Abstract
The goal was to develop a method to detect pesticide adducts in tryptic digests of butyrylcholinesterase in human plasma from patients poisoned by pesticides. Adducts to butyrylcholinesterase in human serum may serve as biomarkers of pesticide exposure because organophosphorus and carbamate pesticides make a covalent bond with the active site serine of butyrylcholinesterase. Serum samples from 5 attempted suicides (with dichlorvos, Aldicarb, Baygon and an unknown pesticide), and from 1 patient who accidentally inhaled dichlorvos were analyzed. Butyrylcholinesterase was purified from 2 ml serum by ion exchange chromatography at pH 4, followed by procainamide affinity chromatography at pH 7. The purified butyrylcholinesterase was denatured, digested with trypsin, and the modified peptide isolated by HPLC. The purified peptide was analyzed by multiple reaction monitoring in the QTRAP 4000 mass spectrometer. This method successfully identified the pesticide-adducted butyrylcholinesterase peptide in 4 patients whose butyrylcholinesterase was inhibited 60–84%, but not in 2 patients whose inhibition levels were 8 and 22%. It is expected that low inhibition levels will require analysis of larger serum plasma volumes. In conclusion, a mass spectrometry method for identification of exposure to live toxic pesticides has been developed, based on identification of pesticide adducts on the active site serine of human butyrylcholinesterase.
Keywords: dichlorvos, Aldicarb, chlorpyrifos oxon, mass spectrometry, butyrylcholinesterase, pesticide poisoning biomarker
Introduction
Butyrylcholinesterase is highly reactive with organophosphorus pesticides (OP) and carbamates. These poisons make a covalent bond with the active site serine, thus inhibiting the activity of butyrylcholinesterase. The inhibited butyrylcholinesterase in human plasma and serum is an indicator of exposure to cholinesterase inhibitors such as OP and carbamates. However, measurement of inhibition levels does not distinguish between exposure to pesticides and exposure to Alzheimer drugs such as tacrine and donepezil.(Darvesh and others 2003).
A sensitive method for detection of nerve agent exposure is GC-mass spectrometry of the nerve agent released by treatment of plasma with 2 M potassium fluoride (Jakubowski and others 2004; Van Der Schans and others 2004). Another method used electrospray ionization in a Q-TOF mass spectrometer to identify the sarin-adducted butyrylcholinesterase peptide isolated from victims of the Tokyo subway attack (Fidder and others 2002). The key step in the analysis by Fidder et al. is the microscale purification of BChE from 0.5 to 1 ml plasma samples. A high level of purification of BChE from plasma is required before the mass spectrometer can detect the modified peptide. It is difficult to obtain sufficient quantities of purified BChE from 1–2 ml of human serum, where the BChE concentration is 4 μg per ml, corresponding to 50 nanomolar, considering that the protein concentration in human serum is 50,000 micrograms per ml.
A method similar to that introduced by Fidder et al has been adapted in the present report to analyze blood samples from patients suspected to have been poisoned by pesticides. Our method introduces two additional purification steps, thus resulting in high quality MSMS spectra. In addition, we use multiple reaction monitoring for detection of the modified BChE peptide. Multiple reaction monitoring of carbofuran-labeled BChE purified from patients exposed to carbofuran has been previously reported by us (Li and others 2009).
Methods
Serum
Serum samples from five attempted suicides and one accidentally poisoned individual were provided by Dr. Ivan Ricordel, Paris Police. The samples were shipped on dry ice and stored at −80°C.
BChE activity assay
BChE activity was measured with 1 mM butyrylthiocholine in 0.1 M potassium phosphate pH 7.0 in the presence of 0.5 mM dithiobisnitrobenzoic acid by measuring the increase in absorbance at 412 nm at 25°C. The reaction rate in delta absorbance per min was converted to μmoles per min using the extinction coefficient E=13600 M−1cm−1 (Ellman and others 1961). One unit of activity is defined as one μmole of substrate hydrolyzed per min.
Purification of BChE from dichlorvos poisoned serum by ion exchange at pH 4 and procainamide affinity gel
The purification method that was developed for 70 –100 L of serum was scaled down to process small clinical serum samples (Lockridge and others 2005). Two ml of the strong anion exchanger Q-Sepharose fast flow (cat. no.17-0510-04 Amersham BioSciences, Piscataway, NJ) were washed with binding buffer (20 mM sodium acetate, 1 mM EDTA, pH 4.0) until the supernatant reached pH 4.0 and the conductivity was 0.3 millisiemens. Then 1.5 to 2 ml serum, diluted 20 fold with binding buffer to reduce the salt concentration, was added to the equilibrated Q-Sepharose in a 50 ml plastic tube. The tube was gently rocked at 4 C for 4–16 h. Binding to Q-Sepharose was monitored by measuring BChE activity in the supernatant. After 85–90% of the BChE had bound, the gel was packed into a 10 ml column, and washed with binding buffer until the absorbance of the eluant had dropped to 0.04 at 280 nm. About 15 ml of binding buffer was needed for the wash step. BChE was eluted with 6 ml of 0.05 M NaCl in 20 mM sodium acetate, 1 mM EDTA, pH 4.0. Fractions of 1 ml were collected. Fractions with BChE activity were pooled and further purified on a procainamide affinity column. Q-Sepharose purified the BChE about 70 fold.
Procainamide-Sepharose gel, custom made by Dr. Yacov Ashani (Grunwald and others 1997), had procainamide attached through a 6-carbon spacer to Sepharose 4B at a ratio of 34 micromoles of procainamide per ml gel. A 0.4 ml aliquot of procainamide gel was packed into a 1.5 ml microfuge spin column and equilibrated with 2 ml of 20 mM potassium phosphate pH 7.0 buffer. The partially purified BChE was loaded on the column by gravity flow. The column was washed 4 times with 1 ml of 20 mM potassium phosphate pH 7.0 buffer. Washing buffer was removed by briefly centrifuging the column. Contaminating proteins were eluted by washing 4 times with 1 ml of 0.2 M NaCl in 20 mM potassium phosphate pH 7.0 buffer. BChE was eluted twice with 1 ml of 1M sodium chloride in 20 mM potassium phosphate pH 7.0. The affinity column increased the purity of BChE about 10 fold. BChE purity was increased 700 fold after Q-Sepharose and procainamide gel purification.
Purification of BChE from carbamate (Aldicarb, Baygon), and unknown organophosphate poisoned sera by procainamide affinity gel
Purification speed was critical for carbamate poisoned samples because the carbamates, Aldicarb and Baygon, are easily released from intact BChE. A one-step purification method on procainamide affinity gel was chosen because this step took 15 min from thawing the serum to elution (Li and others 2009). In contrast, ion exchange purification took at least 6 hours. 1.5 to 2 ml of serum was allowed to flow through a 0.4 ml column of pre-equilibrated procainamide gel by gravity. Wash steps used brief centrifugation to speed the process. BChE was loaded and eluted by gravity flow.
Trypsin digestion of purified BChE
Following purification, the BChE was immediately boiled for 10 min. This denaturation step prevented decarbamylation of the active site serine and unfolded the BChE protein. The BChE (in 2 ml) was digested with 20μl of 0.4μg/ μl porcine trypsin (V5113, Promega) for 18 h at 37°C.
HPLC purification of the tryptic BChE peptides
The modified active site peptide of BChE was purified using a Phenomenex Prodigy, 5μm C18 column on a Waters HPLC system. Peptides were eluted with a 60 min gradient starting at 0.1% trifluoroacetic acid, and ending at 60% acetonitrile, 0.1% trifluoroacetic acid, at a flow rate of 1 ml/min. One ml fractions were collected. A 1μl aliquot from each 1 ml fraction was analyzed by MADLI-TOF mass spectrometry (Applied Biosystems) to identify fractions containing the unlabeled BChE active site peptide. The unlabeled active site peptide of mass 2928.5 amu was detectable by MALDI-TOF and was used as a marker to estimate the elution position of the modified peptide. The modified BChE peptide was not detectable by MALDI-TOF when the starting material was 2 ml serum. However, the elution position could be estimated from previous studies with 0.2 mg of highly purified BChE covalently modified on Serine 198 by various agents. The unlabeled active site peptide elutes between 32–36% acetonitrile, while covalently modified active site peptides elute between 32 and 41% acetonitrile (Gilley and others 2009; Li and others 2009). HPLC fractions were dried in a vacuum centrifuge and dissolved in 50μl of 5% of acetonitrile, 0.1% formic acid in preparation for analysis on the QTRAP 4000 mass spectrometer. If the offline HPLC purification step was omitted, OP-adducted BChE peptide was not detected in the mass spectrometer.
Multiple reaction monitoring (MRM) on the QTRAP 4000 mass spectrometer
5μl of HPLC-purified, tryptic BChE peptides were injected onto a Vydac C18 polymeric rev-phase nanocolumn for a second phase of HPLC separation. Peptides were separated with a 90 min linear gradient from 0 to 60% acetonitrile, 0.1% formic acid and electrosprayed through a fused silica emitter directly into the QTRAP 4000, a hybrid quadrupole linear ion trap mass spectrometer (Applied Biosystems). The mass spectrometer was calibrated on selected fragments from the MSMS spectrum of Glu-Fibrinopeptide B. The MSMS data were collected and processed using Analyst 1.4.1 software (Applied Biosystems). The QTRAP 4000 was operated in MRM mode. Details of the MRM method can be found in Results and Discussion.
Results
Pesticide structures and the adducts they form with BChE
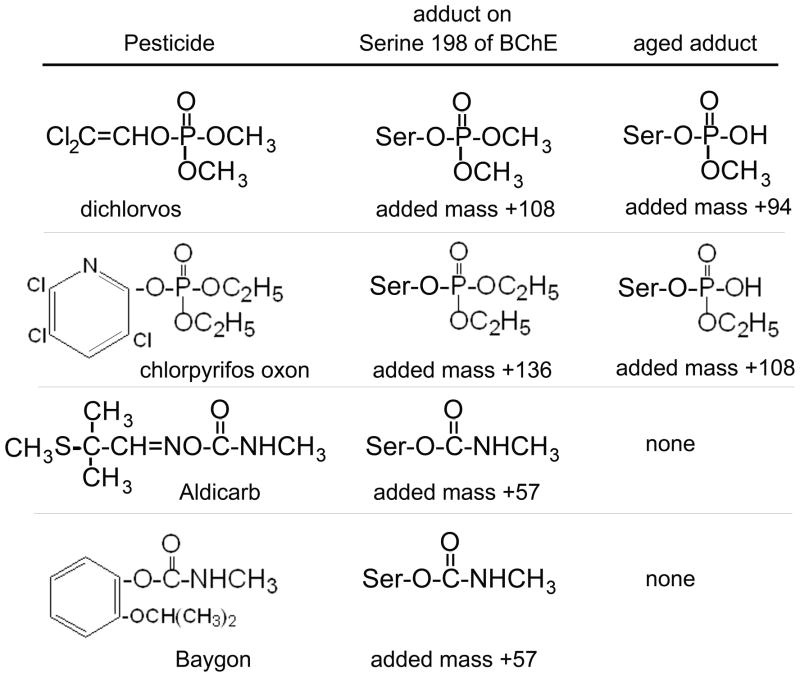
The structures of the OP and carbamate pesticides (the suspect poisons) are shown in Figure 1. The poisons make a covalent bond with the active site serine of BChE to make the adducts shown in the middle column of Figure 1. The initial OP adducts release an alkyl group in a process called “aging” to yield the structures shown in the right-hand column of Figure 1. The entries for the carbamate adducts are listed as “none” in the column labeled “aged adduct” because carbamate adducts do not age. The mass added from each pesticide is large compared to the error tolerance of 1 amu in the QTRAP mass spectrometer.
Figure 1.
Structures of pesticides and the adducts formed by covalent binding to Serine 198 of human BChE. Dichlorvos and aged chlorpyrifos oxon adducts have the same added mass of +108. Aldicarb and Baygon give the same BChE adduct with an added mass of +57. The unlabeled active site peptide of BChE produced by digestion with trypsin has a monoisotopic mass of 2928.5 amu.
The aging reaction is catalyzed by amino acid residues in the active site of BChE including His 438 and Glu 197 (Nachon and others 2005). Dimethoxyphosphate adducts age with a half-life of 3.9 h, whereas diethoxyphosphate adducts age with a half-life of 11.6 h at pH 7.0, 25°C (Masson and others 1997; Worek and others 1999). The carbamate adducts spontaneously reactivate with a half-life of about 2 h (Li and others 2009), but they do not age.
BChE inhibition levels in patient sera
Table 1 shows that three patients were poisoned with dichlorvos (2,2-dichlorovinyl dimethyl phosphate), one with Aldicarb (2-methyl-2-(methylthio)propionaldehyde O-methylcarbamoyloxime), one with Baygon (isopropoxyphenyl methyl carbamate) and one with an unknown OP. The serum samples analyzed in this work came from blood drawn 7–22 hours after the patients were poisoned. BChE activity in patient sera was inhibited 8–97%. The patient with the lowest level of inhibition (8%) was a 15 year old female who accidentally inhaled dichlorvos. The other 5 patients drank the pesticide in attempted suicide. All patients survived. Patients whose BChE was inhibited 80% or more survived with the aid of mechanical ventilation and treatment with atropine and pralidoxime. The last column in Table 1 indicates that our mass spectrometry analysis identified the adducted BChE peptide in 4 of the 6 samples. The samples with minor BChE inhibition (8–22%) did not yield enough adducted BChE peptide from 2 ml serum for detection in the mass spectrometer.
Table 1.
BChE activity in sera from poisoned individuals and from controls
| poison | Time between poisoning and blood draw | Days in hospital | BChE activity units/ml | Inhibition of BChE % | Labeled BChE peptide found |
|---|---|---|---|---|---|
| dichlorvos | 10 hours | 11 | 0.41 | 84 | yes |
| dichlorvos | 9 hours | 2 | 0.50 | 80 | yes |
| dichlorvos | 11 hours | 1 | 2.30 | 8 | no |
| Aldicarb | 7 hours | 3 | 0.07 | 97 | yes |
| Baygon | 22 hours | 3 | 1.96 | 22 | no |
| Unknown OP | Unknown | Unknown | 0.95 | 62 | yes |
| none | none | none | 2.50 | 0 | no |
The name of the poison was established from the reports of family members who found bottles of pesticide in the home. The unknown OP was identified in the present work as chlorpyrifos oxon from mass spectrometry of the BChE adduct.
Information for multiple reaction monitoring (MRM) to detect BChE adducts
The mass spectrometry method selected for detection of BChE adducts was the multiple reaction monitoring method (MRM). This method instructs the mass spectrometer to look only for the specified masses and to ignore all other ions. This increases the sensitivity of detection by deleting background signals. To use MRM one must know the possible parent ion masses. The mass of the parent ion can be calculated from the structure of the pesticide and its known reaction with BChE. Organophosphorus and carbamate pesticides make a covalent bond with Serine 198 of BChE. After digestion with trypsin, the peptide that includes Serine 198 has the sequence SVTLFGES198AGAASVSLHLLSPGSHSLFTR (Lockridge and others 1987; Lockridge and La Du 1986). A list of the 49 tryptic peptides of human BChE can be obtained from the Protein Prospector website of the University of California, San Francisco http://prospector.ucsf.edu/prospector/mshome.htm, by searching for tryptic peptides in accession number gi116353 in the NCBI nonredundant database, after deleting the 28 amino acids in the signal peptide.
In addition one must know which product ion masses (called transition ions) are consistently intense in the MSMS spectrum. From our previous work with OP-labeled and carbamate-labeled pure BChE (Gilley and others 2009; Li and others 2009) we knew that the y9 ion at 1001.5 amu and the y10 ion at 1088.5 amu are reliable indicators of both the labeled and unlabeled BChE active site peptide. Therefore, the y9 and y10 ions were selected as MRM transition ions. Table 2 lists the masses of the quadruply charged parent ions and the transition ions used for MRM.
Table 2.
Multiple Reaction Monitoring (MRM) transitions for the BChE active site peptide
| BChE Active site peptide SVTLFGESAGAASVSLHLLSPGSHSLFTR | Charge | Parent ion m/z | transition to y9 | transition to y10 |
|---|---|---|---|---|
| unlabeled BChE peptide | 4 | 733.8 | 1001.5 | 1088.5 |
| dichlorvos labeled; added mass +108 | 4 | 760.3 | 1001.5 | 1088.5 |
| aged dichlorvos labeled; added mass +94 | 4 | 756.8 | 1001.5 | 1088.5 |
| aged chlorpyrifos oxon labeled; added mass +108 | 4 | 760.3 | 1001.5 | 1088.5 |
| Aldicarb/Baygon labeled; added mass +57 | 4 | 747.6 | 1001.5 | 1088.5 |
Theoretical masses in the QTRAP mass spectrometer are listed. Masses of the quadruply charged parent ions are average masses, while those of the singly charged y9 and y10 ions are monoisotopic masses. The monoisotopic mass of the singly charged unlabeled peptide is 2928.5 amu. The accession number for human butyrylcholinesterase in the NCBI nonredundant database is gi:116353. This sequence includes 28 amino acids of the signal peptide, so that Ser 198 is numbered Ser 226.
Detection of the monomethoxyphosphate adduct of BChE produced by exposure to dichlorvos
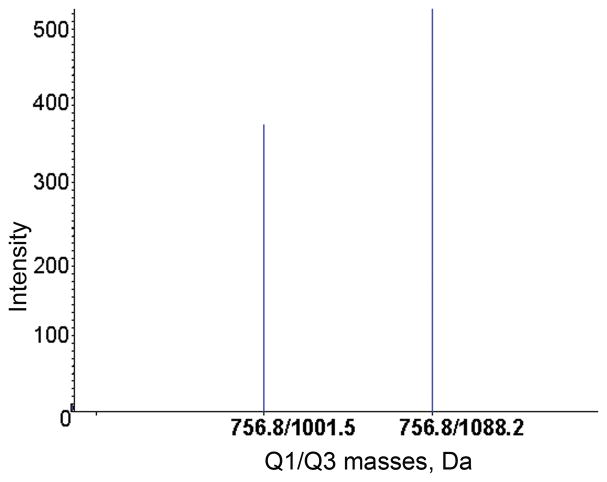
An example of the signal detected by the MRM method for one of the patient samples is given in Figure 2. The parent ion mass at 756.8 m/z and the product ion masses at 1001.5 and 1088.2 amu were found in this sample. These values correspond to the aged dichlorvos adducted BChE tryptic peptide in Table 2.
Figure 2.
MRM transitions for the aged dichlorvos labeled tryptic BChE peptide. The quadruply-charged parent ion has a mass to charge ratio of 756.8 m/z and yields singly-charged product ions at 1001.5 and 1088.2 amu. Q1 is the mass of the parent ion. Q3 is the mass of the product ion.
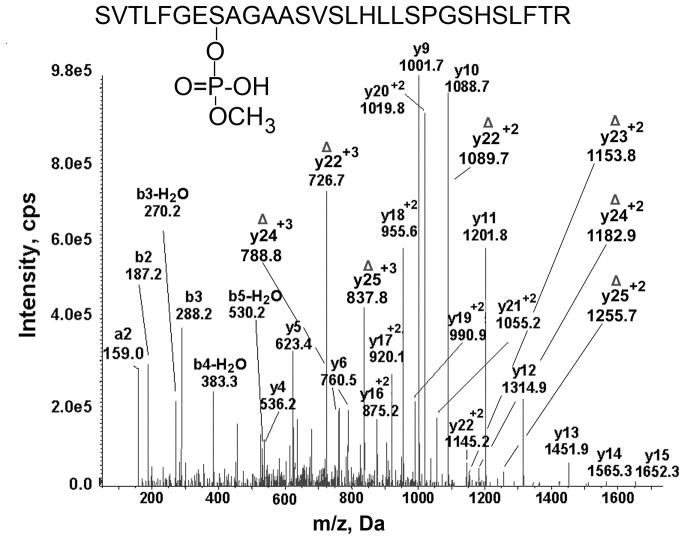
The MRM hit automatically triggered acquisition of the MSMS spectrum shown in Figure 3. The MSMS spectrum proves that the peptide has the amino acid sequence of the BChE active site tryptic peptide SVTLFGES198AGAASVSLHLLSPGSHSLFTR and that monomethoxyphosphate is covalently bound to the active site Serine 198. The singly charged y ion series from y4 to y15 and the doubly charged y ion series from y13+2 to y22+2 support the peptide sequence. The mass of the parent ion (756.6 m/z) is consistent with a monomethoxyphosphate adduct on this peptide and supports the conclusion that the patient was poisoned by dichlorvos.
Figure 3.
MSMS spectrum of the aged dichlorvos labeled tryptic peptide of BChE derived from serum of a dichlorvos poisoned patient. The quadruply charged parent ion has 756.6 m/z. The y9 and y10 transition ions are present. In addition a series of b and y ions are present that fit the masses of the active site tryptic peptide of BChE. The doubly-charged y22+2 ion at 1145.2 m/z carries the aged dichlorvos (monomethoxyphosphate) on Ser 198. The triply and doubly charged ions labeled with the symbol Δ contain dehydroalanine in place of Ser 198. They have lost the OP plus a molecule of water during collision with gas molecules in the mass spectrometer. Facile loss of the organophosphorus agent plus a molecule of water to produce dehydroalanine is a characteristic feature of modified serine. The masses of the ions labeled with the symbol Δ support the conclusion that the modified residue is Ser 198.
Support for modification of Serine 198 by monomethoxyphosphate comes from the doubly charged y22 ion at 1145.2 m/z; this mass includes the mass of aged dichlorvos. Additional support for modification of Serine 198 are the seven ions marked with the symbol Δ to indicate the presence of dehydroalanine in place of the active site serine. The collision energy in the mass spectrometer releases the organophosphorus agent plus a molecule of water from the OP-modified serine to produce dehydroalanine in place of serine (Fidder and others 2002). No serine other than Serine 198 in peptide SVTLFGES198AGAASVSLHLLSPGSHSLFTR is modified. This conclusion is supported by the masses of the b and y ions in Figure 3.
Human BChE covalently modified by dimethoxyphosphate ages with a half-life of 3.9 hours (Worek and others 1999). The blood sample was drawn 10 h after exposure, a time interval that allowed greater than 75% (>2 half-lives) of the modified BChE to undergo aging. It is not surprising therefore, that the modified BChE detected in Figure 3 is the aged dichlorvos adduct.
The second dichlorvos poisoned patient with 80% inhibition of plasma BChE, also yielded the MRM and MSMS spectra shown in Figures 2 and 3, thus providing evidence that the poison was dichlorvos. However the patient whose plasma BChE was inhibited only 8% did not yield enough dichlorvos-adducted BChE for detection in the mass spectrometer. It is expected that very low levels of exposure will require more than 2 ml of plasma to confirm the diagnosis by mass spectrometry.
MRM is a valuable component of our method because it allows analysis to be directed at specific peptides that may be present in low yield. The ability to dismiss peptides that do not meet the MRM selection criteria allows the mass spectrometer to focus on just those peptides that are of interest. However, despite the sensitivity and selectivity of the MRM method, confirmation of the peptide’s identity by analysis of its MSMS spectrum is essential to avoid false positive identifications, especially when dealing with unknown samples. Reliance on MRM identification alone, as is done in a clinical setting, requires that the target be very well characterized (including reproducible liquid chromatography elution times). The myriad of parent ion/product ion possibilities that exist when analyzing a sample from a patient who has been exposed to an unknown OP or carbamate is too complex to rely on MRM identification alone.
Identification of aged CPO adducted BChE peptide in serum from the patient poisoned with an unknown pesticide
Serum from the patient poisoned with an unknown pesticide had 40% of normal BChE activity. The fact that the pesticide inhibited the BChE activity suggested that the poison was either an OP or a carbamate. The sample was handled as if the poison were a carbamate. This meant the BChE purification was limited to use of the procainamide affinity column, followed by HPLC purification of the tryptic peptide. Omission of the pH 4 ion exchange chromatography step yields a less purified BChE. A lower fold purification is associated with interference by contaminating ions when the sample is analyzed in the mass spectrometer, and therefore a lower quality MSMS spectrum.
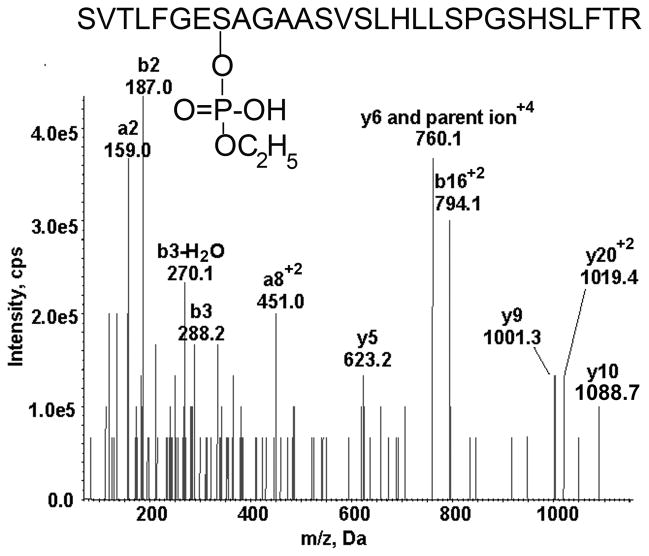
The MSMS spectrum triggered by the MRM method is shown in Figure 4. The quadruply charged parent ion at 760.1 m/z and the y9 and y10 transition ions are present. According to Table 2 these values are consistent with either unaged dichlorvos or aged chlorpyrifos oxon adducts. We interpreted the adduct in Figure 4 to be the aged chlorpyrifos oxon adduct because aging of dichlorvos is so rapid (half-life 3.9 h) that finding the unaged dichlorvos-adducted BChE seemed unlikely.
Figure 4.
MSMS spectrum of the aged chlorpyrifos oxon labeled BChE tryptic peptide from serum taken from the patient exposed to an unknown poison. The quadruply charged parent ion of 760.1 m/z is consistent with aged chlorpyrifos oxon labeled BChE peptide.
The masses of the ions in Figure 4 support the identity of the peptide as the active site peptide of BChE. As expected, the number and intensity of ions in Figure 4 are not as high as those in Figure 3 where the BChE peptide had undergone more extensive purification before the protein was denatured and digested.
Identification of carbamate adducted BChE peptide in patient poisoned by Aldicarb
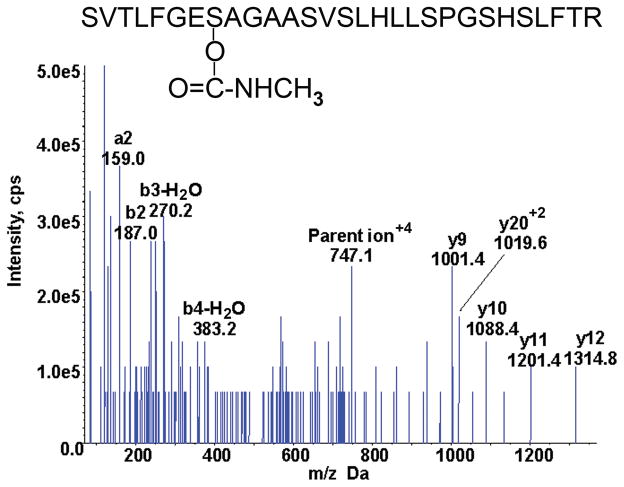
The MRM triggered MSMS spectrum of the adducted BChE peptide isolated from 2 ml serum of the patient poisoned with Aldicarb is shown in Figure 5. The mass of the parent ion and the masses of the y9 and y10 ions are consistent with the masses in Table 2 for the BChE active site peptide labeled with carbamate. The other ions whose masses are indicated in Figure 5 support the assignment of the peptide as the BChE active site peptide. The ion series is incomplete as it is missing the covalently modified serine at y22 and b8. The BChE would need to be more highly purified to generate a more complete mass spectrum. This is a difficult task because of the instability of the carbamate adduct.
Figure 5.
MSMS spectrum of the Aldicarb labeled BChE tryptic peptide from serum taken from the Aldicarb poisoned patient. The quadruply charged parent ion has a mass to charge ratio of 747.1 m/z.
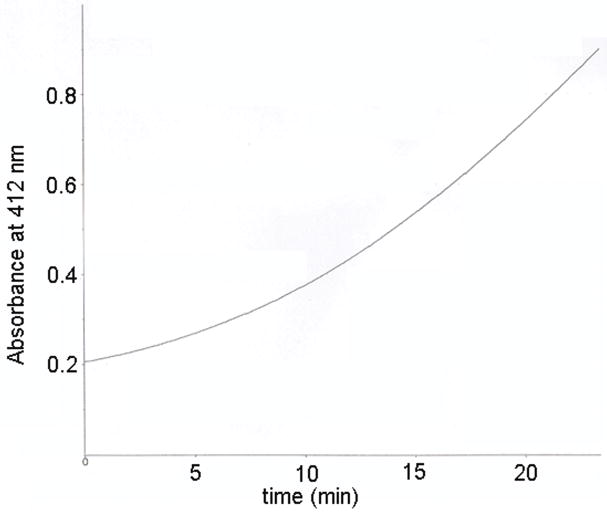
The instability of carbamate-adducted BChE is illustrated in Figure 6 where a 10 μl sample of serum from the patient poisoned with Aldicarb was assayed for BChE activity. The increase in absorbance at 412 nm was not linear with time. Rather it became faster as time elapsed, giving an upward curve. This behavior is characteristic of an increase in enzyme activity with time. After 23 min the activity had increased 4.8 fold over the activity at time zero. The increase in BChE activity can be explained by spontaneous decarbamylation of the active site serine. Spontaneous reactivation is a problem because our goal is to detect covalently modified BChE. Denaturation of the BChE protein stops spontaneous reactivation as previously demonstrated for a carbofuran poisoned plasma sample (Li and others 2009). To maximize the amount of carbamylated BChE peptide available for analysis it is important to freeze the serum or plasma sample immediately after the blood draw, to rapidly purify the BChE protein after the frozen plasma has thawed, and to denature the BChE immediately after it has been purified.
Figure 6.
Spontaneous reactivation of Aldicarb-inhibited BChE. BChE activity in Aldicarb poisoned sera was measured with 1 mM butyrylthiocholine in 1 ml of 0.1 M potassium phosphate pH 7.0 in the presence of 0.5 mM dithiobisnitrobenzoic acid by recording increase in absorbance at 412nm. BChE activity was 0.07 units/ml at the beginning of the measurement and was 0.34 units/ml at 23 min.
Discussion
Limitations of the method
The major limitation of this mass spectrometry method is the need to purify BChE from plasma. Before it becomes practical to use mass spectrometry for detection of pesticide exposure, it will be necessary to devise a simple purification method that yields pure BChE in one step. Mass spectrometers are expensive and require highly skilled personnel to operate them, but mass spectrometers are already used in American hospitals for routine clinical assays. A clinician reviewer of this manuscript pointed out that a clinician will treat a patient based on the symptoms, and will not wait for information on the identity of the poison. We agree with this assessment, but point out that understanding the progress of the illness may require knowing the identity of the poison. For example Eddleston has found that patients poisoned with fenthion have few symptoms initially, but many more die from fenthion than from chlorpyrifos poisoning (Eddleston and others 2005). Eddleston suggests that patients might benefit from management protocols developed for particular organophosphorus agents, a suggestion that requires knowing the identity of the poison.
Another limitation of the method is that it cannot distinguish between pesticide exposures that yield adducts with an identical mass. For example, paraoxon-ethyl and chorpyrifos oxon-ethyl both form diethoxyphosphate adducts with an added mass of +136. The reaction of BChE with Aldicarb, carbofuran, Baygon, carbaryl, aldoxycarb, formetanate, methiocarb, methomyl, oxamyl, propoxur, physovenine, and physostigmine results in identical adducts with an added mass of +57.
Advantages of the method
The adduct mass distinguishes classes of pesticides. OP pesticide adducts have masses that are distinct from masses of carbamate pesticide adducts and these are distinct from OP nerve agent adducts. The presence of a BChE adduct is proof that the person was exposed to live agent, as opposed to a metabolite or degradation product. Only the live agent is capable of binding covalently to BChE. The Centers for Disease Control and Prevention reported that 96% of the 1997 Americans in their study contained pesticide metabolites in their urine (Barr and others 2005). This information does not reveal whether Americans are exposed to live agent or simply to harmless pesticide degradation products.
Exposure to nerve agents, organophosphorus pesticides, and carbamate pesticides can cause similar toxic symptoms and result in similar levels of butyrylcholinesterase inhibition. Our mass spectrometry method distinguishes between these classes of poison. Knowledge of the type of cholinesterase inhibitor to which a person was exposed could be useful to forensic toxicologists. It would aid in tracing the source of the inhibitor.
Epidemiologists have linked OP exposure to risk of Parkinson and Alzheimer Disease in old age (Baldi and others 2003; Hancock and others 2008). Gulf War Illness in veterans of the 1991 Persian Gulf War has been linked to OP exposure (Toomey and others 2009). Depression and cognitive impairment have also been linked to OP exposure (Beseler and others 2008). Laboratory evidence proving exposure to live agent would strengthen these associations.
Conclusion
A mass spectrometry method to identify exposure to organophosphorus and carbamate pesticides has been developed. The method identifies pesticides covalently bound to butyrylcholinesterase in human blood. Success depends on achieving high purification of butyrylcholinesterase from serum, thus enabling detection of the modified peptide. This is the first report to identify dichlorvos, chlorpyrifos oxon, and Aldicarb adducts on butyrylcholinesterase in the blood of patients hospitalized for pesticide overdose.
Acknowledgments
Supported by NIH grant U01 NS058056, NIH Cancer Center Support grant CA036727, US Army Medical Research & Materiel Command W81XWH-07-2-0034, French Procurement Agency DGA/PEA 08co501 and Agence Nationale pour la Recherche ANR-06-BLAN-0163. Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
Abbreviations
- BChE
butyrylcholinesterase
- MSMS
tandem mass spectrometry
Contributor Information
Bin Li, Email: binli@unmc.edu.
Ivan Ricordel, Email: ivan.ricordel@wanadoo.fr.
Lawrence M. Schopfer, Email: lmschopf@unmc.edu.
Frédéric Baud, Email: baud.frederic@wanadoo.fr.
Bruno Mégarbane, Email: bruno-megarbane@wanadoo.fr.
Patrick Masson, Email: pmasson@unmc.edu.
Oksana Lockridge, Email: olockrid@unmc.edu.
References
- Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157(5):409–14. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD, et al. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res. 2005;99(3):314–26. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Beseler CL, Stallones L, Hoppin JA, Alavanja MC, Blair A, Keefe T, Kamel F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ Health Perspect. 2008;116(12):1713–9. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvesh S, Walsh R, Kumar R, Caines A, Roberts S, Magee D, Rockwood K, Martin E. Inhibition of human cholinesterases by drugs used to treat Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17(2):117–26. doi: 10.1097/00002093-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366(9495):1452–9. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: mass spectrometric analysis of phosphylated human butyrylcholinesterase. Chem Res Toxicol. 2002;15(4):582–90. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Gilley C, Macdonald M, Nachon F, Schopfer LM, Zhang J, Cashman JR, Lockridge O. Nerve Agent Analogues That Produce Authentic Soman, Sarin, Tabun, and Cyclohexyl Methylphosphonate-Modified Human Butyrylcholinesterase. Chem Res Toxicol. 2009 doi: 10.1021/tx900090m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald J, Marcus D, Papier Y, Raveh L, Pittel Z, Ashani Y. Large-scale purification and long-term stability of human butyrylcholinesterase: a potential bioscavenger drug. J Biochem Biophys Methods. 1997;34(2):123–35. doi: 10.1016/s0165-022x(97)01208-6. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, Scott BL, Vance JM, Scott WK. Pesticide exposure and risk of Parkinson's disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski EM, McGuire JM, Evans RA, Edwards JL, Hulet SW, Benton BJ, Forster JS, Burnett DC, Muse WT, Matson K, et al. Quantitation of fluoride ion released sarin in red blood cell samples by gas chromatography-chemical ionization mass spectrometry using isotope dilution and large-volume injection. J Anal Toxicol. 2004;28(5):357–63. doi: 10.1093/jat/28.5.357. [DOI] [PubMed] [Google Scholar]
- Li H, Ricordel I, Tong L, Schopfer LM, Baud F, Megarbane B, Maury E, Masson P, Lockridge O. Carbofuran poisoning detected by mass spectrometry of butyrylcholinesterase adduct in human serum. J Appl Toxicol. 2009;29(2):149–55. doi: 10.1002/jat.1392. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM, Winger G, Woods JH. Large scale purification of butyrylcholinesterase from human plasma suitable for injection into monkeys; a potential new therapeutic for protection against cocaine and nerve agent toxicity. J Med CBR Def. 2005;3 doi: 10.1901/jaba.2005.3-nihms5095. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge O, Bartels CF, Vaughan TA, Wong CK, Norton SE, Johnson LL. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987;262(2):549–57. [PubMed] [Google Scholar]
- Lockridge O, La Du BN. Amino acid sequence of the active site of human serum cholinesterase from usual, atypical, and atypical-silent genotypes. Biochem Genet. 1986;24(5–6):485–98. doi: 10.1007/BF00499101. [DOI] [PubMed] [Google Scholar]
- Masson P, Froment MT, Bartels CF, Lockridge O. Importance of aspartate-70 in organophosphate inhibition, oxime re-activation and aging of human butyrylcholinesterase. Biochem J. 1997;325 ( Pt 1):53–61. doi: 10.1042/bj3250053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachon F, Asojo OA, Borgstahl GE, Masson P, Lockridge O. Role of water in aging of human butyrylcholinesterase inhibited by echothiophate: the crystal structure suggests two alternative mechanisms of aging. Biochemistry. 2005;44(4):1154–62. doi: 10.1021/bi048238d. [DOI] [PubMed] [Google Scholar]
- Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, Lyons MJ, Henderson WG, Kang HK, Eisen SA, Murphy FM. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J Int Neuropsychol Soc. 2009;15(5):717–29. doi: 10.1017/S1355617709990294. [DOI] [PubMed] [Google Scholar]
- Van Der Schans MJ, Polhuijs M, Van Dijk C, Degenhardt CE, Pleijsier K, Langenberg JP, Benschop HP. Retrospective detection of exposure to nerve agents: analysis of phosphofluoridates originating from fluoride-induced reactivation of phosphylated BuChE. Arch Toxicol. 2004;78(9):508–524. doi: 10.1007/s00204-004-0568-x. [DOI] [PubMed] [Google Scholar]
- Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch Toxicol. 1999;73(1):7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]