Abstract
Background and purpose:
Cyclooxygenase 2 (COX-2) is involved in inflammatory bowel disease, but the effect of flavonoids at the intestinal epithelial level is unknown. We aimed to characterize the effect and structure-activity relationship of nine selected flavonoids on COX-2 expression in intestinal epithelial cell (IEC)18 cells. We also investigated the signal transduction pathway(s) responsible for the effects observed.
Experimental approach:
Intestinal epithelial cell 18, a non-tumour cell line with intestinal epithelial phenotype, was used. COX-2 was measured by Western blot and the involvement of the NF-κB pathway assessed by Western blot, pharmacological inhibition, luciferase reporter assays and nuclear translocation experiments.
Key results:
The effect of flavonoids on COX-2 expression depended on the experimental conditions tested [non-stimulated and lipopolysaccharide (LPS)-stimulated]. Flavonoids caused an increase in COX-2 expression and NF-κB-dependent gene transcription under basal conditions. Conversely, under LPS stimulation flavonoids increased, decreased or did not affect COX-2 levels depending on the specific type. Variable effects were observed on extracellular signal regulated kinase/p38/c-Jun N-terminal kinase phosphorylation and p50/65 nuclear translocation.
Conclusion and implications:
The effect of flavonoids on COX-2 expression depended on the balance of the interference with IκB-α phosphorylation and other signalling targets, and therefore depends on the experimental conditions and on the type of flavonoids. This is expected to result in different effects in inflammatory conditions. In general, flavonoids may limit epithelial COX-2 expression in inflammatory conditions while favouring it when inflammation is not present.
Keywords: flavonoids, cyclooxygenase 2, NF-κB, intestinal epithelial cells
Introduction
The intestinal mucosa, the innermost layer of the intestine, plays an important physiological role by mediating water and nutrient transport and acting as interphase with the complex luminal milieu, which comprises a combination of diverse bacteria and their products as well as derivative products of the diet. The luminal flora present a formidable challenge to the mucosa, which is met efficiently by a state of mild leukocyte infiltration which has been referred to as ‘physiological inflammation’. The surface epithelium serves as the mucosal frontier, by constituting a physical as well as an immunological barrier to microorganism access. Thus intestinal epithelial cells (IECs) express various immune receptors, traditionally believed to be expressed primarily by myeloid cell lineages (Reinecker and Podolsky, 1995) and, accordingly, they can produce a wide array of immunomodulatory substances such as cytokines and complement factors. Specific perturbation of the intestinal epithelium can lead to intestinal inflammation; in fact, cytokine production from IECs is enough to cause inflammation in vivo (Ohtsuka et al., 2001). In addition, defects in epithelial permeability may facilitate antigen penetration and subsequent intestinal inflammation, as has been proposed for Crohn's disease
Intestinal epithelial cells express cyclooxygenase (COX)-2 when stimulated by pro-inflammatory factors, including lipopolysaccharide (LPS), tumour necrosis factor-α, oxidative stress, etc. (Longo et al., 1998). Cyclooxygenases are rate limiting enzymes in the biosynthesis of a number of eicosanoids such as PGE2 from arachidonic acid and other precursors. Their main product in IECs appears to be PGE2, followed by PGF2α and PGD2 (Eckmann et al., 1997; Longo et al., 1998). COX-2 induction evokes a dramatic increase in eicosanoid release compared with the basal COX-1 dependent production. COX-1 and COX-2 have similar structures but with an important difference in the tunnel through which arachidonic acid gains access to the active site of the enzyme. This structural difference explains the particular selectivity of each isoform. COX-2-derived prostaglandins have been classically assigned a pro-inflammatory effect, restricted in time and space to local inflammatory responses. However, it is well known that COX-2 is constitutively expressed in some cell types, including endothelial and macula densa cells. This explains many of the adverse effects of the COX-2 selective inhibitors (coxibs). Basal expression of COX-2 in IECs is typically low (Singer et al., 1998). The effects of prostaglandins in the intestine include enhanced water and ionic secretion, contractility and vasodilation (MacNaughton and Cushing, 2000; Fornai et al., 2005). Thus the role of epithelial COX-2 apparently conforms to the classical paradigm. However, COX inhibitors (coxibs and non-steroidal anti-inflammatory drugs (NSAIDs) in general) are notoriously ineffective in the management of inflammatory bowel disease and they may actually precipitate inflammatory relapse of these chronic conditions. In fact, epithelial prostaglandins seem to be involved in the resolution of inflammation and the healing process (Wallace, 2006) as well as in intestinal homeostasis (Newberry et al., 1999). In particular, prostaglandin (PG)E2 acting on EP2 or EP4 receptors has been involved in these effects (Stenson, 2007; receptor nomenclature follows Alexander et al., 2009). Hence it is possible that agents that promote COX-2 induction could be useful in the therapy of inflammatory bowel disease by hastening the resolution of the inflammatory process. On the other hand, COX-2-derived prostaglandins have been involved in colorectal cancer, and there is some evidence that COX inhibition may have chemopreventive effects. Colorectal cancer is a known risk of long standing inflammatory bowel disease, although COX-2 induction is probably only one of several mechanisms. Thus COX-2 must be regarded as a double-edged sword with both beneficial and detrimental effects in the intestine.
Among the inflammatory bowel diseases, ulcerative colitis and Crohn's disease have received special attention because of their poorly understood etiology and pathophysiology and their unsatisfactory management. Treatment is largely pharmacological and of empirical nature, based on immunomodulatory drugs (corticoids, tacrolimus, azathioprine, infliximab) and aminosalicylates, all of which have significant adverse effects and are not effective in all patients. Flavonoids are polyphenolic compounds of natural origin which are a significant part of the diet. Flavonoids exhibit a wide array of pharmacological activities, including anti-inflammatory, anticancer and radical scavenging properties (Ballester et al., 2006). There is evidence of the anti-inflammatory properties of these compounds, including intestinal anti-inflammatory activity (Sanchez de Medina et al., 1996; Galvez et al., 1997; Mochizuki and Hasegawa, 2004; Kwon et al., 2005). There have been several in vitro studies investigating the inhibitory activity of flavonoids on pro-inflammatory mediator production in different cell lines, mainly macrophages or monocytes such as RAW 264.7 (Xagorari et al., 2001; Comalada et al., 2006) and J774.1 (Blonska et al., 2004) cells, as well as primary splenocytes (López-Posadas et al., 2008). However, few studies have examined their potential effect on the epithelium and little information about the mechanism of action of these flavonoids is available. Here we report the effects and structure-activity relationship of nine different flavonoids (see Table 1) on COX-2 expression in IEC18 cells, a non-tumour model IEC line. The different categories of flavonoids assayed differ mainly in the presence or absence of a double bond between C2 and C3, the 3-hydroxyl, and the position of the phenol group (also known as B ring). The substitutions in these basic structures give rise to the different flavonoid compounds.
Table 1.
Structural features of the flavonoids tested
| Chemical formula | Name | Substitution | |||
|---|---|---|---|---|---|
| Flavonols | 5 | 7 | 3′ | 4′ | |
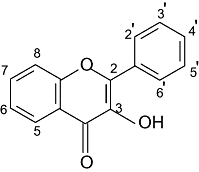 |
Kaempferol | OH | OH | H | OH |
| Quercetin | OH | OH | OH | OH | |
| Flavones | 5 | 7 | 3′ | 4′ | |
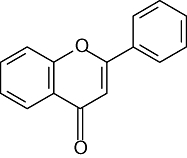 |
Apigenin | OH | OH | H | OH |
| Chrysin | OH | OH | H | H | |
| Diosmetin | OH | OH | OH | OCH3 | |
| Luteolin | OH | OH | OH | OH | |
| Isoflavones | 5 | 7 | 3′ | 4′ | |
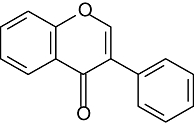 |
Daidzein | H | OH | H | OH |
| Genistein | OH | OH | H | OH | |
| Flavanones | 5 | 7 | 3′ | 4′ | |
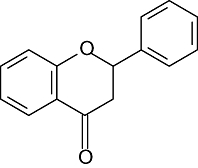 |
Hesperetin | OH | OH | OH | OCH3 |
Methods
Cell lines and culture conditions
IEC18 cells (passages 20–32) were obtained from the Cell Culture service of the University of Granada and were cultured in Dulbecco's modified Eagle's medium containing fetal calf serum (10%), 2 mM L-glutamine, 100 U·mL−1 penicillin, 0.1 mg·mL−1 streptomycin and 2.5 µg·mL−1 amphotericin B. Cells were seeded in 78 cm2 plates to confluence and cultured at 37°C in a 5% CO2-air atmosphere. The culture medium was changed every 2 days. In all the experiments, except where indicated, we followed the same protocol. Flavonoids were dissolved in DMSO to make stock solutions and added to cell culture medium to a final DMSO concentration <0.1% 1 h before the addition of LPS (from Escherichia coli 055:B5).
Viability assay
Cells were cultured in 24-well culture plates to confluency and treated with the indicated flavonoids for 24 h, after which cells were stained with crystal violet as previously described (Bonnekoh et al., 1989) to measure cell viability. Cells were first washed with PBS and then stained and fixed with 0.2% crystal violet in 2% ethanol during 30 min at room temperature. After four washes with PBS, the cells were scraped with 1% SDS for 30 min and then harvested and centrifuged at 3000 g during 5 min. Finally, the colour intensity was quantitated using a Bio-Rad 680XR microplate reader at 540 nm. Each assay condition was performed in at least three independent experiments and the results were represented as mean (% of cell viability) ± SEM.
Assay for lactate dehydrogenase (LDH) release
Cell toxicity was quantitatively assessed by the measurement of LDH, released from damaged cells in the extracellular medium 24 h after flavonoid exposure. Cells were treated with flavonoids exactly as in the COX-2 expression experiments. Samples were centrifuged at 3000 g for 10 min at 4°C. Measurement was carried out in a 96 well-plate by adding 30 µL of the sample and 80 µL of β-NADH (145 mM) in sodium phosphate buffer (50 mM, pH 7.5). After 5 min of incubation at 37°C, 20 µL of sodium pyruvate (25 mM) were added and pyruvate dependent β-NADH disappearance was monitored at 340 nm using a Bio-Rad 680XR microplate spectrophotometer. Values are expressed as U·mL−1.
Extraction of nuclear proteins
Cell monolayers were culured in 75 cm2 flasks. Flavonoids were added 1 h before LPS (1 µg·mL−1) or vehicle (water). Whole cell homogenates were obtained 30 min after LPS/vehicle stimulation. Monolayers were collected in PBS with freshly added phosphatase inhibitors (125 mM NaF, 250 mM β-glycerophosphate, 250 mM p-nitrophenyl phosphate, 25 mM NaVO3). Cells were scraped and the suspension was transferred to a 15 mL Falcon tube and centrifuged at 300 g for 5 min at 4°C. The pellet was resuspended in ice-cold hypotonic buffer (20 mM HEPES, 5 mM NaF, 10 µM Na2MoO4 and 0.1 mM EDTA, pH = 7.5). After incubation on ice for 15 min, 0.5% Igepal CA-630 was added and the suspension was mixed by gentle pipetting. Samples were then centrifuged for 30 s at 14000 g. The supernatant was collected as cytoplasmic extract and the nuclear pellet was resuspended in lysis buffer (provided by Active Motif, Rixensart, Belgium) and rocked on ice for 30 min on a shaking platform prior to being centrifuged for 10 min at 14000 g. Protein concentration in nuclear extracts was measured by the bicinchoninic acid assay (Smith et al., 1985), using bovine serum albumin as standard. The supernatant (nuclear cell extract) was aliquoted and stored at −80°C until measurement. The samples were either analysed by Western blot or subjected to TransAM measurement (Active Motif, Rixensart, Belgium), which detects different NF-κB subunits in microtiter plates labelled with NF-κB target sequence DNA oligomers.
Western blot
Cell samples were washed with cold PBS and homogenized in cold lysis buffer containing 1% Igepal CA-630, 20 mM HEPES-Na, 10 mM EGTA, 40 mM β-glycerophosphate, 25 mM MgCl2 and 2 mM sodium orthovanadate (pH = 7.5) with freshly added protease inhibitors (phenyl-methylsulphonyl fluoride, aprotinin, leupeptin, 1,10-phenanthroline). The protein content was measured as above. Samples were boiled for 5 min in Laemli buffer and separated by SDS-PAGE. After transferring to nitrocellulose (COX-2) or PVDF membranes, a Ponceau red incubation was performed to check for equal loading. Membranes were blocked for 1.5 h at room temperature in Tris-buffered saline-0.1% Tween-20 (TBS-T) containing 5% (w/v) nonfat dry milk and then incubated with TBS-T containing 5% BSA and the primary antibody at 4°C overnight. The dilutions of antibodies used were: 1:1000 for phospho-p38, phospho-(stress-activated protein kinase/c-Jun N-terminal kinase; SAPK/JNK) and phospho-Akt (Cell Signaling, Beverly, MA, USA); 1:2500 for phospho-(inhibitor of κB; IκB)-α (Cell Signaling) and extracellular signal regulated kinase (ERK; Sigma); 1:3000 for COX-2 (Cayman Technologies); and 1:500 for p50 and p65 (Santa Cruz Biotechnology). After three washes of 5 min with TBS-T, peroxidase-conjugated anti-mouse (phospho-ERK) or anti-rabbit (phospho-p38, phospho-SAPK/JNK, COX-2, p50, p65 and phospho-IκB-α) IgG was used as secondary antibody. Then, enhanced chemiluminiscence (Perkin Elmer™, Life Sciences, Boston, MA, USA) detection was performed. Densitometry was carried out with NIH software (Image J).
Transfection assays
IEC18 cells were transfected by the lipofectamine method with a plasmid encoding luciferase under the control of either an NF-κB or a TATA-like promoter (pTAL, control; Clontech, Palo Alto, CA, USA). Transfected cells were selected by G418 (Invitrogen, Carlsbad, CA, USA) resistance, which was cotransfected in a separate plasmid in a 10:1 ratio. Luciferase activity was measured with a Lumat LB9507 Luminometer.
Statistical analysis
All results are expressed as mean ± SEM. Differences among means were tested for statistical significance using one way analysis of variance and a posteriori least significance tests. P-values <0.05 were considered significant. All analyses were carried out with SigmaStat 2.03 (Jandel Corporation, San Rafael, CA, USA).
Materials
Except where indicated, all chemicals were obtained from Sigma (Madrid, Spain).
Results
Effect of flavonoids on cell viability
Crystal violet staining was used to assess the impact of flavonoids (50 µM) on cell viability. No effect was detected (Figure S1) and flavonoids were considered non-toxic to IEC18 cells at this concentration.
Effect of flavonoids on basal COX-2 expression
Cyclooxygenase 2 expression in IEC18 cells was assessed by Western blot. In basal conditions neither isoflavones nor the flavanone hesperetin showed any effect. Flavones and flavonols increased COX-2 expression, except in the case of diosmetin (Figure 1). The effect of flavones was relatively minor compared with the effect of flavonols. Thus kaempferol and quercetin almost doubled the expression of the enzyme (P < 0.05). Luteolin evoked a twofold increase, with smaller effects for apigenin and chrysin (P < 0.05 in all cases).
Figure 1.
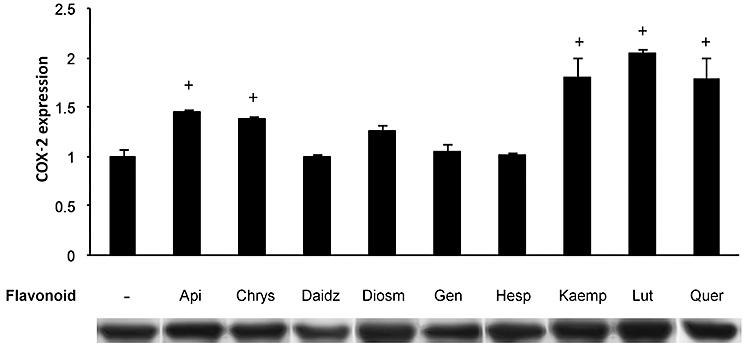
Flavonoid effect on basal COX-2 expression. IEC18 were cultured with nine different flavonoid compounds (50 µM) for 24 h. Whole cell proteins were isolated and separated using acrylamide gels, and COX-2 expression was assessed by Western blot. Data are representative of three different experiments. Results were expressed as fold increase comparing with non-treated cells (mean ± SEM). +P < 0.05 versus control. Here and in following the figures, the flavonoids are indicated as follows: Api, apigenin; Chrys, chrysin; Daidz, daidzein; Diosm, disometin; Gen, genistein; Hesp, hesperidin; Kaemp, kaempferol; Lut, luteolin; Quer, quercetin. COX, cyclooxygenase; IEC, intestinal epithelial cell.
In order to understand the regulation that flavonoids exert over COX-2 expression, we studied the activation of NF-κB, a transcription factor involved in the regulation of expression of multiple genes that participate in immunity and inflammation, cell proliferation and apoptosis, including inducible COX (Jobin et al., 1998). NF-κB is activated in response to several external stimuli, including interleukins, growth factors, viral and bacterial infections, physical factors (e.g. UV light), and LPS. The main transduction pathway leading to NF-κB activation, the classical pathway, involves Ser32 phosphorylation of the inhibitor protein IκB-&alpha, which in the absence of stimuli is bound to NF-κB, preventing its migration to the nucleus (see Figure 8). Quercetin was selected as a representative active flavonoid for further testing. Despite its inducing effect on COX-2 expression, IκB-α was not phosphorylated at all by the flavonoid (Figure 2A). Quercetin, however, elicited the nuclear translocation of NF-κB p50 (but not p65) as efficiently as LPS, as shown by Western blot analysis (Figure 2A). Conversely, LPS evoked both p50 and p65/RelA translocation. Thus LPS and quercetin produce distinct effects on IEC18 cells. In order to assess whether other NF-κB proteins are involved in the transcriptional regulation of COX-2, we used a variant ELISA kit to measure the possible translocation of all five members to the nucleus. Quercetin did not induce the translocation of other subunits to the nucleus (not shown). We also assessed the phosphatidyl inositol 3-kinase (PI3K)/Akt pathway by examining Akt phosphorylation, as this is an alternative route to NF-κB stimulation. LPS augmented Akt phoshorylation in a Bay11-7082 independent way, while quercetin actually inhibited basal Akt phosphorylation (Figure 2B). Thus quercetin is unlikely to induce COX-2 acting on this pathway.
Figure 8.
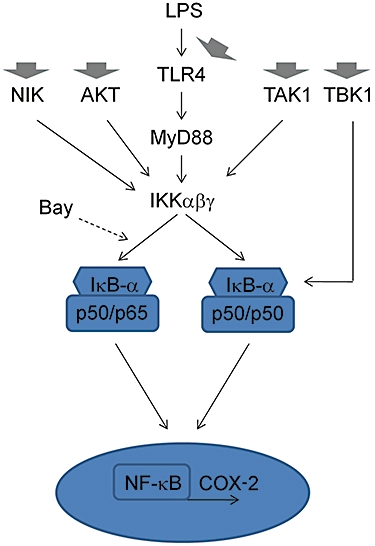
Proposed mechanism of action of COX-2 modulation. The canonical NF-κB pathway activated by LPS via TLR4 is shown. Bay 11-7082 may favour the formation of p50/p50 rather than p65/p50 dimers under LPS stimulation, while quercetin (and perhaps other flavonoids) increases only p50/p50 in basal conditions. Flavonoids may act at different levels (thick arrows) to reduce IκB-α phosphorylation after LPS treatment while at the same time modulating other pathways. This scheme is a simplification of the pathways involved. Normal arrows indicate activation, broken arrows denote inhibition. COX, cyclooxygenase; LPS, lipopolysaccharide; NIK, NF-κB-inducing kinase; TAK, transforming growth factor-β-activated kinase; TBK, Traf family member-associated NF-κB activator (TANK)-binding kinase 1.
Figure 2.
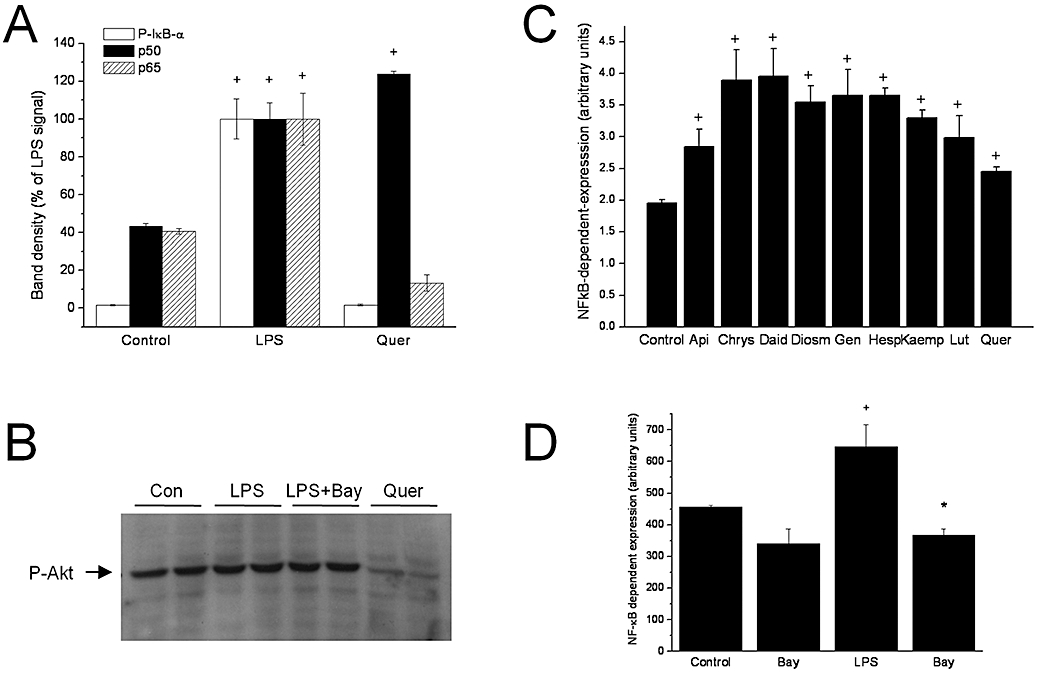
Flavonoid effects on the NF-κB pathway. (A) Effect of LPS and quercetin on IκB-α phosphorylation and p50/p65 nuclear translocation in IEC18 assessed by Western blot. (B) Effect of LPS (with and without Bay11-7085 pretreatment) and quercetin on Akt phosphorylation in IEC18 cells by Western blot. (C) Effect of flavonoids on NF-κB dependent gene expression in a luciferase reporter IEC18 system. (D) Effect of LPS (with and without Bay11-7085 pretreatment) on NF-κB dependent gene expression. +P < 0.05 versus control; *P < 0.05 versus LPS. IEC, intestinal epithelial cell; LPS, lipopolysaccharide.
We additionally examined the effect of flavonoids on NF-κB dependent gene expression in a luciferase reporter IEC18 system. All the compounds tested increased the luciferase signal, albeit to a different extent, ranging from approximately twofold for chrysin and daidzein to only 26% for quercetin (Figure 2C). LPS produced a relatively minor effect in comparison, which was fully reversible by Bay11-7082 pretreatment, as expected (Figure 2D).
Effects of flavonoids on LPS-induced COX-2 expression
We sought to determine the effect of flavonoids when COX-2 was induced by pro-inflammatory stimuli. To this end, cells were treated with vehicle or flavonoids and after 1 h exposed to 1 µg·mL−1 LPS. As expected, LPS increased COX-2 immunoreactivity (twofold over the control cells, Figure 3). The most remarkable effect of all flavonoids was the dramatic increase in COX-2 expression brought about by diosmetin. Chrysin and apigenin also increased COX-2 immunoreactivity, but to a lower extent. In contrast, all other flavonoids except genistein, i.e. flavonols, the flavanone hesperitin and the isoflavone daidzein, failed to augment COX-2 but actually tended to produce the opposite effect, showing COX-2 levels intermediate between those of quiescent and LPS-treated cells. Thus the effects of flavonoids are different depending on the cell status. We additionally examined the concentration dependent effects in the case of apigenin and daidzein (Figure 3D). Apigenin exhibited an apparent trend for higher induction of COX-2 at 100 µM, while daidzein essentially did not affect COX-2 expression regardless of flavonoid concentration.
Figure 3.
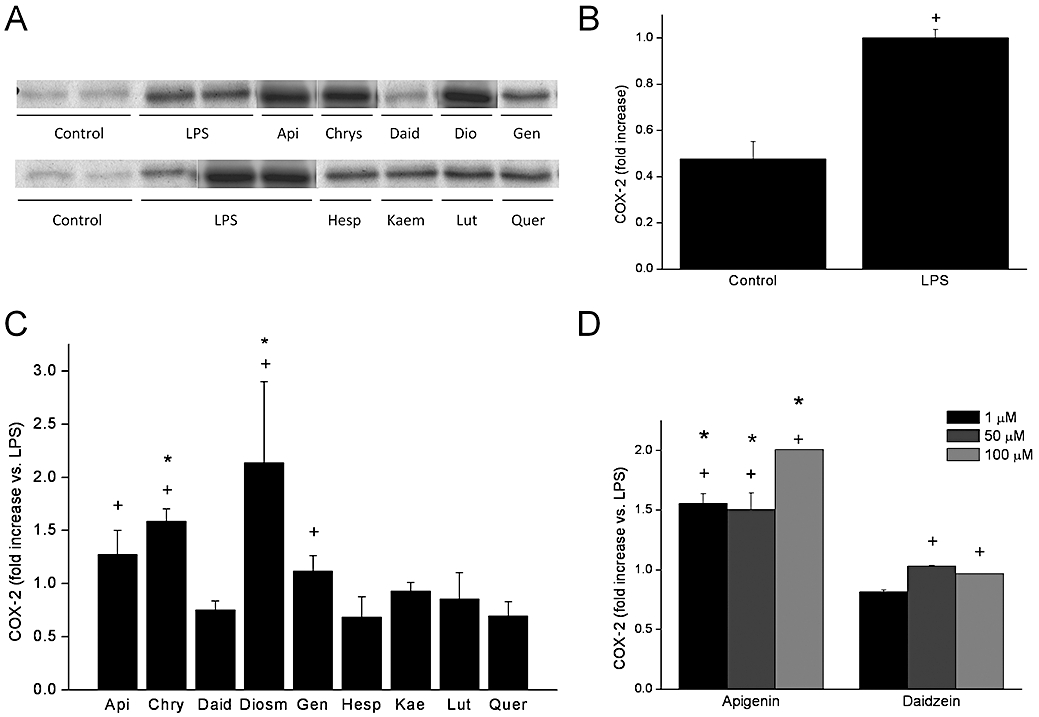
Flavonoid effect on LPS-induced COX-2 expression. (A) Effect of flavonoids on COX-2 expression elicited. by LPS. (B) Quantitation of LPS effect. (C) Quantitation of flavonoid effects. (D) Concentration response for apigenin and daidzein. COX-2 expression was measured on LPS-stimulated cells. After 1 h of flavonoid treatment (50 µM except where indicated), cells were stimulated with LPS (1 µg·mL−1) during 24 h. Expression of COX-2 was analysed by Western blot in protein samples obtained. Data were expressed as fold increase comparing with LPS-stimulated and non-treated cells (mean ± SEM). +P < 0.05 versus control; *P < 0.05 versus LPS. COX, cyclooxygenase; LPS, lipopolysaccharide.
Flavonoid effect on IκB-α phosphorylation
As expected, LPS induced rapid (30 min) phosphorylation of IκB-α, which was fully prevented by the specific inhibitor Bay 11-7082 at a concentration of 10 µM (Figure 4). Several flavonoids inhibited IκB-α phosphorylation, including quercetin, hesperetin, genistein and apigenin, all of which inhibited completely the effect of LPS at this level (Figure 4). Diosmetin and luteolin showed phosphorylation levels intermediate between those of the control and LPS groups. Chrysin, daidzein and kaempferol had no effect whatsoever.
Figure 4.
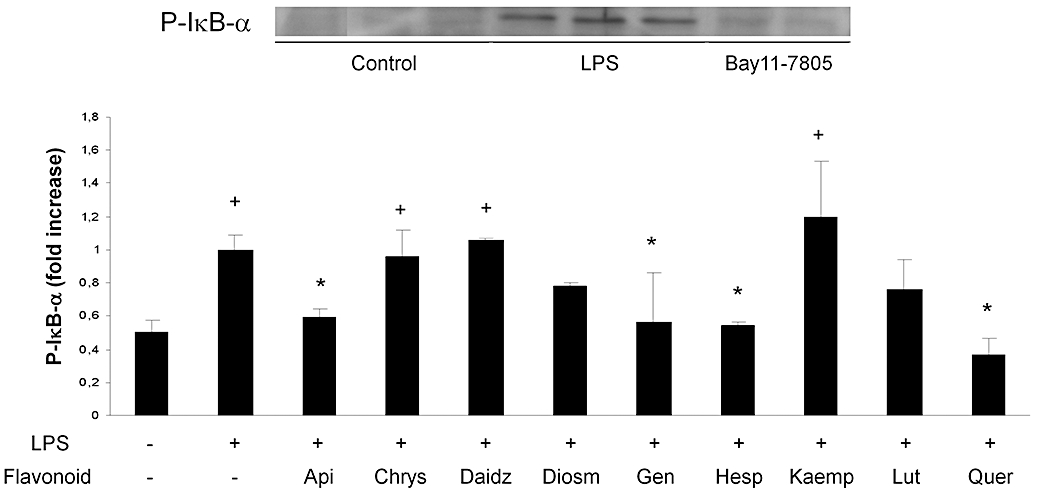
Effect of flavonoids on IκB-α phosphorylation. Cytoplasmic proteins were used to assess IκB-α phosphorylation. After 1 h of flavonoid treatment (50 µM), cells were stimulated with LPS (1 µg·mL−1) for 30 min. IκB-α phosphorylation was analysed by Western blot in protein samples obtained. Top: representative Western blot of the effect of LPS ± Bay11-7085 on IκB-α phosphorylation. Bottom: quantitation of flavonoid effects. Data were expressed as fold increase comparing with LPS-stimulated and non-treated cells (mean ± SEM). +P < 0.05 versus control; *P < 0.05 versus LPS. LPS, lipopolysaccharide.
Effect of flavonoids on NF-κB p50 and p65 nuclear translocation
Subsequent to IκB-α phosphorylation, the protein is ubiquitinated and then degraded by proteasomal machinery, leaving NF-κB dimers free to translocate to the nucleus and exert their transcriptional actions. In enterocytes, NF-κB dimers are composed chiefly of p50 and p65, usually as heterodimers (Inan et al., 2000; Jobin and Sartor, 2000). Hence we focused on this step of the NF-κB pathway by assessing p50/p65 presence in nuclear extracts. As expected, p50 and p65 immunoreactivity was markedly increased (three- and twofold, respectively, P < 0.05) 30 min after LPS stimulation (Figures 2 and 5). Thus the NF-κB classical or canonical pathway appears to be fully operative in IEC18 cells. Surprisingly, however, the inhibitor Bay 11-7802 only blocked partially (∼40%) p50/p65 migration in response to LPS, suggesting that there are non-redundant alternative activation pathways in response to LPS. Even more surprisingly, LPS-evoked COX-2 expression was significantly higher in the presence than in the absence of Bay11-7082 (Figure 6). This was a consistent effect, as it was detected in three different occasions.
Figure 5.
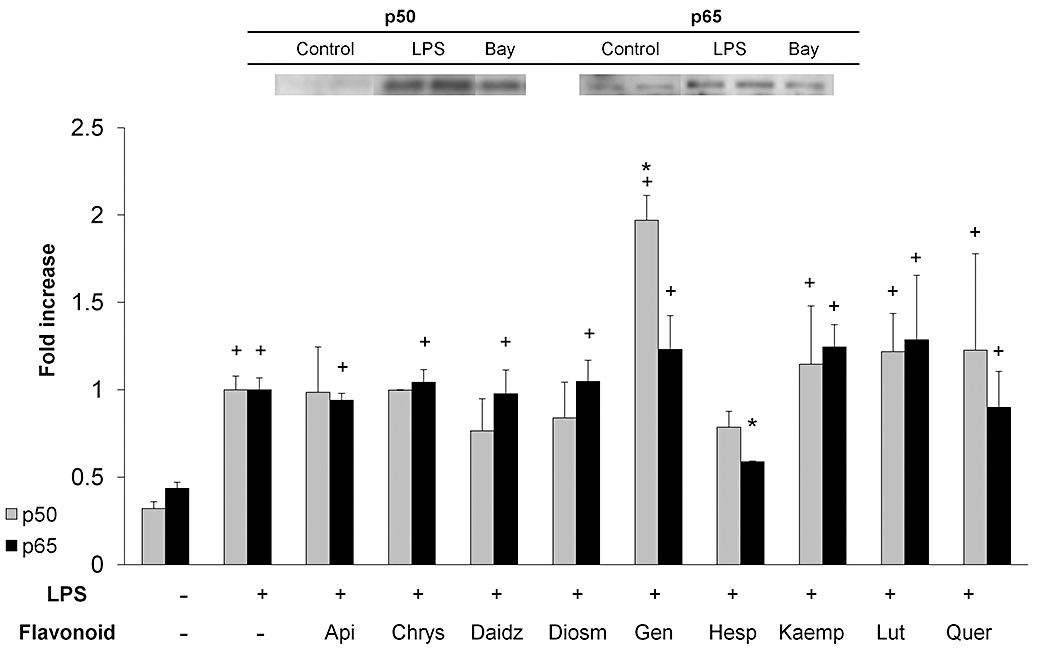
Effect of flavonoids on NF-κB p50 and p65 nuclear translocation. Cells were treated with or without flavonoids (50 µM) and stimulated with LPS for 30 min. Nuclear protein extracts were analysed by Western blot. Top: representative Western blot of the effect of LPS ± Bay11-7085 on p50/p65 translocation. Bottom: quantitation of flavonoid effects. Data are expressed as mean ± SEM. +P < 0.05 versus control; *P < 0.05 versus LPS. LPS, lipopolysaccharide.
Figure 6.
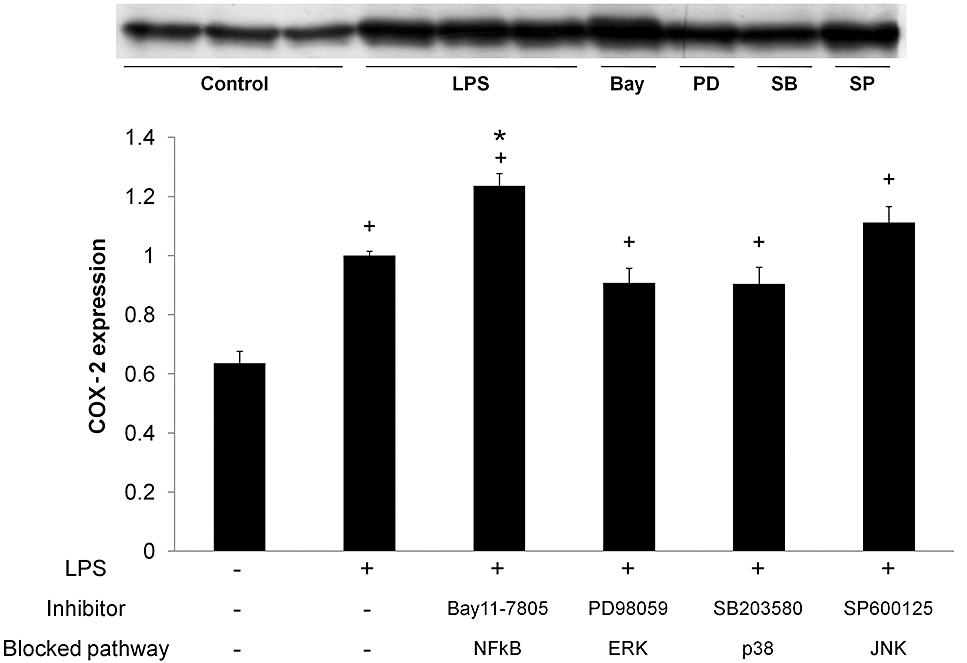
Effect of inhibitors of NF-κB and MAPK on COX-2 expression. After 1 h of inhibitor treatment, cells were stimulated with LPS (1 µg·mL−1) for 24 h. Expression of COX-2 was analysed by Western blot in protein samples obtained. Data were expressed as fold increase comparing with LPS-stimulated and non-treated cells (mean ± SEM). +P < 0.05 versus control; *P < 0.05 versus LPS. COX, cyclooxygenase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase.
This lack of correlation between the inhibition of IκB-α phosphorylation and NF-κB nuclear translocation was also observed with flavonoids, which generally did not modify the effect of LPS on p50/p65, with the sole exception of genistein, which enhanced migration of p50 to the nucleus, and hesperetin, which had the opposite effect on p65.
Effect of flavonoids on mitogen-activated protein kinase (MAPK) signalling pathways
The MAPKs are serine/threonine specific protein kinases that respond to extracellular stimuli and regulate various cellular activities, such as gene expression, proliferation, differentiation, cell survival and apoptosis. To date, four distinct groups of MAPKs have been characterized in mammals. Here we studied the effect of flavonoids on three of the most studied MAPK pathways: ERK, JNK and p38/SNPK. All three of them were strongly activated upon 30 min of LPS stimulation (Figure 7, top), and phosphorylation was partially prevented when a specific inhibitor of ERK (PD098059), JNK (SP600125) or p38 (SB-203580) was applied, reflecting blockade of autophosphorylation.
Figure 7.
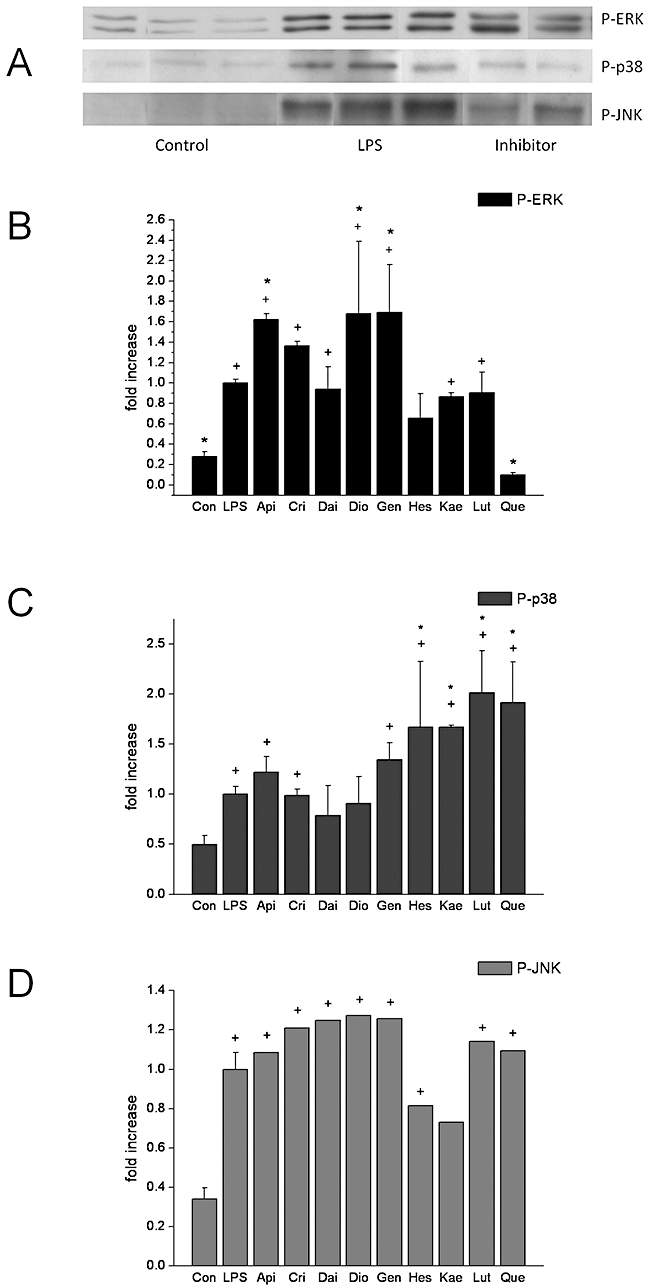
Effect of flavonoids on MAPK. Effect of flavonoids on MAPK. Cells were treated with or without flavonoids (50 µM) for 1 h and stimulated with LPS for 30 min. Western blot analysis was used to assess phosphorylation of three main MAPK: ERK, p38 and JNK. (A) representative Western blot of the effect of LPS ± MAPK inhibitor. (B, C, D) quantitation of flavonoid effects on ERK, p38 and JNK respectively. Data are expressed as mean ± SEM. +P < 0.05 versus control; *P < 0.05 versus LPS. JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase.
c-Jun N-terminal kinase phosphorylation was unaffected by flavonoids. ERK phosphorylation was enhanced by the flavones diosmetin and apigenin (chrysin did not reach statistical significance), but not luteolin, and the isoflavone genistein, but not daidzein (Figure 7, bottom). The increase was ∼60%. Interestingly, quercetin exhibited a complete inhibition of ERK phosphorylation, while the other flavonol, kaempferol, had no effect.
Next we studied the effect of the selected flavonoids on p38 phosphorylation. The results show that the flavonols, quercetin and kaempferol, the flavone luteolin and the flavanone hesperetin were able to increase p38 phosphorylation (P < 0.05), whereas all other flavonoids were inactive (Figure 7, bottom).
Unexpectedly, however, none of the MAPK inhibitors affected LPS-induced COX-2 expression (Figure 4). Hence the effects of flavonoids on these signalling pathways are unlikely to be relevant for the modulation of COX-2, although they must affect other molecular endpoints of LPS.
Discussion and conclusions
The prevalence and burden of chronic inflammatory conditions, including inflammatory bowel disease, is increasing in the last few years. COX-2, the enzyme that catalyses the limiting step in the biosynthesis of prostaglandins in inflammatory sites, is a very interesting drug target because it has a role both in the development of the inflammatory response and in its recovery. The former is the basis of therapeutic interventions in inflammatory/painful conditions with NSAIDs and COX-2 selective inhibitors (referred to as Coxibs). Coxibs allow a better profile of gastric safety, although they have important cardiovascular adverse effects. Both NSAIDs and Coxibs appear to be deleterious for intestinal inflammation, and it is now widely accepted that prostaglandins, in particular PGE2, are essential in the control of epithelial proliferation and apoptosis (Stenson, 2007). For instance, epithelial proliferation is diminished in dextran sulphate sodium colitis induced in COX-2−/− mice but rescued by exogenous PGE2 administration (Brown et al., 2007). In addition, the prostaglandin production profile changes during the different phases of inflammation. Thus PGE2 is initially increased, while PGD2 is the main COX-2-derived mediator in the later stages, corresponding with the healing process (Zamuner et al., 2003). It has been suggested that the latter may play an anti-inflammatory role (Ajuebor et al., 2000). It should be noted that COX-1 is also involved in prostaglandin generation in inflammation, and other eicosanoids such as lipoxins may exert anti-inflammatory/tissue repair functions (Weylandt et al., 2007).
Based on these assumptions, it may be argued that the modulation of COX-2 expression may constitute a novel therapeutic strategy in inflammatory bowel disease. Flavonoids are natural compounds which are consumed as part of the normal human diet and exhibit intestinal anti-inflammatory activity, as demonstrated by ourselves and other groups. This effect has been ascribed to their anti-oxidative properties and on actions on different cell types involved in the inflammatory reaction, such as macrophages (Comalada et al., 2006), lymphocytes (López-Posadas et al., 2008) and enterocytes (Ruiz et al., 2007), and the inhibition of enzymes such as COX-2 itself. However, to the best of our knowledge the effects and structure-activity relationship for COX-2 induction in IECs had not been studied hitherto. We selected IEC18 cells because they constitute a non-tumorigenic cell line. COX-2 is expressed at very low levels in quiescent IEC18 but is readily induced by pro-inflammatory stimuli including oxidative stress and LPS.
The flavonoid structure consists of three phenolic rings, A, B and C, with a variety of substituents (see Table 1). Based on modifications of this basic structure, there are many subgroups or families of flavonoids. We have studied the effects of nine different flavonoids in order to identify structural requirements for each of the main activities examined (see Table 2). Flavonoids exerted distinct effects on COX-2 expression depending on the experimental setting. Thus all flavonols and flavones tested induced COX-2 in the basal state, with the exception of the methylated derivative diosmetin. The magnitude of this increase was similar to that induced by LPS and thus it must be considered relevant. The double bond between positions 2 and 3, the 2-position of the C ring and the presence of an intact 4′-OH group are the major determinants of activity, while 3- and 3′-hydroxylation are apparently without effect on this biological activity.
Table 2.
Summary of flavonoid activities
|
Basal |
LPS-induced |
|||||||
|---|---|---|---|---|---|---|---|---|
| COX-2 | NF-κB luciferase | COX-2 | IκBα-P | p50/p65 translocation | ERK-P | p38-P | JNK-P | |
| Flavonols | ||||||||
| Kaempferol | + | ++ | = | = | =/= | = | + | = |
| Quercetin | + | + | = | − | =/= | −−− | + | = |
| Flavones | ||||||||
| Apigenin | + | + | + | − | =/= | + | = | = |
| Chrysin | + | +++ | ++ | = | =/= | = | = | = |
| Diosmetin | = | ++ | +++ | = | =/= | + | = | = |
| Luteolin | + | + | = | = | =/= | = | + | = |
| Isoflavones | ||||||||
| Daidzein | = | +++ | = | = | =/= | = | = | = |
| Genistein | = | ++ | + | − | +/= | + | = | = |
| Flavanone | ||||||||
| Hesperetin | = | ++ | = | − | =/− | − | + | = |
In the two first columns the effect of flavonoid treatment is compared with basal effect (no stimulation) and in the rest of the columns it is compared with the LPS-treated control. Only statistically significant effects are indicated.
=, no significant modification; +, mild increase; ++, moderate increase; +++, great increase; −, moderate inhibition; −−−, great inhibition; COX, cyclooxygenase; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide.
In contrast, most flavonoids, including kaempferol, quercetin and luteolin, which exhibited the greatest induction effect in quiescent cells, did not increase COX-2 levels as induced by LPS and actually tended to decrease them, as they were not significantly different from the control. Conversely, chrysin and diosmetin had the opposite, enhancing effect. Flavones are the only flavonoids that favour COX-2 expression under LPS stimulation, with the important exception of luteolin (perhaps because hydroxylation may be detrimental). This suggests that 4′-methoxylation is important for this activity. In addition, the 2-3 double bond is necessary for activity (diosmetin vs. hesperetin), as well as the lack of 3-OH characteristic of flavonols. We focused on the mechanistic aspects of flavonoid modulation of COX-2 expression.
Previous studies have shown that JNK, p38 and ERK1/2 (Cario et al., 2000) and NF-κB (Kim et al., 2005; Cheon et al., 2006) are involved in LPS activation and signal transduction in IECs. In particular, COX-2 has been reported to be regulated by NF-κB at the posttranscriptional level and by p38 MAPK in IECs (Grishin et al., 2006; Yan et al., 2006). Thus we examined the effects of flavonoids on these signalling pathways. The results obtained were to a great extent surprising. Thus, our data indicate that: (i) flavonoids show structure-dependent effects on ERK/p38/JNK phosphorylation; (ii) several flavonoids prevent IκB phosphorylation completely; (iii) none of the flavonoids prevented p50/p65 nuclear translocation, and genistein actually increased it; and (iv) flavonoids generally augment NF-κB dependent gene transcription (luciferase reporter data). The structural requirements for some of these activities can be delineated. For inhibition of IκB-α phosphorylation, the only structural features required that can be clearly established are the presence of 4′-OH (apigenin vs. chrysin) and 5-OH (genistein vs. daidzein), while the position of the B ring (genistein vs. apigenin) and 4′-methoxylation (diosmetin vs. luteolin) are irrelevant. The 2-3 double bond seems to impair the activity (diosmetin vs. hesperetin). The contribution of the 3-OH group is obscure, because it favours inhibition in one case (quercetin vs. luteolin), but hinders it in the other (kaempferol vs. apigenin).
Similarly, the double bond 2-3 (diosmetin vs. hesperetin), B ring hydrolylation (apigenin vs. chrysin) and the 5-OH (genistein vs. daidzein) appear to promote ERK phosphorylation, while the 2/3 position of the B ring seems to be indifferent (genistein vs. apigenin). Double hydroxylation in the B ring impairs the effect (luteolin vs. apigenin) unless one of them is methoxylated (diosmetin). On the other hand, the 3-OH skews activity towards inhibition (kaempferol vs. apigenin), which is much enhanced by 3′-hydroxylation. For p38 inhibition, the 2- instead of the 1-hydroxyl group in the B ring (luteolin vs. apigenin) and 3-hydroxylation (kaempferol vs. apigenin) or, alternatively, total hydroxylation, are the key structural features for activity. Interestingly, the 2-3 double bond seems to oppose to this effect (diosmetin vs. hesperetin), suggesting that higher increases in p38 phosphorylation are feasible. The influence of the regular or iso position of the B ring cannot be assessed based on current data.
The NF-κB-related data are particularly intriguing, because flavonoids have been usually shown to down-regulate this pathway (Comalada et al., 2006). Because NF-κB translocation and transcriptional regulation takes place even with total blockade of IκB-α phosphorylation, it must be assumed that IEC18 cells do not depend on the classical pathway for the regulation of NF-κB target genes. Alternative routes have been long known to exist in some cell types (Kawai and Akira, 2007). We examined the possible role of the Akt pathway, but it is apparently not involved. An additional unexpected result was the finding that Bay 11-7082, a specific inhibitor of the classical pathway, increased COX-2 expression in spite of complete inhibition of IκB-α phosphorylation. Bay 11-7082 is considered an inhibitor of IκB kinase (IKK)-β/α, but it can also potentially activate p38, JNK1 and tyrosine phosphorylation (Pierce et al., 1997; Gasparian et al., 2009). It has been shown recently that the composition of the NF-κB dimers which translocate to the nucleus may be affected by pharmacological modulation. Thus, blockade of the proteasome inhibits the formation of both p50/p65 and p50/p50 dimers, while IKK blockade only decreases the heterodimer (Gasparian et al., 2009). Indeed, p65 translocation was reduced to a greater extent than that of p50 by Bay 11-7085 in our study (Figure 5). Because quercetin only augmented p50 nuclear levels and it also increased basal COX-2 expression in basal conditions, increased translocation of p50/p50 homodimers may account for this effect in both cases. Although this form of NF-κB is commonly associated with repression of transcription, it has also been reported to activate transcription. Conversely, quercetin (and possibly other flavonoids) would tend to decrease LPS-evoked COX-2 transcription in part through the effect on not only IκB-α phosphorylation but also Akt and perhaps other targets, some of which are shown in Figure 8. For instance, quercetin has been shown to down-regulate signalling through Toll-like receptor 4 (TLR4) via changes in lipid rafts (Kaneko et al., 2008). Ultimately, the overall effect of flavonoids on COX-2 expression and NF-κB driven transcription would depend on the balance between the different molecular targets. Further support for this hypothesis comes from the poor correlation between inhibition of IκB-α phosphorylation and COX-2 expression.
Alternatively, the paradoxical effect of Bay 11-7082 may be interpreted to indicate a dual role of NF-κB on COX-2 expression, including an inhibitory influence in addition to the known stimulatory effect. This is an unlikely possibility. On the other hand, none of the MAPK inhibitors, which have been previously shown to work effectively in multiple cell types including IEC18 cells (Grishin et al., 2006; Yan et al., 2006), had any effect on COX-2. Thus it is unlikely that these pathways are involved in the regulation of COX-2 expression.
Whatever the exact mechanism, it is clear that flavonoids modulate COX-2 expression with effects depending on flavonoid structure and co-stimuli. The effect in vivo is difficult to predict, but we may speculate that some flavonoids may enhance COX-2 expression and prostaglandin generation in normal or minimally inflammatory conditions but have no effect or even down-regulate it in conditions of intense oxidative stress, as in full blown inflammatory reactions.
Acknowledgments
This work was supported by grants from the Instituto de Salud Carlos III (PI051625 and PI051651) and the Ministry of Science and Innovation (SAF2008-01432 and AGL2008-04332). The CIBERehd is funded by the Instituto de Salud Carlos III. IB and RLP are funded by the Ministry of Science and Innovation. CM is funded by CIBERehd. The group is member of the Network for Cooperative Research on Membrane Transport Proteins (REIT), co-funded by the Ministerio de Educación y Ciencia, Spain and the European Regional Development Fund (Grant BFU2007-30688-E/BFI).
Glossary
Abbreviations
- COX
cyclooxygenase
- IEC
intestinal epithelial cell
- IKK
IκB kinase
- JNK1
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NSAIDs
non-steroidal anti-inflammatory drugs
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effects of flavonoids on viability of IEC18 cells. IEC18 cells were cultured with nine different flavonoids (50 μM) or the positive control for cell toxicity, anisomycin (Anis), for 24 h and processed as described in Methods. Results were expressed as percent-increase comparing with vehicle-treated cells (mean ± SEM). +P < 0.05 versus control.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Ajuebor MN, Singh A, Wallace JL. Cyclooxygenase-2-derived prostaglandin D(2) is an early anti-inflammatory signal in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G238–G244. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester I, Camuesco D, Gálvez J, Sánchez de Medina F, Zarzuelo A. Flavonoids and inflammatory bowel disease. Ars Pharmaceutica. 2006;47:5–21. [Google Scholar]
- Blonska M, Bronikowska J, Pietsz G, Czuba ZP, Scheller S, Krol W. Effects of ethanol extract of propolis (EEP) and its flavones on inducible gene expression in J774A.1 macrophages. J Ethnopharmacol. 2004;91:25–30. doi: 10.1016/j.jep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Bonnekoh B, Wevers A, Jugert F, Merk H, Mahrle G. Colorimetric growth assay for epidermal cell cultures by their crystal violet binding capacity. Arch Dermatol Res. 1989;281:487–490. doi: 10.1007/BF00510085. [DOI] [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- Cheon JH, Kim JS, Kim JM, Kim N, Jung HC, Song IS. Plant sterol guggulsterone inhibits nuclear factor-kappaB signaling in intestinal epithelial cells by blocking IkappaB kinase and ameliorates acute murine colitis. Inflamm Bowel Dis. 2006;12:1152–1161. doi: 10.1097/01.mib.0000235830.94057.c6. [DOI] [PubMed] [Google Scholar]
- Comalada M, Ballester I, Bailon E, Sierra S, Xaus J, Galvez J, et al. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Eckmann L, Stenson WF, Savidge TC, Lowe DC, Barrett KE, Fierer J, et al. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. Bacterial entry induces epithelial prostaglandin h synthase-2 expression and prostaglandin E2 and F2alpha production. J Clin Invest. 1997;100:296–309. doi: 10.1172/JCI119535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai M, Blandizzi C, Colucci R, Antonioli L, Bernardini N, Segnani C, et al. Role of cyclooxygenases 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut. 2005;54:608–616. doi: 10.1136/gut.2004.053322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez J, Cruz T, Crespo E, Ocete MA, Lorente MD, Sanchez de Medina F, et al. Rutoside as mucosal protective in acetic acid-induced rat colitis. Planta Med. 1997;63:409–414. doi: 10.1055/s-2006-957723. [DOI] [PubMed] [Google Scholar]
- Gasparian AV, Guryanova OA, Chebotaev DV, Shishkin AA, Yemelyanov AY, Budunova IV. Targeting transcription factor NFB: comparative analysis of proteasome and IKK inhibitors. Cell Cycle. 2009;8:1559–1566. doi: 10.4161/cc.8.10.8415. [DOI] [PubMed] [Google Scholar]
- Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, et al. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol. 2006;176:580–588. doi: 10.4049/jimmunol.176.1.580. [DOI] [PubMed] [Google Scholar]
- Inan MS, Tolmacheva V, Wang QS, Rosenberg DW, Giardina C. Transcription factor NF-kappaB participates in regulation of epithelial cell turnover in the colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1282–G1291. doi: 10.1152/ajpgi.2000.279.6.G1282. [DOI] [PubMed] [Google Scholar]
- Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- Jobin C, Morteau O, Han DS, Balfour Sartor R. Specific NF-kappaB blockade selectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology. 1998;95:537–543. doi: 10.1046/j.1365-2567.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Takimoto H, Sugiyama T, Seki Y, Kawaguchi K, Kumazawa Y. Suppressive effects of the flavonoids quercetin and luteolin on the accumulation of lipid rafts after signal transduction via receptors. Immunopharmacol Immunotoxicol. 2008;30:867–882. doi: 10.1080/08923970802135690. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kim JS, Narula AS, Jobin C. Salvia miltiorrhiza water-soluble extract, but not its constituent salvianolic acid B, abrogates LPS-induced NF-kappaB signalling in intestinal epithelial cells. Clin Exp Immunol. 2005;141:288–297. doi: 10.1111/j.1365-2249.2005.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KH, Murakami A, Tanaka T, Ohigashi H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem Pharmacol. 2005;69:395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Longo WE, Panesar N, Mazuski J, Kaminski DL. Contribution of cyclooxygenase-1 and cyclooxygenase-2 to prostanoid formation by human enterocytes stimulated by calcium ionophore and inflammatory agents. Prostaglandins Other Lipid Mediat. 1998;56:325–339. doi: 10.1016/s0090-6980(98)00058-6. [DOI] [PubMed] [Google Scholar]
- López-Posadas R, Ballester I, Abadía A, Suárez MD, Zarzuelo A, Martínez-Augustin O, et al. Effect of flavonoids on rat splenocytes, a structure-activity relationship study. Biochem Pharmacol. 2008;76:495–506. doi: 10.1016/j.bcp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- MacNaughton WK, Cushing K. Role of constitutive cyclooxygenase-2 in prostaglandin-dependent secretion in mouse colon in vitro. J Pharmacol Exp Ther. 2000;293:539–544. [PubMed] [Google Scholar]
- Mochizuki M, Hasegawa N. Therapeutic efficacy of pycnogenol in experimental inflammatory bowel diseases. Phytother Res. 2004;18:1027–1028. doi: 10.1002/ptr.1611. [DOI] [PubMed] [Google Scholar]
- Newberry RD, Stenson WF, Lorenz RG. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- Ohtsuka Y, Lee J, Stamm DS, Sanderson IR. MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut. 2001;49:526–533. doi: 10.1136/gut.49.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Reinecker HC, Podolsky DK. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1995;92:8353–8357. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz PA, Braune A, Holzlwimmer G, Quintanilla-Fend L, Haller D. Quercetin inhibits TNF-induced NF-kappaB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J Nutr. 2007;137:1208–1215. doi: 10.1093/jn/137.5.1208. [DOI] [PubMed] [Google Scholar]
- Sanchez de Medina F, Galvez J, Romero JA, Zarzuelo A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J Pharmacol Exp Ther. 1996;278:771–779. [PubMed] [Google Scholar]
- Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stenson WF. Prostaglandins and epithelial response to injury. Curr Opin Gastroenterol. 2007;23:107–110. doi: 10.1097/MOG.0b013e3280143cb6. [DOI] [PubMed] [Google Scholar]
- Wallace JL. COX-2: a pivotal enzyme in mucosal protection and resolution of inflammation. ScientificWorldJournal. 2006;6:577–588. doi: 10.1100/tsw.2006.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weylandt KH, Kang JX, Wiedenmann B, Baumgart DC. Lipoxins and resolvins in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:797–799. doi: 10.1002/ibd.20109. [DOI] [PubMed] [Google Scholar]
- Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181–187. [PubMed] [Google Scholar]
- Yan SR, Joseph RR, Wang J, Stadnyk AW. Differential pattern of inflammatory molecule regulation in intestinal epithelial cells stimulated with IL-1. J Immunol. 2006;177:5604–5611. doi: 10.4049/jimmunol.177.8.5604. [DOI] [PubMed] [Google Scholar]
- Zamuner SR, Warrier N, Buret AG, MacNaughton WK, Wallace JL. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714–1720. doi: 10.1136/gut.52.12.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


