Abstract
Background and purpose:
The present study evaluated the role of CB2 receptors in the regulation of depressive-like behaviours. Transgenic mice overexpressing the CB2 receptor (CB2xP) were challenged with different types of acute and chronic experimental paradigms to evaluate their response in terms of depressive-like behaviours.
Experimental approach:
Tail suspension test (TST), novelty-suppressed feeding test (NSFT) and unpredictable chronic mild stress tests (CMS) were carried out in CB2xP mice. Furthermore, acute and chronic antidepressant-like effects of the CB2 receptor-antagonist AM630 were evaluated by means of the forced swimming test (FST) and CMS, respectively, in wild-type (WT) and CB2xP mice. CB2 gene expression, brain-derived neurotrophic factor (BDNF) gene and protein expressions were studied in mice exposed to CMS by real-time PCR and immunohistochemistry, respectively.
Key results:
Overexpression of CB2 receptors resulted in decreased depressive-like behaviours in the TST and NSFT. CMS failed to alter the TST and sucrose consumption in CB2xP mice. In addition, no changes in BDNF gene and protein expression were observed in stressed CB2xP mice. Interestingly, acute administration of AM630 (1 and 3 mg·kg−1, i.p.) exerted antidepressant-like effects on the FST in WT, but not in CB2xP mice. Chronic administration of AM630 for 4 weeks (1 mg·kg−1; twice daily, i.p.) blocked the effects of CMS on TST, sucrose intake, CB2 receptor gene, BDNF gene and protein expression in WT mice.
Conclusion and implications:
Taken together, these results suggest that increased CB2 receptor expression significantly reduced depressive-related behaviours and that the CB2 receptor could be a new potential therapeutic target for depressive-related disorders.
Keywords: CB2 receptor, depression, antidepressant, brain-derived neurotrophic factor, unpredictable chronic mild stress, behaviour, transgenic mice
Introduction
The limited efficacy of current antidepressant treatments justifies the development of alternative drugs. Recent pharmacological and genetic findings indicate that the endocannabinoid system is a target closely related to the regulation of mood disorders. In fact, genetic inactivation of CB1 receptors results in increased corticosterone levels under both basal conditions and in response to stressful stimuli (Steiner et al., 2008a,b;). Moreover, the cannabinoid CB1-receptor antagonists SR141716A (rimonabant) and AM251 have demonstrated antidepressant-like effects in animal models of depression (Shearman et al., 2003; Tzavara et al., 2003; Griebel et al., 2005; Witkin et al., 2005). In addition, 5-HT, noradrenergic (NA) and dopamine levels in the prefrontal cortex increase after the administration of rimonabant (Tzavara et al., 2003; Griebel et al., 2005; Witkin et al., 2005; Need et al., 2006). However, rimonabant has been paradoxically linked to increased risk of anxiety, depression and suicidal thoughts in the treatment of obesity disorders in humans (Le Foll et al., 2009; Moreira et al., 2009). Cannabinoid CB1 receptor-agonists and fatty acid amide hydrolase (FAAH) inhibitors induce antidepressant-like effects in the forced swimming test (FST) in rats (Adamczyk et al., 2008), which supports the idea of a pivotal role of the endocannabinoid system in the pathogenesis of depression. Chronic treatment with these drugs promotes neurogenesis in the hippocampus and enhanced central 5-HT and NA transmission (Bambico and Gobbi, 2008). In addition, different animal models of depression reveal significant increases in CB1 receptor density and function in the prefrontal cortex (Hill et al., 2008; Rodriguez-Gaztelumendi et al., 2009) that can be reversed by chronic fluoxetine treatment (Rodriguez-Gaztelumendi et al., 2009). Clinically, a significant up-regulation of CB1 receptor density and CB1 receptor-stimulated G-protein activation is found in the prefrontal cortex of depressive suicide victims (Hungund et al., 2004).
Initially, CB2 receptors were first identified in the rat spleen and leukocyte subpopulation in humans (Munro et al., 1993; Galiegue et al., 1995). In addition, CB2 receptors were identified in the brain only under pathological conditions such as senile plaques in Alzheimer's disease (Benito et al., 2003), activated microglial cells/macrophages in multiple sclerosis (Yiangou et al., 2006), the spinal cord in amyotrophic lateral sclerosis (Yiangou et al., 2006) and in the vicinity of tumours (Guzman et al., 2001). CB2 receptors were recently found under normal conditions in the brainstem of rat, mouse and ferret (Van Sickle et al., 2005). Further studies in the rat have identified a wide distribution of CB2 receptors in different brain areas, including the spinal nucleus, hippocampus, olfactory nucleus, cerebral cortex, amygdala, striatum, thalamus and cerebellum (Gong et al., 2006; Onaivi, 2006). The identification of CB2 receptor gene expression in these brain regions predicts the role of these receptors in a wide variety of physiological functions. In fact, the presence of CB2 receptors in the thalamus, dorsal root ganglion neurones and microglia or neurones in the spinal cord seems to be involved in the antinociceptive activity of a CB2 receptor-selective agonist (Pertwee, 2009). In addition, its expression in microglial cells in the central nervous sytem has been associated with a variety of inflammatory processes (Cabral et al., 2008; Lunn et al., 2008). Interestingly, the presence of CB2 receptors in areas related to the response to stress, anxiety and depression, such as hippocampus and amygdala, suggests that they may be involved in the regulation of mood disorders.
Interestingly, a reduction of CB2 receptors in the striatum, midbrain and hippocampus was reported in animal models of depression (Onaivi et al., 2008). Conversely, intracerebroventricular microinjection of cannabinoid CB2 antisense oligonucleotide induces anxiolytic-like effects (Onaivi et al., 2006). In addition, an association between cannabinoid CB2 receptor polymorphism Q63R has been detected in Japanese depressed and alcoholic subjects (Onaivi et al., 2008).
In the current study, the responses of transgenic mice overexpressing this receptor (CB2xP mice) to acute [tail suspension test (TST), novelty-suppressed feeding test (NSFT)] and chronic depressogenic-like stimuli [chronic mild stress test (CMS) procedure] were evaluated. In addition, acute (FST) and chronic (CMS procedure) effects of AM630 were also examined. Furthermore, the influence of CB2 receptors on hippocampal brain-derived neurotrophic factor (BDNF) expression was examined in CB2xP mice and AM630-treated wild-type (WT) mice exposed to CMS.
The results found led us to speculate that overexpression of CB2 receptors produces an endophenotype resistant to acute and chronic depressive-like behaviours and basal alterations in BDNF gene expression in the hippocampus. In addition, acute and chronic treatment with AM630 resulted in antidepressant-like effects and reversed the reduction of CB2 receptor gene expression, BDNF gene and protein expression induced in the hippocampus of mice exposed to CMS.
Methods
All nomenclature of receptors mentioned in the present manuscript conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Animals
Male mice overexpressing CB2 receptors (CB2xP) on a swiss ICR congenic background, were prepared in our laboratory as described elsewhere (Racz et al., 2008b), and their corresponding littermates, swiss ICR (WT mice) were used in all experiments. Male CB2 knockout (CB2−/−) on a C57BL/6J congenic background (kindly provided by Nancy E. Buckley, Cal State Polytechnic Univ., Pomona, CA, USA) were used. CB2−/− founders were crossed with outbred CD1 (Charles River, France) background. Homozygotes from CB2xP and CB2−/− were selected for our experiments. Mice were housed in groups. At the beginning of the experiments, mice were between 2 and 3 months old and weighed 25–35 g. All animals were kept at controlled temperature (23 ± 2°C) and light conditions (light/dark cycle switching at 8.00 h and 20.00 h). All studies were conducted in compliance with Spanish Royal Decree 223/1998 of 14 March (BOE. 8 18), the Ministerial Order of 13 October 1989 (BOE 18) and European Council Directive of 24 November 1986 (86/609/EEC) regulating the care of experimental animals.
Drugs
AM630 (6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone) was obtained from Tocris (Biogen, Madrid, Spain) and dissolved in DMSO : Tween 80 : distilled water (1:1:8) immediately before use (Pertwee et al., 1995; Hosohata et al., 1997). In acute experiments, the drug was administered at 1 and 3 mg·kg−1 (0.3 mL, i.p.) 30 min before the corresponding experimental test. At the end of the 4-week CMS, mice were assigned to different groups, so the initial coat state and body weight were equivalent in all groups. The drug was given twice daily (9.00h and 18.00 h) at 1 mg·kg−1 (0.3 mL, i.p.) for 4 weeks.
Behavioural analyses
All the experiments were analysed by observers blind to treatment and genotype.
TST
Mice were individually suspended by the tail at the edge of a lever suspended above the table top (the distance to the table surface was 35 cm), affixed with adhesive tape placed approximately 1–2 cm from the tip of the tail (Vaugeois et al., 1997). The duration of immobility was measured for 6 min. In this situation, mice develop escape-orientated behaviours interspersed with temporally increasing bouts of immobility.
NSFT
The NSFT was used to measure anxiety-induced hyponeophagia, the inhibition of ingestion and approach to food when exposed to an anxiety-provoking novel environment. The testing apparatus consisted of a square, transparent methacrylate cage 40 × 40 × 50 cm (Surget et al., 2008). At the time of testing, a single pellet of food was placed on a white paper platform in the centre of the cage. Before the experiment, mice were deprived of food for 24 h, and then each individual mouse was placed in the corner of the apparatus. The latent time before the mouse started to eat the pellet was recorded up to a period of 5 min. Once the mice started to eat, the total amount of food pellet (g) was measured during a period of 5 min. Anxiogenics and anxiolytics increase and decrease, respectively, approach and feeding latency (Bodnoff et al., 1988; Gross et al., 2000).
FST
The FST has been used as a model predictive of antidepressant action (Porsolt et al., 1977). Briefly, each mouse was placed for 15 min in a vertical Plexiglas cylinder (height 25 cm diameter 18 cm) containing water to a depth of 15 cm at 25 ± 1°C (Cryan et al., 2002). After 24 h, animals were placed in the cylinder again and the duration of immobility was measured for a period of 5 min. Only active swimming, not floating movements, was taken into account for the immobility measurement.
Chronic unpredictable mild stress
Mice were subjected to CMS for a period of 7–8 weeks (Willner et al., 1992b; Willner, 1997). Mice were subjected several times a day to one or more of the following stressful stimuli (stressors): wet cage, food deprivation, restraint stress, period of stroboscopic illumination (150 flashes·min−1), inversion of light/dark cycle, tilted cage (45°) and loud noise (90–105 db). All stressors and/or sequences were applied at different time points to avoid habituation and to add an element of unpredictability to the stressor (Table 1).
Table 1.
Chronic unpredictable mild stress procedure
| Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday | |
|---|---|---|---|---|---|---|---|
| Week 1 | 10–12 h Loud noise 14–17 h Stroboscopic illumination 18–19 h Restraint stress | 10–11 h Restraint stress 14–16 h Loud noise 19–8.30 h Food deprivation | 10–13.30 h Stroboscopic illumination 16–17 h Restraint stress 19–8.30 h (+1 day) Tilted cage | 10–11 h Restraint stress 14–16 h Loud noise 19–8.30 h Wet cage | 10–13 h Stroboscopic illumination 16–17 h Restraint stress 19–19 h (+1 day) Tilted cage | 19–8.30 h Food deprivation | 8.30–8.30 h (+1 day) Inversion light/dark cycle |
| Week 2 | 10–11 h Restraint stress 14–17 h Stroboscopic illumination 19–8.30 h (+1 day) Wet cage | 10–13.30 h Stroboscopic illumination 16–17 h Restraint stress 19–8.30 h (+1 day) Tilted cage | 10–12 h Loud noise 14–17 h Stroboscopic illumination 18–19 h Restraint stress | 8.30–13 h Behavioural test = Light/dark box 15–17 h Loud noise 18–19 h Restraint stress | 10–13.30 h Stroboscopic illumination 19–8.30 h (+1 day) Food deprivation | 8.30–19 h Inversion light/dark cycle | 19–8.30 h (+1 day) Wet cage |
| Week 3 | 10–12 h Loud noise 14.30–15.30 h Restraint stress 19–8.30 h (+1 day) Tilted cage | 10–11 h Restraint stress 14–17 h Stroboscopic illumination 17.30–19.30 h Loud noise | 10–12 h Loud noise 14–15 h Restraint stress 19–8.30 h (+1 day) Food deprivation | 8.30–13 h Behavioural test = Elevated plus maze 15–18 h Stroboscopic illumination 19–8.30 h (+1 day) Wet cage | 10–13.30 h Stroboscopic illumination 15–16 h Restraint stress 17–19 h Loud noise | 8.30–19 h Inversion light/dark cycle | 19–8.30 h (+1 day) Food deprivation |
| Week 4 | 8.30–13 h Behavioural test = Tail suspension test 14–16 h Loud noise 18–19 h Restraint stress | 10–13.30 h Stroboscopic illumination 16–17 h Restraint stress 19–8.30 h (+1 day)Tilted cage | 10–11 h Restraint stress 14–17 h Stroboscopic illumination 19–8.30 h (+1 day) Wet cage | 10–12 h Loud noise 15–18 h Stroboscopic illumination | 10–12 h Loud noise 16–17 h Restraint stress 19–19 h (+1 day) Tilted cage | 19–8.30 h (+1 day) Food deprivation | 8.30–8.30 h (+1 day) Inversion light/dark cycle |
| Week 5 | 10–11 h Restraint stress 14–16 h Loud noise 19–8.30 h (+1 day) Wet cage | 11–14.30 h Stroboscopic illumination 16–18 h Loud noise | 10–11 h Restraint stress 14–16 h Loud noise 19–8.30 h (+1 day) Tilted cage | 11–14.30 h Stroboscopic illumination 16–17 h Restraint stress 19–8.30 h (+1 day) Wet cage | 10–12 h Loud noise 14–17 h Stroboscopic illumination 18–19 h Restraint stress | 8.30–18.00 h Tilted cage 19–8.30 h (+1 day) Food deprivation | 8.30–8.30 h (+1 day) Inversion light/dark cycle |
| Week 6 | 10–12 h Loud noise 16–17 h Restraint stress 19–8.30 h (+1 day) Wet cage | 10–13.30 h Stroboscopic illumination 16–17 h Restraint stress 19–8.30 h (+1 day) Tilted cage | 10–12 h Loud noise 14–15 h Restraint stress 19–8.30 h (+1 day) Food deprivation | 10–11 h Restraint stress 14–16 h Loud noise 19–13 h (+1 day) Food and water deprivation | 13–14 h Behavioural test = Sucrose intake |
Sucrose consumption test
Sucrose intake (1% sucrose solution) was measured after 18 h of food and water deprivation during a period of 1 h (Li et al., 2007). Consumption of sucrose solution was estimated simultaneously in the control and experimental groups by comparing bottle weight before and after the 1-h window. Sucrose intake was expressed as mg sucrose·g−1 body weight.
Analysis of CB2 receptor and BDNF gene expressions
Mice were decapitated 12–14 h after the last administration of AM630 or exposure to stressful stimuli. Brains were removed from the skull and frozen over dry-ice and stored at −80°C until the day of the assay. Brain sections of 500 µm were cut at different levels containing the regions of interest according to Paxinos and Franklin (Paxinos and Franklin, 2001). Sections were mounted on slides and stored at −80°C. One section of each level was dissected following the method described by Palkovits (Palkovits, 1983). Total RNA was obtained from brain punches using Biozol® Total RNA extraction reagent (Bioflux, Inilab, Madrid, Spain). After DNAse digestion, reverse transcription was carried out following the instructions of the manufacturer (Epicentre, Tech. Corp., Madison, Wisconsin). CB2 receptor (Mm 00438286_m1) and BDNF (Mm00432069_m1) gene expression were measured using Taqman Gene Expression assay (Applied Biosystems, Madrid, Spain) as a double-stranded DNA-specific fluorescent dye and performed on the ABI PRISM 7700 Real Time Cycler (Applied Biosystems, Madrid, Spain). The reference gene used was 18S rRNA, detected using Taqman ribosomal RNA control reagents. Briefly, the data for each target gene were normalized to the endogenous reference gene, and the fold change in target gene abundance was determined using the 2−ΔΔCt method (Livak and Schmittgen, 2001). This quantitative method involves comparing the Ct values of the samples of interest with a control or calibrator, such as an untreated sample or RNA from normal tissue. The Ct values of both the calibrator and the samples of interest were normalized to an appropriate endogenous housekeeping gene.
Immunohistochemistry
After 12–14 h of the last AM630 administration and/or exposure to stressful stimuli, CB2xP and WT mice (n = 3–5 per group) were anaesthetized with ketamine/xylacine (2:1 v/v, 0.2 mL, i.p.), and intracardially perfused with 200 mL of 4% paraformaldehyde in phosphate buffer (PB; 0.1 M, pH 7.4). Brains were dissected, postfixed in the same fixative solution overnight at 4°C, frozen and cut into coronal 50-µm sections using a vibratome. For CB2 receptors, floating sections were pre-incubated with 50 mM sodium citrate pH 9, for 30 min at 80°C, washed three times with phosphate-buffered saline (PBS; 0.1 M, pH 7.3). Sections were then incubated with 1% hydrogen peroxide in PBS for 20 min at room temperature to inhibit endogenous peroxidase, and washed three times with PBS. They were incubated for 1 h in 10% normal goat serum (NGS) in PBS and 0.3% triton X-100, at room temperature. Sections then were incubated with primary CB2 receptor antibody obtained from Cayman Chemicals (MI, USA), diluted 1:500 in PBS + 0.3% triton X-100, overnight at room temperature, rinsed, incubated for 1 h at room temperature in a 1:500 dilution of biotinylated goat anti-rabbit secondary antibody (Vector, Burlingame, CA, USA) in PBS + 0.3% triton X-100, rinsed, incubated in extravidin-peroxidase (Sigma-Aldrich, Madrid, Spain), diluted 1:2000 in PBS + 0.2% triton X-100, for 1 h at room temperature, rinsed, and finally incubated in a solution containing 0.05% diaminobenzidine-nickel (DAB-Ni) (Sigma-Aldrich, Madrid, Spain) and 0.003% hydrogen peroxide for colour deposition. Sections were mounted on coated slides, dehydrated, cover slipped, viewed and photographed using Zeiss and Leitz microscopes and a Nikon digital camera. Images were edited using Photoshop (vCS3; Adobe systems) and quantified using the public domain, Java-based image processing program, for image acquisition after background subtraction. Brain sections from CB2−/− mice (kindly provided by Nancy E. Buckley, Cal State Polytechnic Univ., Pomona, CA, USA) were used to control for primary antibody specificity.
For BDNF, floating sections were washed three times with PBS, and then incubated with 1% hydrogen peroxide in methanol: PBS (1:1) for 15 min at room temperature to inhibit endogenous peroxidase, washed three times with PBS + 0.2% triton X-100 (PBS-T), incubated for 1 h in 10% NGS in PBS-T at room temperature. The sections were then rinsed and incubated in primary BDNF antibody obtained from Chemicon (Temecula, CA, USA), diluted 1:100 in PBS-T, overnight at 4°C. The sections were rinsed, incubated for 1.5 h at room temperature in 1:200 dilution of biotinylated goat anti-rabbit secondary antibody (Vector, Burlingame, CA, USA) in PBS-T. The sections were rinsed again, incubated in extravidin-peroxidase (Sigma-Aldrich, Madrid, Spain) diluted 1:500 in PBS-T for 1.5 h at room temperature. Finally, sections were rinsed and incubated in a solution containing 0.05% diaminobenzidine (Sigma-Aldrich, Madrid, Spain) and 0.003% hydrogen peroxide for colour deposition. Sections were mounted and analysed as described above.
Statistical analyses
Statistical analyses were performed using Student's t-test when comparing two groups and one-way or two-way analysis of variance followed by the Student–Newman–Keul's test when comparing three or four groups. Differences were considered significant if the probability of error was less than 5%. Data are presented as mean ± SEM. SigmaStat 3.1 software was used for all statistical analyses.
Results
Characterization of CB2 receptor expression
Analysis of CB2 receptor gene expression
The analysis of the different brain punches in WT mice by Rt-PCR revealed the presence of CB2 receptor gene expression in almost all the nuclei examined. The results are expressed with respect to those in the CPu, which was considered to be 100% (arbitrarily). CB2 receptor gene expression was significantly higher in Acc (137%), Amy (107%), VMN (90%), Sn (224.62%) and MnR (84%) compared with CPu (see Table 2 and Figure 1A). Interestingly, CB2xP mice exhibited significantly increased CB2 receptor mRNA levels in all the regions analysed compared with WT mice [Student's t-test, CPu t =−3.047, P < 0.011, 11 d.f. (150%); Acc t =−4.590, P = 0.001, 9 d.f. (180%); Cg t =−6.125, P < 0.001, 9 d.f. (199%); Amy t =−1.573, P = 0.05, 9 d.f. (64%); Hipp t =−2.464, P = 0.027, 14 d.f. (158%); VMN t =−2.863, P < 0.001, 9 d.f. (126%); ARC t =−2.184, P = 0.05, 9 d.f. (157%); Sn t =−3.36, P = 0.006, 11 d.f. (278%); VTA t = 3.786, P = 0.003, 10 d.f. (100%); DR t = 2.548, P = 0.031, 9 d.f. (50%); MnR t = 2.987, P = 0.014, 10 d.f. (57%)] (n = 6–7 per group) (Figure 1A).
Table 2.
CB2 gene expression (relative to CPu) in wide-type mice
| Regions | Student's t-test |
|---|---|
| Acc | t = −3.099, P < 0.011, 10 df* (137%) |
| Cg | t = −0.688, P < 0.505, 11 df |
| Amy | t = −2.410, P < 0.037, 10 df* (107%) |
| Hipp | t = −0.226, P < 0.825, 13 df |
| VMN | t = −2.849, P < 0.017, 10 df* (90%) |
| ARC | t = −1.847, P < 0.092, 11 df |
| Sn | t = −3.125, P < 0.010, 11 df* (224%) |
| VTA | Non detected |
| DR | t = −0.568, P = 0.582, 10 df |
| MnR | t = −2.190, P = 0.050, 11 df* (84%) |
Values from different brain regions of wild-type (WT) mice that are significantly different from CPu of WT mice.
Acc, nucleus accumbens; Amy, amygdala; ARC, arcuate nucleus of hypothalamus; Cg, cingulated cortex; CPu, caudate-putamen nucleus; Hipp, hippocampus; MnR, medial raphe; Sn, substantia nigra; VMN, ventromedial nucleus of hypothalamus; VTA, ventral tegmental area; DR, dorsal raphe.
Figure 1.
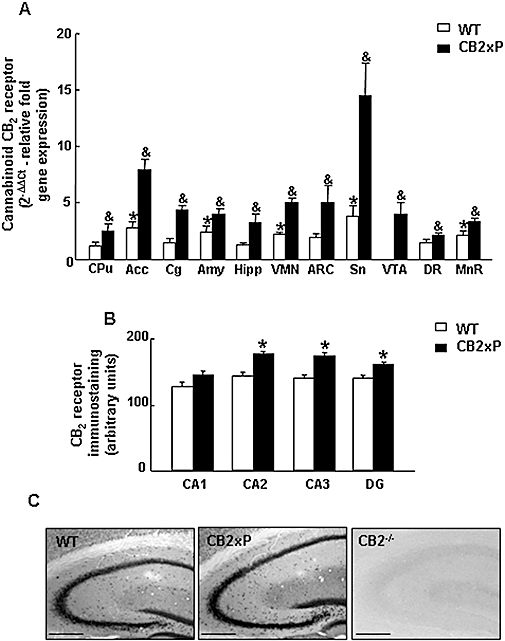
CB2 receptor gene expression in CB2xP and WT mice. (A) Relative CB2 receptor gene expression in different brain nuclei of CB2xP and WT mice by Rt-PCR (CPu: caudate-putamen nucleus, Acc: nucleus accumbens, Cg: cingulated cortex, Amy: amygdale, Hipp: hippocampus, VMN: ventromedial nucleus of hypothalamus, ARC: arcuate nucleus of hypothalamus, Sn: substantia nigra, VTA: ventral tegmental area, DR: dorsal raphe, MnR: medial raphe). Relative expression was calculated using the ΔΔCt method. Columns represent the means and vertical lines represent the SEM of relative CB2 receptor gene expression. *Values from different brain regions of WT mice that are significantly different from the CPu of WT mice. &Values from CB2xP mice in each brain region that differ significantly from the same region of WT mice (Student's t-test, P < 0.05) (n = 6–7 per group). (B) CB2 receptor immunostaining in different fields of the hippocampus in CB2xP and WT mice. DG: dentate gyrus. *Values from CB2xP mice that are significantly different from the same areas in WT mice (Student's t-test, P < 0.05) (n = 4 per group). (C) Representative immunostaining images for CB2 receptor protein in the hippocampus of WT, CB2xP and CB2−/− mice (Negative control). Bar represents 1 mm. CB2xP mice, transgenic mice overexpressing the CB2 receptor; WT, wild-type.
CB2 receptor protein expression in the hippocampus
The results revealed an increased expression of CB2 receptor protein in different fields of the hippocampus (CA2, CA3 and DG) in CB2xP mice compared with WT mice (Student's t-test CA2 t = 5.451, P = 0.002, 6 d.f.; CA3 t = 4.278, P = 0.005, 6 d.f.; DG t = 3.937, P = 0.008, 6 d.f.) (n = 4 per group) but no differences were detected in the CA1 field (Student's t-test t = 1.642, P = 0.152, 6 d.f.) (n = 4 per group) (Figure 1B,C). No immunostaining was detected in CB2−/− (Figure 1C).
Behavioural analyses
The responses of CB2xP and WT mice to acute and chronic anxiogenic and depressogenic-like stimuli were evaluated using TST, NSFT and CMS tests. CB2xP mice showed significantly less duration of immobility compared with WT mice during the TST (Student's t-test, t = 5.723, P < 0.001, 20 d.f.) (n = 12–14/group) (Figure 2A). CB2xP mice had a significantly shorter latency (Student's t-test, t = 2.463, P = 0.0023, 20 d.f.) and increased consumption of food pellets (g) compared with WT mice during NSFT (Student's t-test, t = −3.711, P = 0.001, 21 d.f.) (n = 11–12 per group) (Figure 2B).
Figure 2.
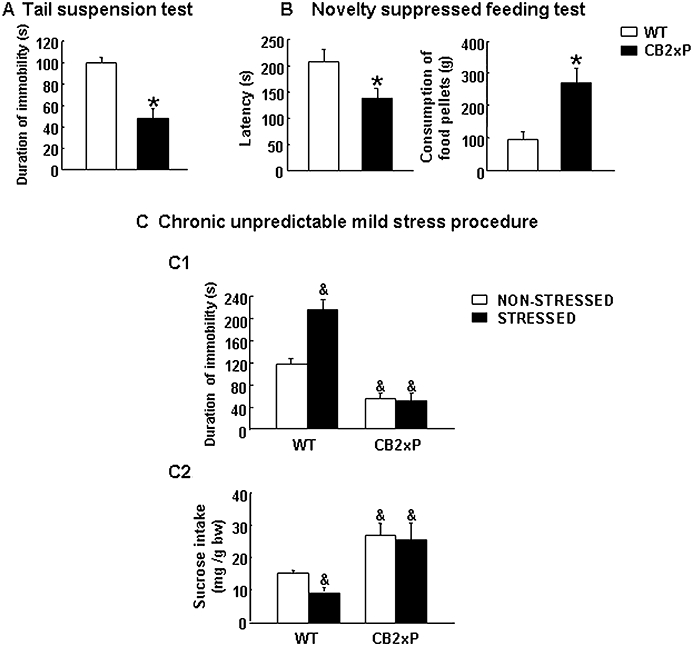
Assessment of depressive-like behaviours in CB2xP and WT mice in tail suspension (A), novelty-suppressed feeding (B) and chronic unpredictable mild stress paradigms (C). Columns represent the means and vertical lines SEM of (A) duration of immobility (s), (B) time of latency (s) and food pellet consumption (g) in 11–14 mice. *Values from CB2xP mice that differ significantly from WT mice (Student's t-test, P < 0.05). During the CMS procedure, depressive-like behaviours were evaluated using tail suspension (5 weeks) (C1) and sucrose intake tests (7 weeks) (C2) (See Methods). Columns represent the means and vertical lines represent the SEM of time of immobility (s) (C1) and sucrose intake (mg sucrose·g−1 body weight) (C2) (n = 12–16 mice per group). &Values from stressed WT mice, non-stressed and stressed CB2xP mice that are significantly different from those of non-stressed WT mice (P < 0.05, Student's t-test). Bw, body weight; CB2xP mice, transgenic mice overexpressing the CB2 receptor; CMS, chronic mild stress tests; WT, wild-type.
In CMS, depressive-like behaviours were evaluated at different time points using the TST (5 weeks) and sucrose intake tests (7 weeks). In the TST, stressed WT mice exhibited significant longer duration of immobility than non-stressed WT mice. In contrast, no differences were observed between stressed and non-stressed CB2xP mice (two-way anova followed by Student–Newman–Keul's: genotype F(1,61) = 18.109, P < 0.001; stress F(1,61) = 4.244, P = 0.044; genotype × stress F(1,61) = 5.564, P = 0.022) (n = 12–16 per group) (Figure 2C1).
At the end of 7 weeks of CMS, stressed WT mice displayed a significant reduction of sucrose intake (mg sucrose·g−1 body weight) compared with non-stressed WT mice. Interestingly, CMS failed to produce any alteration in the sucrose intake of CB2xP mice (two-way anova followed by Student–Newman–Keul's: genotype F(1,21) = 162.846, P < 0.001; stress F(1,21) = 15.419, P < 0.001; genotype × stress F(1,21) = 13.057, P = 0.002) (n = 12–16/group) (Figure 2C2).
Analysis of BDNF in CB2xP and WT mice exposed to CMS
The expression of BDNF in the hippocampus, especially in the dentate gyrus (DG), is down-regulated in response to chronic stress (Gronli et al., 2006). In the present study, the expression of the BDNF gene in the hippocampus of the CB2xP and WT mice exposed to CMS was measured by real-time PCR. As expected, stressed WT mice showed a significant reduction of BDNF gene expression compared with non-stressed WT mice. In contrast, CMS failed to produce any alteration in the expression of the BDNF gene in stressed CB2xP mice. Interestingly, showed significant the basal levels of BDNF mRNA were significantly higher in CB2xP mice than in WT mice (two-way anova followed by Student–Newman–Keul's: genotype F(1,29) = 67.337, P < 0.001; stress F(1,29) = 6.365, P = 0.018; genotype × stress F(1,29) = 0.026) (n = 6–8 per group) (Figure 3A).
Figure 3.
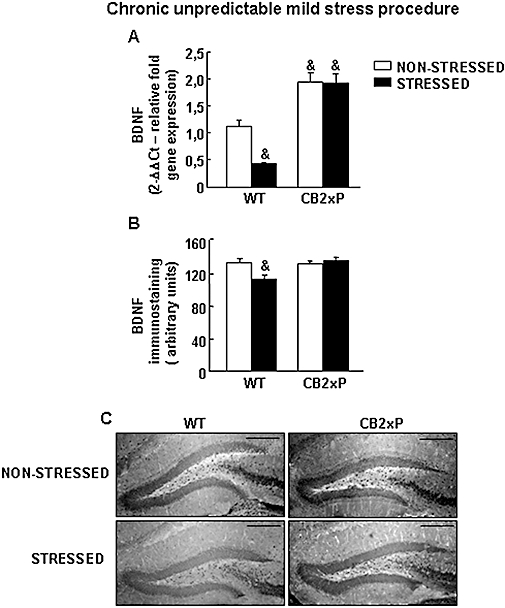
Brain-derived neurotrophic factor (BDNF) gene and protein expression in CB2xP and WT mice exposed to CMS. (A) Relative BDNF mRNA levels in the hippocampus of CB2xP and WT mice exposed to CMS. Columns represent the means and vertical lines represent the SEM of relative BDNF mRNA gene expression. &Values from non-stressed WT mice, stressed and non-stressed CB2xP mice that differ significantly from the non-stressed WT group (two-way anova followed by Student–Newman–Keul's: P < 0.05) (n = 5–7 per group). (B) Quantification of BDNF immunostaining in the hippocampus of CB2xP and WT mice exposed to CMS. Columns represent the means and vertical lines represent the SEM of relative BDNF protein (arbitrary units). &Values from stressed WT mice that are significantly different from non-stressed WT mice (two way anova, followed by Student–Newman–Keul's, P < 0.05) (n = 6–8 per group). (C) Representative immunostaining pictures for BDNF protein in hippocampus of CB2xP and WT mice exposed to CMS. Bar represents 1 mm. CB2xP mice, transgenic mice overexpressing the CB2 receptor; CMS, chronic mild stress tests; WT, wild-type.
The expression of BDNF protein was significantly reduced in the DG of stressed WT mice compared with non-stressed WT mice. In contrast, no alterations were found in CB2xP mice (two-way anova followed by Student–Newman–Keul's: genotype F(1,32) = 4.192, P = 0.050; stress F(1,32) = 3.355, P = 0.077; genotype × stress F(1,32) = 7.381, P = 0.011) (n = 5–6 per group) (Figure 3B,C).
Acute and chronic effects of cannabinoid CB2 receptor antagonist
The effects of acute AM630 administration on the response to depressogenic-like stimuli were evaluated in CB2xP and WT mice using FST. The results revealed that AM630 significantly decreases the duration of immobility in WT mice at 1 and 3 mg·kg−1 (one way anovaF(2,18) = 15.506, P < 0.001) (n = 7–9 per group) (Figure 4A, left panels). In contrast, AM630 failed to produce any change in CB2xP mice (one-way anovaF(2,12) = 0.120, P = 0.888) (n = 6–8 per group) (Figure 4A, right panels).
Figure 4.
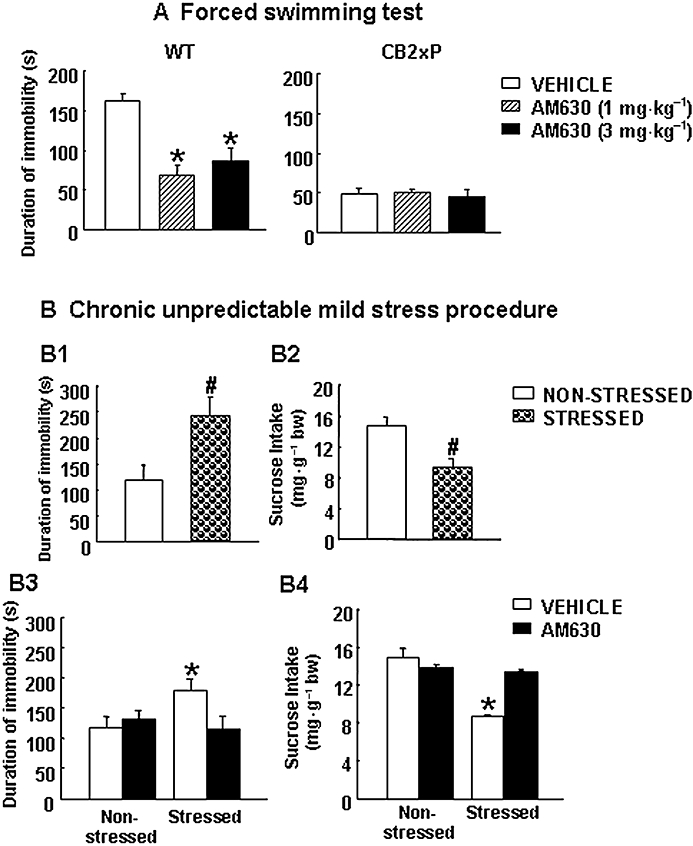
Acute and chronic effects of treatment with the cannabinoid CB2 receptor-antagonist AM630. (A) Acute dose-response effects of AM630 on forced swimming test in WT and CB2xP mice. Columns represent the means and vertical lines represent the SEM of duration of immobility (s). *Values from AM630-treated WT group (1 and 3 mg·kg−1, i.p.) that differ significantly from those of the vehicle-treated WT group (one-way anova followed by Student–Newman–Keul's: P < 0.05) (n = 6–8). (B) Chronic effects of AM630 treatment in WT mice exposed to the CMS paradigm. The depressive-like behaviours were evaluated in weeks 3 and 4 of the CMS paradigm, before chronic AM630 treatment using the tail suspension (B1) and sucrose intake (B2) tests, respectively (n = 24), and after chronic AM630 treatment using the tail suspension (B3) and sucrose intake (B4) tests, respectively (n = 9–11) (see Methods). Columns represent the means and vertical lines the SEM of duration of immobility (s) (B1, B3) and sucrose intake (mg sucrose·g−1 body weight) (B2, B4). #Values from the stressed WT group that differ significantly from those of the non-stressed WT group. *Values from the vehicle-treated stressed WT group that differ significantly from those of the vehicle-treated non-stressed WT group (two-way anova followed by Student–Newman–Keul's: P < 0.05). Bw, body weight; CB2xP mice, transgenic mice overexpressing the CB2 receptor; CMS, chronic mild stress tests; WT, wild-type.
In addition, the effects of chronic AM630 administration on the response to depressogenic-like stimuli were evaluated in WT mice exposed to CMS. Before administration of AM630, depressive-like behaviours were corroborated at different time points during CMS using the TST (3rd week) and sucrose intake tests (4th week). The results showed that the duration of immobility was significantly increased in stressed WT compared with non-stressed WT mice (Student's t-test, t = −2.664, P = 0.011, 33 d.f.) (n = 24 per group) (Figure 4B1). Sucrose intake was significantly reduced in stressed WT mice (Student's t-test, t = 4.766, P = 0.009, 4 d.f.) (n = 24 per group) (Figure 4B2). These results support the notion that the stressful situations during CMS did cause depressive-like behaviours.
Once CMS was established, chronic treatment with AM630 was started. In weeks 3 and 4 after the initiation of treatment, depressive-like behaviours were evaluated using the TST and the sucrose intake test respectively. As expected, vehicle-treated stressed WT mice had a longer duration of immobility compared with vehicle-treated non-stressed WT mice. In contrast, no differences were found between AM630-treated stressed WT mice and vehicle-treated non-stressed WT mice. In addition, the administration of AM630 failed to produce any alteration in non-stressed WT mice (two-way anova followed by Student–Newman–Keul's: stress F(1,30) = 1.562, P = 0.222; drug F(1,30) = 1.879, P = 0.182; stress × drug F(1,30) = 4.358, P = 0.046) (n = 9–11 per group) (Figure 4B3).
In week 4, vehicle-treated stressed WT mice exhibited a significant reduction in sucrose intake compared with vehicle-treated non-stressed WT mice. In contrast, AM630-treated stressed mice showed no differences in sucrose intake compared with non-stressed WT mice. On the other hand, the administration of AM630 failed to produce any alteration in non-stressed WT mice (two-way anova followed by Student–Newman–Keul's: stress F(1,21) = 42.042, P < 0.001; drug F(1,21) = 13.395, P = 0.002; stress × drug F(1,21) = 31.908, P < 0.001) (n = 9–11 per group) (Figure 4B4). These results suggest that 4 weeks of AM630 treatment resulted in a significant antidepressant-like effect.
Analysis of CB2 receptor and BDNF expression in WT mice exposed to the CMS procedure and treated with AM630
The expression of the CB2 receptor gene was significantly reduced in vehicle-treated stressed WT mice. Interestingly, this reduction was blocked by the administration of AM630 (two-way anova followed by Student–Newman–Keul's: stress F(1,29) = 0.291, P = 0.594; drug F(1,29) = 5.708, P = 0.594; stress × drug F(1,29) = 8.468, P = 0.007) (n = 6–9 per group) (Figure 5A).
Figure 5.
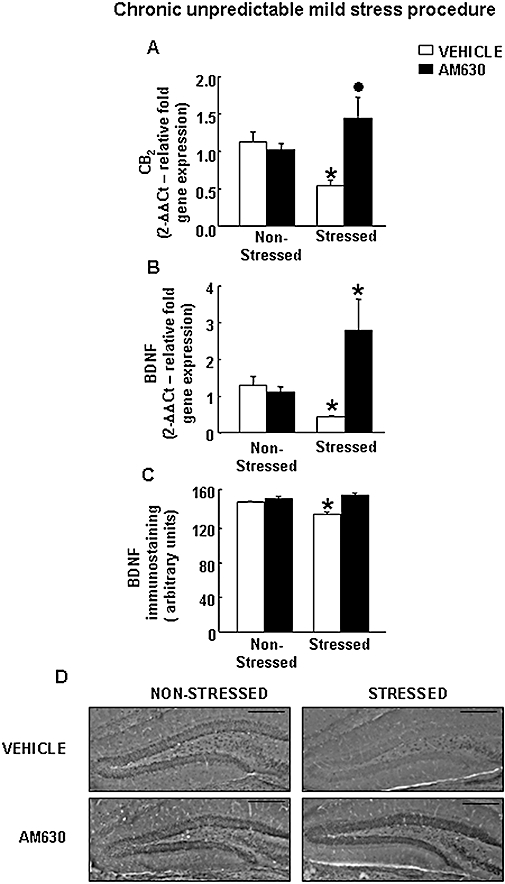
Effects of chronic AM630 treatment on CB2 receptor and BDNF in WT mice exposed to CMS. (A–B) Relative CB2 (A) and BDNF (B) mRNA levels in the hippocampus of AM630-treated and vehicle-treated WT mice exposed to CMS. Columns represent the means and vertical lines the SEM of relative CB2 and BDNF mRNA gene expression. *Values from the vehicle-treated stressed WT and AM630 stressed WT groups that differ significantly from those of the vehicle-treated non-stressed WT group. • Values from AM630-treated stressed WT mice that differ significantly from the vehicle-treated stressed WT group (two-way anova followed by Student–Newman–Keul's: P < 0.05) (n = 6–11 per group). (C) BDNF immunostaining in the hippocampus of AM630-treated and vehicle-treated WT mice exposed to CMS. Columns represent the means and vertical lines the SEM of relative BDNF protein (arbitrary units). *Values from the vehicle-treated stressed WT group that differ significantly from the vehicle-treated non-stressed WT group (two-way anova followed by Student–Newman–Keul's: P < 0.05) (n = 5–6 per group). (D) Representative immunostaining images for BDNF protein in the hippocampus of AM630-treated and vehicle-treated WT stressed and non-stressed mice. Bar represents 1 mm. BDNF, brain-derived neurotrophic factor; CMS, chronic mild stress tests; WT, wild-type.
The expression of the BDNF gene was significantly reduced in vehicle-treated stressed WT compared with vehicle-treated non-stressed WT mice. In contrast, treatment with AM630 significantly blocked the reduction of BDNF gene expression induced by CMS compared with the vehicle-treated stressed group. Interestingly, administration of AM630 per se failed to alter the level of expression of the BDNF gene (two-way anova followed by Student–Newman–Keul's: stress F(1,26) = 4.931, P = 0.037; drug F(1,26) = 40.393, P < 0.001; stress × drug F(1,26) = 17.310, P < 0.001) (n = 10–11 per group) (Figure 5B).
Consistent with these results, the level of expression of the BDNF protein was reduced in the DG of vehicle-treated stressed WT mice compared with that in vehicle-treated non-stressed WT mice. AM630 administration completely blocked this reduction in the level of BDNF protein induced by CMS. AM630 administration alone did not alter the level of BDNF protein (two-way anova followed by Student–Newman–Keul's: stress F(1,35) = 1.913, P = 0.176; drug F(1,35) = 13.148, P < 0.001; stress × drug F(1,35) = 17.656, P < 0.001) (n = 5–6 per group) (Figure 5C,D).
Discussion
The results of the present study provide unequivocal evidence that the CB2 receptor is involved in the regulation of depressive-like behaviours. This assumption is supported by several observations: (i) the presence of basal CB2 receptor gene expression in areas related to stress and depression in WT mice; (ii) overexpression of CB2 receptors produced a behavioural endophenotype resistant to acute and chronic depressogenic-like stimuli; (iii) CB2xP mice had a higher level of expression of the BDNF gene in the hippocampus; and (iv) treatment with AM630 blocked or significantly reduced signs of depressive-like behaviour and CB2 receptor and BDNF loss in the hippocampus after chronic exposure to depressogenic-like stimuli in WT mice.
The present study demonstrates, for the first time, the distribution of CB2 receptor gene expression in different brain nuclei of WT mice under normal conditions (CPu, Acc, Cg, Amy, Hipp, VMN, ARC, SN, DR and MnR). The identification of CB2 receptor gene expression in these brain regions predicts the role of these receptors in a wide variety of physiological functions. For instance, functional expression of these receptors in areas related to stress, anxiety and depression, such as the Amy, Hipp, DR and MnR, further supports their potential role in the regulation of anxiety and depressive-like disorders.
To evaluate the role of CB2 receptors in the regulation of depressive-like behaviour, CB2xP mice were used in a variety of experimental paradigms. Previous studies evaluating the role of this receptor in the regulation of neuropathic pain have partially described these transgenic CB2xP mice (Racz et al., 2008a,b;). In the present study, CB2 receptor gene expression was enhanced in the different brain nuclei analysed (CPu, Acc, Cg, Amy, Hipp, VMN, ARC, SN, VTA, DR and MnR) of CB2xP mice. Furthermore, a significant increase of CB2 receptor protein in the DG, CA3 and CA2 of the hippocampus was also found in CB2xP mice.
The analyses of the TST and NSFT results revealed that CB2xP mice displayed a behavioural endophenotype resistant to acute depressogenic-like stimuli. This response was characterized by a shorter immobility time, shorter latency and more abundant food consumption by CB2xP mice in TST and NSFT, respectively.
The effects of chronic depressogenic-like stimuli were examined by exposing CB2xP mice to CMS. This animal model of depression involved the presentation of repeated unpredictable mild stressors for several weeks (Papp et al., 1996; Willner, 1997). Following this exposure, the animals exhibited persistent reduction of their responsiveness to pleasurable stimuli, such as a palatable sucrose solution (Willner et al., 1992a,b; D'Aquila et al., 1994; Forbes et al., 1996). CMS produced depressive-like behavioural alterations in WT mice (shorter duration of immobility in TST and reduced sucrose intake). In contrast, exposure to CMS failed to produce any alteration in the CB2xP mice. Interestingly, CB2xP mice (under basal conditions) had higher sucrose intake levels than WT mice. Although the precise mechanisms underlying these basal differences remain to be elucidated, it is tempting to speculate that CB2xP mice may present neuroendocrine and/or peripheral alterations that could disrupt the homeostatic regulation of glucose, therefore modifying the appetite of these mice.
Exposure to CMS decreased neurogenesis and produced alterations in the dendrite remodelling process in the hippocampus (Manji et al., 2001; Nestler et al., 2002a,b; Coyle and Duman, 2003). In this respect, BDNF plays an important role in adult neurogenesis, modulating the plasticity and survival of adult neurones and glia cells (Huang and Reichardt, 2001). Converging evidence from various sources confirms a reduction of hippocampal BDNF in rodents exposed to CMS (Li et al., 2007; 2008; Toth et al., 2008). Moreover, clinical studies show a reduction of hippocampal neurogenesis in patients with mood disorders (Sheline et al., 1996; Sheline, 2000). Consistent with these findings, our results revealed that CMS reduced hippocampal BDNF gene and protein expression in stressed WT mice. In contrast, CMS failed to alter the levels of BDNF gene and protein expression in CB2xP mice. Indeed, CB2xP mice had a higher level of hippocampal BDNF gene expression than WT mice. The exact nature of the molecular alterations that occur in CB2xP mice is still unknown. However, these findings point to the CB2 receptor as being an important target involved in the ‘normalization’ of reduced BDNF expression in the hippocampus of mice exposed to CMS.
If the overexpression of CB2 receptors results in molecular adaptations that are associated with an endophenotype resistant to acute and chronic depressogenic-like stimuli, it can be hypothesized that pharmacological manipulation of these receptors by administration of a cannabinoid CB2 receptor-antagonist could produce similar effects in WT mice. This assumption is supported by the fact that chronic treatment with an antagonist results in an increase in the number of receptors antagonized (Neisewander et al., 1989; Patel et al., 2002). Hence, chronic treatment with the cannabinoid CB2r-antagonist (AM630) may lead to an increase in the expression of CB2 receptors thus mimicking the phenotype of CB2xP mice.
The acute administration of AM630 (1 and 3 mg·kg−1) shortened the duration of immobility (FST) in WT mice. These doses had no effect on the motor activity of WT mice in the open-field test (data not shown). Interestingly, AM630 administration did not alter the duration of immobility in CB2xP mice at any of the doses tested. It is possible that the lack of effect of AM630 in CB2xP mice was due to the increased level of CB2 receptor expression in these mice.
Chronic (4 weeks) administration of AM630 reversed the reduction of immobility duration in the TST and the diminished sucrose intake, both induced by CMS. These findings are in contrast with those from previous studies that showed the administration of AM630 did not significantly modify the intake of sucrose solution in CMS (Onaivi et al., 2008). These discrepancies may be due to: (i) individual and species differences between the strains used (BALB/c, Swiss-ICR mice); and (ii) different dosage or pattern of administration of AM630. Onaivi et al. (2008) uses doses of 1 and 3 mg·kg−1 once a day. In contrast, in this study AM630 was administered at a dose of 1 mg·kg−1 twice a day.
Furthermore, CMS resulted in a reduction in the levels of expression of the CB2 receptor gene, BDNF gene and protein in the hippocampus of stressed WT mice. Interestingly, AM630 treatment significantly reversed these reductions. The administration of AM630 increased CB2 receptor and BDNF gene expression in stressed mice, although this alteration did not occur at the protein level. It is important to note the discrepancies observed between BDNF mRNA and protein expressions in this study. Despite several explanations proposed to clarify the lack of correspondence between BDNF gene and protein expression reported by several authors (De Foubert et al., 2004; Jacobsen and Mork, 2004; Mitsukawa et al., 2006), the exact mechanism underlying these differences remains unclear.
The explanation of why the blockade of CB2 receptors increased CB2 receptor and BDNF gene expression only in chronically stressed mice is still not known. It can be hypothesized that AM630 acts differently depending on the level of activity of BDNF and CB2 receptors. Under basal conditions (non-stress), blockade of the receptor did not affect homeostatic regulation between CB2 receptors and BDNF. In contrast, CMS increases the release of endogenous cannabinoid ligands (Gorzalka et al., 2008), which in turn, may tend to down-regulate CB2 receptors and reduce BDNF expression. In fact, these changes may result in hypersensitization of the CB2 receptors and, through an unknown mechanism, could be responsible for the pronounced increase in BDNF after treatment with AM630. Further studies are needed to identify the precise neurochemical mechanisms by which the CB2 receptors regulate BDNF gene expression during stress.
In summary, the results presented here show that the CB2 receptors play a pivotal role in the neurobiology of depressive-related disorders. Overexpression of CB2 receptors resulted in a behavioural endophenotype resistant to depressogenic-like stimuli and modified different targets involved in the neuroplasticity of depressive disorders, such as BDNF. In addition, acute and chronic treatment with AM630 resulted in antidepressant-like effects and reversed the reduction in the levels of expression of the CB2 receptor, BDNF gene and protein induced in the hippocampus of mice exposed to CMS. Taken together, these findings strongly support the role of CB2 receptors in the regulation of depressive disorders and point to the CB2 receptor as a potential new target for the treatment of mood-related disorders.
Acknowledgments
This research was supported by grants from Ministry of Science and Innovation (SAF 2008-01106) and Ministry of Health (RETICS RD06/0001/1004 and PNSD 2007/061). MSGG is a predoctoral fellow of the Ministry of Science and Innovation. JMPO is a postdoctoral fellow of ‘Fundación para la Investigación y Salud de Castilla La Mancha’ (FISCAM). We thank Patricia Rodríguez and Analía Rico for their excellent technical assistance.
Glossary
Abbreviations:
- BDNF
brain-derived neurotrophic factor
- CB2xP mice
transgenic mice overexpressing the CB2 receptor
- CMS
chronic mild stress tests
- FST
forced swimming test
- NSFT
novelty-suppressed feeding test
- TST
tail suspension test
- WT
wild-type
Conflicts of interest
All authors state that they have no biomedical financial interests or potential conflicts of interest.
References
- Adamczyk P, Golda A, McCreary AC, Filip M, Przegalinski E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. 2008;59:217–228. [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 1):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert Opin Ther Targets. 2008;12:1347–1366. doi: 10.1517/14728222.12.11.1347. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. Br J Pharmacol. 2008;153:240–251. doi: 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Duman RS. Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron. 2003;38:157–160. doi: 10.1016/s0896-6273(03)00195-8. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- D'Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav. 1996;60:1481–1484. doi: 10.1016/s0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Guzman M, Sanchez C, Galve-Roperh I. Control of the cell survival/death decision by cannabinoids. J Mol Med. 2001;78:613–625. doi: 10.1007/s001090000177. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, et al. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosohata Y, Quock RM, Hosohata K, Makriyannis A, Consroe P, Roeske WR, et al. AM630 antagonism of cannabinoid-stimulated [35S]GTP gamma S binding in the mouse brain. Eur J Pharmacol. 1997;321:R1–R3. doi: 10.1016/s0014-2999(97)00047-2. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024:183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB(1) antagonists. Psychopharmacology (Berl) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: from brain homeostasis to behavior. Life Sci. 2008;82:934–942. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lunn CA, Reich EP, Fine JS, Lavey B, Kozlowski JA, Hipkin RW, et al. Biology and therapeutic potential of cannabinoid CB2 receptor inverse agonists. Br J Pharmacol. 2008;153:226–239. doi: 10.1038/sj.bjp.0707480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Mombereau C, Lotscher E, Uzunov DP, van der Putten H, Flor PJ, et al. Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders. Neuropsychopharmacology. 2006;31:1112–1122. doi: 10.1038/sj.npp.1300926. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Need AB, Davis RJ, Alexander-Chacko JT, Eastwood B, Chernet E, Phebus LA, et al. The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. Psychopharmacology (Berl) 2006;184:26–35. doi: 10.1007/s00213-005-0234-x. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Nonneman AJ, McDougall SA, Bardo MT. Up-regulation of opiate receptors following chronic naloxone treatment in aged rats. Neurobiol Aging. 1989;10:55–58. doi: 10.1016/s0197-4580(89)80011-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002a;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002b;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2006;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Sejal P, Meozzi PA, Myers L, Tagliaferro P, et al. Methods to study the behavioral effects and expression of CB2 cannabinoid receptor and its gene transcripts in the chronic mild stress model of depression. Methods Mol Med. 2006;123:291–298. doi: 10.1385/1-59259-999-0:291. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M. Punch sampling biopsy technique. Methods Enzymol. 1983;103:368–376. doi: 10.1016/s0076-6879(83)03025-6. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E, Willner P. Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol. 1996;296:129–136. doi: 10.1016/0014-2999(95)00697-4. [DOI] [PubMed] [Google Scholar]
- Patel M, Gomes B, Patel C, Yoburn BC. Antagonist-induced micro-opioid receptor up-regulation decreases G-protein receptor kinase-2 and dynamin-2 abundance in mouse spinal cord. Eur J Pharmacol. 2002;446:37–42. doi: 10.1016/s0014-2999(02)01823-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press. Harcourt Science and Technology Company; 2001. [Google Scholar]
- Pertwee R, Griffin G, Fernando S, Li X, Hill A, Makriyannis A. AM630, a competitive cannabinoid receptor antagonist. Life Sci. 1995;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Racz I, Nadal X, Alferink J, Banos JE, Rehnelt J, Martin M, et al. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J Neurosci. 2008a;28:12136–12145. doi: 10.1523/JNEUROSCI.3402-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Nadal X, Alferink J, Banos JE, Rehnelt J, Martin M, et al. Crucial role of CB(2) cannabinoid receptor in the regulation of central immune responses during neuropathic pain. J Neurosci. 2008b;28:12125–12135. doi: 10.1523/JNEUROSCI.3400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gaztelumendi A, Rojo ML, Pazos A, Diaz A. Altered CB receptor-signaling in prefrontal cortex from an animal model of depression is reversed by chronic fluoxetine. J Neurochem. 2009;108:1423–1433. doi: 10.1111/j.1471-4159.2009.05898.x. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, et al. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008a;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behaviogral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008b;33:1165–1170. doi: 10.1016/j.psyneuen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vaugeois JM, Passera G, Zuccaro F, Costentin J. Individual differences in response to imipramine in the mouse tail suspension test. Psychopharmacology. 1997;134:387–391. doi: 10.1007/s002130050475. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. An animal model of anhedonia. Clin Neuropharmacol. 1992a;15:550A–551A. doi: 10.1097/00002826-199201001-00286. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992b;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Davis RJ, Li X, Nomikos GG. A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders. Trends Pharmacol Sci. 2005;26:609–617. doi: 10.1016/j.tips.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


