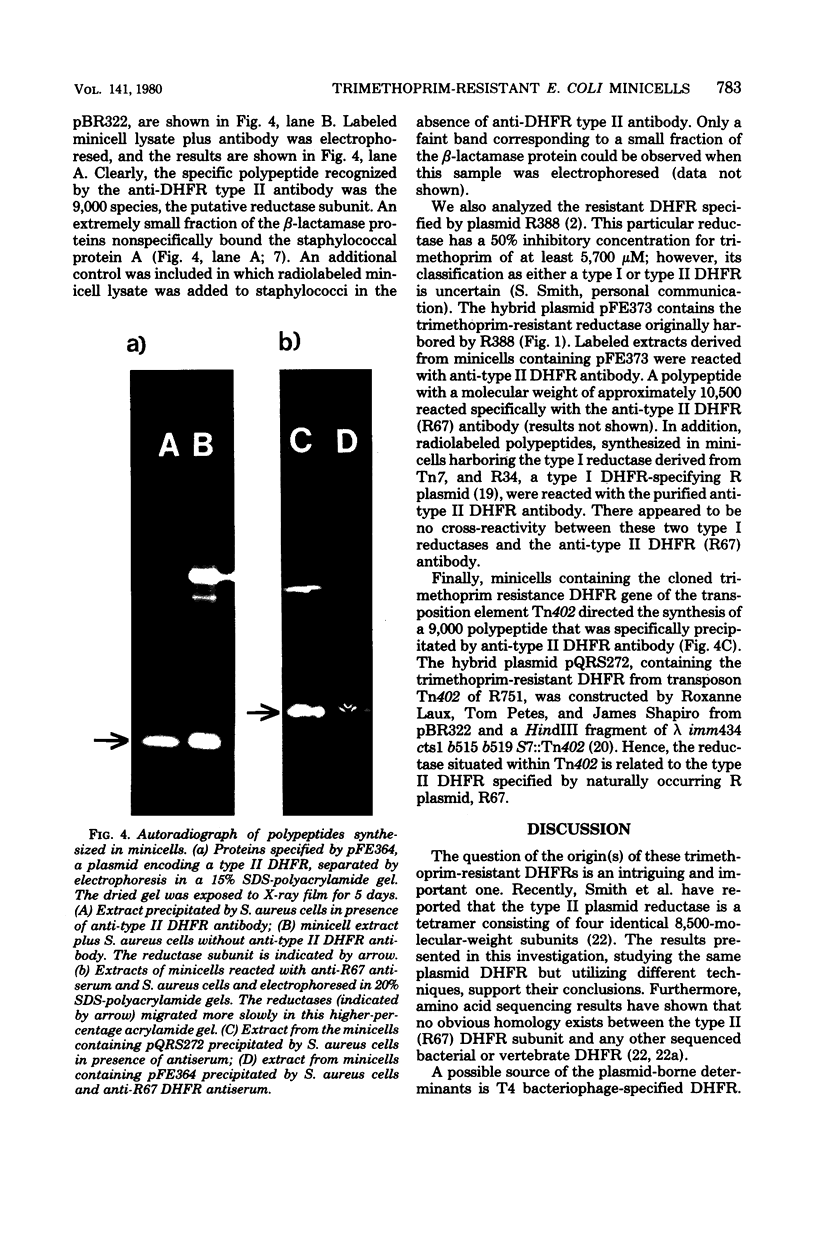
Abstract
Deoxyribonucleic acid fragments containing the structural genes for several trimethoprim-resistant dihydrofolate reductases from naturally occurring plasmids were inserted into small cloning vehicles. The genetic expression of these hybrid plasmids was studied in purified Escherichia coli minicells. The type I dihydrofolate reductase, encoded by plasmid R483 and residing within transposon 7 (Tn7), had a subunit molecular weight of 18,000. The type II dihydrofolate reductase, specified by plasmid R67, had a subunit molecular weight of 9,000. These two enzymes were antigenically distinct in that anti-type II dihydrofolate reductase (R67) antibody did not cross-react with the type I (R483) protein. The trimethoprim-resistant reductase specified by plasmid R388 had a subunit molecular weight of about 10,500 and was immunologically related to the type II (R67) enzyme. A 9,000 subunit of the dihydrofolate encoded by the transposition element Tn402 was also antigenically related to the R67 reductase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acar J. F., Goldstein F. W., Gerbaud G. R., Chabbert Y. A. Plasmides de résistance au triméthoprime: transférabilité et groupes d'incompatibilité. Ann Microbiol (Paris) 1977 Jan;128A(1):41–47. [PubMed] [Google Scholar]
- Amyes S. G., Smith J. T. R-factor trimethoprim resistance mechanism: an insusceptible target site. Biochem Biophys Res Commun. 1974 May 20;58(2):412–418. doi: 10.1016/0006-291x(74)90380-5. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Saul M., Twigg A., Gill R., Sherratt D. Polypeptides expressed in Escherichia coli K-12 minicells by transposition elements Tn1 and Tn3. J Bacteriol. 1979 Apr;138(1):48–54. doi: 10.1128/jb.138.1.48-54.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. J. Changes in protein synthesis on mitomycin C induction of wild-type and mutant CloDF13 plasmids. J Bacteriol. 1977 May;130(2):846–851. doi: 10.1128/jb.130.2.846-851.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Fleming M. P., Datta N., Grüneberg R. N. Trimethoprim resistance determined by R factors. Br Med J. 1972 Mar 18;1(5802):726–728. doi: 10.1136/bmj.1.5802.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobanputra R. S., Datta N. Trimethoprim R factors in enterobacteria from clinical specimens. J Med Microbiol. 1974 May;7(2):169–177. doi: 10.1099/00222615-7-2-169. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kool A. J., Pranger M., Nijkamp H. J. Proteins synthesized by a non-induced bacteriocinogenic factor in minicells of Escherichia coli. Mol Gen Genet. 1972;115(4):314–323. doi: 10.1007/BF00333170. [DOI] [PubMed] [Google Scholar]
- Modrich P., Anraku Y., Lehman I. R. Deoxyribonucleic acid ligase. Isolation and physical characterization of the homogeneous enzyme from Escherichia coli. J Biol Chem. 1973 Nov 10;248(21):7495–7501. [PubMed] [Google Scholar]
- Mosher R. A., DiRenzo A. B., Mathews C. K. Bacteriophage T4 virion dihydrofolate reductase: approaches to quantitation and assessment of function. J Virol. 1977 Sep;23(3):645–658. doi: 10.1128/jvi.23.3.645-658.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattishall K. H., Acar J., Burchall J. J., Goldstein F. W., Harvey R. J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977 Apr 10;252(7):2319–2323. [PubMed] [Google Scholar]
- Shapiro J. A., Sporn P. Tn402: a new transposable element determining trimethoprim resistance that inserts in bacteriophage lambda. J Bacteriol. 1977 Mar;129(3):1632–1635. doi: 10.1128/jb.129.3.1632-1635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O., Widh A. A new dihydrofolate reductase with low trimethoprim sensitivity induced by an R factor mediating high resistance to trimethoprim. J Biol Chem. 1974 Jul 10;249(13):4324–4325. [PubMed] [Google Scholar]
- Smith S. L., Stone D., Novak P., Baccanari D. P., Burchall J. J. R plasmid dihydrofolate reductase with subunit structure. J Biol Chem. 1979 Jul 25;254(14):6222–6225. [PubMed] [Google Scholar]
- Stone D., Smith S. L. The amino acid sequence of the trimethoprim-resistant dihydrofolate reductase specified in Escherichia coli by R-plasmid R67. J Biol Chem. 1979 Nov 10;254(21):10857–10861. [PubMed] [Google Scholar]
- Tennhammar-Ekman B., Sköld O. Trimethoprim resistance plasmids of different origin encode different drug-resistant dihydrofolate reductases. Plasmid. 1979 Jul;2(3):334–346. doi: 10.1016/0147-619x(79)90017-9. [DOI] [PubMed] [Google Scholar]
- Tyler J., Sherratt D. J. Synthesis of E colicins in Escherichia coli. Mol Gen Genet. 1975 Oct 22;140(4):349–353. doi: 10.1007/BF00267325. [DOI] [PubMed] [Google Scholar]
- Zolg J. W., Hänggi U. J., Zachau H. G. Isolation of a small DNA fragment carrying the gene for a dihydrofolate reductase from a trimethoprim resistance factor. Mol Gen Genet. 1978 Aug 4;164(1):15–29. doi: 10.1007/BF00267594. [DOI] [PubMed] [Google Scholar]





