Abstract
The cholinergic input from the lateral dorsal tegmental area (LDTg) modulates the dopamine cells of the ventral tegmental area (VTA) and plays an important role in cocaine taking. Specific pharmacological agents that block or stimulate muscarinic receptors in the LDTg change acetylcholine (ACh) levels in the VTA. Furthermore, manipulations of cholinergic input in the VTA can change cocaine taking. In the current study, the ACh output from the LDTg was attenuated by treatment with the selective muscarinic type 2 (M2) autoreceptor agonist oxotremorine sesquifumarate (OxoSQ). We hypothesized that OxoSQ would reduce the motivation of rats to self-administer both natural and drug rewards. Animals were tested on progressive ratio (PR) schedules of reinforcement for food pellets and cocaine. On test days, animals on food and on cocaine schedules were bilaterally microinjected prior to the test. Rats received either LDTg OxoSQ infusions or LDTg artificial cerebrospinal fluid (aCSF) infusions in a within-subjects design. In addition, infusions were delivered into a dorsal brain area above LDTg as an anatomical control region. OxoSQ microinjection in the LDTg, compared to aCSF, significantly reduced both the number of self-administered pellets and cocaine infusions during the initial half of the session; this reduction was dose-dependent. OxoSQ microinjections into the area just dorsal of the LDTg had no significant effect on self-administration of food pellets or cocaine. Animals were also tested in locomotor activity chambers for motor effects following the above microinjections. Locomotor activity was mildly increased by OxoSQ microinjection into the LDTg during the initial half of the session. Overall, these data suggest that LDTg cholinergic neurons play an important role in modifying the reinforcing value of natural and drug rewards. These effects cannot be attributed to significant alterations of locomotor behavior and are likely accomplished through LDTg muscarinic autoreceptors.
Keywords: cocaine, addiction, acetylcholine, muscarinic, self-administration, motivation
Introduction
Cocaine is a highly addictive drug with severe detrimental physical and mental health effects. Important features of addiction are loss of control over drug use and increased expenditure of time and energy directed toward attaining and using the drug of abuse (Roberts et al., 2007; for review see Koob, 2009). Cocaine addicts lose their ability to control their cocaine intake, and in the process of gaining access to cocaine they will sacrifice their health and social lives (Dackis and O'Brien, 2001). Recent research has significantly improved our understanding of cocaine's mechanisms of action. Cocaine is known to increase dopamine (DA) levels in the shell of the nucleus accumbens (NAc) to levels several fold higher than the increase in dopamine seen in response to natural rewards. Cocaine does this by blocking DA transporter (DAT)-mediated reuptake of DA (Huang et al, 2009, Carboni et al, 2001, Pettit and Justice, 1989). This increase in dopamine, which lingers in the synaptic cleft making it more accessible to both pre- and post-synaptic receptors, partly accounts for the euphoric and addictive properties of cocaine (for review see Nestler, 2005; Kelley and Berridge, 2002). A role for reduced uptake of DA in cocaine reward has been demonstrated in single gene mutant mice (Thomsen et al. 2009a,b). DAT knockout mice have compromised acquisition and maintenance of cocaine self-administration, but not food self-administration (Thomsen et al, 2009a).
DA released in the NAc from the ventral tegmental area (VTA), is critical for the rewarding effects of cocaine (for review see Spanagel and Weiss, 1999). Cocaine, similar to intracranial self-stimulation (ICSS) of the VTA, induces behavioral seeking responses in rats (Wise, 1996). Lesions of the DA terminals in the NAc compromise the self-administration of cocaine (Roberts et al, 1977). Manipulations of VTA DA neurons with pharmacological agents that interact with multiple neurotransmitter systems significantly affect cocaine-induced DA levels in the NAc and cocaine seeking behaviors (for review see Bardo, 1998). Increased acetylcholine (ACh) levels in the VTA in response to cocaine (You et al., 2008) affect both NAc DA levels and reward seeking behaviors, and these effects appear to involve both muscarinic (Forster et al., 2002; Lester et al., 2010) and nicotinic receptors (Clarke and Pert, 1985).
One important source of cholinergic modulation of VTA DAergic neurons arises from the lateral dorsal tegmental nucleus (LDTg). DA cells that are modulated by the LDTg cholinergic input project to the NAc (Omelchenko and Sesack, 2005; 2006). Another much smaller cholinergic modulation of the VTA comes from the pedunculopontine nucleus (PPTg) (Oakman et al., 1995; Yeomans et al., 1993). Lesions of the LDTg, but not PPTg, attenuate the DA elevation in NAc induced by intra-VTA microinjection of neostigmine (Blaha et al, 1996). Agonists of ACh and glutamate receptors are known to activate VTA DA cells and to elevate DA levels in the NAc (Grenhoff et al, 1986; Blaha et al, 1996; Lester et al, 2008). Self-administration experiments, combined with microdialysis methods, have shown that both active (self-administered) and passive cocaine increase ACh levels in the VTA (You et al, 2008); however, the initial peak of ACh in the VTA (which was short in duration) depended upon the history of cocaine self-administration. Passive infusion of cocaine to cocaine-experienced animals produced an initial peak increase in ACh levels in the VTA that was not seen in cocaine naïve animals infused with cocaine for the first time (You et al, 2008). Blocking of muscarinic receptors in the VTA decreased DA levels in the VTA and increased lever pressing for cocaine (You et al, 2008). Thus, muscarinic receptors in the VTA seem to be especially important in cocaine taking, and ACh appears to contribute to the rewarding effects of cocaine. In fact, although the initial peak increase in ACh level was affected by cocaine experience, the more prolonged increase in ACh level was not dependent upon cocaine experience (You et al, 2008). Other studies on nicotinic receptors in the VTA also support the importance of ACh in the escalation of cocaine self-administration. For example, rats trained to self-administer cocaine in short access sessions do not show escalation of cocaine self-administration. However, those trained to self-administer cocaine in extended access sessions do show escalation, and when the nicotinic receptor antagonist mecamylamine was included with cocaine in the intravenous infusion solution during the extended access sessions, the escalation of cocaine self-administration was suppressed (Hansen and Mark, 2007).
Pharmacological manipulation of the LDTg may provide new insights about the role of ACh in cocaine seeking and self-administration. It is known that electrical stimulation of the LDTg leads to a three-component phasic pattern of changes in DA efflux in the NAc that reflects activation of glutamate, nicotinic and muscarinic receptors in the VTA (Forster and Blaha, 2000). The first and third components are likely associated with increased levels of ACh in the VTA, although this has not been directly shown. The second component is associated with hyperpolarization of cholinergic neurons in the LDTg that is mediated by M2 autoreceptors (Forster and Blaha, 2000; Buckley et al, 1988, Vilaro et al., 1992, 1994). In fact patch-clamp studies have shown that ACh hyperpolarizes cholinergic neurons in the LDTg via inwardly rectifying potassium channels (Leonard and Llinas, 1994). Inactivation of M2 autoreceptors in the LDTg with an M2 antagonist can block the hyperpolarizing effects of ACh (Leonard and Llinas, 1994), and increases the release of endogenous ACh (Baghdoyan et al, 1998). Thus, manipulation of M2 autoregulation of cholinergic activity provides a way to further elucidate the functional role of the LDTg in modulating reward pathways, such as the mesocorticolimbic pathway, and particularly the VTA. Oxotremorine sesquifumarate (OxoSQ), a muscarinic agonist, acts preferentially on M2/4 type muscarinic autoreceptors (Fisher et al., 1983; Sanz et al., 1997), which are known to mediate an inhibitory effect on cholinergic neurons (for review see, Calabresi et al., 2000). However, Brauner-Osborne and Brann (1996) showed that, although OxoSQ has the highest affinity for M2 autoreceptors of all available drugs, it is not necessarily exclusively selective to this autoreceptor, and had low EC50 values for M4 and M5 receptors as well in a cell culture assay. It is not known however, whether these other muscarinic receptor subtypes are present in the neuronal populations of LDTg. Regardless, it is possible that OxoSQ could be used to alter cholinergic modulation of the VTA which, should be reflected in altered cocaine seeking behaviors in animals.
Self-administration studies in rats have been performed frequently when addressing mechanisms associated with human drug-seeking behavior. In particular, self-administration on a progressive ratio (PR) schedule is an effective method for measuring the strength of reinforcement of psychostimulant drugs, including cocaine (Richardson and Roberts, 1996; Roberts 1989). In the current study, rats working for food or cocaine self-administration on a PR reinforcement schedule were microinjected with an M2 autoreceptor agonist oxotremorine sesquifumarate (OxoSQ) directly in the LDTg. We hypothesized that suppression of the cholinergic pathway would reduce the reinforcing properties of both natural rewards and cocaine, and would therefore reduce the PR break point (BP) for responding.
Materials and Methods
Animals
Twenty five male Sprague-Dawley rats (Charles River Laboratories), weighing approximately 375 grams were used in these studies. Animals were maintained in hanging Plexiglas® cages (28 × 28 × 18 cm) with ad libitum access to food and water, and in environments with controlled temperature (22°C), and a 12h : 12h light:dark cycle (lights on at 8 am). In the first 10 days, animals were housed in pairs and trained in the operant chambers. After surgeries, they were housed in individual cages. Throughout the experiment rats were maintained according to the “Principles of Laboratory Animal Care”. Experimental procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health & Science University. Experiments were performed during the dark phase for most animals (N = 17), with the exception of 8 animals (as described below).
Drug preparation
Cocaine hydrochloride was obtained from The Research Triangle Institute (Research Triangle Park, NC) under the National Institute on Drug Abuse Drug Supply Program. Cocaine was dissolved in physiological saline and adjusted for pH with NaOH solution to pH 7.0. Oxotremorine sesquifumarate (OxoSQ) was purchased from Sigma (St. Louis, MO) and dissolved in an artificial cerebrospinal fluid (aCSF) consisting of the following (in mM): 120 NaCl, 3.3 KCl, 1.2 CaCl2, 1.0 MgCl2, 25 NaHCO3, 1.2 KH2 PO4, 10 glucose; pH 7.3. OxoSQ solutions were aliquoted in 0.2 ml and stored at -20°C until used.
Operant Training
Operant conditioning chambers (Med Associates Inc.; St. Albans, VT) were housed inside sound attenuating boxes and were equipped with a retractable active lever, an inactive lever, two 4W stimulus lights positioned 2cm above each lever, a 10W house light, a pellet dispenser and a pellet receptacle tray located between the two levers (Hansen and Mark, 2007). A syringe pump for cocaine delivery was located outside of the sound attenuating box. Presses of the active lever resulted in delivery of a reward (either a food pellet or cocaine infusion) and retraction of the lever. Pressing the inactive lever had no scheduled consequence.
Prior to surgery, all animals were trained to lever press for 45mg Noyes food pellets (BioServe; Port Washington, NY), initially on a fixed ratio (FR-1) reinforcement schedule in 3-hr daily sessions. Illumination of the house light and the extension of the active lever into the chamber indicated the start of the session. Active lever presses resulted in the following events: a food pellet was dispensed onto the receptacle tray, and retraction of the active lever, and illumination of the stimulus light above the active lever for five seconds, signaled a time out period (TO), during which reward was unavailable. When an animal earned more than 10 pellets on the FR-1 reinforcement schedule (usually in 1-3 days), the schedule was increased to an FR-3 in the next session, followed by a PR reinforcement schedule in the following session. Maximum session lengths were four hours. Food training for the PR schedule was essential for increasing the number of animals that learned to lever press for cocaine and to decrease the time needed to train the animals after surgery. Complications associated with intravenous (IV) catheter patency and maintenance of head mounts, make it important to minimize the training period. The PR reinforcement schedule required animals to increase lever presses progressively for each successive reward in the following series, within a self-administration session: FR1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901. Sessions on PR schedules were terminated if the animal stopped pressing the active lever for longer than 60 minutes. The last ratio successfully completed was registered as the BP for that session.
When animals achieved a BP of FR25 or higher for food reward, they underwent the following surgeries: intracranial implantation of bilateral microinjection guide cannulae (described below) for the food pellet group, and both the intracranial implants and an IV jugular catheter for the cocaine group. Animals that attained higher than FR-25 were faster to acquire PR behavior after surgery. Food pellet group rats (N=12) were designated to return to food pellet reinforcer sessions, whereas cocaine group animals were (N=13) designated to receive IV cocaine self-administration training. An animal from each group was excluded from the experiment because in each case, their lever presses dropped to zero during the post-surgery sessions. Approximately five days after surgery, both food pellet and cocaine group animals were challenged first with FR-1 schedules on food pellets. The food pellet group was then challenged with PR schedules for food pellet rewards in sessions that were a maximum of 3 hr in duration; these animals tended to receive most of their rewards during the first hour of the session. The cocaine group was challenged with the PR schedule for cocaine infusions. Their IV catheters were connected to an infusion line that administered 0.75 mg/kg/infusion of cocaine over a 4 sec period. Infusions were accompanied by a 20 sec time-out (TO) period to avoid the potential for cocaine overdosing. Catheters were flushed with 0.2 cc saline, 0.1 cc Timentin, and 0.1 cc saline containing heparin immediately before animals were put into the operant chambers, and 0.1 cc saline containing heparin immediately after animals were taken out of the operant chambers. Sessions in the cocaine group lasted for a maximum of 4 hours since animals lever pressed for cocaine gradually and got most of their rewards by the third hour. Sessions for most of the cocaine group animals (N = 8) were conducted beginning 2 hours into the dark phase; however, for technical reasons, a smaller group (N = 5) was run beginning 2 hours into the light phase. Data were combined from the light and dark phase animals since their lever pressing for cocaine did not differ appreciably. BPs in most of these animals were around FR-50 and stabilized within approximately 7 sessions.
Animals that had stabilized PR schedules within ± 3 BP and a minimum of FR-32 were then habituated to the microinjection process on two consecutive days. To lower the microinjectors into the bilateral intracerebral guide cannulae, animals were immobilized by gently wrapping them in a towel. Microinjectors were lowered into the guide cannulae over a 1 min period, approximately 1 min prior to putting the animal into the operant conditioning chamber. Microinjectors penetrated 3.5 mm beyond the guide cannulae into the lateral dorsal tegmental area (LDTg). Nothing was infused on these habituation days, and BP data were collected. The microinfusion experiment was then conducted starting at least two days after the microinjection habituation sessions. The experiment involved the delivery of aCSF or OxoSQ at two doses, 1 nmol and 10 nmol per side, into the LDTg in a counterbalanced order with baseline (i.e. no microinjection) days between infusions. In all cases where microinjections were delivered, injection volume was 0.1 μl per side delivered over 30 sec. In some animals, OxoSQ had sufficient suppressive effects on lever pressing to result in BPs being reached within 70 min of the start of the session; in some animals no lever pressing occurred. This was the case for 4 of the 12 animals after infusion with the 1nmol dose and for 6 of the 12 animals after infusion with the 10nmol dose. For animals that failed to press the active lever, or reached a BP of only FR-1 or FR-2, sessions were immediately restarted on the PR schedule for another 180 min. This practice was followed to show that these animals would lever press once the drug effects from the microinjection had dissipated. The infusions for restarted sessions were accumulated in time bins 90 min and beyond. For most treatments, animals had at least two baseline sessions before receiving the next treatment. Some animals, however, had only one baseline session before treatment if the baseline session did not change in BP from the previous baseline session. To determine whether the effect of OxoSQ was specific to the LDTg, the same animals were also injected with OxoSQ (10 nmol per side) 2.0 mm above the LDTg by lowering the microinjectors 1.5 mm, rather than 3.5 mm, beyond the tip of the guide cannulae. This placed the infusions in the area of the central nucleus of the inferior colliculus and in some, partly in the lateral periaqueductal gray (CIC; Fig 1), a site where OxoSQ was not expected to have an effect on food or cocaine self-administration.
Figure 1.
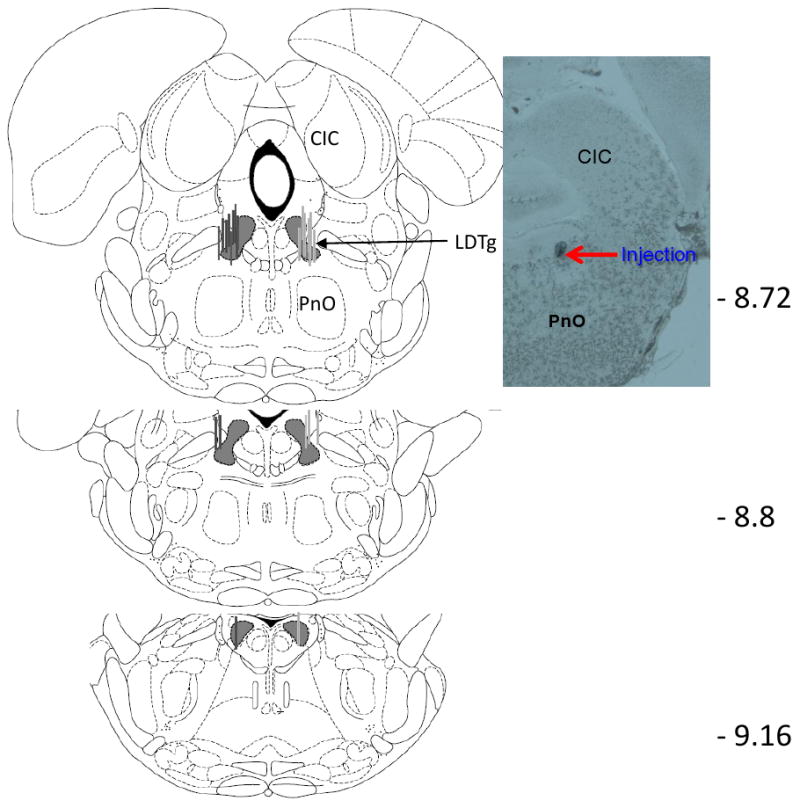
Microinjection sites. Gray lines (food pellet group) and dark lines (cocaine group) around the lateral dorsal tegmental area (LDTg) on the Paxinos and Watson (1998) diagram indicate the areas where microinjectors were placed in all animals used in this study. The numbers on the right show the distance in mm posterior to Bregma. The picture on the right shows a section at -8.72 mm posterior to bregma, and indicates where the tip of the microinjector was positioned in one of the animals, which corresponds with the LDTg. CIC: central nucleus of inferior colliculus; PnO: pontine reticular nucleus, oral part.
Surgery
Cannulae implantation and IV catheterization were performed according to Hansen et al., (2007) and Mark et al., (2006). Briefly, animals were anesthetized with an intraperitoneal injection (0.1 cc/kg) of an anesthetic mixture containing the following: 55.5 mg/cc ketamine, 5.5 mg/cc xylazine, and 1.1 mg/cc acepromazine. The anesthetized animal was then secured in a Cartesian stereotaxic apparatus (Kopf Instrument, Tujunga, CA) using ear bars. Skulls of animals were exposed and bilateral burr holes (5 holes) were drilled to the dura mater in the following skull coordinates of Paxinos and Watson (1998): A- 8.5, L± 1.2, V- 4.0. These skull coordinates allowed microinjectors to reach the LDTg 3.5 mm below the end of the implanted guide cannulae. These coordinates targeted a large portion of the LDTg and especially the medial subdivision of the LDTg, which is thought to contain a large portion of the cholinergic neurons (Wang and Morales, 2009). To ensure penetration of the guide cannulae, the dura mater was punctured with a needle bilaterally through the two burr holes. To anchor the guide cannulae and a U-shaped aluminum shield to the skull, three 5-mm stainless steel screws (size 0-80) were screwed into the remaining burr holes prior to applying dental acrylic. Head shields were used as an attachment point for a tether, which prevented animals from disturbing the infusion line that connected to the IV catheter. The bilateral guide cannulae were 10-mm, 21-gauge stainless steel guide shafts that had a small stainless steel fiber soldered approximately 2 mm below one end.
Intravenous catheterization
Animals were anesthetized prior to catheter implantation, using the same anesthetic cocktail used for cannulation surgery. Catheters were hand-made according to Hansen et al. (2007) and to the method described in detail by Caine et al. (1993), using the specifications for the catheter tubes as described below. CBAS ® heparin-coated PU catheters 3.5FR (Instech laboratories INC, Plymouth Meeting, PA) were used to prolong the life of catheters. Briefly, these tubes were fitted on an L-shaped external guide cannula (Plastics One Inc.; Roanoke, VA), that was then anchored with dental acrylic to a circular piece of propylene mesh (diameter ∼1cm) to form a pedestal. To implant the catheter, the pedestal end of the catheter was threaded subcutaneously between the scapulae, while the open end of the tube was threaded 27 mm into either the left or right jugular vein. Post surgery, the catheters were flushed daily with saline 0.4 cc, 0.2 cc saline containing heparin (70 u/cm3), and 0.1 cc of antibiotic ticarcillin (Timentin®, 100 mg/ cm3; GlaxoSmithKline, Research Triangle Park, NC). The implanted catheters were monitored daily for patency and those suspected of failure (self-administration BP decreased to <50%) were tested according to Mark et al. (2006), with a fast-acting barbiturate Brevital® (methohexital sodium; 2.7 mg/kg in 0.1 ml; Lilly, Indianapolis, IN). Catheters were considered to have failed if animals showed no substantial loss of muscle tone within 5 sec of Brevital® administration.
Microinjectors
Microinjectors were made according to Mark et al. (2006). A silica glass tube (0.15 mm o.d. × 0.037 mm i.d.; Polymicro Tech Inc.) was fitted into a 26-gauge stainless steel shaft and extended 1.5 mm beyond the shaft. Thus, the silica tip reached the ventral coordinate of -7.5 mm into the LDTg. Silica minimized tissue damage and served to preload the drug prior to the injection. The other end of the silica and the 26-ga shaft were press-fit into PE20 tubing (6 cm long) which was loaded with drug. This PE 20 tubing was connected to a longer piece of PE 20 tubing that was filled with water and connected to a 10 ul gas-tight glass syringe (Hamilton; Fisher, St. Louis, MO). The gas-tight syringe was loaded onto a syringe pump (Razel; Braintree Inc., Braintree, MA) that delivered fluid into the brain at a constant rate. 100 nl of the fluid was infused over a 30-sec period. The microinjector was left in the brain for another 30-sec period after delivery of the fluid to allow for fluid diffusion. Microinjectors were then removed, and the syringe pump was turned on for approximately 30 sec to ensure that fluid still flowed through the microinjector. The guide cannulae were plugged with small handmade stainless steel stylets. Animals were put inside operant chambers immediately after this procedure.
Locomotor activity experiment
Eight animals from the food group were used to perform the locomotor activity tests. Microinjections were performed the same way as in food and cocaine groups before the animals were put in the locomotor chambers. The same treatments were delivered before the locomotor activity sessions. Locomotor activity sessions were conducted 2 hours into the dark phase and lasted for 120 min. Five Accuscan automated activity monitors (Accuscan Instruments Inc., Columbus, OH, USA) were used for measuring locomotor activity. The activity monitoring apparatus consisted of three major units: (1) a clear acrylic plastic test cage 40×40×30 cm set inside (2) a monitoring unit equipped with photocell beams and detectors that allowed measurement of total distance in centimeters (cm) via beam breaks; and (3) a black acrylic plastic chamber lined with foam to attenuate external environmental sounds that housed units (1) and (2). Total distance was measured in 5 min time periods.
The experimental procedure involved one, 120-min session of habituation to the locomotor chambers, followed by alternate baseline sessions and test sessions. Animals received microinjections 1 min before every session. Habituation and baseline sessions involved microinjections of aCSF whereas test sessions involved one of the three treatments: aCSF, OxoSQ 1 nmol and OxoSQ 10 nmol per side. Thus a total of 7 microinjections (1 per day) were performed in these animals. The order of the three treatments given to each individual animal was randomized. Two additional sessions involved microinjections in the CIC with aCSF and OxoSQ 10 nmol per side.
Statistical analyses
Repeated measures (GLM 3 SPSS 16.0) analysis of variance (ANOVA) was used to analyze data from all experiments, since each animal received all treatments. OxoSQ effects were tested for interaction with time. Time was binned into 30 min during the first 2 hours for the food and locomotor groups and 3 hours for the cocaine group. Since not all animals continued to press during the entire session, data were too sparse during the last 2-4 time bins to run treatment by time interaction ANOVA. To deal with this issue, separate ANOVA's were performed for each time bin to look for main effects of treatments followed by mean pairwise comparisons using Bonferroni correction. Significance for main effects was adjusted with Huynh and Feldt correction when the sphericity assumption was violated, according to Mauchly's test of sphericity (p < 0.05).
Histology
The experiment was concluded by euthanizing the animals, microinjection of Thionin dye, and rapid freezing of the brains. Animals were euthanized with inhaled isofluorane in enclosed Plexiglas® boxes. Microinjections with 0.25% thionin were performed the same way as microinjections during the experiment, and served the purpose of providing a marker for microinjection location. After microinjection, brains were extracted and rapidly frozen in chilled isopentane solution. The frozen brains were then stored at -80°C. Brains were sliced in 40 micron slices on a cryostat which had an internal temperature of -20°C. Placement of the microinjections was confirmed through the atlas of Paxinos and Watson (1998) using conventional light microscopy at 4-20× (Fig 1). An example photomicrograph showing the LDTg infusion site after multiple infusions at the end of the experiment is shown in Fig 1.
Results
OxoSQ effects on cocaine self-administration
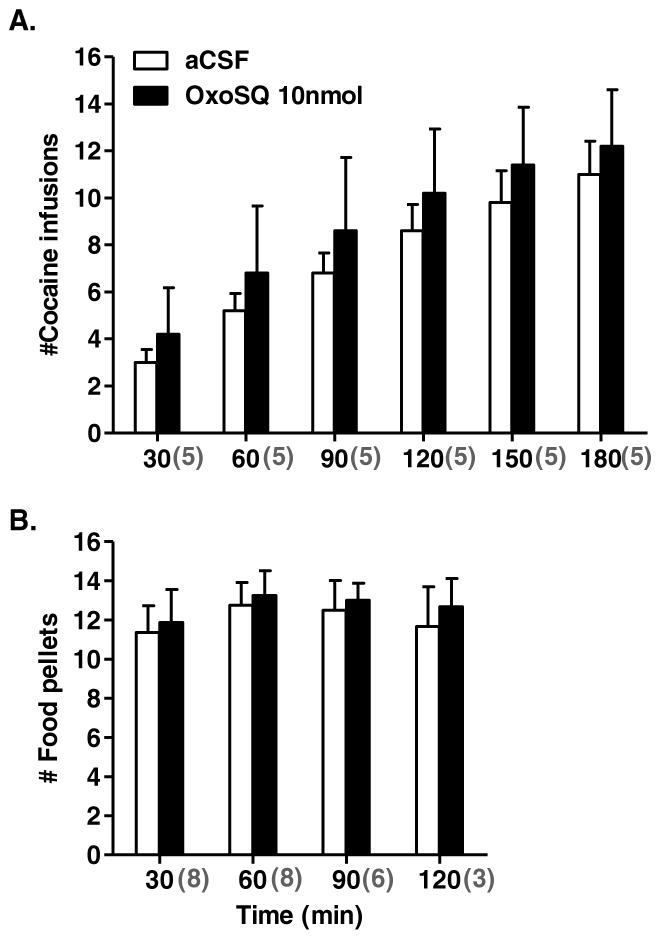
OxoSQ infused into the LDTg suppressed lever pressing for cocaine dose-dependently, mainly during the first 2 hours of the sessions. In some sessions after OxoSQ 10nmol (N = 6 rats) and OxoSQ 1nmol (N = 4 rats) treatment, rats reached BPs of 0 (N= 1 after 1nmol and 4 after 10nmol), 1 (N=2 after 1nmol and 1 after 10nmol) and 2 (N=1 after 1nmol and 1 after 10nmol) within 70 min. These sessions were restarted for an additional 180 min to determine whether OxoSQ had effects early after infusion that abated over time, resulting in increased lever pressing later in the session. The number of cocaine infusions, starting at the 90 min time bin on, cumulatively included infusions from the initial sessions. Repeated measures ANOVAs performed for each time bin, with dose as the repeated measure, showed significant main effects of experimental treatment (Fig 2A) in time bins 60 to 120 min [F60min (2, 22) = 4.8, p < 0.02; F90min (2, 22) = 4.7, p < 0.02; F120min (2, 22) = 5.7, p < 0.01]. Though not statistically significant, a statistical trend [F (2,18) = 3.5, p = 0.061] was observed for the first 30-min bin as well. Pairwise mean comparisons for time bins 60 to 120 min with Bonferroni correction showed that only OxoSQ 10 nmol caused a significant reduction in mean cocaine infusions compared to aCSF (p60min = 0.022, p90min = 0.042, p120min = 0.027) (Fig 2A). When all six time bins were included in the analysis, the time by treatment interaction was not significant [F (5, 41) = 0.9, p = 0.49]. However, the number of animals remaining in the final hour was only nine, resulting in markedly reduced power for the analysis across time. In addition, a majority of animals (9 out of 12; Fig 2A) reached a similar number of infusions by the third hour, regardless of treatment. Repeated measures ANOVAs on BPs, which included only the initial BPs for all animals (including those for which sessions were restarted) showed a significant main effect of treatment [F (2, 22) = 4.13, p = 0.030] on the test day, but not on baseline days (Fig 2B). Bonferroni corrected pairwise mean comparisons detected no significant difference among treatments on the test day, however a strong statistical trend was observed between aCSF and OxoSQ 10 nmol (p = 0.061). The number of cocaine infusions during any of the time bins between baseline sessions and aCSF sessions did not differ appreciably (Fig 2A inset). Similarly, the number of cocaine infusions during any of the time bins between aCSF and CIC microinjection with OxoSQ 10 nmol did not differ significantly (Fig 4A). The ratio of the inactive/active lever presses on average was small at 0.012 ± 0.0016 (Mean ± SE), indicating low levels of inactive lever pressing, and there were no appreciable differences between any of the treatments or baseline sessions with regard to this ratio. This indicates that animals primarily associated the active lever with cocaine infusion rewards.
Figure 2.
Shown in A. is the mean cumulative number of cocaine infusions in 30-min bins, during sessions in which animals (N = 12) received microinjections of aCSF, OxoSQ 1nmol and OxoSQ 10nmol into the lateral dorsal tegmental nucleus (LDTg). Error bars are ± SEM and lines with stars indicate significant differences between means (aCSF, OxoSQ 1nmol and OxoSQ 10nmol) after Bonferroni corrections (p < 0.05). The grey numbers enclosed in parentheses under the x-axis indicate the number of animals in each of the time bins. Graph inset shows the same information as the main graph, during sessions before the three microinjection treatments and after aCSF. Shown in B. is the mean number of infusions (± SEM) corresponding to the break point values in the PR-schedule during the same sessions in A. on test days and baseline sessions before (BL) and after (Post BL) test day session.
Figure 4.
Shown in A. is the mean cumulative number of cocaine infusions in 30-min bins, during session in which animals (N=5) received microinjections of aCSF or OxoSQ 10nmol into the central nucleus of the inferior colliculus (CIC). Error bars are ± SEM. Shown in B. is the mean cumulative number of food pellets in 30-min bins, during session in which animals (N=8) received microinjections of aCSF or OxoSQ 10nmol into the CIC. Sample size is indicated by the grey numbers in parentheses below the x-axis. Error bars are ± SEM.
OxoSQ effects on food self-administration
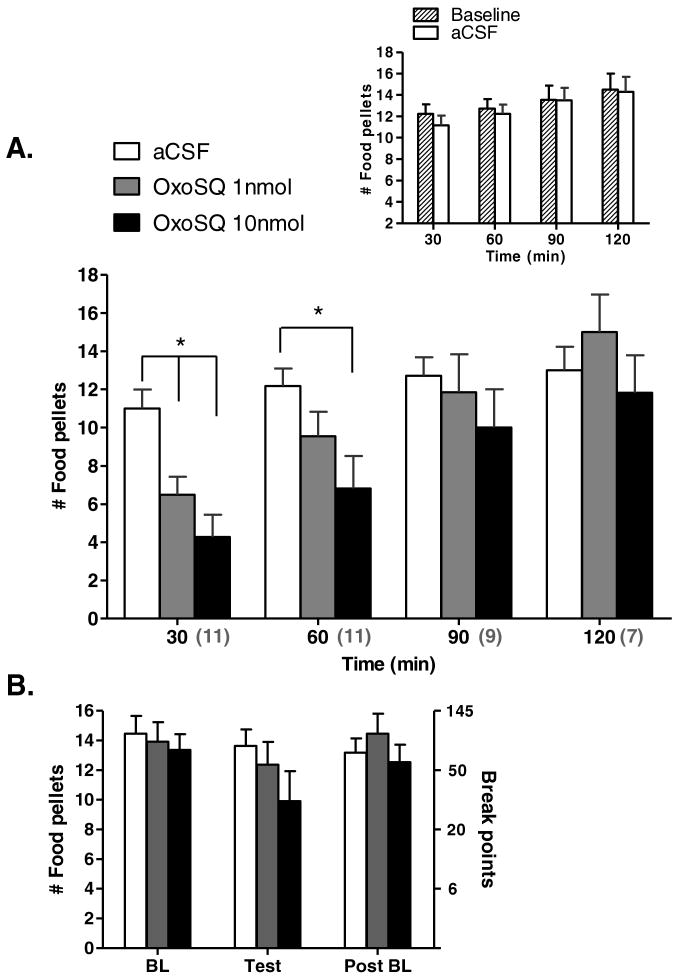
OxoSQ infusion into the LDTg suppressed lever pressing for food pellets primarily during the first 60 min of the session. Unlike the cocaine group, the food pellet group acquired most of their pellet rewards during the first hour of each session, thus any suppression of behavior caused by OxoSQ did not appear to affect the measurement of BP, as it did for cocaine. Repeated measures ANOVAs for each time bin, with dose as the repeated measure, showed a significant main effect of treatment (Fig 3A) for the 30-min [F (2, 20) = 15.5, p = 0.0001] and 60-min time bins [F (2, 20) = 6.6, p = 0.006]. Bonferroni corrected pairwise mean comparisons for the 30-min bin showed that both OxoSQ 1 (p = 0.01) and 10 nmol (p = 0.002) caused a significant reduction in mean number of food pellets compared to aCSF (Fig 3A). The same comparisons for the 60-min bin showed that only OxoSQ 10 significantly affected lever pressing for food pellets compared to aCSF (p = 0.02). When all four time bins were included in the analysis, the time bin by treatment interaction term was significant [F (6, 29) = 2.8, p = 0.03], and supported the largest effect of OxoSQ within the first hour (Fig 3A). Similar to the cocaine study however, the majority of rats in the food pellet study (7 out of 11; Fig 3A) attained a similar number of pellets by the second hour regardless of treatment. Repeated measures ANOVA on BPs showed a trend for a treatment effect [F (2, 20) = 2.6, p = 0.097] on the test day and no appreciable effect on baseline days (Fig 3B). The number of food pellets obtained for any of the time bins between baseline sessions and aCSF sessions did not significantly differ (Fig 3A inset). Similarly, the number of food pellets for any of the time bins between aCSF and CIC microinjection with OxoSQ 10 nmol was not statistically different (Fig 4B). The ratio of the inactive/active lever presses on average was small 0.0066 ± 0.0010 (Mean ± SE) and there were no appreciable differences between any of the treatment or baseline sessions with regard to this ratio. Similar to the cocaine group, this indicates that animals primarily associated the active lever with food pellet rewards.
Figure 3.
Shown in A. is the mean cumulative number of food pellets in 30-min bins, during sessions in which animals (N = 11) received microinjections of aCSF, OxoSQ 1nmol and OxoSQ 10nmol into the lateral dorsal tegmental nucleus (LDTg). Error bars are ± SEM and lines with stars indicate significant differences between means after Bonferroni corrections (p < 0.05). The grey numbers enclosed in parentheses under the x-axis indicate the number of animals in each of the time bins. Graph inset shows the same information as the main graph, during sessions before the three microinjection treatments and after aCSF. Shown in B. is the mean number of food pellets (± SEM) corresponding to the break point values in the PR-schedule during the same sessions in A. on test days and baseline sessions before (BL) and after (PostBL) test day session.
Microinjection effects on locomotor activity
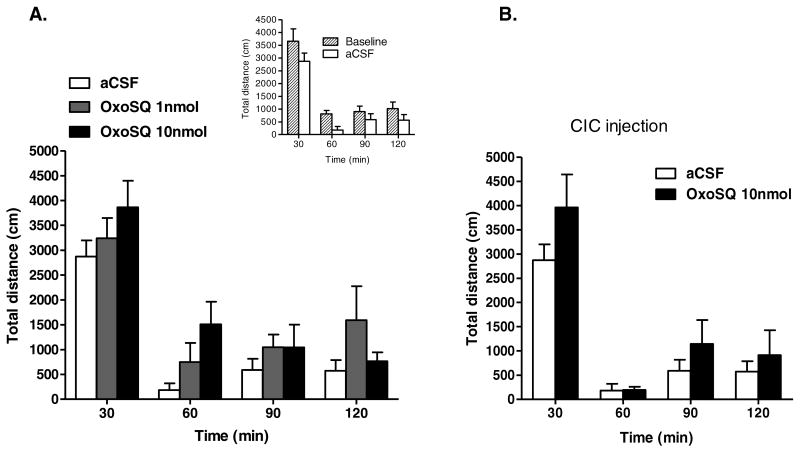
OxoSQ infused into the LDTg mildly increased locomotor activity, when measured as mean total distance traveled in cm. Although repeated measures ANOVA identified a significant main effect of dose (Fig 5A) for the 60-min time bin [F (2, 14) = 4.34, p = 0.034], Bonferroni corrected pairwise mean comparisons for the 60-min bin identified only a statistical trend (p = 0.071) for a difference in activity between OxoSQ 10 nmol and aCSF treated rats (Fig 5A). That any effects on activity were small in magnitude was supported by the lack of a statistically significant interaction of time bin and treatment, when all five time bins were included in the analysis (Fig 5A). The total distance traveled during each of the time bins between baseline sessions and aCSF session did not significantly differ (Fig 5A inset). Similarly, the total distance traveled during any of the time bins between aCSF and microinjection in CIC with OxoSQ 10 nmol was not statistically different (Fig 5B).
Figure 5.
Shown in A. is the mean total distance traveled in centimeters during 120-min sessions in which animals (N = 8) received microinjections of aCSF, OxoSQ 1nmol and OxoSQ 10nmol into the lateral dorsal tegmental nucleus (LDTg). Error bars are ± SEM. The inset shows the same measurements as A., but during baseline sessions before the three treatments and aCSF session. Shown in B. is the same measurement as in A. during sessions in which animals (N = 8) received microinjections of aCSF and OxoSQ 10nmol in the central nucleus of the inferior colliculus (CIC).
Discussion
The current results suggest that activation of M2 receptors in the LDTg suppresses the efficacy of both a natural and a drug reward. OxoSQ suppressed lever pressing for both cocaine and food in a dose-dependent manner, especially during the initial part of the PR schedule sessions (Figs 2 - 4). Lever pressing for cocaine was attenuated for a longer period in response to OxoSQ than was lever pressing for food pellets (compare Figs 2A and 3A). This difference is attributed to the different pattern of lever pressing for cocaine as opposed to food pellets in the absence of drug treatment. For example, cocaine rewards were acquired gradually throughout the first 3 hours of the session (see Fig 2A), whereas most of the pellet rewards were acquired within the first hour (see Fig 3A). Animals resumed their responding for cocaine or food as the effect of OxoSQ waned, which is reflected by lack of a significant effect on BPs for either cocaine or food. OxoSQ did not significantly compromise motor ability of the animals (Fig 5), and the non-significant locomotor stimulant effect seen was shorter lived than the effects on either food or cocaine responding. The effect of OxoSQ could not be attributed to systemic cholinomimetic effects, because OxoSQ was effective in reducing lever pressing when microinjected in the LDTg but not CIC. Further, there was no significant effect of OxoSQ on locomotor activity, when OxoSQ was microinjected in the CIC (Fig 5B).
M2 autoreceptors are important in the regulation of the activity of cholinergic neurons, including those in the LDTg (Calabresi et al., 2000, 1998; Forster and Blaha, 2000; Leonard and Llinas, 1994). Activation of M2 receptors on cell bodies and dendrites of striatal cholinergic interneurons cause inhibitory post-synaptic potentials (IPSPs) via increases in K+ currents combined with decreases in Ca2+ channel conductance (Calabresi et al., 2000, 1998; Yan and Surmeier, 1996). Evidence that M2 receptor activation is associated with induction of IPSPs is also provided by in vivo and in vitro studies in single gene mutant (M2R -/-) mice (for review see Wess et al, 2007). Importantly, blocking M2 receptors in the LDTg with scopolamine or the more selective antagonist, methoctramine, abolishes suppression of cholinergic neurons and thus suppression of DA currents in the NAc (Forster and Blaha, 2000). The possibility that OxoSQ affects M2/4 receptors on non-cholinergic neurons cannot be excluded. Previous work shows that locus coeruleus (LC) noradrenergic neurons interconnect heavily with LDTg neurons (Jones, 1991; Leonard et al., 1995). Interestingly, M2 receptors are present on postsynaptic noradrenergic neurons, and their stimulation results in depolarization of the noradrenergic neurons (Egan and North, 1985). Elevated LC noradrenergic discharge rates are strongly associated with increased arousal (Berridge, 2008). Thus, it is possible that LC activity was affected by OxoSQ through M2 heteroreceptors. It is possible that the mild increases in locomotor activity seen after OxoSQ injections in our animals are attributable to M2 heteroreceptor activation. However, even if other M2/4 heteroreceptors were activated by OxoSQ, we contend that in our study direct microinjection of OxoSQ into the LDTg likely activated M2 autoreceptors on cholinergic neurons and thus suppressed their firing. This suppression is hypothesized to be a central cause of the decreased lever pressing for either cocaine or food. OxoSQ did not decrease lever pressing because it induced depressive-like behaviors, as many cholinomimetics do when administered systemically (Williams and Adinoff, 2008). In fact, OxoSQ at the higher dose used, when injected in the LDTg, slightly increased the activity of animals during the first hour (Fig 5). Of course, we cannot completely rule out the possibility that this non-significant increase in locomotion altered the operant behavior.
Our study supports the view that increased activity from dopaminergic neurons in the VTA that project to the NAc promotes motivated behaviors (for review see Wheeler and Carelli, 2009). Motivated behaviors for obtaining food and cocaine in our study were attenuated by suppression of an excitatory cholinergic input to the VTA. Our findings are strikingly similar to recent findings on OxoSQ's effects on food and cocaine seeking. Systemic injections of OxoSQ suppressed both food and cocaine seeking under a FR schedule in mice (Thomsen et al., 2010). OxoSQ likely affected muscarinic type 2 and 4 receptors in LDTg/PPTg brain areas in addition to other brain areas that have these and other muscarinic receptors. Authors of this study, however, attributed some of the OxoSQ effects on activation of M1 type receptors found in the nucleus accumbens. Similar attenuation of motivated behavior is also supported by recent work on cocaine priming-induced reinstatement of cocaine seeking (Schmidt et al., 2009). Microinjection of an ionotropic glutamate receptor antagonist into the LDTg/PPTg or VTA prior to cocaine priming suppressed lever pressing during cocaine seeking reinstatement trials in rats. This suppression of lever pressing was suggested to reflect reduced glutamatergic activity in a multisynaptic circuit involving glutamatergic input from the medial prefrontal cortex to the LDTg/PPTg, and glutamatergic and cholinergic input from the LDTg/PPTg to the VTA. Similar behavioral results were observed with intra-VTA microinjections of scopolamine, an ACh receptor antagonist. The DA cells of the VTA are innervated by the excitatory cholinergic input from the LDTg (Omelchenko and Sesack, 2006). DA cells in the VTA depolarize in response to muscarine (Lacey et al, 1990) and nicotine (Calabresi et al., 1989), resulting in an increase in DA levels in the NAc (Miller and Blaha, 2005). Muscarinic agonists, such as carbachol in the VTA, are thought to be rewarding since animals learn to lever press for carbachol infusions into the VTA (Ikemoto and Wise, 2002). Conversely, DA levels in the NAc decrease in response to the muscarinic antagonist, scopolamine, and the nicotinic antagonist, mecamylamine, when applied to the VTA (Lester et al., 2008). Electrical stimulation of LDTg changes DA efflux in the NAc in a distinctive three component pattern (Forster and Blaha, 2000). The third and longest component, which is the sustained increase in DA, is mediated via M5 receptors located in the VTA (Forster et al., 2002; Williams and Adinoff, 2008). Electrical activation of the LDTg cholinergic neurons fails to activate the third component in the NAc of M5 receptor-deficient (M5R-/-) mice. A recent study by Lester et al. (2010) supports the role of M5 receptors in mediating the enhancing effects of cocaine on NAc DA efflux evoked by electrical stimulations of the LDTg. Whereas IP injections of cocaine enhanced NAc DA efflux evoked by the electrical stimulations in the LDTg, they failed to do so when scopolamine was also injected into the VTA. In fact, even when DA efflux reached its peak it plummeted to baseline levels if scopolamine was injected into the VTA. Behavioral studies on M5R -/- mice show that M5 receptors are important in cocaine self-administration and conditioned place preference (Fink-Jensen et al., 2003; Thomsen et al, 2005). Cocaine self-administration on an FR-1 schedule was significantly lower in M5R -/-, compared to wild type M5R +/+, mice (Fink-Jensen et al., 2003). Furthermore, in conditioned place preference tests, M5R -/- mice spent less time in contact with a cocaine-paired floor than did wild type mice, suggesting reduced sensitivity to cocaine conditioned reward. Similarly, motivation of M5R -/- mice for self-administering low to medium doses of cocaine, as expressed by BPs on a PR schedule, was significantly lower than in M5R +/- and M5R +/+ mice (Thomsen et al., 2005). Furthermore, M5R -/- and M5R +/- mice were significantly more inefficient than wild type mice in acquiring self-administration behavior, when low doses of cocaine were used. Our study complements this previous work by suggesting a significant role for cholinergic input to VTA dopaminergic neurons during reward seeking behaviors.
Our study also supports previous behavioral and microdialysis studies on the role of ACh in the VTA. You et al (2008) demonstrated that ACh release in the VTA of rats was elevated by both cocaine seeking and cocaine taking. The amount of ACh elevation associated with cocaine taking depended on cocaine training experience, as rats that controlled the self-administration of cocaine had an elevation in ACh during a cocaine self-administration trial, greater than that in rats that passively received cocaine and had no prior cocaine training. During cocaine-seeking, when saline was substituted for cocaine, so that animals could respond for cocaine, but received saline, cocaine trained rats showed higher levels of VTA ACh, compared to saline-trained animals in the same environment. Thus, contextual cues, such as insertion of the active lever, which predicted cocaine for cocaine-trained rats, were important factors for the source of ACh associated with cocaine seeking. On the other hand, ACh release that was sustained for hours after cocaine self-administration was attributed to cocaine taking. ACh effects were mediated mostly by muscarinic receptors, as blocking them in the VTA enhanced lever-pressing for cocaine. In our study, however, the M2 agonist OxoSQ applied in the LDTg suppressed lever pressing for cocaine. This discrepancy may be explained by the fact that You et al. (2008) measured lever pressing under an FR-1 schedule as opposed to a PR schedule. In their study, time-outs (see methods: TO) did not include a retraction of the active lever, and thus lever pressing was counted even though increased pressing had no consequence. Our study, however, confirms the findings of You et al. (2008), with regard to the idea that blocking cholinergic input may reduce the salience of contextual cues that predict reward. This view is also congruent with results from the M5 knock out studies (Thomsen et al., 2005), which showed lowered acquisition of cocaine self-administration in null mutant mice.
In summary, we have shown that the muscarinic autoreceptors of the cholinergic input to the VTA play a significant role in regulating motivated behaviors for either natural rewards or psychostimulants, such as cocaine. Thus, pharmacological targeting of muscarinic receptors could provide an important tool for regulating cocaine seeking. Specific targets could include either the cell bodies in the LDTg or the presynaptic axons from the LDTg innervating DA cells in the VTA. A continued challenge, however, will be to identify ways to feasibly target specific cells types or anatomical regions in human treatment.
Acknowledgments
We would like to thank Dr. Stephen T. Hansen for providing training on all the surgical procedures mentioned in this study, Dr. Ted Benice for his help with Med Associates software, and Tamie Painter for her technical support. We would also like to thank Dr. Suzanne Mitchell for her advice and generous support with lab space and equipment during the locomotor activity experiment. This work was supported by NIH grants R01 DA14639 and T32 DA07262, and P50 DA018165, and by the Department of Veterans Affairs.
List of Abbreviations
- ACh
acetylcholine
- aCSF
artificial cerebrospinal fluid
- BP
break point
- CIC
central nucleus of inferior colliculus
- DA
dopamine
- FR
fixed ratio
- ICSS
intracranial self-stimulation
- IP
intraperitoneal
- IV
intravenous
- LC
locus coeruleus
- LDTg
laterodorsal tegmental nucleus
- M2
muscarinic type 2
- NAc
nucleus accumbens
- OxoSQ
oxotremorine-sesquifumarate
- PPT
pedunculopontine nucleus
- PR
progressive ratio
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
S Shabani, Email: shabanis@ohsu.edu, Oregon Health & Science University, 3181 SW Sam Jackson Park Road L470, Portland, Oregon, U.S.A. 97239-3098, Phone: 503 220 8262 x 56673, Fax: 503 721 1029.
R Foster, Email: fosterr@ohsu.edu, Oregon Health & Science University, 3181 SW Sam Jackson Park Road L470, Portland, Oregon, U.S.A. 97239-3098.
N Gubner, Email: gubnern@ohsu.edu, Oregon Health & Science University, 3181 SW Sam Jackson Park Road L470, Portland, Oregon, U.S.A. 97239-3098.
TJ Phillips, Email: phillipt@ohsu.edu, Oregon Health & Science University, 3181 SW Sam Jackson Park Road L470, Portland, Oregon, U.S.A. 97239-3098; VA Medical Center, Portland VA Medical Center (VAMC), R&D 32, 3710 SW US Veterans Hospital Rd., Portland, OR 97239.
GP Mark, Email: markg@ohsu.edu, Oregon Health & Science University, 3181 SW Sam Jackson Park Road L470, Portland, Oregon, U.S.A. 97239-3098, Phone: 503 494 2680.
References
- Baghdoyan HA, Lydic R, Fleegal MA. M2 muscarinic autoreceptors modulate acetylcholine release in the medial pontine reticular formation. J Pharmacol Exp Ther. 1998;286:1446–1452. [PubMed] [Google Scholar]
- Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner-Osborne H, Brann MR. Pharmacology of muscarinic acetylcholine receptor subtypes (m1-m5): high throughput assays in mammalian cells. Eur J Pharmacol. 1996;295:93–102. doi: 10.1016/0014-2999(95)00639-7. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug selfadministration techniques in animals. In: Sahgal A, editor. Behavioral neuroscience; a practical approach. Oxford University Press; New York: 1993. pp. 117–143. [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Blockade of M2-like muscarinic receptors enhances long-term potentiation at corticostriatal synapses. Eur J Neurosci. 1998;10:3020–3023. doi: 10.1111/j.1460-9568.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci. 2001;21:RC141, 1–4. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Egan TM, North RA. Acetylcholine acts on m2-muscarinic receptors to excite rat locus coeruleus neurones. Br J Pharmacol. 1985;85:733–735. doi: 10.1111/j.1476-5381.1985.tb11070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Klinger PD, Agranoff BW. Muscarinic agonist binding and phospholipid turnover in brain. J Biol Chem. 1983;258:7358–7363. [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- Huang X, Gu HH, Zhan CG. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B. 2009;113:15057–15066. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–9904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56 1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. Noradrenergic locus coeruleus neurons: their distant connections and their relationship to neighboring (including cholinergic and GABAergic) neurons of the central gray and reticular formation. Prog Brain Res. 1991;88:15–30. doi: 10.1016/s0079-6123(08)63797-8. [DOI] [PubMed] [Google Scholar]
- Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther. 1990;253:395–400. [PubMed] [Google Scholar]
- Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Kerman I, Blaha G, Taveras E, Taylor B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J Comp Neurol. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- Lester DB, Miller AD, Blaha CD. Muscarinic receptor blockade in the ventral tegmental area attenuates cocaine enhancement of laterodorsal tegmentum stimulation-evoked accumbens dopamine efflux in the mouse. Synapse. 2010;64:216–223. doi: 10.1002/syn.20717. [DOI] [PubMed] [Google Scholar]
- Lester DB, Miller AD, Pate TD, Blaha CD. Midbrain acetylcholine and glutamate receptors modulate accumbal dopamine release. Neuroreport. 2008;19:991–995. doi: 10.1097/WNR.0b013e3283036e5e. [DOI] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader SL, Berger SP, Bechtholt AJ. Injection of oxotremorine in nucleus accumbens shell reduces cocaine but not food self-administration in rats. Brain Res. 2006;1123:51–59. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Breaking points on a progressive ratio schedule reinforced by intravenous apomorphine increase daily following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1989;32:43–47. doi: 10.1016/0091-3057(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz B, Exposito I, Mora F. M1 acetylcholine receptor stimulation increases the extracellular concentrations of glutamate and GABA in the medial prefrontal cortex of the rat. Neurochem Res. 1997;22:281–286. doi: 10.1023/a:1022486721267. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30:1358–1369. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Conn PJ, Lindsley C, Wess J, Boon JY, Fulton BS, Fink-Jensen A, Caine SB. Attenuation of cocaine's reinforcing and discriminative stimulus effects via muscarinic M1 acetylcholine receptor stimulation. J Pharmacol Exp Ther. 2010;332:959–969. doi: 10.1124/jpet.109.162057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J Neurosci. 2009a;29:1087–1092. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009b;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–8149. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaro MT, Palacios JM, Mengod G. Multiplicity of muscarinic autoreceptor subtypes? Comparison of the distribution of cholinergic cells and cells containing mRNA for five subtypes of muscarinic receptors in the rat brain. Brain Res Mol Brain Res. 1994;21:30–46. doi: 10.1016/0169-328x(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2-selective ligands also recognize M4 receptors in the rat brain: evidence from combined in situ hybridization and receptor autoradiography. Synapse. 1992;11:171–183. doi: 10.1002/syn.890110302. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56 1:149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Mathur A, Tampakeras M. Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci. 1993;107:1077–1087. doi: 10.1037//0735-7044.107.6.1077. [DOI] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Wise RA. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28:9021–9029. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]