Abstract
BACKGROUND AND PURPOSE
Hypertriglyceridaemia is associated with an increased risk of cardiovascular disease. Irbesartan, a well-established angiotensin II type 1 receptor (AT1) blocker, improves hypertriglyceridaemia in rodents and humans but the underlying mechanism of action is unclear.
EXPERIMENTAL APPROACH
Male obese Koletsky (fak/fak) rats, which exhibit spontaneous hypertension and metabolic abnormalities, received irbesartan (40 mg·kg−1·day−1) or vehicle by oral gavage over 7 weeks. Adipocyte-derived hormones in plasma were measured by ELISA. Gene expression in liver and other tissues was assessed by real-time PCR and Western immunoblotting.
KEY RESULTS
In Koletsky (fak/fak) rats irbesartan lowered plasma concentrations of triglycerides and non-esterified fatty acids, and decreased plasma insulin concentrations and the homeostasis model assessment of insulin resistance index. However, this treatment did not affect food intake, body weight, epididymal white adipose tissue weight, adipocyte size and plasma leptin concentrations, although plasma adiponectin was decreased. Irbesartan up-regulated hepatic expression of mRNAs corresponding to peroxisome proliferator-activated receptor (PPAR)α and its target genes (carnitine palmitoyltransferase-1a, acyl-CoA oxidase and fatty acid translocase/CD36) that mediate hepatic fatty acid uptake and oxidation; the increase in hepatic PPARα expression was confirmed at the protein level. In contrast, irbesartan did not affect expression of adipose PPARγ and its downstream genes or hepatic genes that mediate fatty acid synthesis.
CONCLUSIONS AND IMPLICATIONS
These findings demonstrate that irbesartan treatment up-regulates PPARα and several target genes in liver of obese spontaneously hypertensive Koletsky (fak/fak) rats and offers a novel insight into the lipid-lowering mechanism of irbesartan.
Keywords: irbesartan, lipid, peroxisome proliferator-activated receptor, angiotensin II type 1 receptor, obesity
Introduction
The metabolic syndrome is a cluster of conditions arising from many factors including genetic mutation, overnutrition and a sedentary lifestyle. Common components of the metabolic syndrome include abdominal obesity, hypertension, dislipidaemia and insulin resistance. Insulin resistance and type 2 diabetes are associated with abnormalities in lipid and lipoprotein homeostasis, including elevated triglycerides, which increase the risk of cardiovascular disease (Krauss, 2004). Hypertriglyceridaemia is considered to be an important risk factor for atherosclerosis and other cardiovascular complications in patients with type 2 diabetes (Ginsberg, 1996), and may also be associated with premature coronary artery disease (Brunzell, 2007). In addition, elevated plasma concentrations of non-esterified fatty acids (NEFA) have been associated with deterioration of glucose tolerance independent of other markers of insulin resistance that characterize subjects who are at risk from type 2 diabetes (Charles et al., 1997). Prolonged elevations of NEFA in plasma can exacerbate the impairment in glucose homeostasis in individuals with obesity and type 2 diabetes (Saloranta and Groop, 1996) and may stimulate gluconeogenesis, the development of insulin resistance in muscle and liver, and may also impair insulin secretion in genetically predisposed individuals (Bergman and Ader, 2000). It has been suggested that NEFAs are a major link between obesity and insulin resistance/type 2 diabetes (McGarry, 2002; Bays et al., 2004). Therefore, pharmacological treatments that decrease circulating triglycerides and NEFAs may improve insulin resistance and reduce the risk of cardiovascular disease.
Irbesartan, one of the earliest angiotensin II type 1 receptor blocker (ARBs) to enter clinical use, is a well-established and widely used antihypertensive agent. Irbesartan has been shown to decrease plasma triglyceride concentrations in the obese Zucker rat (Janiak et al., 2006; Muñoz et al., 2006) and the corpulent JCR:LA-cp rat (Russell et al., 2009). Large-scale clinical trials have also demonstrated that irbesartan improves metabolic parameters, including plasma triglyceride concentrations, in patients with hypertension and the metabolic syndrome (Kintscher et al., 2007; Parhofer et al., 2007). However, the underlying mechanism of these lipid-lowering effects remains unknown.
The genetically obese Koletsky (fak/fak) rat strain carries a nonsense mutation in the leptin receptor gene (Takaya et al., 1996). The fak mutation results in hyperphagia, obesity, insulin resistance and hyperlipidaemia (Koletsky, 1973; Koletsky and Ernsberger, 1992; Friedman et al., 1997) superimposed on the background of the spontaneously hypertensive lean Koletsky (+/+) littermates. As ARBs are prescribed to the patients with hypertension, the present study investigated the mechanism of the lipid-lowering effect of irbesartan using the obese spontaneously hypertensive Koletsky (fak/fak) rats. The principal findings to emerge were that, irbesartan decreased plasma triglyceride and NEFA concentrations, in addition to decreases in plasma insulin concentrations and the index of homeostasis model assessment of insulin resistance (HOMA-IR). Peroxisome proliferator-activated receptor (PPAR)α and several PPARα-responsive genes were up-regulated in liver, thus increasing the capacity for uptake and oxidation of fatty acids. In contrast, irbesartan did not significantly affect the expression of PPARγ and downstream genes in white adipose tissues, and the genes responsible for fatty acid synthesis in liver. Thus, irbesartan improves hypertriglyceridaemia and high free fatty acid concentrations via a hepatic PPARα pathway in insulin resistant rats with obesity and hypertension.
Methods
Animals, diet and experimental protocol
All animal procedures were in accordance with the ‘Principles of laboratory animal care’ (http://grants1.nih.gov/grants/olaw/references/phspol.htm) and were approved by the Animal Ethics Committee, Kyoto University, Japan.
Male obese Koletsky (fak/fak) rats and their lean (+/+) littermates aged 10–11 weeks were generous gifts from Japan SLC, Inc., Shizuoka, Japan. Rats were housed in a temperature-controlled facility (21 ± 1°C, 55 ± 5% relative humidity) with a 12-h light/dark cycle (2 rats per cage). Animals were allowed free access to water and the standard diet (CLEA Tokyo, Japan) for 1 week before starting the experiments. Rats were divided into three groups (n = 6 per group): lean control (+/+ Irb -), obese control (fak/fak Irb -) and obese with irbesartan treatment (fak/fak Irb +). There was no difference in body weight between two obese groups before treatments. Animals in the fak/fak Irb + group were administered irbesartan (nomenclature follows Alexander et al., 2008) (40 mg·kg−1, a generous gift from Shionogi & Co., Ltd, Japan, suspended in 5% Gum Arabic) by oral gavage once daily (11 h 00 min–12 h 00 min) for 7 weeks. The rats in the +/+ Irb – and fak/fak Irb – groups received vehicle (5% gum arabic) alone. The rats were weighed once every 3–4 days to determine gavage volume and daily food intake was estimated from weekly measurements. Systolic blood pressure (SBP) was measured at Week 1. Blood samples were collected by retro-orbital venous puncture under ether anaesthesia at Week 5 in animals that had been deprived of food for 6 h, for determination of plasma concentrations of triglyceride and NEFA using enzymatic methods (kits from Wako, Osaka, Japan), leptin (Morinaga, Tokyo, Japan) and adiponectin (Otsuka Pharmaceutical, Tokushima, Japan) using commercial ELISAs. Plasma glucose and insulin concentrations were determined using enzymatic (kit from Wako, Osaka, Japan) and ELISA (kit from Morinaga, Tokyo, Japan) methods, respectively, at Week 6 after the rats had been deprived of food for 12 h. The HOMA-IR index was calculated as an indicator of insulin sensitivity according to the following formula: [insulin (µIU·mL−1) × glucose (mM)]/22.5. Animals were weighed at Week 7 and then killed by prompt dislocation of the neck vertebra. Epididymal white adipose tissue (eWAT) and liver were collected and weighed. The gastrocnemius muscle [contains red (mostly type IIa muscle fibres) and white (primarily type IIb fibres) skeletal muscle] was also collected. Segments of each of eWAT, liver and skeletal muscle were snap frozen in liquid nitrogen and stored at −80°C for subsequent determination of triglyceride content and/or gene analysis.
SBP
Systolic blood pressure was measured (2–5 h after administration of irbesartan or vehicle) in conscious rats by a tail-cuff method (MK-2000ST; Muromachi Kikai Co Ltd, Tokyo, Japan). At least six readings were taken for each measurement.
Determination of triglyceride content in liver and skeletal muscle
Triglyceride contents in liver and skeletal muscle were determined as described previously (Oakes et al., 2001). Briefly, 100 mg of tissue was homogenized and extracted with 2 mL of isopropanol. After centrifugation (1000× g), the triglyceride content in the supernatants was determined enzymatically (Wako, Osaka, Japan).
Histological examination
A portion of eWAT or liver was fixed with 10% formalin and embedded in paraffin. Four-micron sections were cut and stained with haematoxylin and eosin for examination of adipose tissue and liver histology (IX-81, Olympus Corporation, Tokyo, Japan). The adipocyte cross-sectional area was measured using an image analysing system (KS 400 Imaging System; Carl Zeiss Vision, Eching, Germany).
Gene expression analysis
Total RNA was isolated from the eWAT, livers and skeletal muscle of individual mice using TRIzol (Invitrogen, Osaka, Japan). Single-stranded cDNA was synthesized from 1 µg of total RNA using SuperScript III First-Strand Synthesis System for RT-PCR, according to the manufacturer's instructions (Invitrogen, Osaka, Japan). Quantitative real-time PCR was performed with an AB 7300 Real-Time PCR System using TaqMan (Applied Biosystems, USA). The sequences of primers and probes (Sigma-Genosys, Japan) used in the present study are shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was chosen as an endogenous control (housekeeping gene).
Table 1.
Primer and probe sequences for real time RT-PCR assays
| Gene | Probe | Primersa |
|---|---|---|
| GAPDH | TTGTGCAGTGCCAGCCTCGTCTCA | f TGTTCTAGAGACAGCCGCATCTT |
| r CCGACCTTCACCATCTTGTCTAT | ||
| PPARγ | CCTGCGGAAGCCCTTTGGTGACT | f TGACCAGGGAGTTCCTCAAAA |
| r AGCAAACTCAAACTTAGGCTCCAT | ||
| FAS | ACCATCTCTGGACCTCAGGCTGCAGT | f TGCCTGCCTGCCACAAC |
| r CTTGCTTTAGCTGCTCCACAAATT | ||
| ACC1 | CAGCACAGCTCCAGATTGCCATGG | f GGTGGCTGATGTCAATCTTCCT |
| r TCATACGAATATCCTTGATCCTAAATAGAG | ||
| CD36 | CTTGGATGTGGAACCCATAACTGGATTC | f CCTAACGAAGATGAGCATAGGACAT |
| r GTTGACCTGCAGTCGTTTTGC | ||
| SCD1 | CCGGGCCCATTCATACACATCGTTCT | f CCTCATCATTGCCAACACCAT |
| r GGCGTGTGTCTCAGAGAACTTG | ||
| SREBP1c | CAAAGCTGAATAAATCTGCTGTCTTGCGCA | f CCTGGTGGTGGGCACTGA |
| r GTGCTGTAAGAAGCGGATGTAGTC | ||
| PPARα | CTGCAAGGGCTTCTTTCGGCGA | f CTATGGAGTCCACGCATGTGAA |
| r TTGTCGTACGCCAGCTTTAGC | ||
| CPT1a | CCCCGCGAATCCGTCCAGC | f GGTTCAAGAATGGCATCATCACT |
| r TCACACCCACCACCACGATA | ||
| ACO | CAGACGGAGATGGGCCACGGAAC | f AAGAACTCCAGATAATTGGCACCTA |
| r TGGTTTCCAAGCCTCGAAGAT | ||
| CPT1b | CGAGCAGTGCCAGACAGCCATCG | f CGGATGCAGTGGGACATTC |
| r CCAGGGCCTTGGCTACTTG | ||
| aP2 | TGGGAGTTGGCTTCGCCACCAG | f TCCAGTGAGAACTTCGATGATTACA |
| r GGCCATACCGGCCACTTT | ||
| Adiponectin | TTCTCTCCAGGAGTGCCATCTCTGCC | f GGACCAAGAACACCTGCGTCT |
| r TCCTGGTCACAATGGGATACC | ||
| DGAT1 | CAGAACTCCATGAAGCCCTTCAAGGACAT | f CAGCAGTGGATGGTCCCTACTAT |
| r AAGAGACGCTCAATGATTCGTG | ||
| GLUT4 | CATCAACGCCCCACAGAAAGTGATTG | f GCTCCCTTCAGTTTGGCTATAACA |
| r GCCAAGTTGCATTGTAGCTCTGT |
Forward primers are designated by f and reverse primers by r.
Sequences: 5′ to 3′.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ACC, acetyl-CoA carboxylase; ACO, acyl-CoA oxidase; CPT, carnitine palmitoyltransferase; DGAT, diacylglycerol acyltransferase; FAS, fatty acid synthase; GLUT, glucose transporter; PPAR, peroxisome proliferator-activated receptor; SCD, stearoyl-CoA desaturase; SREBP, sterol regulatory element-binding protein.
Protein expression was quantified by Western blotting (Lorenzo et al., 2002). Tissue proteins were resolved on 4–12% polyacrylamide gels in the presence of sodium dodecylsulphate, transferred electrophoretically to polyvinylidene difluoride membranes, blocked (in buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 5% bovine serum albumin, 0.1% Tween-20) and incubated at 4°C for 18 h with PPARα-specific antibody (1:300; Santa Cruz, CA, USA). Detection was performed with peroxidase-conjugated secondary antibody, by enhanced chemiluminescence (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Immunoblotting with a monoclonal anti-β-actin antibody (Cell Signaling, Beverly, MA, USA) was conducted to ensure equal protein loading.
Data analysis
All results are expressed as means ± SEM. Data obtained from experiments with three groups of animals (Figures 1–4) were analysed by one-way analysis of variance (anova). If a difference was detected (F-ratio), the Student-Newman-Keuls test was performed to locate the differences between groups. Data obtained from experiments with two groups of animals (Figure 5) were analysed by Student's t-test; P < 0.05 was considered to be statistically significant.
Figure 1.
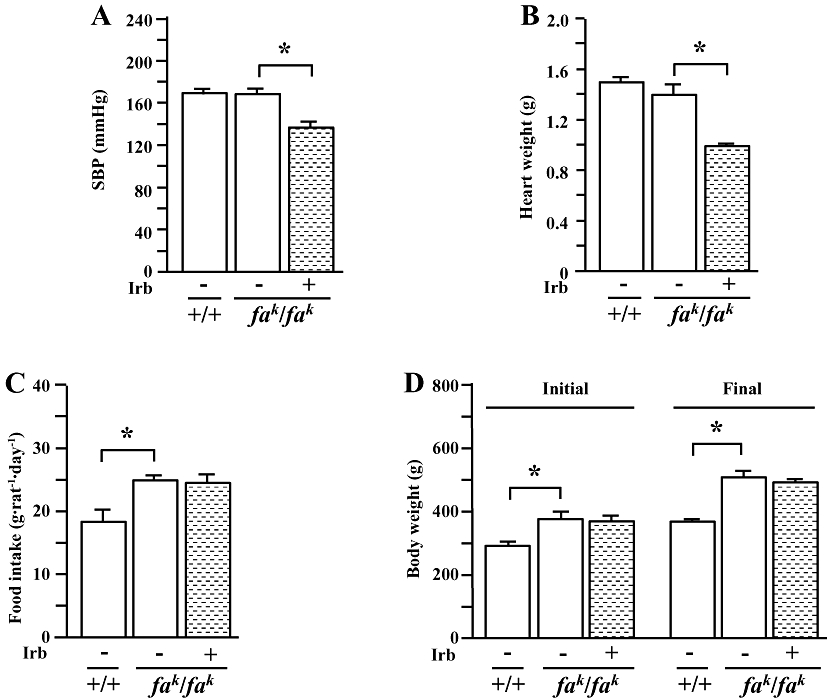
Systolic blood pressure (SBP) (A), heart weight (B), food intake (C) and initial and final body weight (D) in male lean (+/+) and obese (fak/fak) Koletsky rats. Animals received irbesartan (Irb) (40 mg·kg−1·day−1) or vehicle by oral gavage for 7 weeks. SBP was measured with a tail-cuff method at Week 1 after treatment. Food intake over 24 h was determined at Week 3. All values are means ± SEM (n = 6 each group). Irb -: vehicle; Irb +: irbesartan; *P < 0.05.
Figure 4.
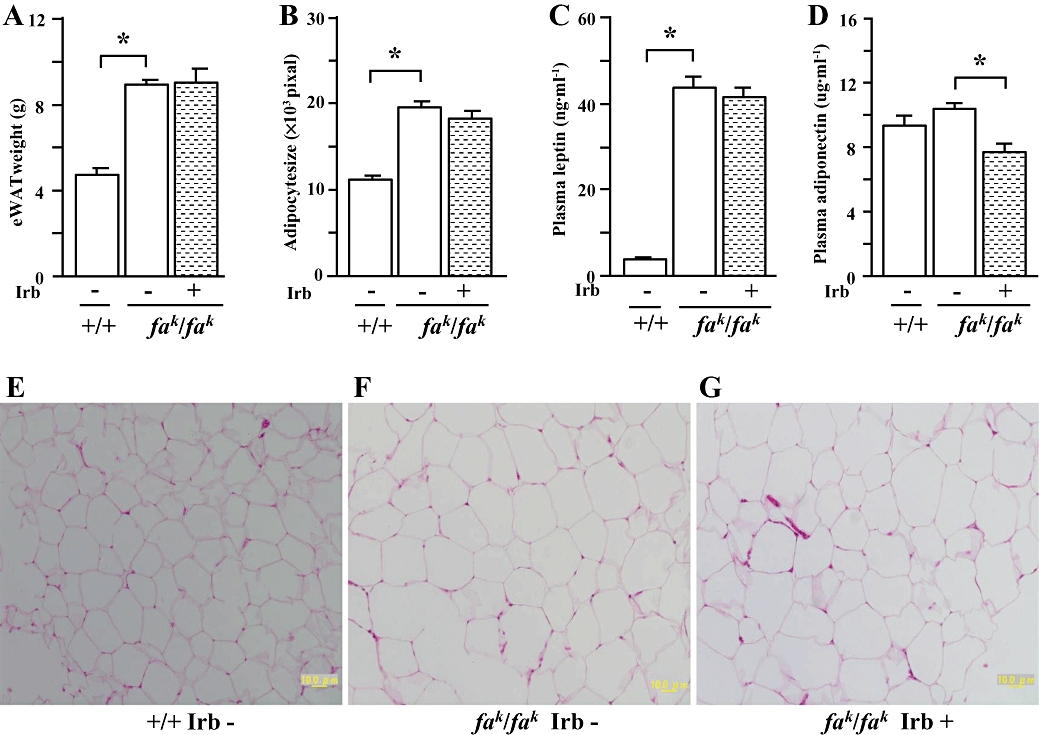
Epididymal white adipose tissue (eWAT) weight (A), adipocyte size (B), fasted (rats deprived of food) plasma concentrations of leptin (C) and adiponectin (D), and representative images showing histology of eWAT (haematoxylin and eosin–staining, X200) (E-G) in male lean (+/+) and obese (fak/fak) Koletsky rats; animals received irbesartan (Irb) or vehicle as described in the legend to Figure 1. All values are means ± SEM (n = 6 each group). Irb -: vehicle; Irb +: irbesartan; *P < 0.05.
Figure 5.
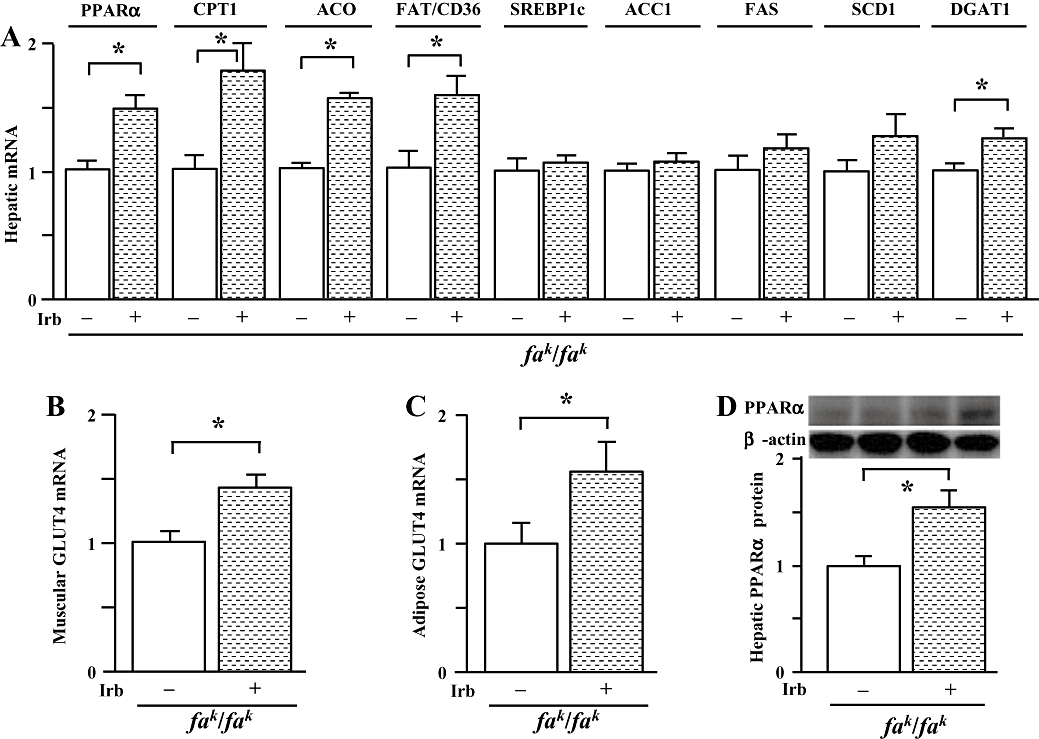
Gene expression profile. (A) mRNAs encoding peroxisome proliferator-activated receptor (PPAR)α, carnitine palmitoyltransferase (CPT)1a, acyl-CoA oxidase (ACO), fatty acid translocase (FAT)/CD36, sterol regulatory element-binding protein (SREBP)1c, acetyl-CoA carboxylase (ACC)1, fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD)1 and diacylglycerol acyltransferase (DGAT)1 (B) GLUT4 mRNA in skeletal muscle (C) GLUT4 mRNA in eWAT and (D) protein expression of PPARα in liver of male obese Koletsky (fak/fak) rats that were either untreated (-) or treated (+) with irbesartan (Irb) as described in Figure 1. Total RNA was isolated from liver, skeletal muscle or eWAT of individual rats using TRIzol. Quantitative PCR results were normalized to GAPDH, while the results from Western blot analysis were normalized to β-actin. Levels in obese control rats were arbitrarily assigned a value of 1. All values are means ± SEM (n = 6 each group). *P < 0.05. eWAT, epididymal white adipose tissue; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT, glucose transporter.
Results
Metabolic abnormalities and effects of irbesartan in obese Koletsky (fak/fak) rats
Animals were genotyped by the supplier. Obese Koletsky (fak/fak) rats appeared to be somewhat larger than their lean littermates; rats of both genotype were hypertensive (SBP: ≈170 mmHg; Figure 1A) compared with normal controls (SBP: ≈120 mmHg). Food intake (Figure 1C) and body weights (Figure 1D) were greater in obese rats than in lean controls, but there was no difference in heart weight between the genotypes (Figure 1B). Irbesartan treatment (40 mg·kg−1) decreased SBP by ∼40 mmHg (Figure 1A) and heart weight (Figure 1B) in obese rats, consistent with its cardiovascular actions. However, this treatment did not significantly affect food intake (Figure 1C) and body weight (Figure 1D).
Compared with lean controls, plasma triglyceride concentrations were higher in obese Koletsky (fak/fak) rats under both fasting (deprived of food) and non-fasting conditions (Figure 2A). Plasma NEFA concentrations were also higher in obese rats under fasting, but not non-fasting, conditions (Figure 2B). Treatment of obese rats with irbesartan for 5 weeks decreased both fasted and non-fasted plasma triglyceride and fasted NEFA concentrations, but it was without effect on non-fasted NEFA concentrations in plasma of obese rats (Figure 2A and B).
Figure 2.
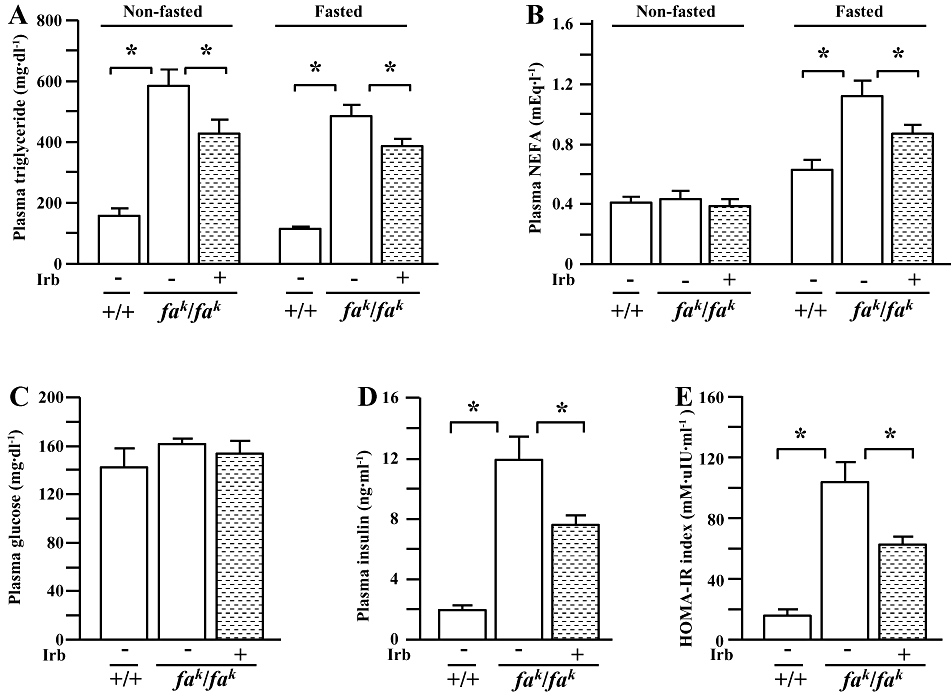
Non-fasted and fasted (rats deprived of food) plasma concentrations of triglyceride (A) and non-esterified fatty acids (NEFA) (B), fasted plasma glucose (C) and insulin (D) concentrations, and the index of the homeostasis model assessment of insulin resistance (HOMA-IR) (E) in male lean (+/+) and obese (fak/fak) Koletsky rats; animals received irbesartan (Irb) or vehicle as described in the legend to Figure 1. All values are means ± SEM (n = 6 each group). Irb -: vehicle; Irb +: irbesartan; *P < 0.05.
In fasted animals, plasma glucose concentrations did not differ between rats of either genotype (Figure 2C). However, plasma insulin concentrations (Figure 2D) and the HOMA-IR index (Figure 2E) were considerably higher in the obese rats than in their lean counterparts. Although irbesartan treatment was without effect on plasma glucose concentration, plasma insulin concentrations and the HOMA-IR index in obese rats were decreased by this treatment (Figure 2D and E).
Hepatomegaly (Figure 3A) was evident in the obese Koletsky (fak/fak) rat; consistent with this finding, hepatic triglyceride content was increased markedly in these animals (Figure 3B and C), compared with corresponding lean controls. Increased vacuolization was evident on histological examination of liver sections from obese rats (Figure 3F) compared with lean rats (Figure 3E), indicative of excess lipid droplet accumulation. In skeletal muscle, triglyceride content was also increased in obese rats (Figure 3D); irbesartan treatment did not significantly alter these parameters (Figure 3A–D and G).
Figure 3.
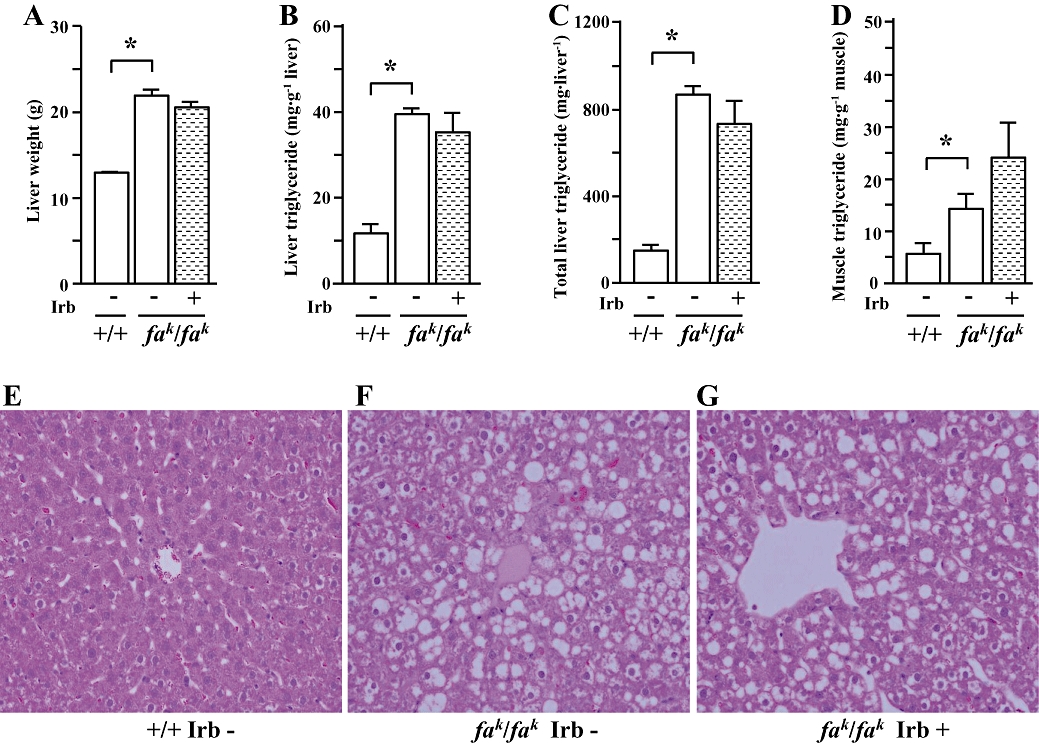
Liver weight (A), triglyceride contents in liver (B, C) and skeletal muscle (D), and representative images showing histology of liver (Hematoxylin and eosin–staining, X200) (E-G) in male lean (+/+) and obese (fak/fak) Koletsky rats; animals received irbesartan (Irb) or vehicle as described in the legend to Figure 1. All values are means ± SEM (n = 6 each group). Irb -: vehicle; Irb +: irbesartan; *P < 0.05.
Compared with lean control rats, eWAT weight (Figure 4A), adipocyte size (Figure 4B and F) and plasma leptin concentrations (Figure 4C) were greater in obese Koletsky rats, but plasma adiponectin concentrations (Figure 4D) were not different between lean and obese animals. In obese rats irbesartan decreased plasma adiponectin concentrations (Figure 4D) but did not affect eWAT weight (Figure 4A), adipocyte size (Figure 4B and G) and plasma leptin concentrations (Figure 4C).
Gene expression profile in obese Koletsky (fak/fak) rats
By real-time PCR obese Koletsky (fak/fak) rats showed a significant increase in hepatic and adipose, but not muscular, expression of GAPDH, compared with lean rats; irbesartan treatment did not significantly affect GAPDH expression (data not shown). Thus, comparisons in gene expression were restricted to obese animals, with or without irbesartan treatment.
Interestingly, irbesartan treatment up-regulated PPARα, carnitine palmitoyltransferase (CPT)1a, acyl-CoA oxidase (ACO) and fatty acid translocase (FAT)/CD36 (Figure 5A) mRNAs in liver of obese Koletsky (fak/fak) rats; the increase in PPARα expression was confirmed at the protein level by Western immunoblotting (Figure 5D). However, irbesartan treatment did not alter hepatic sterol regulatory element-binding protein (SREBP)1c, acetyl-CoA carboxylase (ACC)1, fatty acid synthase (FAS) and stearoyl-CoA desaturase (SCD)1 mRNA expression, but increased the level of diacylglycerol acyltransferase (DGAT)1 mRNA (Figure 5A).
In contrast with these findings in liver, irbesartan treatment did not alter the expression of PPARα, CPT1a, ACO and FAT/CD36 mRNAs in skeletal muscle of obese Koletsky (fak/fak) rats (data not shown). Consistent with decreased HOMA-IR index, irbesartan up-regulated muscular glucose transporter (GLUT)4 expression (Figure 5B).
In white adipose tissue from obese Koletsky (fak/fak) rats, irbesartan treatment did not significantly change the expression of mRNAs encoding PPAR&gamma, aP2, adiponectin, FAS, ACC1, CD36, SCD1, SREBP1c and DGAT1 (data not shown). However, GLUT4 mRNA level was increased by irbesartan treatment (Figure 5C).
Discussion
Consistent with recent clinical findings (Kintscher et al., 2007; Parhofer et al., 2007), the present study demonstrates that irbesartan treatment improves hypertriglyceridaemia and reduces free fatty acid concentrations in obese Koletsky (fak/fak) rats that exhibit hypertension and metabolic abnormalities.
Improvements in plasma lipid concentrations are not produced by all ARBs. Telmisartan, valsartan, candesartan, olmesartan and losartan were without effect on serum triglyceride concentrations in hypertensive patients with metabolic syndrome and/or type 2 diabetes (Bahadir et al., 2007; Ichikawa, 2007; Tomiyama et al., 2007; Nakayama et al., 2008). Genetic blockade of AT1 also failed to decrease serum triglyceride concentrations in mice fed high fat (Kouyama et al., 2005) or methionine-choline deficient diets (Nabeshima et al., 2009). Similarly, telmisartan and valsartan treatment did not alter serum triglyceride concentrations in rats fed a diet that was high in fat and carbohydrate (Sugimoto et al., 2006). Losartan (Xu et al., 2005) and olmesartan (Yokozawa et al., 2009) did not significantly affect serum triglyceride and/or NEFA concentrations in obese Zucker rats. Thus, it appears that mechanisms other than AT1 inhibition may mediate the lipid-lowering actions of irbesartan.
peroxisome proliferator-activated receptor α is a member of the nuclear receptor superfamily. PPARα is predominantly expressed in liver and, to a lesser extent, in skeletal muscle and heart, where it has a crucial role in controlling fatty acid oxidation (Reddy and Hashimoto, 2001). Fibrates are established pharmacological activators of PPARα that decreases circulating triglycerides and improves insulin sensitivity. Interestingly, in the present study irbesartan treatment was found to up-regulate PPARα and several target genes that mediate fatty acid oxidation in liver of obese Koletsky (fak/fak) rats. Furthermore, hepatic expression of FAT/CD36, another PPARα target gene that is important in facilitating cellular fatty acid uptake and ameliorating insulin resistance in rodents and humans (Pravenec et al., 2001; Su and Abumrad, 2009), was also up-regulated by irbesartan. However, irbesartan did not significantly enhance the expression of these genes in skeletal muscle. Irbesartan also did not affect the hepatic expression of the genes that mediate fatty acid synthesis, including SREBP1c, FAS, ACC1 and SCD1, in obese Koletsky (fak/fak) rats. Thus, the present findings suggest that the actions of irbesartan in increasing fatty acid uptake and oxidation by the liver are mediated by a hepatic PPARα pathway and lead to decreased plasma triglyceride and free fatty acid concentrations. However, irbesartan also increased hepatic expression of DGAT1, a key enzyme in triglyceride synthesis (Villanueva et al., 2009). It is possible that the unchanged hepatic lipid content after irbesartan treatment may reflect minimal overall effects on the balance between fatty acid uptake, β-oxidation, esterification and lipid secretion/storage.
In contrast to the situation with PPARα, PPARγ is expressed predominantly in adipose tissue and at only low levels in liver and skeletal muscle. PPARγ-activating ligands improve adipose tissue function by altering fat topography and adipocyte phenotype and by up-regulating genes involved in fatty acid metabolism and triglyceride storage (Sharma and Staels, 2007). Furthermore, PPARγ activation is associated with potentially beneficial effects on the secretion of a range of factors from adipose tissue, including adiponectin, thereby improving insulin sensitivity (Sharma and Staels, 2007). Indeed, adiponectin is an important mediator of the improvement in insulin sensitivity elicited by PPARγ agonists (Sharma and Staels, 2007). The increase in plasma adiponectin concentrations observed after thiazolidinedione therapy is closely associated with a decline in hepatic fat content (Sharma and Staels, 2007). Thus, treatment with rosiglitazone enhances insulin sensitivity, and is accompanied by decreased plasma triglyceride and NEFA concentrations, increased plasma adiponectin and leptin concentrations, and increased eWAT weight in mice fed high fat-containing diets (Stienstra et al., 2008; Kuda et al., 2009). Rosiglitazone treatment also decreased plasma glucose and triglyceride concentrations, but increased plasma adiponectin and leptin concentrations, as well as body weight in obese Zucker rats (Cai et al., 2000; Reifel-Miller et al., 2005). In addition, rosiglitazone stimulated adipose activity of DGAT, a key enzyme catalysing triglyceride synthesis, which is accompanied by a specific increase in the mRNA corresponding to adipose DGAT1, but not DGAT2, in rats (Festuccia et al., 2009). Irbesartan has been shown to enhance PPARγ-dependent 3T3-L1 adipocyte differentiation, as reflected by increases in expression of certain adipogenic marker genes (Benson et al., 2004; Schupp et al., 2004). However, findings from immunohistochemical studies indicated that irbesartan treatment decreased PPARγ expression in the white and brown adipose tissues of obese Zucker rats in a dose-dependent fashion (Di Filippo et al., 2005). In the present study, treatment of obese Koletsky (fak/fak) rats with irbesartan decreased plasma adiponectin concentrations, but was without effect on eWAT weight, adipocyte size and plasma leptin concentrations. In adipose tissue of Koletsky (fak/fak) rats, irbesartan was without effect on expression of PPARγ and its downstream target genes aP2, adiponectin, FAS, ACC1, CD36, SCD1, SREBP1c and DGAT1. Thus, our present findings do not support the contention that irbesartan improves hyperlipidaemia and insulin sensitivity by modulating PPARγ signalling in adipose tissue of obese Koletsky rats. Further investigations are required to evaluate whether irbesartan also modulates expression of PPARβ/&delta, the other PPAR isoform involving lipid metabolism.
Muñoz et al. reported that irbesartan (50 mg·kg−1 for 6 months) decreased lipid accumulation in the liver of obese Zucker rats (Muñoz et al., 2006; Toblli et al., 2008). This group also demonstrated that irbesartan reduced adipocyte size and increased adiponectin expression in eWAT from these animals (Muñoz et al., 2009). However, the present findings demonstrated that irbesartan (40 mg·kg−1 for 7 weeks) did not significantly affect hepatic steatosis, eWAT weight, plasma leptin concentration, adipocyte size or adipose adiponectin gene expression in obese Koletsky (fak/fak) rats. Further, both Russell et al. (2009), using the insulin-resistant JCR:LA-cp rat and the present study found that irbesartan treatment decreased plasma adiponectin concentrations. There are several possible factors that may be responsible for these discrepancies. First, the animal strains are different: although both obese Zucker rats and obese Koletsky rats carry leptin receptor mutations, there are still some important differences. In the fatty Zucker rat the Leprfa gene carries a point mutation in codon 269 that produces an amino acid sequence change adjacent to the ligand-binding domain of the receptor (Chua et al., 1996); these animals are still responsive to leptin (Cusin et al., 1996; Wang et al., 1998; Wildman et al., 2000). In contrast, the Koletsky rat (Leprfak) carries a premature stop codon and the mutant receptor lacks a transmembrane domain. This truncates all known isoforms of the receptor and, unlike the Leprfa mutation, the Leprfak mutation is null (Takaya et al., 1996; Wu-Peng et al., 1997; Wildman et al., 2000). Second, blood pressure differs between the two rat strains: young obese Zucker rats (at least until 18 weeks of age) are normotensive (Muñoz et al., 2006; Toblli et al., 2008). In contrast, obese Koletsky rats are spontaneously hypertensive (SBP ≈ 170 mmHg at 10 weeks of age); which remained in excess of 130 mmHg after irbesartan treatment (Figure 1A). Finally, the different doses of irbesartan (50 mg·kg−1 vs. 40 mg·kg−1) and durations of treatments (6 months vs. 7 weeks) were used in the Zucker and Koletsky rats so that comparisons are not straightforward.
In the present study, irbesartan treatment also decreased plasma insulin concentration and the HOMA-IR index in obese Koletsky rats. The results of quantitative PCR analysis demonstrated that GLUT4 gene expression in adipose tissue and skeletal muscle was increased by irbesartan treatment. These results suggest that insulin sensitivity improves after irbesartan treatment. NEFAs are a major link between obesity and insulin resistance/type 2 diabetes (McGarry, 2002; Bays et al., 2004). Although a decrease in plasma NEFAs by irbesartan treatment may be associated with enhanced insulin sensitivity, the underlying molecular mechanisms require further investigation. It has been demonstrated that angiotensin II decreases local blood flow both in adipose and skeletal muscle tissue of normal-weight and obese subjects (Goossens et al., 2004). Increased muscular blood flow is associated with increased glucose utilization (Baron et al., 1992; Wiernsperger, 1994). Further specific investigations are required to determine the involvement of this increased blood flow in the enhanced insulin sensitivity induced by irbesartan
Acknowledgments
We thank Wufuerjiang Aini for his technical assistance in this project.
Glossary
Abbreviations
- ACC
acetyl-CoA carboxylase
- ACO
acyl-CoA oxidase
- AT1
angiotensin II type 1 receptor
- ARB
angiotensin II type 1 receptor blocker
- CPT
carnitine palmitoyltransferase
- DGAT
diacylglycerol acyltransferase
- eWAT
epididymal white adipose tissue
- FAS
fatty acid synthase
- FAT
fatty acid translocase
- GLUT
glucose transporter
- HOMA-IR
homeostasis model assessment of insulin resistance
- NEFA
non-esterified fatty acids
- PPAR
peroxisome proliferator-activated receptor
- SBP
systolic blood pressure
- SCD
stearoyl-CoA desaturase
- SREBP
sterol regulatory element-binding protein
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 1):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadir O, Uzunlulu M, Oguz A, Bahadir MA. Effects of telmisartan and losartan on insulin resistance in hypertensive patients with metabolic syndrome. Hypertens Res. 2007;30:49–53. doi: 10.1291/hypres.30.49. [DOI] [PubMed] [Google Scholar]
- Baron AD, Bretchel-Hook G, Johnson A, Hardin D. Skeletal muscle blood flow: a possible link between insulin resistance and blood pressure. Hypertension. 1992;21:129–135. doi: 10.1161/01.hyp.21.2.129. [DOI] [PubMed] [Google Scholar]
- Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- Brunzell JD. Clinical practice. Hypertriglyceridemia. N Engl J Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Lister CA, Buckingham RE, Pickavance L, Wilding J, Arch JR, et al. Down-regulation of orexin gene expression by severe obesity in the rats: studies in Zucker fatty and Zucker diabetic fatty rats and effects of rosiglitazone. Brain Res Mol Brain Res. 2000;77:131–137. doi: 10.1016/s0169-328x(00)00041-3. [DOI] [PubMed] [Google Scholar]
- Charles MA, Eschwe'ge E, Thibult N, Claude J-R, Warnet J-M, Rosselin GE, et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101–1106. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- Chua SC, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Cusin I, Rohner-Jeanrenaud F, Stricker-Krongrad A, Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats. Diabetes. 1996;45:1446–1450. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- Di Filippo C, Lampa E, Tufariello E, Petronella P, Freda F, Capuano A. Effects of irbesartan on the growth and differentiation of adipocytes in obese zucker rats. Obes Res. 2005;13:1909–1914. doi: 10.1038/oby.2005.235. [DOI] [PubMed] [Google Scholar]
- Festuccia WT, Blanchard PG, Turcotte V, Laplante M, Sariahmetoglu M, Brindley DN, et al. The PPARgamma agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1327–R1335. doi: 10.1152/ajpregu.91012.2008. [DOI] [PubMed] [Google Scholar]
- Friedman JE, Ishizuka T, Liu S, Farrell CJ, Bedol D, Kaung HL, et al. Reduced insulin receptor signaling in the obese spontaneously hypertensive Koletsky rat. Am J Physiol. 1997;273:E1014–E1023. doi: 10.1152/ajpendo.1997.273.5.E1014. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996;45(Suppl. 1):S27–S30. doi: 10.2337/diab.45.3.s27. [DOI] [PubMed] [Google Scholar]
- Goossens GH, Blaak EE, Saris WH, van Baak MA. Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab. 2004;89:2690–2696. doi: 10.1210/jc.2003-032053. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y. Comparative effects of telmisartan and valsartan on insulin resistance in hypertensive patients with metabolic syndrome. Intern Med. 2007;46:1331–1336. doi: 10.2169/internalmedicine.46.7173. [DOI] [PubMed] [Google Scholar]
- Janiak P, Bidouard JP, Cadrouvele C, Poirier B, Gouraud L, Grataloup Y, et al. Long-term blockade of angiotensin AT1 receptors increases survival of obese Zucker rats. Eur J Pharmacol. 2006;534:271–279. doi: 10.1016/j.ejphar.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Bramlage P, Paar WD, Thoenes M, Unger T. Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: a sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14 200 patients. Cardiovasc Diabetol. 2007;6:12. doi: 10.1186/1475-2840-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletsky S. Obese spontaneously hypertensive rats-a model for study of atherosclerosis. Exp Mol Pathol. 1973;19:53–60. doi: 10.1016/0014-4800(73)90040-3. [DOI] [PubMed] [Google Scholar]
- Koletsky RJ, Ernsberger P. Obese SHR (Koletsky rat): a model for the interactions between obesity and hypertension. In: Sassard J, editor. Genetic Hypertension. London: John Libbey; 1992. pp. 373–375. [Google Scholar]
- Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, et al. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481–3489. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496–1504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- Kuda O, Jelenik T, Jilkova Z, Flachs P, Rossmeisl M, Hensler M, et al. n-3 fatty acids and rosiglitazone improve insulin sensitivity through additive stimulatory effects on muscle glycogen synthesis in mice fed a high-fat diet. Diabetologia. 2009;52:941–951. doi: 10.1007/s00125-009-1305-z. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Ruiz-Ortega M, Suzuki Y, Rupérez M, Esteban V, Sugaya T, et al. Angiotensin III activates nuclear transcription factor-kappaB in cultured mesangial cells mainly via AT(2) receptors: studies with AT(1) receptor-knockout mice. J Am Soc Nephrol. 2002;13:1162–1171. doi: 10.1681/ASN.V1351162. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Muñoz MC, Argentino DP, Dominici FP, Turyn D, Toblli JE. Irbesartan restores the in-vivo insulin signaling pathway leading to Akt activation in obese Zucker rats. J Hypertens. 2006;24:1607–1617. doi: 10.1097/01.hjh.0000239297.63377.3f. [DOI] [PubMed] [Google Scholar]
- Muñoz MC, Giani JF, Dominici FP, Turyn D, Toblli JE. Long-term treatment with an angiotensin II receptor blocker decreases adipocyte size and improves insulin signaling in obese Zucker rats. J Hypertens. 2009;27:2409–2420. doi: 10.1097/HJH.0b013e3283310e1b. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Tazuma S, Kanno K, Hyogo H, Chayama K. Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol. 2009;50:1226–1235. doi: 10.1016/j.jhep.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Watada H, Mita T, Ikeda F, Shimizu T, Uchino H. Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertension. Hypertens Res. 2008;31:7–13. doi: 10.1291/hypres.31.7. [DOI] [PubMed] [Google Scholar]
- Oakes ND, Thalen PG, Jacinto SM, Ljung B. Thiazolidinediones increase plasma-adipose tissue FFA exchange capacity and enhance insulin-mediated control of systemic FFA availability. Diabetes. 2001;50:1158–1165. doi: 10.2337/diabetes.50.5.1158. [DOI] [PubMed] [Google Scholar]
- Parhofer KG, Münzel F, Krekler M. Effect of the angiotensin receptor blocker irbesartan on metabolic parameters in clinical practice: the DO-IT prospective observational study. Cardiovasc Diabetol. 2007;6:36. doi: 10.1186/1475-2840-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravenec M, Landa V, Zidek V, Musilova A, Kren V, Kazdova L, et al. Transgenic rescue of defective Cd36 ameliorates insulin resistance in spontaneously hypertensive rats. Nat Genet. 2001;27:156–158. doi: 10.1038/84777. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Hashimoto T. Peroxisomal β-oxidation and peroxisome proliferatoractivated receptor α: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- Reifel-Miller A, Otto K, Hawkins E, Barr R, Bensch WR, Bull C, et al. A peroxisome proliferator-activated receptor alpha/gamma dual agonist with a unique in vitro profile and potent glucose and lipid effects in rodent models of type 2 diabetes and dyslipidemia. Mol Endocrinol. 2005;19:1593–1605. doi: 10.1210/me.2005-0015. [DOI] [PubMed] [Google Scholar]
- Russell J, Kelly S, Vine D, Proctor S. Irbesartan-mediated reduction of renal and cardiac damage in insulin resistant JCR: LA-cp rats. Br J Pharmacol. 2009;158:1588–1596. doi: 10.1111/j.1476-5381.2009.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloranta C, Groop L. Interactions between glucose and NEFA metabolism in man. Diabetes Metab Rev. 1996;12:15–35. doi: 10.1002/(SICI)1099-0895(199603)12:1<15::AID-DMR153>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- Sharma AM, Staels B. Review: peroxisome proliferator-activated receptor gamma and adipose tissue – understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Müller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Qi NR, Kazdová L, Pravenec M, Ogihara T, Kurtz TW. Telmisartan but not valsartan increases caloric expenditure and protects against weight gain and hepatic steatosis. Hypertension. 2006;47:1003–1009. doi: 10.1161/01.HYP.0000215181.60228.f7. [DOI] [PubMed] [Google Scholar]
- Takaya K, Ogawa Y, Hiraoka J, Hosoda K, Yamori Y, Nakao K, et al. Nonsense mutation of leptin receptor in the obese spontaneously hypertensive Koletsky rat. Nat Genet. 1996;14:130–131. doi: 10.1038/ng1096-130. [DOI] [PubMed] [Google Scholar]
- Toblli JE, Muñoz MC, Cao G, Mella J, Pereyra L, Mastai R. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity. 2008;16:770–776. doi: 10.1038/oby.2007.114. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Yambe M, Yamada J, Motobe K, Koji Y, Yoshida M. Discrepancy between improvement of insulin sensitivity and that of arterial endothelial function in patients receiving antihypertensive medication. J Hypertens. 2007;25:883–889. doi: 10.1097/HJH.0b013e3280149518. [DOI] [PubMed] [Google Scholar]
- Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, Bhanot S, et al. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50:434–442. doi: 10.1002/hep.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hartzell DL, Flatt WP, Martin RJ, Baile CA. Responses of lean and obese Zucker rats to centrally administered leptin. Physiol Behav. 1998;65:333–341. doi: 10.1016/s0031-9384(98)00173-5. [DOI] [PubMed] [Google Scholar]
- Wiernsperger N. Vascular defects in the aetiology of peripheral insulin resistance in diabetes. A critical review of hypotheses and facts. Diabetes Metab Rev. 1994;10:287–307. doi: 10.1002/dmr.5610100305. [DOI] [PubMed] [Google Scholar]
- Wildman HF, Chua SC, Jr, Leibel RL, Smith GP. Effects of leptin and cholecystokinin in rats with a null mutation of the leptin receptor Leprfak. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1518–R1523. doi: 10.1152/ajpregu.2000.278.6.R1518. [DOI] [PubMed] [Google Scholar]
- Wu-Peng XS, Chua SC, Jr, Okada N, Liu SM, Nicolson M, Leibel RL. Phenotype of the obese Koletsky (f)rat due to Tyr763Stop mutation in the extracellular domain of the leptin receptor (LEPR): evidence for deficient plasma-to-csf transport of leptin in both the Zucker and Koletsky obese rat. Diabetes. 1997;46:513–518. doi: 10.2337/diab.46.3.513. [DOI] [PubMed] [Google Scholar]
- Xu ZG, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, et al. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation. 2005;111:1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- Yokozawa J, Sasaki T, Ohwada K, Sasaki Y, Ito JI, Saito T, et al. Down-regulation of hepatic stearoyl-CoA desaturase 1 expression by angiotensin II receptor blocker in the obese fa/fa Zucker rat: possible role in amelioration of insulin resistance and hepatic steatosis. J Gastroenterol. 2009;44:583–591. doi: 10.1007/s00535-009-0042-x. [DOI] [PubMed] [Google Scholar]


