Abstract
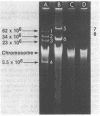
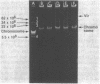
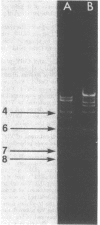
Three wild strains of bovine septicemic Escherichia coli were selected on the basis of their production of a toxin lethal for mice and chickens and their characteristic surface antigen. The transfer of these virulence (Vir) properties from two of the three to recipient E. coli was detected after mating. One Vir plasmid (pJL1) was derepressed for transfer and associated with mobilization of chromosomal markers. The other, pJL2, was repressed. Both plasmids were tagged with transposon Tn5 (kanamycin resistance), and transfer parameters of the tagged plasmids were studied. The Tn5 insertion in pJL2 usually increased transfer efficiency 100-fold. Plasmid pJL1 was classified as a member of the FIV incompatibility group. A pJL1::Tn5 derivative plasmid was incompatible with ColV1. Plasmid pJL2 behaved as an fi+ plasmid. Both plasmids pJL1 and pJL2 had a molecular weight of 92 x 10(6) and were present at about four copies per chromosome; their deoxyribonucleic acid (DNA) structures were not identical on the basis of restriction enzyme analysis. DNA-DNA hybridization revealed a polynucleotide sequence homology of at least 58% between the two plasmids. No plasmids could be detected in one wild or certain laboratory-derived Vir+ E. coli strains.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. A rapid screening test for transfer factors in drug-sensitive Enterobacteriaceae. Nature. 1965 Dec 4;208(5014):1016–1017. doi: 10.1038/2081016a0. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Nature of R-factor replication in the presence of chloramphenicol. Proc Natl Acad Sci U S A. 1975 Feb;72(2):654–658. doi: 10.1073/pnas.72.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Guerry P., Hedges R. W., Datta N. Polynucleotide sequence relationships among plasmids of the I compatibility complex. J Gen Microbiol. 1974 Nov;85(1):65–76. doi: 10.1099/00221287-85-1-65. [DOI] [PubMed] [Google Scholar]
- Grant R. B., Bannatyne R. M., Shapiey A. J. Resistance to chloramphenicol and ampicillin of Salmonella typhimurium in Ontario, Canada. J Infect Dis. 1976 Oct;134(4):354–361. doi: 10.1093/infdis/134.4.354. [DOI] [PubMed] [Google Scholar]
- Grindley J. N., Anderson E. S. I-like resistance factors with the fi+ character. Genet Res. 1971 Jun;17(3):267–271. doi: 10.1017/s0016672300012295. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Palchaudhuri S., Maas W. K. Naturally occurring plasmid carrying genes for enterotoxin production and drug resistance. Science. 1977 Oct 14;198(4313):198–199. doi: 10.1126/science.333581. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N. R124, an fi R factor of a new compatibility class. J Gen Microbiol. 1972 Jul;71(2):403–405. doi: 10.1099/00221287-71-2-403. [DOI] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitton J. S., Anderson E. S. The inhibitory action of transfer factors on lysis of Escherichia coli K12 by phages mu 2 and phi 2. Genet Res. 1970 Oct 2;16(2):215–224. doi: 10.1017/s0016672300002433. [DOI] [PubMed] [Google Scholar]
- Shalita Z., Hertman I., Sarid S. Isolation and characterization of a plasmid involved with enterotoxin B production in Staphylococcus aureus. J Bacteriol. 1977 Jan;129(1):317–325. doi: 10.1128/jb.129.1.317-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley P. L., Gyles C. L., Falkow S. Characterization of plasmids that encode for the K88 colonization antigen. Infect Immun. 1978 May;20(2):559–566. doi: 10.1128/iai.20.2.559-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. L., Maas W. K., Gyles C. L. Isolation and characterization of enterotoxin-deficient mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1384–1388. doi: 10.1073/pnas.75.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. A search for transmissible pathogenic characters in invasive strains of Escherichia coli: the discovery of a plasmid-controlled toxin and a plasmid-controlled lethal character closely associated, or identical, with colicine V. J Gen Microbiol. 1974 Jul;83(0):95–111. doi: 10.1099/00221287-83-1-95. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between different transmissible plasmids introduced by F into the same strain of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):227–285. doi: 10.1099/00221287-62-3-277. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. Further observations on the association of the colicine V plasmid of Escherichia coli with pathogenicity and with survival in the alimentary tract. J Gen Microbiol. 1976 Feb;92(2):335–350. doi: 10.1099/00221287-92-2-335. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transmissible pathogenic characteristics of invasive strains of Escherichia coli. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):601–607. [PubMed] [Google Scholar]
- So M., Heffron F., Falkow S. Method for the genetic labeling of cryptic plasmids. J Bacteriol. 1978 Mar;133(3):1520–1523. doi: 10.1128/jb.133.3.1520-1523.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott R. B. Studies on the chick-lethal toxin of Eecherichia coli. Can J Comp Med. 1973 Oct;37(4):375–381. [PMC free article] [PubMed] [Google Scholar]
- Turscott R. G., Lopez-Alvarez J., Pettit J. R. Studies of Escherichia coli infection in chickens. Can J Comp Med. 1974 Apr;38(2):160–167. [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]