Abstract
Striatal medium spiny neurons (MSNs) receive glutamatergic afferents from the cerebral cortex and dopaminergic inputs from the substantia nigra (SN). Striatal dopamine loss decreases the number of MSN dendritic spines. This loss of spines has been suggested to reflect the removal of tonic dopamine inhibitory control over corticostriatal glutamatergic drive, with increased glutamate release culminating in MSN spine loss. We tested this hypothesis in two ways. We first determined in vivo if decortication reverses or prevents dopamine depletion–induced spine loss by placing motor cortex lesions 4 weeks after, or at the time of, 6-hydroxydopamine lesions of the SN. Animals were sacrificed 4 weeks after cortical lesions. Motor cortex lesions significantly reversed the loss of MSN spines elicited by dopamine denervation; a similar effect was observed in the prevention experiment. We then determined if modulating glutamate release in organotypic cocultures prevented spine loss. Treatment of the cultures with the mGluR2/3 agonist LY379268 to suppress corticostriatal glutamate release completely blocked spine loss in dopamine-denervated cultures. These studies provide the first evidence to show that MSN spine loss associated with parkinsonism can be reversed and point to suppression of corticostriatal glutamate release as a means of slowing progression in Parkinson's disease.
Keywords: cortex, corticostriatal, dendritic remodeling, metabotropic glutamate receptors, mGluR2/3, Parkinson's disease
Introduction
The motor symptoms of Parkinson's disease (PD) are caused by striatal dopamine insufficiency. The dopamine innervation of the striatum arises from neurons in the substantia nigra (SN) and contributes to a characteristic synaptic triad involving SN and cortical afferents and the dendrites of striatal medium spiny neurons (MSNs). The synaptic architecture of these three elements involves a dopamine synapse with the neck of MSN dendritic spines and a corticostriatal terminal synapsing onto the spine head (Bouyer et al. 1984; Freund et al. 1984; Smith et al. 1994). This synaptic arrangement suggests that dopamine modulates the influence of corticostriatal glutamatergic axons on MSNs. Consistent with this inference are data indicating that dopamine D2 heteroreceptors on the terminals of corticostriatal axons tonically inhibit release of glutamate from these axons (Bamford, Robinson, et al. 2004). Thus, in the dopamine-depleted striatum, excess glutamatergic drive from corticostriatal terminals, coupled with other mechanisms intrinsic to the MSNs that are regulated by dopamine (Day et al. 2006), results in hyperexcitable MSNs (Florio et al. 1993; Cepeda et al. 2001). Moreover, the loss of dopamine on the spine neck removes a modulatory influence that determines if cortically derived signals invade the dendritic shaft.
Dendritic spines are remarkably plastic structures, changing in number and shape over time scales ranging from seconds to years (Crick 1982; McKinney 2005; Alvarez et al. 2007; Harms and Dunaevsky 2007; Chen et al. 2009). Long-lasting changes in dendritic spine number have been documented in a number of neuropsychiatric disorders (Ferrante et al. 1991; Kaufmann and Moser 2000; Hill et al. 2006; Kalivas 2009; Tackenberg et al. 2009). Among these disorders is PD, in which striatal dopamine depletion elicits dystrophic changes of MSN dendrites. Both postmortem studies of PD as well as studies in animal models of parkinsonism have reported a marked decrease in MSN spine density (McNeill et al. 1988; Ingham et al. 1989, 1993, 1998; Arbuthnott et al. 2000; Stephens et al. 2005; Zaja-Milatovic et al. 2005; Day et al. 2006; Villalba et al. 2009). While the primary cause of this dendritic remodeling is loss of dopamine signaling through the D2 receptor (Day et al. 2006; Deutch et al. 2007), it appears likely that changes in cortically derived glutamate contribute to the changes in MSN spines. Dopamine replacement treatment in PD patients or in animals with striatal dopamine depletion does not restore spine loss (Stephens et al. 2005; Zaja-Milatovic et al. 2005; Deutch et al. 2007), suggesting that the dopamine receptor is uncoupled from its intracellular effectors. This led us to hypothesize that manipulating corticostriatal glutamate release directly might reverse the MSN spine loss seen in the dopamine-denervated striatum.
Glutamatergic mechanisms are critically involved in determining both dendritic spine development and maintenance (Korkortian and Segal 2001; Passafaro et al. 2003; Lippman and Dunaevsky 2005; McKinney 2005; Bloodgood and Sabatini 2008). For example, glutamatergic signaling through N-methyl-D-aspartate (NDMA) receptors increases intraspinous calcium levels, which determines spine morphology (Segal et al. 2000). These considerations suggest that corticostriatal neurons play a central role in determining the structure of MSN dendrites.
A recent in vitro study that examined the role of corticostriatal projections in dendritic remodeling in the dopamine-denervated striatum reported that complete decortication prevents the development of spine loss on striatal MSNs in organotypic slice cocultures (Neely et al. 2007). However, the ability of decortication to “reverse” spine loss that has already been established, which is more relevant to treatment of PD, has neither been examined in these cultures nor in vivo. We therefore determined in vivo if decortication can reverse or prevent MSN dendritic spine loss in an animal model of parkinsonism.
In order to determine if the loss of MSN spines requires corticostriatal glutamate release and hence if cortical lesions may mitigate spine loss by suppressing glutamate release, we used organotypic slice co-cultures comprised of cortex, striatum, and ventrolateral mesencephalon (including the SN) to assess the effects of a metabotropic mGluR2/3 receptor agonist. Activation of these mGluRs, which are located on the presynaptic terminals of corticostriatal neurons (Testa et al. 1998), dampens glutamate release (Lovinger 1991; Calabresi et al. 1992; Lovinger and McCool 1995).
Materials and Methods
Animals
Adult male Sprague-Dawley rats (Harlan; Indianapolis, IN) were group-housed on a 12:12 light–dark schedule with food and water freely available. All studies were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and under the oversight of the Vanderbilt University Animal Care and Use Committee.
Experimental Design
We first determined if focal lesions of the motor cortex in vivo could reverse spine loss that occurred in response to striatal dopamine denervation and in the next experiment determined if cortical lesions could prevent the development of MSN spine loss.
In the “reversal” experiment, 6-hydroxydopamine (6-OHDA) lesions of nigrostriatal dopamine neurons were made, and then 4 weeks later, when spine loss was established, the motor cortex was lesioned with ibotenic acid (IA). MSN spine loss is present starting about 2 weeks after striatal dopamine depletion and persisting for at least 12 months (Ingham et al. 1989; Deutch et al. 2007). Animals survived for 4 weeks after the cortical lesions before being sacrificed.
In the “prevention” experiment, striatal dopamine depletion was also accomplished by means of 6-OHDA lesions, and during the same surgery, IA was used to lesion the motor cortex. Animals were sacrificed 4 weeks later.
Surgical Manipulations
Animals were deeply anesthetized with isoflurane and burr holes made in the skull overlying the primary motor cortex and SN. Lesions of the motor cortex were performed by injecting 1.0 μL of 45 nM IA (Tocris, Ellisville, MO) into the M1 cortex (anteroposterior [AP]: +0.7; mediolateral [ML]: +2.0, +3.6; dorsoventral [DV]: −2.0, −2.3) at a rate of 200 nL/min. Control (sham) cortical lesions involved incision of the skin and placement of a burr hole.
Striatal dopamine denervation was accomplished by injecting 6-OHDA HBr (4.0 μg/μL free base; Sigma-Aldrich, St. Louis, MO) into 2 sites in the SN (AP: −5.4; ML: +1.0, +2.4; DV: −8.4) in a volume of 1.5 μL at a rate of 100 nL/min.
Organotypic Slice Cultures
Triple-slice cultures consisting of cortex, striatum, and ventrolateral mesencephalon (SN) were prepared from the brains of P1–P2 Sprague-Dawley rats (Harlan) following our previously described method (Neely et al. 2007). Two cultures were plated in each well. At 14 days in vitro (DIV), by which time the MSNs achieve a mature dendritic morphology (see Neely et al. 2007), the dopamine innervation of the striatum was denervated by treatment of the cultures with 15 μM 1-methyl-4-phenylpyridinium (MPP+) (Sigma-Aldrich). This concentration of MPP+ causes a selective loss of dopamine but not other neurons in the cultures (Neely et al. 2007). MPP+ was removed 24 h later and treatment with the mGluR2/3 agonist LY379268 (1.0 μM; Tocris) started, with the agonist being added to the cultures at the time of media changes (every other day over 14 days). Culture medium was collected at 14 DIV (just before MPP+ treatment) and again at 17 DIV and stored at −80 °C until subsequently assayed for the dopamine metabolite homovanillic (HVA) acid as an index of dopamine denervation. The cultures were harvested at 28–30 DIV for analysis of dendritic spine density.
We determined if adding an mGluR2/3 antagonist to cultures would block the ability of the agonist LY379268 to prevent spine loss in dopamine-denervated cultures. At 14–16 DIV, the cultures were treated with MPP+, which was removed 24 h later, at which time either the mGluR2/3 agonist LY379268 (1.0 μM), the antagonist LY341495 (0.2 μM), or both the mGluR2/3 agonist and antagonist were added to the culture media. The concentration of LY379268, which has at least an 80-fold higher affinity for group II mGluRs than other metabotropic glutamate receptors (Schoepp et al. 1999; Marek et al. 2000), was based on in vitro slice data from Marek et al. (2000) and Picconi et al. (2002). The concentration of the antagonist LY341495 (Monn et al. 1999; Schoepp et al. 1999) was also based on the data of Marek et al. (2000). The cultures were treated as described above, with the drugs replaced every other day for 14 days, at which time the cultures were harvested and diOlistically labeled.
Golgi Impregnation
Animals were transcardially perfused with a solution of 2.5% glutaraldehyde (EM Sciences, Hatfield, PA) and 2% paraformaldehyde (VWR, West Chester, PA) in 0.1 M phosphate buffer (pH 7.45). Brains were removed and the forebrains postfixed for 3 h. Coronal sections (150 μm) were cut on a vibrating microtome. The sections were then incubated in 1% osmium tetroxide (EM Sciences) for 40 min, after which sections were transferred to 3.5% potassium dichromate (Sigma-Aldrich) for 16 h in a humid chamber. The sections were then “sandwiched” between glass slides and incubated in the dark in 1% silver nitrate (Sigma-Aldrich) for 4–6 h. Sections were washed in water, mounted on 0.5% gelatin-coated slides, dehydrated, cleared, and coverslipped with DPX (Sigma-Aldrich).
DiOlistic Labeling of Cultures
Cultures were fixed in 1.5% paraformaldehyde in 0.1 M phosphate-buffered saline for 25 min and then diOlistically labeled with the carbocyanine dye CM-DiI (Invitrogen, Carlsbad, CA), following the general protocol of Gan et al. (2000), as modified by Neely et al. (2007, 2009). Cultures were then mounted in Prolong Antifade (Invitrogen).
Dendritic Analyses of Golgi-Labeled MSNs
Microscopic images were acquired by a digital camera coupled to a computer running the cell reconstruction software Neurolucida (Microbrightfield Inc., Williston, VT), using a 63× 1.4 planApo objective. The image was digitally magnified by a factor of 2 to yield a final magnification equivalent to a ×126 objective.
Data from animals with lesions that impinged on the corpus callosum were excluded from subsequent analyses, as were data from animals in which the lesions did not involve layer V, where the majority of cells that innervate the striatum are located.
Golgi-impregnated MSNs in dorsolateral striatum were reconstructed by a person unaware of the treatment conditions of the animals. Neurons were randomly selected from the M1-recipient zone of dorsolateral striatum, provided that the cells had a soma diameter of 12–17 μm and through a ×10 objective appeared to be well impregnated.
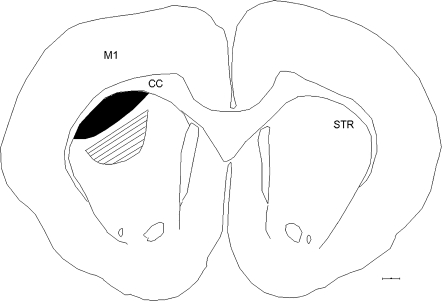
In a pilot experiment, we determined that dendritic spine density in control animals did not differ significantly at distances on the dendritic tree located 60–120 μm from the soma. We therefore measured dendritic spine density on dendritic segments 10–20 μm in length that were located 60–120 μm distal to the MSN soma. Dendritic spine densities were determined on branches of 4 primary dendrites from each reconstructed neuron, with at least 5 MSNs assessed in each animal (an average of 7.7 cells analyzed for each group in the prevention study and an average of 10.0 MSNs for each group analyzed in the reversal experiment). We analyzed MSNs located in the striatal zone that receives inputs from the lesioned motor cortex, as well in a second region, located ventromedial to the M1-innervated sector, that does not receive significant M1 inputs (see Fig. 1).
Figure 1.
MSNs were analyzed in the striatal region that receives afferents from the M1 cortex (black). In addition, we also analyzed MSNs in a region that does not receive a significant density of inputs from the M1 cortex as an internal negative control site (hatched). CC, corpus callosum; STR, striatum. Scale bar, 500 μm.
The striatal domain that received afferents from the lesioned areas of motor cortex was determined by iontophoretically depositing the tracer biotinylated dextran amine (BDA; molecular weight 10 000; Invitrogen) into the M1 cortex and subsequently assessing the distribution of anterogradely labeled axons in the striatum in 4 animals. A 10% BDA solution prepared in 0.1 M sodium phosphate was loaded into fiber-filled glass pipettes (25–30 μm outer diameter) and deposited into M1 (at the same coordinates at which IA was injected) using +5.0 μA pulsed (7 s on/off) current for 10 min. Animals survived for 7–10 days before being sacrificed. The results (data not shown) were consistent with previous reports (Hoffer and Alloway 2001; Ramanathan et al. 2002; Alloway et al. 2006) and indicated that one can reliably define the location in the striatum that receives inputs from a given zone of cortex, although the precise borders of the territory cannot be identified. We therefore assessed MSN dendritic spines in the M1-recipient zone of striatum and as a control site in a region ventromedial to the M1 zone that does not receive a significant density of inputs from the M1 cortex.
Dendritic Spine Analyses in DiOlistically Labeled Cultures
A confocal laser scanning microscope with a ×63 1.4 numerical aperture objective (with a ×2 digital zoom to yield a final magnification of ×126) was used to obtain z-stacks of MSN segments at 0.5-μm intervals. MSN spines were assessed on third and fourth order dendritic segments.
Assessment of Striatal Dopamine Depletion
The use of the Golgi method to impregnate MSNs precludes biochemical measurements of striatal dopamine concentrations. In order to assess the extent of the 6-OHDA lesion, striatal and midbrain sections were processed for tyrosine hydroxylase (TH) immunohistochemistry, using our previously described methods (Bubser et al. 2005). Only rats with ≤3 TH-immunoreactive axon segments per high-powered (×40) field in the dorsal striatum were included in the analyses (see Supplementary Fig. 1); SN dopamine neurons were almost entirely lost (see Supplementary Fig. 1).
In order to determine the extent of MPP+-induced striatal dopamine depletion in the cultures, we measured the concentration of the dopamine metabolite HVA in the culture media both immediately before and 48 h after MPP+ treatment. The medium was analyzed by high performance liquid chromatography with electrochemical detection, following our previously described procedure (Deutch and Cameron 1992). Briefly, 500 μL medium was added to 125 μL of 1 M perchloric acid solution containing 0.2 g/L NA2S2O5 and 0.05 g/L Na2-EDTA. The samples were centrifuged at 23 000 × g for 5 min, injected on a C18 column (Alltech, Deerfield, IL), and HVA measured using a 501A Coulochem detectror (ESA Biosciences, Chemsford, MA). HVA levels were expressed as pmol/mL medium. Because dopamine is rapidly oxidized in the culture media and therefore below detection thresholds in control cultures, we focused on the dopamine metabolite HVA, which is stable. Spine density in MSNs from cultures in which HVA levels were depleted by <70% were not analyzed.
Immunohistochemistry
Animals were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Forebrain and midbrain blocks of tissue were postfixed overnight and then cryoprotected in 30% sucrose for 2–4 days. Coronal sections (40 μm) were cut on a freezing microtome.
To assess the extent of cortical IA or SN 6-OHDA lesions, free-floating brain sections were processed as described previously (see Bubser et al. 2005), using mouse anti-NeuN (1:1000; Chemicon, Temecula, CA) to reveal the borders of the cortical lesions and mouse anti-TH (1:3000; ImmunoStar, Inc., Hudson, WI) to stain dopaminergic neurons.
Fluorojade C Staining
In order to determine if the cortical lesions resulted in any overt transsynaptic cell loss in the striatum, animals received IA injections of the motor cortex and were sacrificed at various times between 2 and 28 days after the cortical lesions. The brains were processed to stain degenerating neurons using Fluorojade C (FJC; Schmued et al. 2005). Animals were perfused with 4% paraformaldehyde and sections cut through the forebrain, mounted on 0.5% gelatin-coated slides, and dried overnight. Briefly, slides were incubated in basic ethanol solution (1% NaOH in 80% ethanol), dehydrated for 2 min in 70% ethanol, and then incubated in 0.06% potassium permanganate for 10 min. Slides were then incubated in 0.001% FJC (Chemicon) for 10 min. Sections were rinsed 3 times in water, dried overnight, and dehydrated and cleared in xylene before being coverslipped with DPX.
Data Analysis
In the in vivo studies, the average spine densities for each cell (determined from the analysis of 4 second- and third-order branches emanating from 4 different primary dendrites) were collapsed to yield a mean MSN spine density. In turn, these mean “per cell” spine densities (which averaged overall 8.8 MSNs/experimental group) were collapsed to generate a mean MSN value for each animal. This latter “per animal” MSN spine density value was used for subsequent statistical analyses by means of two-way analyses of variance (ANOVAs) and subsequent Bonferroni t-tests if warranted by significant main effects or a significant interaction. The degree of MSN spine loss in the non–M1-recipient zone of the striatum was analyzed relative to the 6-OHDA plus decortication group separately.
In the in vitro studies, MSN spine density values/neuron were determined, after which these values were collapsed to yield a single “per culture” value. These per culture values were then analyzed by two-way ANOVAs.
Results
Characterization of M1 Motor Cortex Lesions
IA injections lesioned the M1 motor cortex (see Fig. 2); the lesions impinged medially on the M2 area, with some degree of lateral invasion into the forelimb region of the primary somatosensory cortex (see Fig. 2). Although the lesions sometimes involved areas adjacent to M1, for simplicity sake we will refer to the lesioned region as the M1 area. The IA injections did not result in cavitation, and NeuN staining revealed intact underlying tissue (see Fig. 2). In most cases, the lesion involved layers I–VI, although loss of cells in layer VI was variable.
Figure 2.
Characterization of focal cortical lesions. (A) A representative 150-μm coronal section illustrating the cortical lesion, which in this case spanned layers I–V. (B) The absence of NeuN-positive cells illustrates the loss of cortical neurons in the lesioned area and shows that the underlying tissue is intact. Reconstructions of the largest (black) and smallest (gray) cortical lesions as assessed by the loss of NeuN-like immunoreactive neurons is shown in panel (C). Numbers refer to distance from the bregma skull suture (Paxinos and Watson 2007). Scale bars in panel (A), 200 μm; panel (B), 100 μm; and panel (C), 500 μm.
FJC Staining
IA injections caused extensive neuronal loss in the vicinity of the IA injection, as reflected by a dense aggregation of cortical FJC-positive cells seen at 2 and 4 days postoperatively (see Supplementary Fig. 2). In contrast, at no time point up to 28 days after the IA injection did we observe any FJC-positive cells in the striatum of animals with M1 lesions, indicating that there was no transsynaptic loss of striatal neurons in response to cortical IA injections.
Cortical Lesions Reverse Dopamine Depletion–Induced Spine Loss
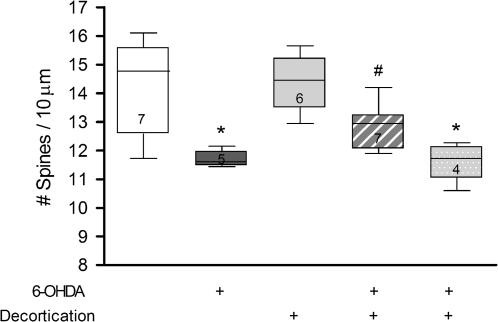
In this experiment, we assessed if decortication could reverse MSN spine loss that was caused by 6-OHDA lesions performed 4 weeks earlier. Cortical lesions significantly attenuated but did not totally reverse established MSN spine loss in the M1-recipient zone (omnibus ANOVA F3,21 = 7.98, P = 0.001), with a significant main effect of dopamine depletion (F1,21 = 20.2, P = 0.0002) but no significant interaction uncovered. Post hoc analyses revealed that striatal dopamine depletion resulted in a significant decrease in spine density (17.9%) compared with that seen in sham-lesioned control animals (P =0.007); cortical lesions alone did not change MSN spine density relative to control (sham lesioned) animals (Fig. 3). However, M1 lesions made 4 weeks after striatal dopamine denervation significantly attenuated the degree of MSN spine loss compared with the 6-OHDA-lesioned group alone (P = 0.008), with a 9.4% decrease in spine density relative to sham-lesioned rats. Thus, animals with striatal dopamine depletion suffered a loss of dendritic spines that was almost 50% less than that seen in animals without cortical lesions (see Fig. 4). The cortical lesions significantly attenuated MSN spine loss only in the M1-recipient zone of the striatum but not in the ventromedially adjacent striatal sector that does not receive significant M1 inputs (see Fig. 3).
Figure 3.
Cortical lesions significantly reverse dopamine depletion–induced MSN spine loss. Cortical lesions attenuated spine loss only in the M1-recipient zone (hatched bar) and not in an adjacent territory (stippled bar). Numbers inside each bar indicate the number of animals/group. *P < 0.01 relative to control animals. #P = 0.0008 relative to 6-OHDA-lesioned animals with intact cortex.
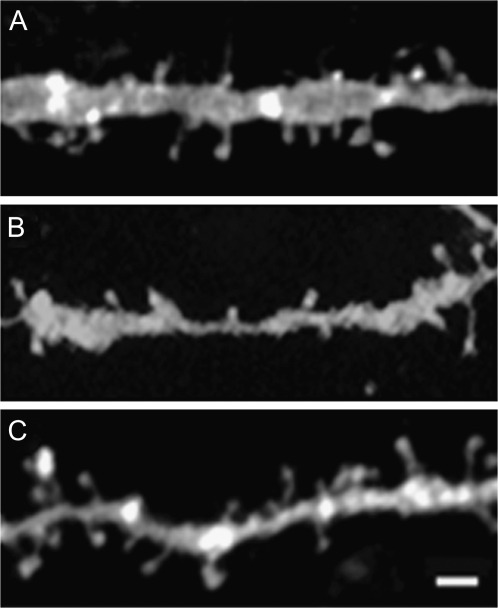
Figure 4.
Photomicrographs of representative Golgi-impregnated MSN dendritic segments are shown for (A) control, (B) 6-OHDA-lesioned, (C) cortically-lesioned, and (D) 6-OHDA- plus cortically-lesioned animals. Scale bar, 4 μm.
Effects of Concurrent Cortical and 6-OHDA Lesions on MSN Spine Density
The omnibus ANOVA for the analysis of the effects of cortical lesions performed at the same time as 6-OHDA lesions was significant (F3,27 = 9.37, P = 0.0002), with a significant main effect of dopamine depletion (F1,27 = 23.88, P < 0.0001) but no significant cortical lesion × 6-OHDA lesion interaction. Post hoc tests revealed that striatal dopamine depletion in animals with an intact cortex significantly decreased MSN spine density in the M1-recipient zone of animals relative to control rats (18.6%, P = 0.0022). Spine loss in animals with both cortical and 6-OHDA lesions averaged 10.3%. Although spine loss was attenuated in the combined cortical + 6-OHDA lesions relative to the 6-OHDA-lesioned group alone, this effect did not reach statistical significance (P = 0.06) (see Fig. 5). In the non–M1-recipient zone of the striatum, there was no attenuation of spine loss (see Fig. 5).
Figure 5.
Cortical lesions performed at the same time as 6-OHDA lesions of nigrostriatal dopamine neurons attenuate MSN spine loss. Cortical lesions attenuated dopamine depletion–induced spine loss only in the M1-recipient zone (hatched bar) and not in an adjacent region of the striatum that does not receive inputs from the motor cortex (stippled bar). Numbers inside each bar indicate the number of animals/group. *P < 0.01 relative to control animals. #P = 0.06 relative to 6-OHDA-lesioned animals with intact cortex.
Metabotropic Glutamate Receptors Regulate Spines
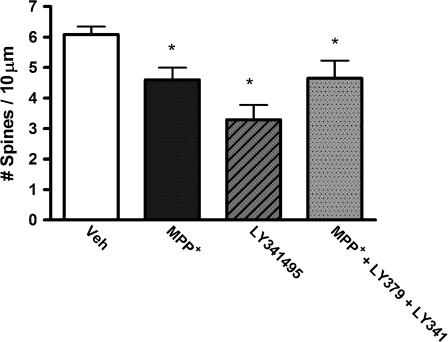
The treatment of cultures with MPP+ caused a marked (89.4%) decrease in the concentration of the dopamine metabolite HVA in the culture medium (t32 = 17.73, P < 0.0001), consistent with extensive striatal dopamine denervation. A two-factor (dopamine innervation × drug treatment) ANOVA was used to assess the effect of the mGluR2/3 agonist on MPP+-induced spine loss, yielding an overall F3,40 = 6.86 (P = 0.0008). Main effects of both dopamine innervation (F1,40 = 5.33, P = 0.0262) and drug treatment (F3,40 = 9.83, P = 0.0032) were uncovered and a strong trend toward an interaction (F1,40 = 4.08, P = 0.0502). Post hoc analyses found a significant decrease (26%) in MSN spine density compared with vehicle-treated cultures (P = 0.0032; see Fig. 6). While treatment with the mGluR2/3 agonist LY379268 had no effect on spine density in MSNs from cultures with an intact striatal dopamine innervation, the mGluR2/3 agonist completely prevented MSN spine loss in MPP+-treated cultures such that spine density was not significantly different from control (intact dopamine innervation + vehicle treated) cultures (see Fig. 6). Representative diOlistically labeled MSN dendritic segments are shown in Figure 7.
Figure 6.
Treatment of slice cultures with the mGlur2/3 agonist LY379268 completely prevented dopamine depletion–induced spine loss. Each symbol represents the mean spine density in a single culture. **P < 0.005 relative to control cultures.
Figure 7.
Photomicrographs of ballistically labeled MSN dendrites. (A) Dendritic segment of MSN from a control culture, (B) MPP+-treated dendrite, and (C) LY379268 plus MPP+–treated dendrite. Scale bar, 2 μm.
In order to determine the specificity of the mGluR2/3 agonist LY379268, we assessed if treatment with the mGluR2/3 antagonist LY341495 would block the actions of the group II metabotropic glutamate receptor agonist. As expected, post hoc analyses revealed that MPP+ treatment significantly decreased spine density (P = 0.0104). The antagonist alone caused a significant decrease in spine density (P = 0.0365), while the combined treatment of the antagonist plus agonist in dopamine-depleted cultures did not significantly differ from the MPP+-treated cultures alone and thus completely reversed the protective effects of the agonist (see Fig. 8).
Figure 8.
Treatment of slice cultures with the mGluR2/3 antagonist LY341495 blocks the effects of the mGluR2/3 agonist LY379268. LY341495 significantly decreased MSN spine density in cultures with an intact dopamine innervation. *P < 0.05 compared with control cultures. **P < 0.05 compared with control cultures.
Discussion
Lesions of the motor cortex reversed the loss of MSN spines that is seen in response to striatal dopamine denervation. This observation provides the first evidence that MSN spine loss, once established, can be reversed. Moreover, treatment with the mGluR2/3 agonist LY379268 completely prevented MSN spine loss in dopamine-denervated cultures, supporting the hypothesis that the mechanism by which cortical lesions attenuate spine loss is through decreased glutamate release from corticostriatal axons.
Effect of Cortical Lesions on MSN Spines in the Intact Striatum
We did not observe any effect of the cortical lesions on MSN spine density in animals with an intact striatal dopamine innervation. In contrast, some previous studies have reported that cortical lesions decrease MSN spine loss (Kemp and Powell 1971; Cheng et al. 1997). However, these earlier studies examined the effects of cortical aspiration lesions, which can easily damage the underlying striatal tissue, either by direct extension or secondary to edema. Moreover, Cheng et al. (1997) noted that the decrease in MSN spine density was transient, being maximal at 10 days postoperatively and returning to baseline levels by 20 days after the lesion, consistent with resolution of edematous changes. Because we examined animals at 4 weeks after the cortical lesion, we cannot exclude the possibility that there was a transient decrease in spine density in animals with IA cortical lesions that resolved by 28 days postoperatively. However, because dopamine denervation–induced MSN spine loss persists for at least 1 year (Ingham et al. 1989), the reversal of spine loss that we observed after cortical lesions cannot be due to recovery from the dopamine depletion.
To minimize the possibility of striatal damage by cortical lesions, we used IA to lesion the cortex. Although excitotoxic lesions can cause distant neuronal loss, we did not observe FJC-positive MSNs at any time up to 28 days postlesion. Moreover, we found that spine density was unchanged in animals subjected to cortical IA lesions 4 weeks earlier, suggesting that the cortical lesion did not compromise the structural integrity of MSNs. We measured MSN dendritic spine changes at 4 weeks after cortical lesions, that is, at a time point at which there was no evidence of FJC-positive degenerating striatal neurons. We did not determine if striatal cells degenerate after periods longer than 28 days, but there are no published data pointing to such changes in response to cortical lesions in adult animals.
Effect of Cortical Lesions on Spines in Dopamine-Denervated Striatum
A previous study in organotypic slice cocultures found that complete ablation of the cortex performed at the same time as MPP+ treatment to lesion the nigrostriatal dopamine neurons completely prevents MSN spine loss (Neely et al. 2007). In our in vivo prevention experiment, we observed a strong trend that did not reach statistical significance (P = 0.06). Because the in vitro study of Neely et al. (2007) indicated that total decortication completely prevented the development of dendritic spine loss in the dopamine-denervated striatum, and because we found that the mGluR2/3 agonist prevented spine loss from developing, it is likely that the nonsignificant trend we observed in animals with focal cortical lesions represents a type II (false negative) error.
An advantage of organotypic cultures is the ability to completely ablate the cortex, resulting in a near total glutamatergic denervation of the striatum (the thalamic intralaminar nuclei were not included in the cultures). In contrast, complete removal of glutamatergic projections to the striatum in vivo is not feasible, with a substantial glutamatergic innervation from the thalamus as well as some other sites being present. We therefore conducted both in vivo and in vitro studies, with the former examining the effects of a cortical lesion that left intact glutamatergic projections from the thalamus and the contralateral cortex, both of which synapse onto dendritic spines (Freund et al. 1984; Smith et al. 1994; Lacey et al. 2007). Despite not eliminating all glutamatergic inputs, we found that focal cortical lesions in vivo significantly reversed dopamine denervation–induced MSN spine loss and tended to prevent the development of spine loss. The significant but incomplete reversal of spine loss is most likely due to remaining glutamatergic inputs. The vesicular glutamate transporter 2 (VGluT2) is abundantly expressed by thalamostriatal but not corticostriatal neurons (Kaneko and Fujiyama 2002). Raju et al. (2008) found no change in striatal VGluT2 in dopamine-denervated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated primates, consistent with intact striatal glutamate inputs from the thalamus. The other major source of glutamate inputs to the striatum is the cortex. Previous studies have noted that axonal sprouting from the contralateral cortex partially reinnervates the denervated striatum (Napieralski et al. 1996; Hughes-Davis et al. 2005). The presence of some sprouting of inputs from the contralateral cortex, coupled with an intact thalamostriatal glutamatergic system, probably accounts for the significant attenuation but not complete reversal of dopamine depletion–induced spine loss in MSNs. These considerations suggest that our studies with unilateral cortical lesions represent a conservative test of the hypothesis that cortical denervation prevents and reverses spine loss in vivo.
Because there are no clearly defined boundaries that distinguish the M1-recipient zone from adjacent striatal tissue that does not receive M1 inputs, we restricted our analysis to a fairly conservative definition of the striatal M1-recipient zone, based on our anterograde tracer studies as well as published data on the striatal projections of the motor and adjacent cortices (Alloway et al. 2006). The cortical lesion attenuated the loss of dendritic spines only in this M1-recipient zone of the striatum. Spine densities of MSNs located in an area of the striatum ventromedial to the M1-innervated region did not differ significantly from those seen in animals with 6-OHDA lesions but an intact motor cortex. These observations argue that we were able to define accurately the striatal territory receiving inputs from the lesioned cortex.
Mechanism of Action of Cortical Lesions
A variety of in vivo and in vitro data suggest that striatal dopamine depletion increases glutamate release from corticostriatal terminals (Florio et al. 1993; Meshul et al. 1999; Cepeda et al. 2001; Bamford, Robinson, et al. 2004; Bamford, Zhang, et al. 2004; Day et al. 2006). Dendritic spine formation and maintenance are determined largely by changes in intracellular calcium levels, including NMDA-driven increases in calcium levels. Accordingly, we hypothesized that one mechanism that contributes to dopamine depletion–induced MSN spine loss is the loss of tonic dopaminergic inhibition of the D2 heteroreceptor on corticostriatal terminals, leading to increased glutamate release (Bamford, Zhang, et al. 2004), which in turn increases intraspinous calcium and culminates in spine loss. Thus, we anticipated that cortical lesions would reduce excess glutamate release from cortical axons and thereby attenuate MSN spine loss.
Group II metabotropic glutamate (mGluR2/3) receptors, which are located presynaptically on corticostriatal terminals (Testa et al. 1998), are release-modulating autoreceptors. We therefore treated organotypic slice co-cultures with a mGluR2/3 receptor agonist to dampen glutamate release from disinhibited corticostriatal axons. In mature cultures, there are both extensive cortical and midbrain dopamine projections to the striatum (Neely et al. 2007; Snyder-Keller et al. 2008), and one sees both spontaneous and cortically evoked activity of MSNs (Plenz and Kitai 1998; Snyder-Keller et al. 2008), indicating intact functional connections between the cortex and striatum in the cultures. The use of these cultures allowed us to determine specifically if the loss of cortical glutamate, as opposed to glutamatergic afferents from other areas such as the thalamus, was responsible for preventing spine loss.
Treatment of dopamine-denervated cultures with LY379268 completely prevented the spine loss. It appears likely that the ability of LY379268 to block MSN spine loss is due to actions at mGluR2/3 sites. LY379268 is a preferential agonist at mGluR2/3 receptors (Schoepp et al. 1999; Imre 2007). The agonist inhibits forskolin-stimulated cyclic adenosine monophosphate accumulation in cells expressing mGluR2/3 receptors with a half-maximal effective concentration of <6 nM but has very weak actions at mGluR4 and mGluR8 receptors (EC50 > 2 μM) and no significant actions at other mGluRs (Monn et al. 1999; Imre 2007). In addition, we found that the mGluR2/3 antagonist LY341495 completely reversed the protective effects of the agonist on MSN spines in the dopamine-depleted striatum. Moreover, treatment of cultures containing an intact nigrostriatal dopamine system with the antagonist was sufficient to cause MSN spine loss, supporting the hypothesis that MSN spine loss results from excessive glutamate release from corticostriatal axons.
Recently, Seeman et al. (2008) have suggested that mGluR2/3 agonists, including LY379268, are partial agonists at D2 receptors. Because dopamine denervation–induced MSN dendritic spine loss is not reversed by levodopa treatment of rodents (Deutch et al. 2007), and because MSN spine loss is seen in postmortem striatal samples from PD patients who had received chronic treatment with levodopa and/or dopamine agonists (Stephens et al. 2005; Zaja-Milatovic et al. 2005), the attenuation of spine loss in animals treated with the mGluR2/3 agonist is probably not due to any actions of LY379268 at dopamine receptors.
Group II metabotropic receptor agonists have been reported to afford protection against MPTP-induced striatal dopamine loss (Battaglia et al. 2003). However, we started treatment with LY379268 after treatment with MPP+, rather than administering the mGluR2/3 agonist prior to or together with MPP+. Moreover, we observed extensive striatal dopamine depletion in cultures treated with LY379268, as reflected by an almost 90% decrease in media concentrations of HVA, indicative of striatal dopamine denervation.
Taken together, these data strongly suggest that the ability of LY379268 to attenuate MSN spine loss is due to actions at mGluR2/3 receptors and not some off-target action of the drug. Moreover, the observation that treatment of the cultures with an mGluR2/3 antagonist in cultures with an intact striatal dopamine innervation elicited spine loss is consistent with the hypothesis that cortical lesions protect against dopamine depletion–induced MSN spine loss by reducing glutamate release from corticostriatal terminals.
It is likely that there are sources of glutamate in addition to corticostriatal axons that contribute to MSN spine loss. Extracellular glutamate levels involve not only synaptically released glutamate but also glutamate release from astrocytes through the cystine–glutamate exchanger as well as glutamate uptake through high-affinity glutamate transporters, including the astrocytic transporter GLT-1 (EAAT2) (see Kalivas 2009). Because group II mGluRs are expressed by astrocytes (Testa et al. 1994), it is possible that the effects of the mGluR2/3 agonist are mediated in part by astrocytic glutamate transporters. In addition, factors extrinsic to MSNs (such as certain trophic factors) and intrinsic to striatal cells (such as L-type voltage-gated calcium channels) may also play important roles.
Relation to Motor Deficits in Parkinsonism
Our data on the effects of mGluR2/3 agonists point to derangements in corticostriatal glutamate systems as being responsible for dendritic remodeling in the dopamine-denervated striatum. This has significant implications for the treatment of PD, in which MSNs undergo extensive spine loss (Stephens et al. 2005; Zaja-Milatovic et al. 2005). We have previously suggested that MSN spine loss may be related to progression in PD and to the resistance to the full therapeutic benefits of dopamine replacement that is seen in later stages of the illness (Marsden and Parkes 1977; Lesser et al. 1979; Rinne 1981; Clissold et al. 2006; see also Deutch 2006).
Surprisingly, few studies have examined the effects of mGluR2/3 agonists on parkinsonian motor deficits (Murray et al. 2002; Feeley Kearney and Albin 2003). Murray et al. (2002) found that intraventricular administration of the mGluR2/3 agonist LY379268 dose dependently reversed reserpine-induced akinesia. In contrast, Ossowska et al. (2007) did not observe any benefit of intrastriatal injections of a different mGluR2/3 agonist, 2R,4R-APDC on haloperidol-induced motor deficits; it is not clear if this is because of the acute nature of the dopamine blockade achieved with haloperidol.
In contrast to studies with group I mGluR antagonists, recent data suggest that mGluR 2/3 agonists do not attenuate levodopa-induced dyskinesias (Rylander et al. 2009). Because the animals in our study were not treated with levodopa or other dopamine agonists, the attenuation of spine loss that we observed is not related directly to dyskinesias. Several studies have pointed to D1-expressing (direct pathway) MSNs as being critical to the development and maintenance of dyskinesias (Bordet et al. 2000; Carta et al. 2008; Berthet et al. 2009; Darmopil et al. 2009). In contrast, rodent studies indicate that MSN dendritic spine loss in the dopamine-depleted striatum occurs in D2-expressing MSNs (Rodriguez and Pickel 1999; Day et al. 2006; Deutch et al. 2007), although a recent study in the primate suggests that spines are also lost on D1-expressing cells (Villalba et al. 2009). Because Golgi impregnation of neurons precludes the determination of the type of MSN, we cannot ascertain if the changes we observed occur in indirect- or direct-pathway MSNs. Future studies using different methods that permit evaluation of spine changes in specific types of MSNs will be required to untangle changes in direct- and indirect-pathway MSNs.
Conclusions
To the best of our knowledge, this is the first demonstration of reversing the loss of MSN dendritic spines once the spine loss is established. Dopamine replacement in both animal models of parkinsonism and in PD patients does not reverse MSN spine loss. Our data indicate that cortical lesions do reverse the structural changes in MSNs, suggesting that treatments that target corticostriatal projections may be a useful intervention in PD. However, clinical trials of ionotropic glutamate receptor antagonists in parkinsonism have been disappointing. These trials focused on symptom reduction and did not assess disease (or symptom) progression. Our data suggest that modulation of glutamatergic transmission through metabotropic glutamate receptors, specifically mGluR2/3 agonists, may be warranted in studies aimed at slowing progression in PD by attenuating MSN dendritic spine loss. Our studies emphasize the importance of extended corticofugal circuits in PD, rather than a more short-sighted focus on only the striatum.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Mental Health (NIMH), National Institutes of Health (F31 NS060174 to B.G.G., RO1 MH077298 to A.Y.D., PO1 NS44282 to A.Y.D.); Eunice Kennedy Shriver National Institute of Child Health and Human Development for core support (P30 HD15052); National Parkinson Foundation Center of Excellence at Vanderbilt.
Supplementary Material
Acknowledgments
The authors are indebted to Drs Jennifer Blackford, Michael Bubser, and Brian Mathur for helpful comments and advice. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS or NIMH of the National Institutes of Health or the National Parkinson Foundation. Conflict of Interest: None declared.
References
- Alloway KD, Lou L, Nwabueze-Ogbo F, Chakrabarti S. Topography of cortical projections to the dorsolateral neostriatum in rats: multiple overlapping sensorimotor pathways. J Comp Neurol. 2006;499:33–48. doi: 10.1002/cne.21039. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Ridenour DA, Sabatini BL. Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. J Neurosci. 2007;27:7365–7376. doi: 10.1523/JNEUROSCI.0956-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott GW, Ingham CA, Wickens JR. Dopamine and synaptic plasticity in the neostriatum. J Anat. 2000;196:587–596. doi: 10.1046/j.1469-7580.2000.19640587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45(2):155–166. doi: 10.1016/s0028-3908(03)00146-1. [DOI] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci. 2009;29:4829–4835. doi: 10.1523/JNEUROSCI.5884-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Regulation of synaptic signaling by postsynaptic, non-glutamate receptor ion channels. J Physiol. 2008;586(6):1475–1480. doi: 10.1113/jphysiol.2007.148353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci. 2000;12:2117–2123. doi: 10.1046/j.1460-9568.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302:267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur J Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Bernardi G. Activation of quisqualate metabotropic receptors reduces glutamate and GABA-mediated synaptic potentials in the rat striatum. Neurosci Lett. 1992;139:41–44. doi: 10.1016/0304-3940(92)90852-x. [DOI] [PubMed] [Google Scholar]
- Carta AR, Frau L, Pinna A, Pontis S, Simola N, Schintu N, Morelli M. Behavioral and biochemical correlates of the dyskinetic potential of dopaminergic agonists in the 6-OHDA lesioned rat. Synapse. 2008;62:524–533. doi: 10.1002/syn.20527. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernández J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, et al. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Chen W, Prithviraj R, Mahnke AH, McGloin KE, Tan JW, Gooch AK, Inglis FM. AMPA glutamate receptor subunits 1 and 2 regulate dendrite complexity and spine motility in neurons of the developing neocortex. Neuroscience. 2009;159:172–182. doi: 10.1016/j.neuroscience.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HW, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp Neurol. 1997;147:287–298. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- Clissold BG, McColl CD, Reardon KR, Shiff M, Kempster PA. Longitudinal study of the motor response to levodopa in Parkinson's disease. Mov Disord. 2006;21:2116–2121. doi: 10.1002/mds.21126. [DOI] [PubMed] [Google Scholar]
- Crick F. Do spines twitch? Trends Neurosci. 1982;5:44–46. [Google Scholar]
- Darmopil S, Martín AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009;66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sampson AR, Mugnaini E, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Deutch AY. Striatal plasticity in parkinsonism: dystrophic changes in medium spiny neurons and progression in Parkinson's disease. J Neural Transm. 2006;70(Suppl):67–70. doi: 10.1007/978-3-211-45295-0_12. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46(1):49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism and Related Disord. 2007;13:S251–S258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley Kearney JA, Albin RL. mGluRs: a target for pharmacotherapy in Parkinson disease. Exp Neurol. 2003;184(Suppl 1):S30–S36. doi: 10.1016/s0014-4886(03)00391-1. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kowall NW, Richardson EP., Jr Proliferative and degenerative changes in striatal spiny neurons in Huntington's disease: a combined study using the section-Golgi method and calbindin D28k immunocytochemistry. J Neurosci. 1991;11:3877–3887. doi: 10.1523/JNEUROSCI.11-12-03877.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio T, Di Loreto S, Cerrito F, Scarnati E. Influence of prelimbic and sensorimotor cortices on striatal neurons in the rat: electrophysiological evidence for converging inputs and the effects of 6-OHDA-induced degeneration of the substantia nigra. Brain Res. 1993;619:180–188. doi: 10.1016/0006-8993(93)91610-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Dunaevsky A. Dendritic spine plasticity: looking beyond development. Brain Res. 2007;1184:65–71. doi: 10.1016/j.brainres.2006.02.094. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Alloway KD. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas of rodents. J Comp Neurol. 2001;439:87–103. doi: 10.1002/cne.1337. [DOI] [PubMed] [Google Scholar]
- Hughes-Davis EJ, Cogen JP, Jakowec MW, Cheng HW, Grenningloh G, Meshul CK, McNeill TH. Differential regulation of the growth-associated proteins GAP-43 and superior cervical ganglion 10 in response to lesions of the cortex and substantia nigra in the adult rat. Neuroscience. 2005;135(4):1231–1239. doi: 10.1016/j.neuroscience.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res. 1993;93:17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42(4):243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: a study with the Golgi method. Philos Trans R Soc Lond B Biol Sci. 1971;262:429–439. doi: 10.1098/rstb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Korkotian E, Segal M. Regulation of dendritic spine motility in cultured hippocampal neurons. J Neurosci. 2001;21:6115–6124. doi: 10.1523/JNEUROSCI.21-16-06115.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J Neurosci. 2007;27:4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser RP, Fahn S, Snider SR, Cote LJ, Isgreen WP, Barrett RE. Analysis of the clinical problems in parkinsonism and the complications of long-term levodopa therapy. Neurology. 1979;29:1253–1260. doi: 10.1212/wnl.29.9_part_1.1253. [DOI] [PubMed] [Google Scholar]
- Lippman J, Dunaevsky Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47–57. doi: 10.1002/neu.20149. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Trans-l-amino-cylcopentane-1,3-dicarboxylic acid (t-ACPD) decreases synaptic excitation in rat striatal slice through a presynaptic action. Neurosci Lett. 1991;129:17–21. doi: 10.1016/0304-3940(91)90710-b. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson's disease. Lancet. 1977;1(8007):345–349. doi: 10.1016/s0140-6736(77)91146-1. [DOI] [PubMed] [Google Scholar]
- McKinney RA. Physiological roles of spine motility: development, plasticity and disorders. Biochem Soc Trans. 2005;33:1299–1302. doi: 10.1042/BST0331299. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson's disease. Brain Res. 1988;455:148–152. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr., Wright RA, Johnson BG, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, O'Neill MJ. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson's disease. Pharmacol Biochem Behav. 2002;73:455–466. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- Napieralski JA, Butler AK, Chesselet MF. Anatomical and functional evidence for lesion-specific sprouting of corticostriatal input in the adult rat. J Comp Neurol. 1996;373:484–497. doi: 10.1002/(SICI)1096-9861(19960930)373:4<484::AID-CNE2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149(2):457–464. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely MD, Stanwood GD, Deutch AY. Combination of diOlistic labeling with retrograde tract tracing and immunohistochemistry. J Neurosci Methods. 2009;184:332–336. doi: 10.1016/j.jneumeth.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, Wolfarth S, Pilc A. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids. 2007;32:179–188. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam (The Netherlands): Elsevier Inc.; 2007. [Google Scholar]
- Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P. Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain. 2002;125:2635–2645. doi: 10.1093/brain/awf269. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J Neurosci. 1998;18:266–283. doi: 10.1523/JNEUROSCI.18-01-00266.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22:8158–8169. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne UK. Treatment of Parkinson's disease: problems with a progressing disease. J Neural Transm. 1981;51:161–174. doi: 10.1007/BF01664013. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Pickel VM. Enhancement of N-methyl-D-aspartate (NMDA) immunoreactivity in residual dendritic spines in the caudate-putamen nucleus after chronic haloperidol administration. Synapse. 1999;33:289–303. doi: 10.1002/(SICI)1098-2396(19990915)33:4<289::AID-SYN6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330:227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Seeman P, Caruso C, Lasaga M. Dopamine partial agonist actions of the glutamate receptor agonist LY354,740 and LY379,268. Synapse. 2008;62(2):154–158. doi: 10.1002/syn.20482. [DOI] [PubMed] [Google Scholar]
- Segal I, Korkotian I, Murphy DD. Dendritic spine formation and pruning: common cellular mechanism? Trends Neurosci. 2000;23(2):53–57. doi: 10.1016/s0166-2236(99)01499-x. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Snyder-Keller A, Tseng KY, Lyng GD, Graber DJ, O'Donnell P. Afferent influences on striatal development in organotypic cocultures. Synapse. 2008;62:487–500. doi: 10.1002/syn.20518. [DOI] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, et al. Evidence of a breakdown of corticostriatal connections in Parkinson's disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Tackenberg C, Ghori A, Brandt R. Thin, stubby or mushroom: spine pathology in Alzheimer's disease. Curr Alzheimer Res. 2009;6:261–268. doi: 10.2174/156720509788486554. [DOI] [PubMed] [Google Scholar]
- Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB., Jr. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson's disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.