Abstract
Mental illness can include impaired abilities to express emotions or respond to the emotions of others. Speech provides a mechanism for expressing emotions, by both what words are spoken and by the melody or intonation of speech (prosody). Through the perception of variations in prosody, an individual can detect changes in another's emotional state. Prosodic features of mouse ultrasonic vocalizations (USVs), indicated by changes in frequency and amplitude, also convey information. Dams retrieve pups that emit separation calls, females approach males emitting solicitous calls, and mice can become fearful of a cue associated with the vocalizations of a distressed conspecific. Since acoustic features of mouse USVs respond to drugs and genetic manipulations that influence reward circuits, USV analysis can be employed to examine how genes influence social motivation, affect regulation, and communication. The purpose of this review is to discuss how genetic and developmental factors influence aspects of the mouse vocal repertoire and how mice respond to the vocalizations of their conspecifics. To generate falsifiable hypotheses about the emotional content of particular calls, this review addresses USV analysis within the framework of affective neuroscience (e.g. measures of motivated behavior such as conditioned place preference tests, brain activity, and systemic physiology). Suggested future studies include employment of an expanded array of physiological and statistical approaches to identify the salient acoustic features of mouse vocalizations. We are particularly interested in rearing environments that incorporate sufficient spatial and temporal complexity to familiarize developing mice with a broader array of affective states.
Keywords: animal communication, bioacoustic communication, ultrasonic vocalizations, mood, empathy, affective disorders, schizophrenia, autism, addiction
Introduction
Many of life's enriching experiences occur within a social context; with the ability to share enjoyment, to grasp how another individual feels, or to see the world from the perspective of another. In a variety of developmental disabilities and neurological diseases, these capacities are diminished or lost (Hagerman et al. 1986; Lord et al. 2000; Testa et al. 2001; Abdi and Sharma 2004; Ruby and Decety 2004; Orbelo et al. 2005; O'Keeffe et al. 2007; Freeman et al. 2009). To elucidate how these deficits emerge in development, adulthood or aging, emotional experiences cannot be directly measured. Rather, changes in behaviors are used as a metric to infer changes in emotion. For example, the level of fear experienced by a person cannot be measured but the quiver of a fearful voice can be detected. Scientists can also measure behavioral indications of empathy; how one individual responds to the emotional expressions of another individual. For instance, in response to hearing the quiver in a voice, an individual might feel compassion, fearful, vindicated, frustrated or angry. In turn, these emotions can engender various behavioral responses, such as expression of consoling words, flight from the scene, pursed lips, or a confrontation with the dangerous stimulus. The purpose of this review is to explore how mouse vocalizations convey emotional information, how mice respond to these vocalizations, and how we can infer affective states from changes in the mouse vocal repertoire.
Prosody
Vocalizations provide highly salient cues that can indicate changes in emotion. Emotional information can be provided by what words are spoken; from the meanings of the words themselves. “That feels great!” “You're making me frustrated.” “That dude gives me the creeps.” We know the meaning of these statements and we often express an emotional response to them. But emotions are also expressed by how words are spoken. This quality of speech, which is called ‘prosody’ and is often referred to as intonation, is central to communication. Prosody is measured by variations in the pitch (notes), energy (loudness), duration, and the duration of spoken words and the timing between them.
Prosody can be classified into three functional groups. Grammatical prosody entails changes in emphasis on particular syllables, or changes in pitch, throughout a sentence that can help us distinguish meanings. For example, applications of stress on one of two syllables in a word can help us to differentiate a dry, treeless ecosystem (des’-ert) from the pleasant plate of food at the end of a meal (des-sert’). An upward trend in pitch through a sentence enables us to discern a question from an assertion. The soup bowl is in the kitchen. The soup bowl is in the kitchen? Pragmatic prosody is used to help us put emphasis on an important idea, sentence or word in a sentence, and thus reveals our focus and intentions. For example, enhanced duration and loudness of a particular word can indicate its importance within a sentence. “I don't want the brown coat, I want the DOWN coat”). A different function of pragmatic prosody is that it signals the context, roles, and relationships between speakers. People speak to infants in “motherese” or “infant-directed talk” in a voice that they do not use with older children (Fernald, 1989). People speak with a different tone their employer than their intimate partner. Affective prosody is responsive to internal emotional experiences, such as sadness, anger, fatigue, annoyance, concern, contentment, relief, excitement, or victory. For instance, high levels of frequency modulation, in which there are broad variations in the level of pitch, from syllable to syllable, can be associated with excitement. A minimal level of frequency modulation, or monotone, can indicate boredom or fatigue. Prosody is also responsive to social context. Adults talk with old friends and new acquaintances with different vocal patterns. The variability of speech within the context of changes in emotion or social context is referred to as emotional or affective prosody.
Affective Prosody and Mental Disorders
Autistic individuals have been shown to assign stress to the wrong syllables of a word (Baltaxe, 1984, Baltaxe & Guthrie, 1987, Shriberg et al., 2001) and children with high-functioning autism can have difficulties modulating the pitch and controlling the volume of their speech (Shriberg et al., 2001). Some aspects of autistic speech incorporate all three features of the diagnosis; repetitive behaviors, deficits in the ability to express emotions and deficits in communication. For instance, some children with autism repeat the use of certain sounds, syllables, or words more than typically developing children (Shriberg et al., 2001) or fail to use appropriate patterns of intonation to communicate. Prosody can be monotonic, minimally pitched or energy modulated, or it can be amplified in pitch range or even singsong-like (Amorosa, 1992), masking dynamics in emotional status. Autistic language may not be appropriately attuned to context, or ‘machine-like’ (Fine et al., 1991). Overall, speech impairments in autism are generally more prevalent in the expression of pragmatic or affective prosody than grammatical prosody (Shriberg et al., 2001). Individuals with an autism diagnosis can have impairments in the ability to understand the spoken language and gestures of others and infants can show these deficits (Golan et al., 2007, Kuhl et al., 2005, Rutherford et al., 2002). The ability to respond to the emotional expressions of others can be deficient among people with autism (Silani et al., 2008, Smith, 2009). In this regard, autistic children also show deficits in their abilities to use prosodic cues to disambiguate sentence meaning (Diehl et al., 2008). These deficits can extend to inabilities to interpret intonations that denote liking or disliking (Mccann et al., 2007).
Schizophrenia, mania and depression can also be significantly impaired in the ability to comprehend emotional prosody, while schizophrenia can additionally feature substantial deficits in the ability to express affective prosody (Murphy & Cutting, 1990). Schizophrenia can feature deficits in expressive and receptive prosody (Bach et al., 2009, Leentjens et al., 1998, Leitman et al., 2006), including paranoid (Bach et al., 2009) and flat affect (Alpert et al., 2000) schizophrenia. For instance, individuals with flat affect schizophrenia employ normal word patterns to express emotions (frequency of words describing pleasurable or distressful experiences), but they communicate these experiences with less prosodic inflection (Alpert et al., 2000). Like the autism disorders, there are many forms of schizophrenia and they do not all demonstrate deficits in the expressive prosody (Cohen & Docherty, 2005, Wan et al., 2008). Receptivity to emotional expressions of others can be impaired in schizophrenia (Derntl et al., 2009, Fujiwara et al., 2008, Haker & Rossler, 2009), particularly in males who are responding to emotions that have negative valence (Bozikas et al., 2006, Pijnenborg et al., 2007, Scholten et al., 2008). In addition to schizophrenia, responsiveness to affective prosody can be impaired in depression (Emerson et al., 1999, Uekermann et al., 2008), multiple sclerosis (Beatty et al., 2003), Parkinson's disease (Benke et al., 1998), alcoholism (Monnot et al., 2002, Monnot et al., 2001, Uekermann & Daum, 2008, Uekermann et al., 2005) and following exposure to MDMA or ‘ecstasy’ (Yip & Lee, 2006).
Mouse Vocalizations
To model deficits in expressive and receptive prosody, there must be evidence that features of mouse vocalizations typically associate with specific affective states, such as distress, or reward, or anticipation of reward and whether these features can be sensitive to differences in specific alleles or genetic background. Mice have the capability of vocalize across a broad range of frequencies that extend from as low as the human-audible range (when we hear squeaks) to well into the ultrasound range, above the limit of human hearing (20,000 cycles per second (hertz) = 20 kHz). Audible squeaks are produced by laboratory mice in stressful and painful situations (Whitney & Nyby, 1983) such as during handling and restraint (Whitney, 1969), grid-shock test (Winter, 1965), or during aggressive encounters (Gourbal et al., 2004, Houseknecht, 1968). In reproductive contexts, human-audible squeaks are produced by females when a sexually motivated male is interacting with a non-receptive female (Sales, 1972).
Over the last 40 years, mouse vocalizations have been studied mostly within two social contexts: adult male vocalizations emitted in response to exposure to cues of estrous females and pup vocalizations emitted when they are separated from the nest. Initial analyses of these calls were limited to determining the rate of call production within a particular bandwidth of frequencies and how mice responded to these calls. More recently, analyses include classification of discrete vocalizations based upon their differences in frequency (pitch) and energy (loudness).
Adult male-female interactions
Adult vocalizations have been primarily studied within reproductive contexts. Adult males emit USVs when exposed to a female partner or to her odor cues (Maggio et al., 1983, Nyby, 2001, Whitney & Nyby, 1979). During courtship, the male vocalizes and approaches the female (Maggio et al., 1983, Whitney & Nyby, 1979). The relationship between male USV production and female behavior suggests that USVs predict mating opportunities rather than aggression. Female responses to these USVs include attenuated aggressive behaviors, approach, and more frequent expression of mating postures (Sewell, 1969). In a two compartment choice test, females approach tethered males that are vocalizing versus males that are surgically rendered incapable of vocalizing. If both males are rendered unable to vocalize, but one of the males is placed next to an ultrasound generator producing 70 kHZ ultrasounds, females prefer to reside in the compartment containing the male with the sound generator (Pomerantz et al., 1983).
During these male-female social interactions, males emit two types of vocalizations (Maggio et al., 1983, Nyby & Whitney, 1978, Wang et al., 2008b, Whitney & Nyby, 1979). One is a highly modulated pure tone with a mean frequency of around 70 kHz and an intensity of 40 dB (Sales & Pye, 1974). The second is a pure tone around 40 kHz with an intensity of more than 100 dB. The male emits the 70 kHz calls almost continuously prior to the first mount, while he actively sniffs and investigates the female. After the first mount, the number of calls declines, although during the latter stages of copulatory behavior, the male intermixes both 70 and 40 kHz calls immediately before and between mounting bouts (Nyby, 1983, Sales & Pye, 1974, White et al., 1998)
The development of digital sound spectrographic analysis of USVs has allowed researchers to expand analyses of mouse vocalizations to include dynamic changes in mean frequency (pitch). Individual calls can then be classified according to the dominant frequency, as a function of time. This analytical approach was used to assess USVs of adult males that were elicited by odors of receptive adult females (Holy & Guo, 2005). Recordings were collected and calls were classified. For each call, pitch changes were assessed by comparison of sudden punctuations in pitch that occur within a syllable. This analysis revealed distinct clusters of pitch changes, such as upward and downward jumps between comparatively low frequencies (35–50 kHz) and similar transitions from between 70–90 kHz and 55–70 kHz (see Figs. 2E and 2F). There were also calls that lacked any abrupt changes in pitch. Often these calls were emitted in repeated temporal sequences (Holy & Guo, 2005) and changed reproducibly with the succession of approach, mounting, and release behaviors that constitute the mating encounter. Before mounting, males usually called in either flat or continuous frequency-modulated USVs. However, when males mounted females, males shifted to frequency-modulated broken “step-like” USVs with 70-kHz and 40/80 harmonic frequencies until the end of mounting (Wang et al., 2008b).
Figure 2.
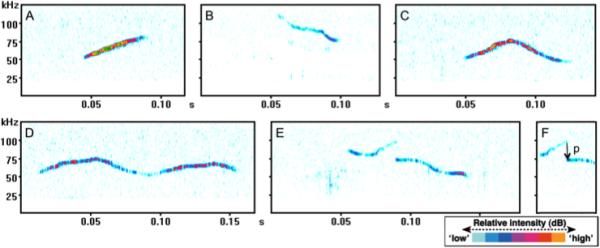
Spectrograms of representative call types emitted by juvenile male C57BL/6J mice (P30) mice reunited in a home cage environment after 24 hours of social isolation. Spectrograms include examples of an upward-modulated call (A), down-ward modulated call (B), chevron (C), complex call (D), and punctuated call (E). The abrupt change in frequency in figure E is a pitch jump (p), indicated by the downward pointing arrow (F). Time (in seconds) is indicated by the X axis, frequency in kHZ is indicated by the Y axis, and relative intensity, or loudness is indicated by color (see key).
Could these male vocalizations be relevant to affective prosody? Inferences that males derive a feeling of reward from social interactions with estrous females are warranted, based upon studies that use conditioned place preference (CPP) testing (and see below for discussion of CPP). Specifically, male mice prefer environments that have been associated with either mating (Kudwa et al., 2005, Popik et al., 2003) or even access to (Pankevich et al., 2006, Pierman et al., 2006) estrous female mice. The changing repertoire of male USVs through the mating process could reflect a shift from anticipation to consumption of sexual reward. In other words, these studies suggest that male mice may emit different peak frequencies that correlate with incentive salience versus hedonic pleasure (see Berridge 2007).
Adult same-sex interactions
During male-male agonistic encounters, two types of calls can be expressed. V-shaped USVs encompass a broad frequency range and are emitted during naso-nasal contacts and body sniffing behaviors that precede fighting. A second set of calls consists of harmonics, or overtones, which are recorded during fighting behavior (Gourbal et al., 2004). These results are in agreement with previous studies (Scott, 1966) including observations that the number of audible vocalizations emitted during a male-male encounter correlates with time fighting and number of attacks (Brain et al., 1980, Morgret & Dengerink, 1972). These audible vocalizations, unlike the USVs emitted during solicitation and mating, are associated with aggression, but share similarities. There are differences in USV structure that are associated with the anticipation of aggression versus participation in aggressive interactions. Taken together, the differences in frequency modulation associated with male anticipation and consumption of sexual versus aggressive encounter indicates that these call patterns respond to differences in the affective nature of the social encounter.
There are fewer studies of vocalizations among female mice. Females express audible low-frequency vocalizations during copulation, particularly when they are not in estrous, consistent with the observation that they tend to avoid sexual encounters with males unless they are reproductively receptive. By contrast, female-female encounters engender USV production, especially when they were engaged in olfactory investigation of the other (Sewell, 1970). Female mice emit a large number of USV, typically emitted at 70 kHz, during the first minutes of social interaction (Gourbal et al., 2004, Moles et al., 2007). The rate of USV production among females appears to be responsive to levels of social motivation, experimentally imposed by food-deprivation, estrous cycle, pregnancy and aging such that USV rates positively correlated with levels of social investigation (Moles et al., 2007). Interestingly, just as estrous females produce fewer calls during same-sex interactions in estrous, brief exposure to a male also inhibits USV production (Maggio & Whitney, 1985).
USVs of Infant Mice and the Maternal Response
Infant mice of postnatal day (PD) 1-4 are born blind and deaf (Fox, 1965), have limited motor abilities, lack fur and subcutaneous fat and cool rapidly when displaced from the nest. When removed from the nest, they emit separation calls that elicit female retrieval. Under conditions of stress, cold and hunger, pups emit calls that engender maternal nest building, licking behaviors, and crouching behaviors that allow for access to teats (Noirot, 1969). Rates of separation calls change with age and peak between PD 3 and PD 7, depending upon background strain (Branchi et al., 1998, Elwood & Keeling, 1982, Hennessy et al., 1980, Scattoni et al., 2008b). By two weeks of age, juvenile mice no longer emit separation calls (Elwood & Keeling, 1982, Noirot, 1966).
As early as 1970, Sewell showed that lactating female wood mice (Apodemus sylvaticus) more frequently enter a compartment containing the loudspeaker emitting the recorded USVs versus background noise or an artificial stimulus (Sewell, 1970). Early studies also showed that lactating Mus musculus females preferentially approach ultrasounds within the frequency range of the natural calls (ranging from 40-80 kHz) versus other ultrasonic sounds (Ehret & Haack, 1981). More recently, a strain-dependent difference in the dynamic relationship between maternal responsiveness and pup calling rate was found. Using a three-compartment test structure, in which the mother could only reach her pups by crossing the central chamber which contained olfactory cues from a potentially infanticidal male, C57BL/6 mothers expressed higher maternal responsiveness versus BALB/c females, and their pups emitted fewer calls than BALB/c pups (D'amato et al., 2005). These data suggest that maternal responsiveness to pup calls, in turn, affects the rate that pups emit ultrasonic vocalizations.
Several studies show that USV production in response to separation and isolation can be a physiological response to a thermal challenge, e.g. a reflexive abdominal compression reaction in response to the cold that helps return venous blood to the heart (Blumberg & Alberts, 1990, Blumberg & Sokoloff, 2001). Additionally, pups vocalize when placed in a warm location (Branchi et al., 2004, Shair et al., 2003, Wohr et al., 2008). In these cases, studies that employ knockout mice with alleles relevant to social bonding and separation distress suggest that separation calls are generated in response to affective changes. For instance, mice that lack a functional oxytocin allele, a molecule that plays a role in social bonding (Ferguson et al., 2000, Ferguson et al., 2002, Kavaliers et al., 2004, Mahler & Berridge, 2009, Petrovic et al., 2008), emit fewer separation calls than their wild-type littermates (Winslow et al., 2000). Rates of separation calls are also influenced by the presence or absence of a mu-opioid receptor (Moles et al., 2004a), which is a well-defined mediator of reward physiology and separation distress in rodents (Mahler & Berridge, 2009, Panksepp et al., 1980).
Spectrographic analysis has revealed that neonatal laboratory mice emit different kinds of USVs in response to varied environmental conditions to which the pup is exposed, such as odor from the nest, social isolation, low temperature, tactile stimulation or odor of an unfamiliar conspecific adult male (Branchi et al., 1998). For instance, isolated pups emit a high percentage of calls that contain abrupt breaks in frequency steps but an equal number of gradually modulated frequency calls relative to pups exposed to the odor of a conspecific unfamiliar, potentially infanticidal, adult male (Branchi et al., 1998). Variations in USV frequency modulation are also sensitive to the age of the pups, such that calls become stereotyped with maturation but remain acoustically distinct from adult USV patterns (Liu et al., 2003).
Mouse pups also produce ‘wriggling’ calls when struggling in the nest, mainly when pushing for the teats during suckling by the mother (Ehret, 1975). These calls release at least three types of maternal behavior that include licking of pups, changes of suckling position, and nest building (Ehret & Bernecker, 1986). Wriggling calls usually consist of a fundamental frequency near 4 kHz and overtones that can extend to a maximum frequency of about 20 kHz (Ehret & Bernecker, 1986, Geissler & Ehret, 2002, Geissler & Ehret, 2004a). These calls elicit a specific neural activity. There is a differential response of C-fos immunoreactivity within the auditory cortex to synthetic wriggling calls played back to nursing females versus similar synthetic wriggling USVs with a temporally displaced harmonic (Geissler & Ehret, 2004b). Interestingly, differences in immunoreactivity were more pronounced in the left hemisphere (Geissler & Ehret, 2004b), consistent with electrophysiological studies (Stiebler et al., 1997) and sharing similarities with human auditory processing (Ehret & Riecke, 2002).
Genetics
Call rates and frequency modulation of male USVs are responsive to genetic manipulations of endogenous reward pathways. For instance, targeted deletion of muscarinic receptor M5 results in an 80% decrease of male USVs production during sexual interaction. Male exposure to amphetamine, a direct ligand of D2 receptors, results in increased USV production, also responsive to targeted disruption of muscarinic receptors (Wang et al., 2008a). Amphetamine can modulate dopamine release in the nucleus accumbens (Yeomans et al., 2001, Yeomans et al., 2000), thereby exerting a substantial role in appetitive behaviors (Berridge, 2007, Ikemoto & Panksepp, 1999, Kelley & Berridge, 2002b). Similarly, infant mice lacking functional mu-opioid receptors express reduced numbers of USVs during maternal separation (Moles et al., 2004b), consistent with evidence that enkephalin interactions with muopioid receptor can mediate social reward and isolation distress (Bertrand et al., 1997, Kalin et al., 1988, Panksepp et al., 1980, Panksepp et al., 1994, Vanderschuren et al., 1995).
Genetic manipulations that do not directly interact with incentive salience or hedonia can also modify call rate and call repertoire. For example, mice lacking functional components of circuits involved in social recognition, which include oxytocin and vasopressin receptor 1b, emit fewer calls (Scattoni et al., 2008a, Winslow & Insel, 2002). Mice that lack functional alleles of genes associated with autistic-like social deficits, such as MECP2 and neuroligin 4 knockout mice, can express a diminished call rate (Jamain et al. 2008; Picker et al. 2006). Classification analysis has also revealed that a spontaneous mutation (BTBR) and a targeted allelic replacement (foxp2 R552H) can influence the pup repertoire (Fujita et al. 2008; Scattoni et al. 2008b; Shu et al. 2005). This recent demonstration that ultrasound vocalizations can be modified by manipulations of foxp2, a gene involved in a familial form of speech impairment in humans and required for normal vocal development in songbirds, suggests that rodents may also be an important model for studying the neural and genetic basis of speech learning and impairments.
Mouse USVs and Affect: Theoretical Considerations
Changes in subjective affective experience can be a proximal cause for changes in behavior. At a basic level, fear can induce freezing behavior, discomfort can induce withdrawal behavior, and anticipation of reward can induce an approach behavior. To the extent that specific aspects of the USV repertoire are associated with particular affective states, USV studies can provide insight to the genetic mechanisms that underlie affect regulation in normal psychological development and in mental illness.
Among humans, individuals make inferences to the quality of another person's experience based upon verbal communication or based upon observations of an individual's behavior. One means of communicating personal experience is through use of words. Self-report is an essential analytical tool for human research and clinical diagnosis; the basis of tremendous advances in psychological and psychiatric medicine. However, self-report has scientific and philosophical limitations. Bertrand Russell (1912) identified the problem: while words or labels are used to describe universal subjective states, it remains unknown whether these labels identify identical internal experiences. For example, two individuals can use the label ‘red’ to describe an apple but the red apple may appear as red-orange to one person and red-purple to another. It is inherently unknown whether both individuals have identical internal experiences, due to perceptual variations, such as differences in vision or brain function. Differences in sex, age, cultural context, and mental illness further contribute to variation in how words label subjective experiences. For instance, post-traumatic stress disorder might impose an atypical sensitivity to stimuli that may be inadequately described with the existing verbal repertoire. Discrete labels used to communicate individual perceptions, no matter how basic or complicated, have limitations in terms of describing internal experience.
To gain greater insight to the affective experience of individuals, new assessment tools have been developed that do not depend upon verbal report. Directionality of eye-gaze (Klin et al., 2002, Wolf et al., 2008) and patterns of brain activity provide insights to how autistic individuals attend to social interactions (Baron-Cohen et al., 1999). Assessments of heart rate variability provide insight to how children experience distress of others (Eisenberg et al., 1988). To gain insight to the etiology of developmental disorders, there are ongoing efforts to identify the psychological experiences that are experiences during mental illness. For example, there is ongoing controversy about whether some forms of autism can result from social anxiety (Kuusikko et al., 2008), deficits in temporal cognition (Boucher et al., 2007) or central coherence (Briskman et al., 2001, Happe et al., 2001). Just as efforts to infer psychological experience can be useful for elucidating mechanisms underlying mental illness, these same issues are important for mouse research. If a knockout mouse expresses low levels of social approach, how do we know whether the targeted allele models autism, versus depression, versus shyness?
Such questions are rather new to behavioral neuroscience. Considerable precedence in the history of philosophy, extending back to the work of Rene Descartes (1637), maintains that animals are ‘automata’ or machine-like, in contrast to ‘sentient’ or soul-full humans. With the advent of cognitive and affective neuroscience, these Cartesian distinctions are no longer relevant, but it is important to be careful about inferences to rodent affective experience (Panksepp, 1998, Schneirla, 1959a). Such inferences require consideration of the theoretical framework of affective neuroscience, including operant, fear, and preference conditioning paradigms, as well as studies of functional anatomy and brain/systems physiology (Panksepp 1998). Such an approach provides working hypotheses for comparative studies of affective prosody that can be subject to falsification.
Operant and place preference conditioning are used to establish anticipation and consumption of rewards (Bardo & Bevins, 2000a, Kelley et al., 2002, Moles et al., 2004a) and aversive conditions can be identified by conditioned fear learning paradigms (Falls et al., 1997, Ledoux et al., 1988, Paylor et al., 1994). Emotional responses to stimuli can also be inferred from studies of functional anatomy, particularly the highly conserved limbic structures in the brain (Maclean, 1990) and the underlying physiological systems that mediate affect regulation, including dopamine, serotonin, corticosterone and endogenous opiates (Panksepp, 1998). Drugs of abuse, such as opiates and amphetamine, are also useful, since they can influence natural reward systems (Abarca et al., 2002, Bardo & Bevins, 2000b, Kelley & Berridge, 2002a, Reith & Selmeci, 1992, Van Ree et al., 1999). Pharmaceuticals that modulate affective states in humans can be employed that influence analogous behaviors in mouse models (Holsboer, 2001, Hunsberger et al., 2007, Kato, 2006, Pezet & Malcangio, 2004, Rupniak et al., 2001).
In summary, assessments of prosody in mice should include measures of neural and behavioral correlates of motivated behavior, to gain greater reliability in inferences to subjective experience. Such assessments are important for translational applications of mouse research to mental illness. However, human vocal patterns are vastly more complex with regards to frequency modulation, timing, overtones, and range of sound produced, and may provide more nuanced emotional content. Studies of human language can assess the temporal associations between prosodic content and referential aspects of language. However, basic elements of prosodic content in mice are measurable and have been associated with rewarding and aversive conditions. Thus, studies of prosody in humans and mice may yield apples-to-apples comparisons useful for subjective inference.
Mouse USVs and Affect: Practical Considerations
Behavioral Associations with USV Production
Juvenile mice emit USVs when meeting a littermate after 24 hours of social isolation. When USVs of juvenile C57BL/6J (B6) and BALBc/J (BALB) mice are compared during reunion, there are clear differences in the kinds of calls that they emit. For example, there are more downward-modulated calls among reunited B6 juveniles and more upward-modulated call-types and chevron call-types among BALB juveniles (see Figure 2 for example spectrograms).
Differences in USV production between genetic variants of mice could be explained as products of discrete genetic contributions to the anatomies of the larynx or labia. Alternatively, there may be particular call-types that are associated with state, such as the motivation to solicit social interaction. To assess the likelihood of these opposing hypotheses, we compared the usage of various call-types by different individuals within a strain that express differences in social approach behaviors. Individual B6 and BALB juveniles express more USVs when they are engaged in more vigorous social approach behaviors (Panksepp et al., 2007). During more vigorous social approach behaviors, B6 mice disproportionally express calls that are of a higher pitch and more downward modulated call-types during vigorous social approach. These differences in B6 vocal repertoire suggest the possibility that different call-types correspond to different degrees of social motivation or function to engender discrete levels of motivation in others. Also important, selective B6 use of particular call-types during higher levels of social interaction was specific to B6 mice. BALB mice that engaged in vigorous social interactions expressed all call-types at a higher rate. This finding indicates that the degree of repertoire flexibility within a strain is also dependent upon genetic background.
Rodents prefer social encounter to isolation, consistent with classical theories of motivated behavior (Bardo & Bevins, 2000b, Berridge & Robinson, 2003, Tzschentke, 1998) that underscore a role for reward and punishment in behavioral approach and withdrawal (Glickman & Schiff, 1967, Schneirla, 1959b, Young, 1959). Mouse responses to the conditioned place-preference (CPP) test demonstrate that they can find social encounters rewarding and social isolation aversive. In a standard CPP experiment, mice are placed in one of two distinct environments. For instance, the beddings of the two environments may be different (one is aspen and the other is paper), the walls may have horizontal versus vertical lines, or the floors may have circular holes in them or exist as a parallel grid. During the conditioning phases of these experiments, the test mouse is housed successively in either one or the other environment. The mouse has access to a putative reward (e.g. peanut butter, chocolate, cocaine, morphine) in one environment but not in the alternate environment. If the mouse finds the test stimulus rewarding, then through repeated association, the environment associated with access to the reward eventually elicits approach behavior. At the end of the conditioning phase, the mouse is placed in a testing structure that does not contain the reward but contains both of the ‘conditioned’ environments. The dependent measure of CPP is the difference in the amount of time spent in the environment associated with the presence of the putative reward versus the environment paired with its absence. If the stimulus is rewarding, the mouse should spend more time in the environment that is paired with it. Among rodents, CPP tests have demonstrated reward processes during different kinds of social encounters, including play (Calcagnetti & Schechter, 1992, Douglas et al., 2004), sexual interactions (Camacho et al., 2004, Jenkins & Becker, 2003), mother–infant bonding (Mattson et al., 2001, Weller et al., 2001) and even aggression (Martinez et al., 1995).
CPP experiments can be helpful in determining whether mouse strains that express different USV repertoires experience different levels of social motivation. For instance, B6 mice show a strong place preference for bedding environments that have been associated with access to age-matched juveniles versus beddings that are paired with social isolation (Panksepp & Lahvis, 2007). These results suggest the possibility that the vocal repertoire of B6 mice may be associated with the degree of social reward. To dissociate the relationships between calls and the patterns change under various levels of social motivation, we can gain insight to useful approaches for studying mouse USVs from insightful experiments of rat vocalizations. Rats emit high frequency (50 kHz) calls when they anticipate a future reward or when they are experiencing the reward, and lower frequency (22 kHz) USVs under aversive condition (Burgdorf et al., 2008a, Knutson et al., 1999, Schwarting et al., 2007, Wohr & Schwarting, 2009). In addition to conditioning experiments, studies with operant tasks indicate that rats will self-administer playbacks of recordings of 50 kHZ ‘trill’ calls but not a 22 kHz calls (Burgdorf et al., 2008b). Similar kinds of evaluations might be useful for interpreting particular mouse USVs. It would be very useful, for example, to know whether there are specific call-types that associate with different conditioned place preference responses to specific drugs. Measures of mouse USVs have the potential to provide insight to real-time fluctuations in affect that we cannot obtain through conditioned place preference or fear conditioning testing. Experiments that take advantage of the differences in social motivation that accompany variations the duration of social isolation or the time of day when social encounter occurs (Panksepp et al., 2008) could be used to discern how call patterns, and responses to them, change with social conditions.
Behavioral and Systemic Responses to Vocalizations
Studies of animal acoustic communication utilize behavioral responses to recordings or ‘playbacks’ of particular kinds of animal calls to ascertain the meaning of various signals. In a series of experiments, we found that mice can become differentially fearful of a tone that is temporally associated with the vocalizations of a distressed mouse. This experiment involves a cue-conditioned fear learning paradigm (Ledoux et al., 1988). A standard fear conditioning procedure entails presenting an animal with a neutral stimulus, such as a tone (conditioned stimulus, CS), forward paired with an aversive stimulus, such as an electrical shock (unconditioned stimulus, UCS). Upon repeated administration of the paired CS-UCS, mice acquire a robust freezing behavior in response to presentation of the CS-only. When B6 and BALB mice observed others undergo this fear conditioning procedure, only B6 mice were capable of learning from the distress of others. B6 also acquired the freezing response to the tone if they simply heard playbacks of the vocalizations of other distressed mice (played back 2-s recordings of the vocalizations of shocked mice) in association with the 30-s tone. Control experiments indicated that the BALB mice could hear the calls, since they responded to an environmental cue (the tone) of an overlapping frequency and energy level (approximately 85 dB). Importantly, heart rate deceleration was expressed in B6 when demonstrator mice were undergoing fear conditioning, but BALB mice did not show heart rate deceleration. Depression of heart rate can be associated with the empathic response to other's distress and occurs among children more capable of detecting distress among others (Eisenberg et al., 2006). These studies indicate that fear can be perceived through vocal signals under the appropriate genetic background.
Brain Activity in Response to Vocalizations
To assess the salience of different call-types, researchers are beginning to examine freezing behavior, heart rate changes, or other behavioral endpoints in response to playbacks of auditory cues, such as distress vocalizations (Liu et al., 2006a) (Daejong Jeon et al., 2010) and see also (Knapska et al., 2006, Sadananda et al., 2008). For instance, variations in USV frequency modulation are sensitive to the age of the pups, with distinct influences on activity of the auditory cortex (Liu et al., 2003). In fact, experience appears to change neural processing of auditory stimuli; maternal auditory processing of pup USVs is distinct from that of naïve females (Liu et al., 2006b, Liu & Schreiner, 2007).
FUTURE DIRECTIONS
USV Analysis
There are a variety of approaches to vocal analysis, ranging from classification schemes to principal components analysis to hidden-Markov approaches. Various software programs have been developed to serve different purposes and each has its own benefits. Software programs can be employed to directly measure ultrasonic vocalizations, such as Avisoft SASLabPro (Panksepp et al., 2007a, Scattoni et al., 2008c, Wohr et al., 2008), others are designed to dissociate calls based upon highly nuanced spectral features, such as continuity and entropy (Tchernichovski et al. 2001), or identify calls in low signal-to-noise conditions (Mellinger, 2009). Investigators also utilize their own MATLAB software programs to analyze frequency modulation in mouse USVs (Liu et al., 2006a). Pattern recognition in USV sequence is also aided by the application of advanced statistical approaches (Ren et al. 2009). In this regard, future studies will likely utilize a variety of software approaches to obtain different kinds of information from calls, ranging from unsophisticated classifications of relatively similar call-types (Panksepp et. al. 2007) to more careful discriminations of quite subtle changes in the frequency modulation within a single call type. Differences in affective content might then be discerned by use of playbacks in combination with measures of brain activity, heart rate changes, behavioral responses, or other parameters that allow us to make inferences to affective response.
Complex environments
The rodent has long been considered a mammalian substitute for the human patient (Beach, 1950), but when concentrating on a single, increasingly exploited, lab species, it has to be taken in mind that such a species is the “ultimate” product (a variable genetic pool fragmented in a variety of local populations) of species-specific phylogenetic and epigenetic processes. Laboratory mice have been strongly selected for husbandry in a small cage, where there are limited opportunities for temporal and geographic variations in food access, social encounter, temperature, diurnal rhythms, encounter with predators, or exploration. By contrast, feral mice, by definition strictly associated to human settlements (Bronson, 1979), show highly variable adaptive strategies that are not promoted by standard cage housing. It is likely that laboratory mice are thus not raised to vocalize in response to the more complex variety of social-emotional states that accompanies a multifaceted environment. In this regard, wild-derived mice provide potentially useful opportunities to dissociate USV patterns (Musolfa et al., 2010, Kalcounis-Rueppell 2010). A truly, genuine, comparative approach should be based on a much broader range of mouse experiences and representative species: the search for functional and/or structural invariance could be a fruitful “zoo-semeiotic” perspective (sensu Sebeok (Sebeok, 1972). Studies with feral mice, as well as other feral rodent species might provide a high degree of fidelity between call-type and affective state.
More substantive vocal behaviors might also be revealed by naturalistic or semi-naturalistic settings (Alleva et al., 1994, Portfors 2007). While mouse vocalizations are sensitive to targeted loss-of-function mutations in genes involved in affect regulation, language development, social bonding, and autism, we may gain greater insight to how these mutations modify call patterns by expanding mouse developmental histories. Genetic variants can be raised in complex settings to expand their emotional experience and vocal repertoire. Rearing could entail geographic and temporal variation, such as random temporal access to food rewards, such as peanut butter, and stressors, such as cat urine, within a complex 3-dimensional environment. Mice raised in environments that incorporate rewarding and aversive stimuli, in the context of more complex social paradigms, might provide a useful model for delineating relationships between affective states and specific call types.
SUMMARY
Assessments of frequency modulation provide researchers with an ability to study USVs in a fashion that has translational relevance to prosodic deficits in mental illness. Measures of mouse USVs have the potential to provide a window to real-time changes in affective state that are not accessible by conditioned place preference or fear conditioning tests.
Inferences about call meaning should be considered within the context of ethologically relevant behaviors or traditional assessments of reward and aversion (e.g. conditioning experiments) and though the use of heart rate monitoring, assessments of brain activity, and other physiological indicators of affective response.
Mutant genetic models that are relevant to reward neurobiology, language, autism, and social bonding can express abnormal USV patterns. Use of multiple statistical approaches for bioacoustic analysis, including clustering and hidden Markov approaches, can help elucidate associations between USV structure and function.
By rearing mice in more complex environments, researchers might also gain greater insight to the discrete functions individual calls or call sequences within the diversity of the vocal repertoire.
Figure 1.
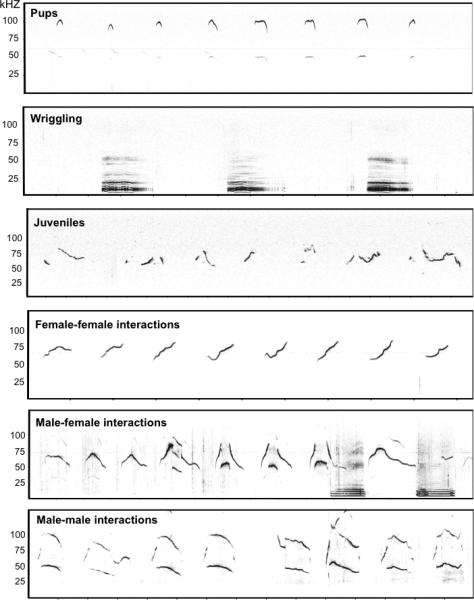
Spectrograms of call sequences differ among mice of varied ages and social contexts. Pup separation calls were collected from an 8-day-old mouse of the C57BL/6J strain approximately 1 minute after removal from the nest and placement in a soundproof chamber at 23 °C. Wriggling calls were recorded from pups within the litter of the C57BL/6J strain approximately 1 minute after placing the microphone above the nest. Juvenile calls were recorded approximately 30 seconds after two male C57BL/6J mice (PD30) were reunited in a home cage environment after 24 hours of social isolation. Calls emitted during female-female interactions were collected approximately 20 seconds after an intruder female was inserted into the cage of a resident female, both mice of the C57BL/6J strain after 3 days of social isolation. Calls emitted during male-female interactions were collected approximately 20 seconds after an adult female of the C57BL/6J strain was inserted into the cage of an adult male mouse of the C57BL/6J strain in his home cage environment. Calls emitted during male-male interactions were collected approximately 20 seconds after an intruder male of the C57BL/6J strain was inserted into the cage of a resident male mouse of the C57BL/6J strain in his home cage environment after 3 days of social isolation.
ACKNOWLEDGEMENTS
The authors wish to thank Jan Van Santen and Lois Black of Oregon Health and Science University for communicating their insights to prosodic communication. We also want to acknowledge the Waisman Center at the University of Wisconsin. This work was supported by R01 funding from the National Institute of Drug Abuse (DA022543).
REFERENCES
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva E, Fasolo A, Lipp HP, Nadel L, Ricceri L. Behavioural brain research in naturalistic and semi-naturalistic settings. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1994. [Google Scholar]
- Alpert M, Rosenberg SD, Pouget ER, Shaw RJ. Prosody and lexical accuracy in flat affect schizophrenia. Psychiatry Res. 2000;97:107–118. doi: 10.1016/s0165-1781(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Amorosa H. Disorders of vocal signaling in children. In: Papousek U, Jurgens U, Papousek M, editors. Nonverbal vocal communication: Comparative and developmental approaches. Cambridge University Press; Cambridge, UK: 1992. pp. 192–204. [Google Scholar]
- Bach DR, Buxtorf K, Grandjean D, Strik WK. The influence of emotion clarity on emotional prosody identification in paranoid schizophrenia. Psychol Med. 2009;39:927–938. doi: 10.1017/S0033291708004704. [DOI] [PubMed] [Google Scholar]
- Baltaxe CA. Use of contrastive stress in normal, aphasic, and autistic children. J Speech Hear Res. 1984;27:97–105. doi: 10.1044/jshr.2701.97. [DOI] [PubMed] [Google Scholar]
- Baltaxe CA, Guthrie D. The use of primary sentence stress by normal, aphasic, and autistic children. J Autism Dev Disord. 1987;17:255–271. doi: 10.1007/BF01495060. [DOI] [PubMed] [Google Scholar]
- Bardo M, Bevins R. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000a;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000b;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Beach FA. The snark was a boojum. American Psychologist. 1950:115–124. [Google Scholar]
- Beatty WW, Orbelo DM, Sorocco KH, Ross ED. Comprehension of affective prosody in multiple sclerosis. Mult Scler. 2003;9:148–153. doi: 10.1191/1352458503ms897oa. [DOI] [PubMed] [Google Scholar]
- Benke T, Bosch S, Andree B. A study of emotional processing in Parkinson's disease. Brain Cogn. 1998;38:36–52. doi: 10.1006/brcg.1998.1013. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: The case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Smadja C, Mauborgne A, Roques BP, Dauge V. Social interaction increases the extracellular levels of [Met]enkephalin in the nucleus accumbens of control but not of chronic mild stressed rats. Neuroscience. 1997;80:17–20. doi: 10.1016/s0306-4522(97)00136-x. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: an acoustic byproduct of laryngeal braking? Behav Neurosci. 1990;104:808–817. doi: 10.1037//0735-7044.104.5.808. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev. 2001;108:83–95. doi: 10.1037/0033-295x.108.1.83. [DOI] [PubMed] [Google Scholar]
- Boucher J, Pons F, Lind S, Williams D. Temporal cognition in children with autistic spectrum disorders: Tests of diachronic thinking. Journal of Autism and Developmental Disorders. 2007;37:1413–1429. doi: 10.1007/s10803-006-0285-9. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Anezoulaki D, Giannakou M, Andreou C, Karavatos A. Impaired perception of affective prosody in schizophrenia. J Neuropsychiatry Clin Neurosci. 2006;18:81–85. doi: 10.1176/jnp.18.1.81. [DOI] [PubMed] [Google Scholar]
- Brain PF, Benton D, Cole C, Prowse B. A device for recording submissive vocalizations of laboratory mice. Physiol Behav. 1980;24:1003–1006. doi: 10.1016/0031-9384(80)90165-1. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Puopolo M, Alleva E. Neonatal Behaviors Associated with Ultrasonic Vocalizations in Mice (Mus musculus): A Slow-Motion Analysis. Developmental Psychobiology. 2004;44:37–44. doi: 10.1002/dev.10150. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic vocalizations by infant laboratory mice: a preliminary spectrographic characterization under different conditions. Dev Psychobiol. 1998;33:249–256. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Briskman J, Happe F, Frith U. Exploring the cognitive phenotype of autism: Weak “central coherence” in parents and siblings of children in autism: II. Real-life skills and preferences. Journal of Child Psychology and Psychiatry. 2001;42:309–316. [PubMed] [Google Scholar]
- Bronson FH. The reproductive ecology of the house mouse. Q Rev Biol. 1979;54:265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008a;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. Journal of Comparative Psychology. 2008b;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Camacho F, Sandoval C, Paredes RG. Sexual experience and conditioned place preference in male rats. Pharmacol Biochem Behav. 2004;78:419–425. doi: 10.1016/j.pbb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Docherty NM. Effects of positive affect on speech disorder in schizophrenia. J Nerv Ment Dis. 2005;193:839–842. doi: 10.1097/01.nmd.0000188963.16870.27. [DOI] [PubMed] [Google Scholar]
- D'Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35:103–112. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Daejong Jeon D, Sangwoo Kim S, Mattu Chetana M, Daewoong Jo D, H Earl Ruley HE, Shih-Yao Lin S-Y, Rabah D, Kinet J-P, Hee-Sup Shin H-S. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neuroscience. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Finkelmeyer A, Toygar TK, Hulsmann A, Schneider F, Falkenberg DI, Habel U. Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res. 2009;108:197–206. doi: 10.1016/j.schres.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Diehl JJ, Bennetto L, Watson D, Gunlogson C, McDonough J. Resolving ambiguity: a psycholinguistic approach to understanding prosody processing in high-functioning autism. Brain Lang. 2008;106:144–152. doi: 10.1016/j.bandl.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Ehret G. Schallsignale der Hausmaus (Mus Musculus). Behaviour. 1975:38–56. [Google Scholar]
- Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behaviour. Anim Behav. 1986:821–830. [Google Scholar]
- Ehret G, Haack B. Categorical perception of mouse pup ultrasound by lactating females. Naturwissenschaften. 1981;68:208–209. doi: 10.1007/BF01047208. [DOI] [PubMed] [Google Scholar]
- Ehret G, Riecke S. Mice and humans perceive multiharmonic communication sounds in the same way. Proc Natl Acad Sci U S A. 2002;99:479–482. doi: 10.1073/pnas.012361999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Bustamante D, Mathy RM, Miller PA, Lindholm E. Differentiation of vicariously induced emotional reactions in children. Developmental Psychology. 1988;24:237–246. [Google Scholar]
- Eisenberg N, Fabes RA, Spinard TL. Prosocial development. In: Eisenberg N, editor. Handbook of child psychology. John Wiley & Sons Inc.; New Jersey: 2006. pp. 646–718. [Google Scholar]
- Elwood RW, Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev Psychobiol. 1982;15:221–227. doi: 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- Emerson CS, Harrison DW, Everhart DE. Investigation of receptive affective prosodic ability in school-aged boys with and without depression. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:102–109. [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner JG, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behav Neurosci. 1997;111:855–861. [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Frontiers in Neuroendocrinology. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Fernald A. Intonation and communicative intent in mothers’ speech to infants: is the melody the message? Child Dev. 1989;60:1497–1510. [PubMed] [Google Scholar]
- Fine J, Bartolucci G, Ginsberg G, Szatmari P. The use of intonation to communicate in pervasive developmental disorders. J Child Psychol Psychiatry. 1991;32:771–782. doi: 10.1111/j.1469-7610.1991.tb01901.x. [DOI] [PubMed] [Google Scholar]
- Fox M. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Shimizu M, Hirao K, Miyata J, Namiki C, Sawamoto N, Fukuyama H, Hayashi T, Murai T. Female specific anterior cingulate abnormality and its association with empathic disability in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1728–1734. doi: 10.1016/j.pnpbp.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Time-critical integration of formants for perception of communication calls in mice. Proc Natl Acad Sci U S A. 2002;99:9021–9025. doi: 10.1073/pnas.122606499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Auditory perception vs. recognition: representation of complex communication sounds in the mouse auditory cortical fields. Eur J Neurosci. 2004a;19:1027–1040. doi: 10.1111/j.1460-9568.2004.03205.x. [DOI] [PubMed] [Google Scholar]
- Geissler DB, Ehret G. Auditory perception vs. recognition: representation of complex communication sounds in the mouse auditory cortical fields. European Journal of Neuroscience. 2004b;19:1027–1040. doi: 10.1111/j.1460-9568.2004.03205.x. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Schiff BB. A biological theory of reinforcement. Psychol Rev. 1967;74:81–109. doi: 10.1037/h0024290. [DOI] [PubMed] [Google Scholar]
- Golan O, Baron-Cohen S, Hill JJ, Rutherford MD. The ‘Reading the Mind in the Voice’ test-revised: a study of complex emotion recognition in adults with and without autism spectrum conditions. J Autism Dev Disord. 2007;37:1096–1106. doi: 10.1007/s10803-006-0252-5. [DOI] [PubMed] [Google Scholar]
- Gourbal BE, Barthelemy M, Petit G, Gabrion C. Spectrographic analysis of the ultrasonic vocalisations of adult male and female BALB/c mice. Naturwissenschaften. 2004;91:381–385. doi: 10.1007/s00114-004-0543-7. [DOI] [PubMed] [Google Scholar]
- Haker H, Rossler W. Empathy in schizophrenia: impaired resonance. Eur Arch Psychiatry Clin Neurosci. 2009;259:352–361. doi: 10.1007/s00406-009-0007-3. [DOI] [PubMed] [Google Scholar]
- Happe F, Briskman J, Frith U. Exploring the cognitive phenotype of autism: Weak “central coherence” in parents and siblings of children with autism: I. Experimental tests. Journal of Child Psychology and Psychiatry. 2001;42:299–307. [PubMed] [Google Scholar]
- Hennessy MB, Li J, Lowe EL, Levine S. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol. 1980;13:573–584. doi: 10.1002/dev.420130603. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Prospects for antidepressant drug discovery. Biol Psychol. 2001;57:47–65. doi: 10.1016/s0301-0511(01)00089-8. [DOI] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseknecht CR. Sonographic analysis of vocalizations of three species of mice. Journal of Mammalogy. 1968;49:555–560. [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003;43:503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Kalcounis-Rueppell MC, Petric R, Briggs JR, Carney C, Marshall MM, Willse JT, Rueppell O, Ribble DO, Crossland JP. Differences in Ultrasonic Vocalizations between Wild and Laboratory California Mice (Peromyscus californicus). PLoS ONE. [Electronic Resource] 2010;5(4) doi: 10.1371/journal.pone.0009705. doi: 10.1371/journal.pone.0009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kato T. The role of mitochondrial dysfunction in bipolar disorder. Drug News & Perspectives. 2006:597–602. doi: 10.1358/dnp.2006.19.10.1068006. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Agmo A, Pfaff DW. Olfactory-mediated parasite recognition and avoidance: linking genes to behavior. Hormones & Behavior. 2004;46:272–283. doi: 10.1016/j.yhbeh.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002a;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002b;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, Kaczmarek L, Werka T. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3858–3862. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66:639–643. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- Kudwa A, Dominguez-Salazar E, Cabrera D, Sibley D, Rissman E. Dopamine D5 receptor modulates male and female sexual behavior in mice. Psychopharmacology. 2005;180:206–214. doi: 10.1007/s00213-005-2150-5. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila M-L, Ebeling H, Pauls DL, Moilanen I. Social anxiety in high-functioning children and adolescents with autism and Asperger syndrome. Journal of Autism and Developmental Disorders. 2008;38:1697–1709. doi: 10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leentjens AF, Wielaert SM, van Harskamp F, Wilmink FW. Disturbances of affective prosody in patients with schizophrenia; a cross sectional study. J Neurol Neurosurg Psychiatry. 1998;64:375–378. doi: 10.1136/jnnp.64.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med. 2006;36:1075–1083. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. European Journal of Neuroscience. 2006a;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006b;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. Journal of the Acoustical Society of America. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: role in paleocerebral functions. Plenum Press; New York: 1990. [DOI] [PubMed] [Google Scholar]
- Maggio JC, Maggio JH, Whitney G. Experience-based vocalization of male mice to female chemosignals. Physiol Behav. 1983;31:269–272. doi: 10.1016/0031-9384(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Maggio JC, Whitney G. Ultrasonic vocalizing by adult female mice (Mus musculus). J Comp Psychol. 1985;99:420–436. [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. The Journal of Neuroscience. 2009;29:6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Guillen-Salazar F, Salvador A, Simon VM. Successful intermale aggression and conditioned place preference in mice. Physiol Behav. 1995;58:323–328. doi: 10.1016/0031-9384(95)00061-m. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- McCann J, Peppe S, Gibbon FE, O'Hare A, Rutherford M. Prosody and its relationship to language in school-aged children with high-functioning autism. Int J Lang Commun Disord. 2007;42:682–702. doi: 10.1080/13682820601170102. [DOI] [PubMed] [Google Scholar]
- Mellinger DK. Noise-resistant acoustic measurements implemented in user-friendly software. J. Acoust. Soc. Am. 2009;125:2737–2737. [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C, D'Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004a;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004b;304:1983–1986. doi: 10.1126/science.1095943. [see comment] [DOI] [PubMed] [Google Scholar]
- Monnot M, Lovallo WR, Nixon SJ, Ross E. Neurological basis of deficits in affective prosody comprehension among alcoholics and fetal alcohol-exposed adults. J Neuropsychiatry Clin Neurosci. 2002;14:321–328. doi: 10.1176/jnp.14.3.321. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon S, Lovallo W, Ross E. Altered emotional perception in alcoholics: deficits in affective prosody comprehension. Alcohol Clin Exp Res. 2001;25:362–369. [PubMed] [Google Scholar]
- Morgret MK, Dengerink HA. The squeal as an indicator of aggression in mice. Behavior Research Methods and Instrumentation. 1972;4:138–140. [Google Scholar]
- Murphy D, Cutting J. Prosodic comprehension and expression in schizophrenia. J Neurol Neurosurg Psychiatry. 1990;53:727–730. doi: 10.1136/jnnp.53.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolfa K, Hoffmanna F, Penna DJ. Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Animal Behaviour. 2010;79:757–764. [Google Scholar]
- Noirot E. Ultra-sounds in young rodents. I. Changes with age in albino mice. Anim Behav. 1966;14:459–462. doi: 10.1016/s0003-3472(66)80045-3. [DOI] [PubMed] [Google Scholar]
- Noirot E. Changes in responsiveness to young in the adult mouse. V. Priming. Animal Behaviour. 1969;17:542–546. doi: 10.1016/0003-3472(69)90161-4. [DOI] [PubMed] [Google Scholar]
- Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–134. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- Nyby J, Whitney G. Ultrasonic communication of adult myomorph rodents. Neuroscience & Biobehavioral Reviews. 1978;2:1–14. [Google Scholar]
- Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; Boca Raton, Florida: 2001. pp. 3–18. [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiology & Behavior. 2006;87:781–788. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective Neuroscience: The foundations of human and animal emotions. Oxford University Press; Oxford: 1998. [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S. Brain opioids and mother-infant social motivation. Acta Paediatr Suppl. 1994;397:40–46. doi: 10.1111/j.1651-2227.1994.tb13264.x. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Jochman K, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE [Electronic Resource] 2007;2 doi: 10.1371/journal.pone.0000351. doi:10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Wong JC, Kennedy BC, Lahvis GP. Differential entrainment of a social rhythm in adolescent mice. Behav Brain Res. 2008;195:239–245. doi: 10.1016/j.bbr.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of Neuroscience. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin Ther Targets. 2004;8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- Pierman S, Tirelli E, Douhard Q, Baum MJ, Bakker J. Male aromatase knockout mice acquire a conditioned place preference for cocaine but not for contact with an estrous female. Behavioural Brain Research. 2006;174:64–69. doi: 10.1016/j.bbr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pijnenborg GH, Withaar FK, Bosch RJ, Brouwer WH. Impaired perception of negative emotional prosody in schizophrenia. Clin Neuropsychol. 2007;21:762–775. doi: 10.1080/13854040600788166. [DOI] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Rygula R, Bisaga A, Bespalov A. Effects of memantine, an NMDA receptor antagonist, on place preference conditioned with drug and nondrug reinforces in mice. Behavioural Pharmacology. 2003;14:237–244. doi: 10.1097/00008877-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic volcaizations in laboratory rats and mice. JAALAS. 2007;46:28–34. [PubMed] [Google Scholar]
- Reith ME, Selmeci G. Cocaine binding sites in mouse striatum, dopamine autoreceptors, and cocaine-induced locomotion. Pharmacol Biochem Behav. 1992;41:227–230. doi: 10.1016/0091-3057(92)90087-v. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Carlson EJ, Webb JK, Harrison T, Porsolt RD, Roux S, de Felipe C, Hunt SP, Oates B, Wheeldon A. Comparison of the phenotype of NK1R-/- mice with pharmacological blockade of the substance P (NK1 ) receptor in assays for antidepressant and anxiolytic drugs. Behav Pharmacol. 2001;12:497–508. doi: 10.1097/00008877-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Baron-Cohen S, Wheelwright S. Reading the mind in the voice: a study with normal adults and adults with Asperger syndrome and high functioning autism. J Autism Dev Disord. 2002;32:189–194. doi: 10.1023/a:1015497629971. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Wohr M, Schwarting RKW. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neuroscience Letters. 2008;435:17–23. doi: 10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Sales G, Pye D. Ultrasonic communication by animals. Chapman & Hall Ltd.; London: 1974. [Google Scholar]
- Sales GD. Ultrasound and mating behaviour in rodents with some observations on other behavioural situations. Journal of Zoology. 1972;168:149–164. [Google Scholar]
- Scattoni M, McFarlane H, Zhodzishsky V, Caldwell H, Young W, Ricceri L, Crawley J. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behavioural Brain Research. 2008a;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008b;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, Crawley JN. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behavioural Brain Research. 2008c;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneirla TC. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In: Jones MR, editor. Nebraska symposium on motivation. Univer. Nebraska Press; 1959a. pp. 1–42. [Google Scholar]
- Schneirla TC. An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. In: Jones MR, editor. Nebraska symposium on motivation. University of Nebraska Press; Lincoln: 1959b. pp. 1–42. [Google Scholar]
- Scholten MR, Aleman A, Kahn RS. The processing of emotional prosody and semantics in schizophrenia: relationship to gender and IQ. Psychol Med. 2008;38:887–898. doi: 10.1017/S0033291707001742. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Jegan N, Wohr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behavioural Brain Research. 2007;182:208–222. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Scott JP. Agonistic behavior of mice and rats: a review. Am Zool. 1966;6:683–701. doi: 10.1093/icb/6.4.683. [DOI] [PubMed] [Google Scholar]
- Sebeok TA. Perspectives in zoosemiotics. Mouton; The Hague: 1972. [Google Scholar]
- Sewell GD. Ultrasound in small mammals. University of London; 1969. [Google Scholar]
- Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Developmental Psychobiology. 2003;42:206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- Shriberg LD, Paul R, McSweeny JL, Klin AM, Cohen DJ, Volkmar FR. Speech and prosody characteristics of adolescents and adults with high-functioning autism and Asperger syndrome. J Speech Lang Hear Res. 2001;44:1097–1115. doi: 10.1044/1092-4388(2001/087). [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3:97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Smith A. The empathy imbalance hypothesis of autism: A theoretical approach to cognitive and emotional empathy in autistic development. The Psychological Record. 2009:273–294. [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. Journal of Comparative Physiology A Sensory Neural & Behavioral Physiology. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Abdel-Hamid M, Lehmkamper C, Vollmoeller W, Daum I. Perception of affective prosody in major depression: a link to executive functions? J Int Neuropsychol Soc. 2008;14:552–561. doi: 10.1017/S1355617708080740. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: An encounter of biology, psychology, and medicine. Pharmacol Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- Vanderschuren LJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Research. 1995;680:148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- Wan MW, Penketh V, Salmon MP, Abel KM. Content and style of speech from mothers with schizophrenia towards their infants. Psychiatry Res. 2008;159:109–114. doi: 10.1016/j.psychres.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS ONE [Electronic Resource] 2008a;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008b;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A, Tsitolovskya L, Gispan IH, Rabinovitz S. Examining the role of cholecystokinin in appetitive learning in the infant rat. Peptides. 2001;22:1317–1323. doi: 10.1016/s0196-9781(01)00458-2. [DOI] [PubMed] [Google Scholar]
- White NR, Prasad M, Barfield RJ, Nyby JG. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol Behav. 1998;63:467–473. doi: 10.1016/s0031-9384(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Whitney G, Nyby J. Cues that elicit ultrasounds from adult male mice. American Zoologist. 1979;19:457–463. [Google Scholar]
- Whitney G, Nyby J. Sound communication among adults. In: Willot JF, editor. The auditory psychology of the mouse. Charles C Thomas; Springfield: 1983. pp. 98–129. [Google Scholar]
- Whitney GD. Vocalization of mice: a single genetic unit effect. J Hered. 1969;60:337–340. doi: 10.1093/oxfordjournals.jhered.a108009. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Winter CA. The physiology and pharmacology of pain. In: de Stevens G, editor. Analgesics. Academic; New York: 1965. [Google Scholar]
- Wohr M, Dahlhoff M, Wolf E, Holsboer F, Schwarting RK, Wotjak CT. Effects of genetic background, gender, and early environmental factors on isolation-induced ultrasonic calling in mouse pups: An embryo-transfer study. Behavior Genetics. 2008;38:579–595. doi: 10.1007/s10519-008-9221-4. [DOI] [PubMed] [Google Scholar]
- Wohr M, Schwarting RK. Ultrasonic communication in rats: Effects of morphine and naloxone on vocal and behavioral responses to playback of 50-khz vocalizations. Pharmacology, Biochemistry and Behavior Sep. 2009 doi: 10.1016/j.pbb.2009.09.008. No Pagination Specified. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Tanaka JW, Klaiman C, Cockburn J, Herlihy L, Brown C, South M, McPartland J, Kaiser MD, Phillips R, Schultz RT. Specific impairment of face-processing abilities in children with autism spectrum disorder using the Let's Face It! skills battery. Autism Research. 2008;1:329–340. doi: 10.1002/aur.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans J, Forster G, Blaha C. M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sciences. 2001;68:2449–2456. doi: 10.1016/s0024-3205(01)01038-4. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Takeuchi J, Baptista M, Flynn DD, Lepik K, Nobrega J, Fulton J, Ralph MR. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. The Journal of Neuroscience. 2000;20:8861–8867. doi: 10.1523/JNEUROSCI.20-23-08861.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip JT, Lee TM. Selective impairment of sadness and disgust recognition in abstinent ecstasy users. Neuropsychologia. 2006;44:959–965. doi: 10.1016/j.neuropsychologia.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Young PT. The role of affective processes in learning and motivation. Psychol Rev. 1959;66:104–125. doi: 10.1037/h0045997. [DOI] [PubMed] [Google Scholar]


