a zone in medial PPC (Fig. 5A). A DY injection near the center of this zone labeled a modest number of cells due to a small injection volume. More than half of the labeled cells were in PA (Fig. 5B), and the second densest concentration of labeled cells was in motor thalamus (39%), primarily in VLp followed by VLx. A few labeled cells were in LP.
Defense Zone
In case 08-03, the defense zone was lateral and slightly rostral to the reach zone (Fig. 4A). DY was injected into the rostrolateral aspect of this PPC defense zone. The small number of labeled cells were primarily (47%) in PA (Fig. 4B). The second densest concentration of labeled cells was in VP (32%), with more cells in VPL than in VPM. A few cells were identified in LP (Table 2).
Grasp Zone
A grasp zone was identified in the most rostral/lateral aspect of PPC in 2 cases. In case 08-09, DY was injected near the center of the grasp zone. The densest concentration of labeled cells (33%) was in PA. The number of cells labeled in VP was comparable to that labeled in PA and was primarily in VPL (Table 2). A proportion of labeled cells (16%) was in the motor thalamus, primarily in VLx. The concentration of cells labeled in LP was slightly less than that identified in the motor thalamus (Fig. 3B). In case 07-118, an injection of CTB near the center of the grasp zone (Fig. 5A) confirmed the cell-labeling pattern from the previous injection. The densest concentration (27%) of labeled cells was in PA (Table 2). The proportion of labeled cells in the motor thalamus (40%) was equally distributed between VLx and VLp (Fig. 5B). The proportion of cells labeled in VP was approximately the same as in PA with denser concentrations in VPL. Unlike the previous injection, the number of cells labeled in LP was negligible.
PPC Organization (Owl Monkeys)
Reach and defense zones were organized approximately parallel to the lateral sulcus as in squirrel monkeys. A grasp representation zone was only identified in a single case, and it was separated laterally from the defense zone by unresponsive sites (Fig. 6A).
PPC Thalamocortical Connections (Owl Monkey)
The distributions of labeled cells from 9 tracer injections in PPC are summarized in Table 2. The general pattern of connections was similar to that observed in squirrel monkeys. Labeled cells were mostly concentrated in LP and PA. Cells were also labeled in motor thalamus and to a lesser extent in VP, PM, and PL.
Reach Zone
A reach zone was identified in the most caudomedial aspect of PPC in all owl monkeys and was injected in 4 cases. In case 08-45, CTB was injected in the caudal aspect of the reach zone. The densest concentration of labeled cells was in LP (37%). Nevertheless, the number of cells labeled in the motor thalamus was nearly 42% (Table 2). Those cells were primarily concentrated in VLx near its border with VLd and LP (Fig. 6B). The remainder of the cells labeled in the motor thalamus was in VLd and to a lesser extent in VLp and VApc. A small proportion of labeled cells were in intralaminar nuclei, primarily CL. Another small proportion of cells was identified in PA and PM. In case 07-85, DY was injected into the rostral/lateral aspect of the reach zone (Fig. 8A) and revealed a distribution of labeled cells similar to the previous injection. The densest concentrations of labeled cells were in LP (21%) and PA (22%). Nevertheless, nearly 34% of labeled cells were in the motor thalamus with the densest concentration in VLx (Table 2). Most of the remaining cells labeled in motor thalamus were equally distributed between VLd and VLp (Fig. 8B). A small proportion of labeled cells was present in adjacent MD. A proportion of labeled cells was in VP (15%) and was primarily localized to VPL. In case 08-41, FR was injected into the center of the reach zone (Fig. 9A). The distribution of labeled cells was comparable with the previous 2 injections despite the modest number of cells labeled (Table 2). Nearly 50% of labeled cells were in the motor thalamus (Fig. 9B). The densest concentration was in VLd (38%), which might have been a rostral extension of the cells labeled in LP that comprised the second densest concentration of labeled cells (36%). A few cells were labeled in PM. In case 07-103, CTB was injected near the center of this reach zone (Fig. 7A). The densest concentration (27%) of labeled cells was in PA (Table 1). Nevertheless, nearly 55% of labeled cells were in the motor thalamus with the densest concentration in VLp being comparable with that in PA (Fig. 7B). The second densest concentration of labeled cells within the motor thalamus was equally distributed between VLd and VLx. A few cells were labeled in VApc. A small number of cells was labeled in intralaminar nuclei, MD, LP, and PL.
Defense Zone
A defense zone was lateral and slightly rostral to the reach zone in all owl monkeys and was injected in 4 cases. In case 08-45, FB was injected near the center of the defense zone (Fig. 6A). A modest number of cells was labeled likely due to a limited volume of injected FB (Table 2). The densest concentration of labeled cells was in LP (35%). Nevertheless, nearly 54% of labeled cells were in the motor thalamus (Table 2), and they were primarily concentrated in VLx near its border with VLd (Fig. 6B). A smaller proportion of labeled cells was approximately distributed between VLd and VLp. A few cells were labeled in PA. In case 07-77, DY was injected near the center of the defense zone (Fig. 10A) and revealed a similar distribution of labeled cells to the previous injection. The densest concentration of labeled cells was in LP (60%). Nearly 30% of labeled cells were in the motor thalamus (Table 2), and they were concentrated in VLd as well as VLx near its border with VLd and LP (Fig. 10B). A few cells were labeled in the intralaminar nuclei, and they were primarily in CL. In case 07-103, FB was injected into the medial aspect of the defense zone. Nearly 41% of labeled cells were in the motor thalamus (Table 2) with the densest concentration in VLp and only a small proportion in VLx (Fig. 7B). The second densest concentration of labeled cells was in PA (30%). A small proportion of labeled cells was identified in VPS (17%). The border separating VPS from VLp was not clear in at least one thalamic section leaving open the possibility that a small proportion of labeled cells was mischaracterized in VPS or VLp. An even smaller concentration of cells was labeled in LP (8%). A few labeled cells were identified in PL. In case 08-41, CTB was injected into the caudal aspect of the defense zone (Fig. 9A). Nearly 55% of labeled cells were in the motor thalamus with the densest concentration in VLd (Table 2). A slightly smaller proportion of labeled cells was in VLx near its border with VLd (Fig. 9B). A markedly smaller proportion of labeled cells was in VLp also near the VLd border. A few cells were labeled in CL. Comparable concentrations of labeled cells were identified in LP (14%), PA (12%), and PM (11%).
Grasp Zone
In cases 08-45, a grasp zone was identified in the most lateral aspect of PPC (Fig. 6A) and was injected with a small volume of FR, which labeled a limited number of cells (Table 2). Although cells were primarily labeled in PA (30%), nearly 37% of cells were in VP, with the densest concentration in VPL (Fig. 6B). A proportion of labeled cells (22%) was in LP.
Topography of Thalamocortical Connections
Injecting multiple tracers in each monkey revealed the thalamic nuclei that project to functional zones within PPC and M1/PM. In addition, the topography of thalamocortical projections could also be compared across functional zones. The topographical organization of PPC thalamocortical connections was most apparent in squirrel monkey cases 08-09 and 07-118 (Figs. 3B and 5B, respectively) because PPC injections in those cases were spatially segregated. The general distribution of labeled cells was such that cells labeled from injections into medial PPC (reach zone and forelimb-to-body zone) were dorsal and slightly lateral to cells labeled from lateral PPC injections (grasp zone). Injections into the PPC reach zone and PPC defense zone in case 08-03 were spatially closer (Fig. 4B). Nevertheless, cells labeled from the reach zone injection were mostly dorsal to those labeled from the defense zone injection. Three PPC injections in owl monkey case 08-45 confirmed the topographical organization observed in squirrel monkeys. Cells labeled from the reach zone injection were dorsal to those labeled from the defense zone injection, which were in turn dorsal to those labeled from the grasp zone injection (Fig. 6B). Similarly in owl monkey case 07-103, cells labeled from the reach zone injection were primarily dorsal to those labeled from the defense zone injection (Fig. 7B). Although the topographic organization of labeled cells was less obvious in case 08-41, some of the cells labeled from the reach zone injection were lateral and dorsal to most cells labeled from the defense zone injection. In the main, cells labeled from PPC injections in both species occupied a more dorsal and slightly lateral position, than cells labeled from M1/PM injections. Perhaps the most apparent exception was for proportions of cells labeled from injections into the PPC grasp zone partially overlapping in VLx/VLp with cells labeled from injections into caudal M1/PM (Figs. 3B and 5B).
Summary of Thalamocortical Connections
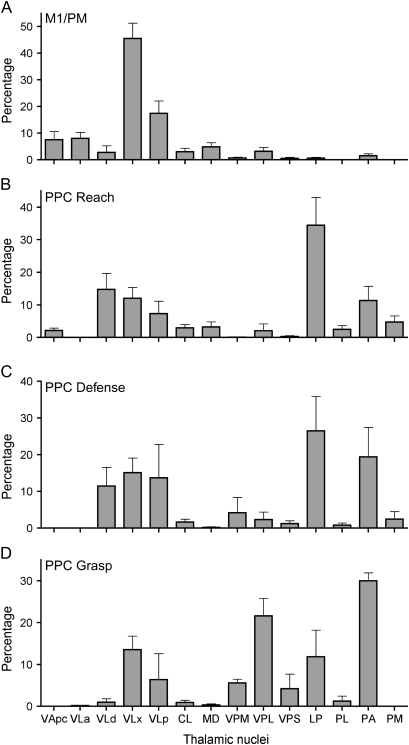
The densities of thalamocortical connections with PPC and M1/PM zones are summarized in Figure 11. Connection patterns were similar for squirrel and owl monkeys and results from the 2 species were pooled for the summary. Twelve injections into M1/PM showed that it was primarily connected to VL divisions. Nearly half of the connections were with VLx and the second densest projection was from VLp. Connections with VLd, VLa, and VApc were comparatively weaker. Connections with intralaminar nuclei and MD were even less dense. The weakest connections were with VPL and PA. Fifteen injections into PPC showed that its zones were most densely connected with dorsal and posterior thalamic nuclei. The caudomedial reach zone was primarily connected to LP followed by motor thalamus (VLd, VLx, and VLp). The next densest connections were with PA. Additional connections were with intralaminar nuclei, VApc, CL, MD, VPL, PL, and PM. Connections of the more lateral and slightly rostral defense zone were similar to those of the PPC reach zone. The densest connections were with LP followed by PA. Connections with motor thalamus were nearly equally distributed between VLd, VLx, and VLp. The PPC defense zone received denser projections from VP than the PPC reach zone. Connections with CL, PL, and PM were minimal. The most lateral PPC grasp zone was most densely connected with PA. Connections with LP were less dense than for the PPC reach and defense zones. Projections from the motor thalamus were from VLx and VLp and only minimally from VLd. Connections with VP were denser for the PPC grasp zone than for the reach and defense zones, particularly for VPL. Minimal connections were with CL, MD, and PL.
Figure 11.
Histogram summaries of the distribution of cells labeled in thalamic nuclei from 3 squirrel monkeys and 5 owl monkeys. The mean percentage of labeled cells and corresponding standard errors of the mean were calculated from the distributions presented in Tables 1 and 2. The number of injections into each functional zone is listed in the same tables. Thalamic nuclei included in the histograms contained at least a mean of 2% of the labeled cells for any given functional zone.
Discussion
Thalamocortical connections of PPC were investigated in squirrel and owl monkeys. PPC zones were identified according to forelimb movements evoked with long-train intracortical electrical stimulation. Retrograde tracers were injected into PPC zones and in M1/PM for comparison. M1/PM was primarily connected to VL divisions of the motor thalamus, whereas PPC zones were primarily connected to LP and PA. The most interesting finding of the present study was the additional input from motor and somatosensory thalamus that differentially projected to PPC zones. In addition, the caudomedial to rostrolateral topographical organization of PPC zones was reflected in a dorsoventral pattern of thalamocortical connections.
PPC Organization
Electrical stimulation evoked multijoint forelimb movements from PPC zones in similar locations in squirrel and owl monkeys. Functional zones were approximately parallel to the lateral sulcus and included a caudomedial reach zone and a more lateral and slightly rostral defense zone. A grasp zone was less consistently identified lateral to the defense zone. Sites that evoked other forelimb movements bordered those 3 zones, but their numbers were comparatively lower and their distributions were less consistent. Both the reach and the defense zones were too caudal to the central sulcus to be part of somatosensory cortex. The same cannot be ascertained for the grasp zone because its proximity to the central sulcus leaves open the possibility that it partially overlapped the anterior parietal cortex, for example, areas 1 and 2 (Merzenich et al. 1978; Pons and Kaas 1985).
Long-train electrical stimulation of PPC has only been reported in galagos and macaque monkeys. The absence of an IPS in the present New World monkeys complicates comparison of the present results to those published reports. Furthermore, PPC microstimulation in macaques has been limited to the lateral bank (Thier and Andersen 1996) and fundus (Cooke et al. 2003) of the IPS. Thus, we will refer to relevant results from single unit recordings in PPC of behaving macaque monkeys to compensate for the dearth of PPC stimulation studies.
A reach zone was identified in caudomedial PPC in squirrel and owl monkeys. This relative location is similar to the reach zone evoked from caudomedial PPC in galagos (Stepniewska et al. 2005; Stepniewska, Cerkevich, et al. 2009; Stepniewska, Fang, and Kaas 2009). Single-unit recordings in macaque monkeys showed that neural activity in segments of the medial bank of the IPS, known as the medial intraparietal area (MIP), is predictive of forelimb extension during a reach (Johnson et al. 1996). Unit activity in the dorsal aspect of the parietal–occipital area adjacent to MIP is also sensitive to reaching (Battaglia-Mayer et al. 2000). The 2 regions have been collectively coined the parietal reach region (Cohen and Andersen 2002), and the present PPC reach zone may be partly or wholly homologous to it. The caveat for this interpretation is the assumption of faithful overlap between the actions encoded by neural activity and actions evoked with electrical stimulation from the same group of neurons, that is, neurons active during reaching also evoke reaching movements during electrical stimulation under anesthesia.
A defense zone was adjacent and lateral to the PPC reach zone. Defensive forelimb movements were evoked from a relatively similar location in galagos (Stepniewska et al. 2005; Stepniewska, Cerkevich, et al. 2009; Stepniewska, Fang, and Kaas 2009). Aggressive face movements in the present study also paralleled the organization in galagos. Defensive forelimb movements and aggressive face gestures have also been evoked from the fundus of the IPS in macaque monkeys (Cooke et al. 2003), a region coined the VIP. Given the relative position of the present defense zone and its constellations of movements, it is likely homologous to the defense zones in galagos and macaque monkeys.
A grasp/wrist supination zone was lateral to the defense zone, albeit less consistently identified than the reach and defense zones, in squirrel and owl monkeys. Although grasping movements were not reported from PPC electrical stimulation in galagos, a small zone of hand-to-mouth movements was identified lateral to the defense zone (Stepniewska et al. 2005). Thus, there is spatial overlap between the present grasp zone and the hand-to-mouth zone in galagos. Moreover, recent results from galagos have identified a small grasp zone slightly rostral to the hand-to-mouth zone (Stepniewska, Cerkevich, et al. 2009; Stepniewska, Fang, and Kaas 2009). There have been no reports of grasping movements evoked with electrical stimulation from PPC in macaque monkeys. Nevertheless, single-unit activity within rostral aspects of the lateral bank of the IPS overlapped hand shaping for grasping (Sakata et al. 1995). Temporary deactivation of the same region impaired digit shaping to the contours of target objects (Gallese et al. 1994). This grasp sensitive zone has been coined the anterior intraparietal area (AIP), and the grasp zone identified in the present monkeys may be its homolog. Again, this interpretation assumes faithful correspondence between actions encoded by single units in behaving monkeys and those evoked with electrical stimulation under anesthesia.
Forelimb movements evoked from PPC with electrical stimulation were likely driven indirectly through activation of parietal–frontal networks. Short-train microstimulation (10–18 pulses in 50 ms), effective in evoking muscle twitches from M1/PM, did not evoke movements from PPC. This is not surprising considering corticospinal projections arising in PPC are sparse by comparison to projections from M1/PM and limited to area 5 as well as rostral aspects of the medial bank of the IPS (Nudo and Masterton 1990; Galea and Darian-Smith 1994; Matelli et al. 1998). The effectiveness of long-train electrical stimulation (150 pulses in 500 ms) in evoking forelimb movements from PPC suggests the recruitment of distant targets. It is unlikely that movements were evoked due to indiscriminate current spread for at least 2 reasons. First, PPC zones were separated from M1/PM by somatosensory cortex, which was mostly unresponsive to microstimulation. Second, there were subtle variations in forelimb movements evoked within a PPC zone and more substantial shifts in forelimb movements across functional zones. Both reasons diminish the possibility that indiscriminate current spread prompted movements from PPC, in which case a continuous map joining PPC and M1/PM as well as homogenous forelimb responses from electrical stimulation sites, would have been expected. The most parsimonious explanation for movements evoked from PPC zones is that electrical stimulation activated specific neuroanatomical channels linking PPC with M1/PM. Supporting anatomical evidence in owl monkeys (Stepniewska et al. 1993, 2006) and galagos (Stepniewska, Cerkevich, et al. 2009; Stepniewska, Fang, and Kaas 2009) shows strong connections between M1/PM and the approximate location of PPC zones identified here. Parietal–frontal networks have been shown in macaque monkeys with tracer injections in M1/PM as well as with PPC injections (Strick and Kim 1978; Petrides and Pandya 1984; Matelli et al. 1998; Luppino et al. 1999; Lewis and Van Essen 2000; Tanne-Gariepy et al. 2002).
M1/PM Thalamocortical Connections
Thalamocortical connection patterns of M1/PM were similar for squirrel and owl monkeys. Connections were primarily with VL and to a much lesser extent rostral motor thalamus. The results are consistent with previous studies in New World monkeys (Stepniewska et al. 1994a, 1994b, 2007), Old World monkeys (Strick 1975; Matelli et al. 1989; Darian-Smith et al. 1990), and prosimian galagos (Fang et al. 2006). This connection pattern is likely conserved in other phylogenetic orders considering it has been documented for motor cortex in rats (Donoghue and Parham 1983; Alder 1988) and cats (Strick 1970; Larsen and Asanuma 1979).
Electrical stimulation mapping and tracer injections into M1/PM were included in the present study for 2 reasons: first, to confirm that the electrical stimulation parameters reliably identified zones within M1/PM and can therefore be used to map PPC—indeed, the present thalamocortical connections demonstrate that tracers were injected into traditional M1/PM regions identified with cytoarchitecture and short-train microstimulation (Asanuma and Rosen 1972; Strick and Peterson 1978; Sessle and Wiesendanger 1982; Gould et al. 1986; Donoghue et al. 1992; Nudo et al. 1992; Stepniewska et al. 1993); second, for comparing the thalamocortical connections of PPC zones with matching counterparts in M1/PM.
PPC Thalamocortical Connections
Relating the present PPC thalamocortical connections to previous work is constrained by the same limitations of comparing the present neurophysiological results to existing reports. With the exception of one report on titi monkeys (Padberg and Krubitzer 2006), the thalamocortical connections of PPC have not been studied in New World monkeys. Comparisons to the better-documented PPC connections of macaque monkeys are complicated because cortical landmarks (e.g., IPS) that guided tracer injections in those studies are absent in the present species. Thus, the locations of the present tracer injections are described according to the forelimb movements evoked from each PPC zone to facilitate comparisons to other studies.
In general, the present results showed that the densest thalamic projections to PPC zones were from LP and PA. Additional connections were with motor thalamus, somatosensory thalamus, and other pulvinar nuclei. This is in agreement with reports showing that the densest thalamocortical connections to area 5 and the medial bank of the IPS (also known, respectively, as PE and PEa) are from LP and pars oralis division (PO) of pulvinar (Jones et al. 1979; Schmahmann and Pandya 1990; Cappe et al. 2007).
The most caudomedial PPC zone (reach) received its densest connections from LP. Dense input from LP has been consistently reported for PPC, especially area 5 in macaque monkeys (Jones et al. 1979; Schmahmann and Pandya 1990; Cappe et al. 2007; Padberg et al. 2009). However, dense LP projections were not reported for area 5 in titi monkeys (Padberg and Krubitzer 2006). The discrepancy should be interpreted with caution because the dearth of LP connections was reported from only 2 tracer injections into the hand representation of area 5 identified by multiunit recordings. Those injections might have been somatotopically incongruent with injections into the present reach zone identified with electrical stimulation.
The PPC reach zone received additional inputs from PA and in one injection VPL, which imply direct somatosensory inputs from the thalamus. Although PA projections are consistent with the thalamic input of area 5 in Old and New World monkeys, they also characterize somatosensory areas of the anterior parietal cortex. For example, PA input to area 2, in macaque monkeys (Pons and Kaas 1985; Schmahmann and Pandya 1990). Nevertheless, the distance separating injections into the PPC reach zone from the central sulcus precludes the possibility of tracer spread into anterior parietal cortex and suggests that the present projections likely parallel those reported for area 5. Additional inputs to PPC reach zone, albeit minimal by comparison, were from the lateral (PL) and medial (PM) pulvinar. Reports from Old and New World monkeys have shown PL projections to be related to vision. Nevertheless, cells labeled in PL from the present injections were likely rostral to those labeled in PL by injections into the dorsolateral area, which is also known as V4 (Weller et al. 2002). In addition, the dorsomedial division of PL is known to have connections with PPC (Stepniewska 2004). Projections from PM are considered multisensory. Diversity of input modality to the PPC reach zone may be central to accurate forelimb trajectory during reaching. The convergence of thalamic input from multiple nuclei further supports the assertion that injections were caudal to somatosensory cortex.
The PPC reach zone received projections from the motor thalamus, primarily VLd, VLx, and VLp. The collective density of this input rivaled that from LP in owl monkeys but was less intense in squirrel monkeys. It is possible that the density of these connections may have been overestimated due to difficulties in identifying thalamic borders. For example, discerning the border between VLd and LP in coronal sections was challenging in instances of insufficient staining contrast and was inferred from surrounding nuclei. Identifying the ventral border of LP was occasionally complicated for the same reasons. Thus, the possibility exists that labeled cells were mischaracterized in VLd, VLx, or VLp, although they belonged to LP. Nevertheless, this only raises concerns about a small proportion of cells labeled in the motor thalamus. Although cells labeled in VL from PPC reach zone injections were separate from those labeled by M1/PM injections, the close proximity of the 2 populations of cells suggests similarities between the connections of PPC with motor thalamus and the M1/PM counterpart. VL divisions receive dense projections from deep cerebellar nuclei and are generally considered the node that provides cortical motor regions with cerebellar feedback (Alexander et al. 1986; Sakai et al. 2000).
A defense zone was lateral and slightly rostral to the PPC reach zone. Thalamocortical connections of the PPC defense zone were more thoroughly investigated in owl monkeys and were similar to those of the PPC reach zone. The densest connections were with LP, and a slightly weaker projection was from PA. Direct somatosensory input was also apparent from VP projections, which were relatively denser than those that reached the PPC reach zone. Nevertheless, injections were too distant from the central sulcus to have spread to the anterior parietal somatosensory cortex. Cases that received injections in the reach and defense zones showed that input to the defense zone arose from neurons that were generally ventral to those that projected to the reach zone. The slight spatial offsetting of thalamic cells projecting to the reach and dense zones suggests that they may represent different sequences of forelimb movements that can be evoked from the same cortical area, likely area 5. The PPC defense zone received minimal input from PL and PM reflecting various sensory modalities. The importance of such input to a cortical zone involved in protecting the body can be easily envisaged. Dense connections with VL divisions rivaled those with LP and highlight the association of this PPC zone with the motor system.
A grasp zone was identified lateral and slightly rostral to the defense zone. Although the grasp zone was more thoroughly investigated in squirrel monkeys, a comparable pattern of thalamocortical connections was revealed for the 2 species, which were also distinct from the pattern of connections of the other PPC zones. Perhaps the most distinguishing feature of the grasp zone was the limited number of cells labeled in LP. Pulvinar projections were primarily from PA and characterized the bulk of the grasp zone input. In addition, VP connections with the PPC grasp zone were denser than with the other PPC zones. The distribution of cells labeled in VLx/VLp from PPC grasp zone injections partially overlapped cells labeled from injections into caudal M1/PM (squirrel monkey case 08-09 and 07-118), whereas they were spatially segregated from cells labeled from more rostral M1/PM injections (squirrel monkey case 08-09 and owl monkey case 08-45). This overlap may be a feature of the relative proximity of the corresponding injections. It may also reflect a closer thalamocortical relationship between PPC grasp and caudal M1/PM than is the case for other functional zones.
The spatial location of the PPC grasp zone and its pattern of thalamocortical connections complicate assignment to a conventional cortical area. The limited number of labeled cells in LP is inconsistent with the dense thalamocortical connections of area 5 in macaque monkeys (Jones et al. 1979; Schmahmann and Pandya 1990; Cappe et al. 2007). Limited LP connections are consistent however with the results from a small number of injections in area 5 of titi monkeys (Padberg and Krubitzer 2006) as well as connection patterns of the inferior parietal lobe in macaque monkeys (Schmahmann and Pandya 1990). Even though the pattern of connections of the PPC grasp zone is similar to that reported for area 2 in macaque monkeys (Pons and Kaas 1985), electrophysiological and neuroanatomical investigations of the same region in titi monkeys have been interpreted as evidence for area 5 and not area 2 (Padberg et al. 2005; Padberg and Krubitzer 2006). Tentatively, repealing those 2 areas as equivalents of the PPC grasp zone limits the possibilities to area 1 and area 7b. Dense inputs from VP, and particularly VPS from the present injections, are consistent with cell-labeling patterns after area 1 injections in titi monkeys (Coq et al. 2004; Padberg and Krubitzer 2006). Dense inputs from PA to the present grasp zone is also consistent with area 1 connections. However, projections from VL divisions may reflect injections into area 1, area 7b, or both. As explained earlier, if the sequence of forelimb movements evoked with electrical stimulation is a faithful representation of the actions that drive neural activity, then the present PPC grasp zone likely corresponds to 7b or AIP in macaque monkeys. In this case, the present projections from VL divisions may be the link revealed with transynaptic tracing methods that connects deep cerebellar nuclei with AIP via VL (Clower et al. 2005). Nevertheless, the present PPC grasp zone appears to share thalamocortical connection patterns with both anterior (areas 1 and 2) and posterior (areas 5 and 7b) parietal cortex.
The density of the present VL projections to PPC zones rivaled those from LP. Reports in macaque monkeys have generally shown that LP projections to PPC are much denser than the VL counterpart. Nevertheless, the relative density of VL projections to PPC varies between reports. For example, Schmahmann and Pandya (1990) estimate substantial projections from VL to PE and PEa. That those connections arise from dorsal VL (VLps and VLc) further supports the present results showing that cells labeled in motor thalamus were primarily concentrated in VLd. Nevertheless, a separate investigation of PE and PEa showed only weak connections with VL (Cappe et al. 2007). The similarities and discrepancies in the findings may reflect differences in somatotopic locations of the injections. Indeed, the present results show that caudomedial PPC (reach zone) is less densely connected to VL than rostrolateral PPC (grasp zone). Another source of discrepancy between the present results and macaque monkeys (Cappe et al. 2007) may be related to species differences.
Convergence of PPC Thalamocortical Connections
The rostrocaudal extent of the thalamocortical connection origins was markedly different for M1/PM and PPC. Thalamic input to M1/PM was primarily from VL divisions. Only a marginal number of cells were labeled more caudally in VP or PA. In contrast, thalamic inputs to PPC zones were distributed across a longer rostrocaudal axis. The near even distribution of labeled cells in several thalamic nuclei (LP, VL, VP, and pulvinar) from PPC injections suggests a convergence of thalamic input onto PPC. The wide range of modalities from this converging input likely reflects higher order processing in PPC as compared with M1/PM. A similar contrast has been demonstrated between the concentrated input from VP to anterior parietal cortex (areas 3b, 1, and 2) and the wide convergence of thalamic inputs to area 5 (Padberg et al. 2009).
Conclusions
Electrical stimulation with long trains of current proved reliable for identifying PPC zones involved in action. The pattern of thalamocortical connections distinguished the individual PPC zones (Fig. 11). In addition to thalamocortical connections with dorsal and posterior nuclei of the thalamus, PPC zones received projections from motor and sensory thalamus. The caudomedial to rostrolateral arrangement of the functional zones was paralleled by a reduction in projections from LP and increases in the density of projections from PA and VP. Dense inputs from VL reached all PPC functional zones. Motor and sensory inputs are consistent with the proposed role of this region of PPC in generating actions.
Funding
National Institutes of Health (NS16446 to J.H.K and NS055843 to I.S.); Natural Sciences and Engineering Research Council of Canada (postdoctoral fellowship to O.A.G).
Acknowledgments
We are grateful to Laura Trice for assistance with histology and Mary Feurtado for pre- and postoperative animal care. Conflict of Interest: None declared.
References
- Alder LD. Thalamic connectivity of rat somatic motor cortex. Brain Res Bull. 1988;20:333–348. doi: 10.1016/0361-9230(88)90063-9. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Rosen I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14:243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferraina S, Mitsuda T, Marconi B, Genovesio A, Onorati P, Lacquaniti F, Caminiti R. Early coding of reaching in the parietooccipital cortex. J Neurophys. 2000;83:2374–2391. doi: 10.1152/jn.2000.83.4.2374. [DOI] [PubMed] [Google Scholar]
- Cappe C, Morel A, Rouiller EM. Thalamocortical and the dual pattern of corticothalamic projections of the posterior parietal cortex in macaque monkeys. Neuroscience. 2007;146:1371–1387. doi: 10.1016/j.neuroscience.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–562. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Taylor CS, Moore T, Graziano MS. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci U S A. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coq JQ, Qi H, Collins CE, Kaas JH. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey (Callicebus moloch) J Comp Neurol. 2004;476:368–387. doi: 10.1002/cne.20237. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Darian-Smith I, Cheema SS. Thalamic projections to sensorimotor cortex in the macaque monkey: use of multiple retrograde fluorescent tracers. J Comp Neurol. 1990;299:17–46. doi: 10.1002/cne.902990103. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Parham C. Afferent connections of the lateral agranular field of the rat motor cortex. J Comp Neurol. 1983;217:390–404. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Emmers R, Akert K. A stereotaxic atlas of the brain of the squirrel monkey. Madison (WI): The University of Wisconsin Press; 1963. [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. The thalamic connections of motor, premotor, and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti) J Comp Neurol. 2006;143:987–1020. doi: 10.1016/j.neuroscience.2006.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H. Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport. 1994;5:1525–1529. doi: 10.1097/00001756-199407000-00029. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Burish BJ, Kaas JH. Comparison of thalamocortical connections of frontal and posterior parietal cortex in New World primates. 2008 Abstract 78.5. Society for Neuroscience 38th annual meeting. Washington, DC. [Google Scholar]
- Gould H, Jr., Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. New York: Plenum Press; 1985. [Google Scholar]
- Jones EG. The thalamus. Cambridge (MA): Cambridge University Press; 2007. [Google Scholar]
- Jones EG, Wise SP, Coulter JD. Differential thalamic relationships of sensory-motor and parietal cortical fields in monkeys. J Comp Neurol. 1979;183:833–881. doi: 10.1002/cne.901830410. [DOI] [PubMed] [Google Scholar]
- Larsen KD, Asanuma H. Thalamic projections to the feline motor cortex studied with horseradish peroxidase. Brain Res. 1979;172:209–215. doi: 10.1016/0006-8993(79)90533-x. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Luppino G, Murata A, Govoni P, Matelli M. Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4) Exp Brain Res. 1999;128:181–187. doi: 10.1007/s002210050833. [DOI] [PubMed] [Google Scholar]
- Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J Comp Neurol. 1998;402:327–352. [PubMed] [Google Scholar]
- Matelli M, Luppino G, Fogassi L, Rizzolatti G. Thalamic input to inferior area 6 and area 4 in the macaque monkey. J Comp Neurol. 1989;280:468–488. doi: 10.1002/cne.902800311. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Sur M, Chia-Sheng L. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in “S1” in the owl monkey (Aotus trivirgatus) J Comp Neurol. 1978;181:41–74. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord, III: sites of origin of the corticospinal tract. J Comp Neurol. 1990;296:559–583. doi: 10.1002/cne.902960405. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski J. The thalamus of Macaca Mulatta. Basel (Switzerland): Krager; 1952. [Google Scholar]
- Padberg J, Cerkevich C, Engle J, Rajan AT, Recanzone G, Kaas JH, Krubitzer L. Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb Cortex. 2009;9:2038–2064. doi: 10.1093/cercor/bhn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Disbrow E, Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do New World monkeys have an area 2? Cereb Cortex. 2005;12:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Padberg J, Krubitzer L. Thalamocortical connections of anterior and posterior parietal cortical areas in New World titi monkeys. J Comp Neurol. 2006;487:416–435. doi: 10.1002/cne.21005. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Connections of area 2 of somatosensory cortex with the anterior pulvinar and subdivisions of the ventroposterior complex in macaque monkeys. J Comp Neurol. 1985;240:16–36. doi: 10.1002/cne.902400103. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Stepniewska I, Kaas JH. Pallidal and cerebellar afferents to pre-supplementary motor area thalamocortical neurons in the owl monkey: a multiple labeling study. J Comp Neurol. 2000;417:164–180. [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomical investigation of projections from thalamus to posterior parietal cortex in the rhesus monkey: a WGA-HRP and fluorescent tracer study. J Comp Neurol. 1990;295:299–326. doi: 10.1002/cne.902950212. [DOI] [PubMed] [Google Scholar]
- Sessle BJ, Wiesendanger M. Structural and functional definition of the motor cortex the monkey (Macaca fascicularis) J Physiol. 1982;323:245–265. doi: 10.1113/jphysiol.1982.sp014071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I. The pulvinar complex. In: Kaas JH, Collins CE, editors. The primate visual system. Boca Raton (FL): CRC Press; 2004. pp. 53–80. [Google Scholar]
- Stepniewska I, Cerkevich C, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: iI. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci U S A. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: i. Functional zones identified by microstimulation. J Comp Neurol. 2009;517:765–782. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (M1) of owl monkeys. J Comp Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Architectonic subdivisions of the motor thalamus of owl monkeys: Nissl, acetylcholinesterase, and cytochrome oxidase patterns. J Comp Neurol. 1994a;349:536–557. doi: 10.1002/cne.903490404. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Thalamic connections of the primary motor cortex (M1) of owl monkeys. J Comp Neurol. 1994b;349:558–582. doi: 10.1002/cne.903490405. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Ipsilateral cortical connections of dorsal and ventral premotor areas in New World owl monkeys. J Comp Neurol. 2006;495:691–708. doi: 10.1002/cne.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Preuss TM, Kaas JH. Thalamic connections of the dorsal and ventral premotor areas in New World owl monkeys. Neuroscience. 2007;147:727–745. doi: 10.1016/j.neuroscience.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Strick PL. Cortical projections of the feline thalamic nucleus ventralis lateralis. Brain Res. 1970;20:130–134. doi: 10.1016/0006-8993(70)90162-9. [DOI] [PubMed] [Google Scholar]
- Strick PL. Multiple source of thalamic input to the primate motor cortex. Brain Res. 1975;88:372–377. doi: 10.1016/0006-8993(75)90402-3. [DOI] [PubMed] [Google Scholar]
- Strick PL, Kim CC. Input to primate motor cortex from posterior parietal cortex (area 5). I. Demonstration by retrograde transport. Brain Res. 1978;157:325–330. doi: 10.1016/0006-8993(78)90035-5. [DOI] [PubMed] [Google Scholar]
- Strick PL, Peterson JB. Multiple representations in the primate motor cortex. Brain Res. 1978;154:366–370. doi: 10.1016/0006-8993(78)90707-2. [DOI] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation suggests two different forms of representation of head-centered space in the intraparietal sulcus of rhesus monkeys. Proc Natl Acad Sci U S A. 1996;93:4962–4967. doi: 10.1073/pnas.93.10.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenman CL, Reiner A, Honing MG. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods. 1992;41:239–254. doi: 10.1016/0165-0270(92)90089-v. [DOI] [PubMed] [Google Scholar]
- Weller RE, Steele GE, Kaas JH. Pulvinar and subcortical connection of dorsal lateral visual cortex in monkeys. J Comp Neurol. 2002;450:215–240. doi: 10.1002/cne.10298. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Corticothalamic connections of the posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1985;237:408–426. doi: 10.1002/cne.902370309. [DOI] [PubMed] [Google Scholar]