Abstract
The effects of immunization with the second-generation cocaine immunoconjugate GND-keyhole limpet hemocyanin (KLH) or with the anti-cocaine mAb GNC92H2 were assessed in a model of acute cocaine-induced locomotor activity. After i.p. administration of cocaine⋅HCl (15 mg/kg), rats were tested in photocell cages, and stereotypy was rated to determine preimmunization drug response (baseline). Experimental animals were subjected to an immunization protocol with GND-KLH or treated with the mAb GNC92H2. Rats were then challenged with systemic cocaine, and their locomotor responses were again measured. Active immunization with GND-KLH produced a 76% decrease in the ambulatory measure (crossovers) in the experimental group and a 12% increase in the control group compared with baseline values. Also, stereotypic behavior was significantly suppressed in the vaccinated animals. Decreases in both measures were seen in the experimental group on two subsequent challenges. The maximum effect was observed at the time of the second challenge with a dramatic 80% decrease in crossovers. Treatment with GNC92H2 resulted in a 69% decrease in crossovers compared with baseline. This effect persisted across two additional challenges over 11 days with decreases of 46–47%. In contrast, the control group showed increases of up to 28%. Significant differences between groups were observed in the stereotypic measure in all three challenges. The results indicate that these immunopharmacotherapeutic agents have significant cocaine-blockade potential and therefore may offer an effective strategy for the treatment of cocaine abuse.
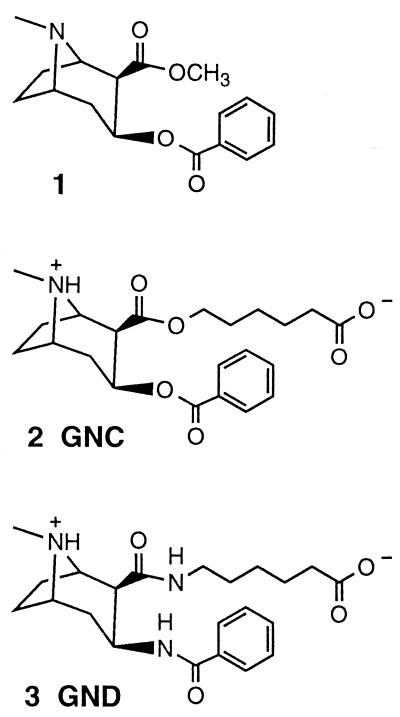
The abuse of cocaine (structure 1 in Fig. 1) has reached epidemic proportions in the United States, representing a major and increasing threat to public health (1). At present, there is no suitable medication for the treatment of this illness. Despite the many advances in the understanding of the biochemistry of action of cocaine, the development of antagonists, agonists, and antidepressants for pharmacotherapeutic and psychotherapeutic interventions has had limited success in both animal models and clinical studies (2–4). Because these therapeutic means are designed to block the central neurochemical effects of cocaine, their actions are nonselective and thus generate unwanted secondary effects (5). Immunopharmacotherapy, wherein antibodies are used to neutralize the drug, offers a possible alternative. Rather than targeting the receptors in the brain, antibodies obstruct partitioning of cocaine from the blood. This approach has the advantage of operating by means of an endogenous response that is independent of the central nervous system, thus circumventing the problem of neurotoxicity.
Figure 1.
Structures of cocaine (1) and the haptens GNC (2) and GND (3) used to form the immunoconjugates for vaccination studies.
Earlier studies showed that anti-morphine (6) and anti-heroin (7) antibodies could suppress opiate-induced effects. In the last decade, this strategy has been explored as a possible treatment for cocaine abuse. Work from this laboratory demonstrated that active immunization with a keyhole limpet hemocyanin (KLH) immunoconjugate derived from the hapten 2 (code-named GNC) (Fig. 1) suppressed the psychostimulant effects of cocaine in rats and resulted in an 80% decrease of brain cocaine levels compared with controls (8). Others tested the effects of a similar approach on the analgesic and reinforcing properties of cocaine with relative success (9–11). However, these studies have raised concerns regarding the efficacy of the conjugate (12), antibody surmountability (13, 14), and the choice of schedules of reinforcement (14). Recently, these concerns were addressed in the report of a series of studies from this laboratory, where both active (GNC-KLH) and passive (mAb GNC92H2) immunization were tested in a rat model of cocaine relapse (15). It was found that immunization with GNC-KLH or GNC92H2 significantly inhibited the reinforcing value and, when combined, the intake of self-administered cocaine. These findings lend support to the hypothesis that antibody-based therapy could be useful in the treatment of cocaine abuse. To further examine this hypothesis, we sought alternative haptens that might provide a more effective immunogen. In an effort to obtain a more potent, long-lasting anti-cocaine immune response, a second-generation hapten 3 (code-named GND) was designed and synthesized (16).
Cocaine is known to be a powerful psychostimulant, and this effect is believed to result from actions on dopamine neurons in the striatum and ventral forebrain (17). One measure of this effect is the increased locomotor activity and stereotypic behavior in the rat that is dose-dependent. Acute patterns of behavior in which characteristic behaviors such as sniffing, rearing, biting, and gnawing are repeated with abnormal frequency (18). Thus, motor behavior provides a sensitive and reproducible measure of cocaine action in rats under standardized conditions.
The present study examines the efficacy of active immunization with the second-generation cocaine immunoconjugate GND-KLH on the psychomotor effects of acute cocaine. To provide a mechanistic rationale, passive immunization with the mAb GNC92H2 was also tested.
Materials and Methods
Animals.
Male Wistar rats (n = 32; Charles River Breeding Laboratories) weighing 200–225 g on arrival were housed in groups of two in a humidity- and temperature-controlled (22°C) vivarium on a 12-h light/dark cycle (lights on at 10 p.m.) with free access to food and water. All behavioral procedures were performed during the light cycle to promote low levels of motility during testing sessions. Before behavioral testing, each rat was handled by the experimenter (10 min). The study consisted of two separate experiments.
Cocaine Immunoconjugate GND-KLH.
The hapten 3 was synthesized and coupled to the carrier protein KLH as described (8, 16).
Monoclonal Antibody GNC92H2.
The GNC-KLH conjugate (8) was mixed with an MPL + TDM adjuvant system (Ribi Immunochem), and the vaccine emulsion was used to immunize mice (strain 129 GIX+) that were not older than 3 months of age. The first injection (200 μg) contained the immunoconjugate (100 μg based on KLH) and adjuvant (50 μg) reconstituted in PBS. The injection was administered i.p. A booster injection was given 2 weeks later in a similar fashion. An eyebleed of anesthetized mice 7–10 days later was used to assess the titer. In this case, a titer of ∼25,000 indicated that no further i.p. vaccine was necessary. A final tail vein i.v. injection of GNC-KLH (50 μg) in PBS (150 μl) was given 1 month after the final i.p. boost. Three days later, the spleen was fused with a myeloma cell line (X63-Ag8.653) to produce hybridomas according to standard techniques (19, 20) with some modifications developed in our laboratory. The hybridomas were cloned into 96-well plates and screened against GNC-BSA conjugate by immunosorbent assay (ELISA) during the cloning process. Each member of a final panel of 19 mAbs was then assessed for binding to cocaine and cocaine metabolites in solution by using equilibrium dialysis (3H-labeled compounds). The mAb GNC92H2 emerged as the clone with the most favorable overall properties of specificity and affinity for cocaine (15).
Surgical Procedure.
Rats in the first experiment (passive immunization with GNC92H2) were deeply anesthetized under chronic vapor halothane (1.0–1.5%) and implanted with chronic indwelling catheters in the jugular vein as described (21). All animals were allowed to recover for a minimum of 7 days before self-administration training. The patency of each catheter was maintained by flushing daily with heparinized (30 USP units/ml) sterile physiological saline. The integrity of each catheter was tested periodically (on observed erratic performance) by infusion of 0.1 ml of Brevital sodium (1% methohexital sodium), which resulted in pronounced loss of muscle tone within 3 s of i.v. injection.
Behavioral Procedures.
Locomotor activity was measured in a bank of 16 wire cages, each cage 20 cm high × 25 cm wide × 36 cm long, with two horizontal infrared beams across the long axis 2 cm above the floor. Total photocell beam interruptions and crossovers, the number of times breaking one photocell beam was followed directly by breaking the other photocell beam, were recorded by a computer every 10 min. Background noise was provided by a white noise generator.
Before the immunization series, each rat was habituated to the photocell cages overnight, and before drug injection the rats were habituated again for 90 min. To determine preimmunization drug response (baseline), animals received an i.p. injection of 15 mg/kg cocaine⋅HCl mixed in saline solution (bolus 1 ml/kg), and their locomotor responses were measured during a 90-min session. Based on locomotor activity scores, animals were assigned to the experimental or control group in ranking order.
Stereotypic behavior (sniffing and rearing) was rated for 10 s every 10 min as described (22). Data were arranged in contingency tables in the following way. For each response category and for each 10-min interval, the number of rats showing a particular category was tabulated. The degree of heterogeneity in each contingency table was then calculated by a likelihood ratio method (see Statistical Analysis).
On challenge days, animals received an i.p. injection of isotonic saline (bolus 1 mg/kg) and were habituated for 90 min before the drug injection. Locomotor activity was measured during habituation and the testing session as described above. In both experiments, animals were subjected to a cocaine challenge (15 mg/kg i.p.) on the 3rd, 7th, and 12th day after the last booster (GND-KLH) or passive immunization treatment (GNC92H2).
Immunization Procedures.
Active immunization.
Three days after the preimmunization cocaine injection, rats were immunized with i.p. injections of a 400-μl bolus of a Ribi adjuvant (MPL-TDM) containing 250 μg of GND-KLH or KLH in 100 mM PBS, pH 7.4. This initial inoculation was followed by boosts at 21 and 35 days. The last boost was administered without adjuvant; 7 days later blood was collected by tailbleed, and serum samples were analyzed by ELISA as described (8) by using GND-BSA-coated plates.
Passive immunization.
Five days after the preimmunization cocaine injection, rats received an i.v. bolus injection of 90 mg/kg of the mAb GNC92H2 in PBS (2.3 ml of a 13 mg/ml mAb solution) or the control rat IgG (Sigma) in PBS (3 ml of a 10 mg/ml solution) 30 min before the onset of the test session.
Statistical Analysis.
Locomotor activity data were analyzed by subjecting 10-min total means for locomotor activity to a two-factor analysis of variance (ANOVA) (group × time) with repeated measures on the within-group factor, time. Individual means comparisons for the main treatment effects were analyzed by using a Newman–Keuls a posteriori test. Stereotyped behavior data were analyzed by a likelihood ratio method, the “information statistic” (23, 24).
Results
Active Immunization with GND-KLH.
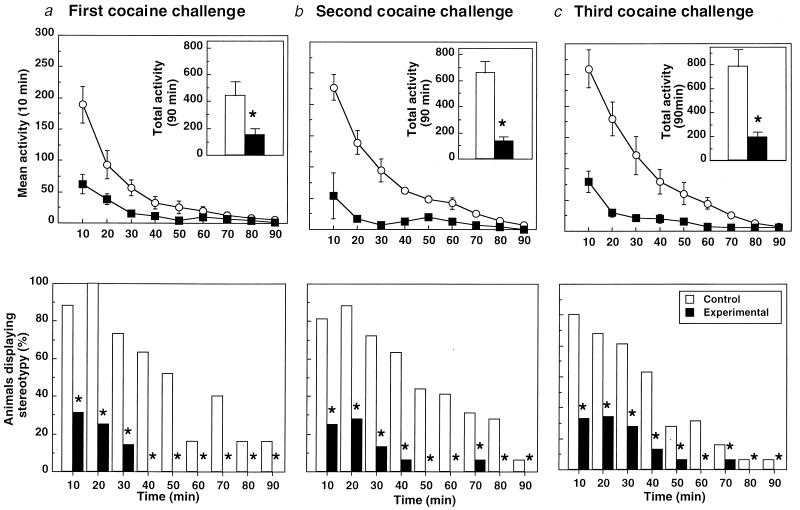
The average weight of the animals on completion of the study was 402 ± 36 g (n = 16). Anti-cocaine peak antibody titers were 1:25,000. The effects of GND-KLH on the psychomotor effects of cocaine can be seen in Fig. 2. Once the rats were habituated to the photocell apparatus, saline injection produced only transient arousal (lasting less than 20 min) followed by relative inactivity. Preimmunization baseline values (control, 487.5 ± 110.2; experimental, 635.2 ± 165.7). At the first challenge, there was a significant statistical difference between the control and experimental groups in both mean activity [Fig. 2a Upper: control, 443.3 ± 104; experimental, 154.1 ± 41.7; F(1,14) = 14.01, P < 0.002] with a significant main effect of treatment × time interaction [F(1,8) = 8.25, P < 0.0001]. Analysis of simple main effects revealed a significant difference between the GND-KLH and control KLH-treated groups at 10–30 and 50 min into the session. Differences in stereotyped behavior between groups were also significant (Fig. 2b Lower: 2Î = 99.35, df = 1, 9). Both psychomotor measures achieved significance at the time of the second cocaine challenge [locomotor: Fig. 2a Upper: control, 659.3 ± 33.2; experimental, 133.3 ± 37.0; F(1,14) = 24.98, P < 0.0002; with significant main effects of treatment × time interaction F(1,8) = 12.22, P < 0.001; stereotypy: Fig. 2b Lower, 2Î = 98.2, df = 1, 9; P < 0.01]. According to simple main effects analysis, differences between groups were greater throughout the first 70 min of the testing session. The difference in both measures between the groups persisted into the last cocaine challenge [locomotor: Fig. 2c Upper: control, 787.1 ± 144; experimental, 189.3 ± 48.3; F (1,14) = 25.26, P < 0.0002; with significant main effects of treatment × time interaction F(1,8) = 14.54, P < 0.0001; stereotypy: Fig. 2c Lower, 2Î = 91.5, df = 1, 9; P < 0.01]. Mean activity curves were significantly different during the first 80 min of the session (simple effects, P < 0.05).
Figure 2.
Locomotor activity (crossovers; Upper) and stereotyped behavior (sniffing and rearing; Lower) after i.p. injection of cocaine (15 mg/kg) after immunization with GND-KLH. The figure shows the response to postimmunization cocaine challenge on the 3rd (a), 7th (b), and 12th (c) day after the last immunization boost. Upper values represent means ± SEM of 16 animals (n = 8). *, P < 0.01; ANOVA, significant difference between groups. Lower data represent the percentage of incidence of the observed behavior. *, P < 0.05.
Passive Immunization with GNC92H2.
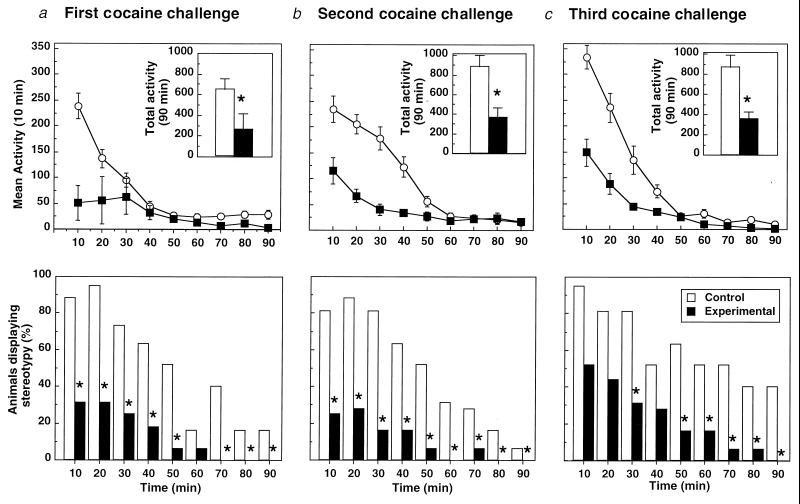
The average weight of animals on completion of the study was 338 ± 26 g (n = 16). The effects of GNC92H2 on the psychomotor effects of cocaine can be seen in Fig. 3. Once the rats were habituated to the photocell apparatus, saline injection produced only transient arousal (lasting less than 20 min) followed by relative inactivity. Baseline values were control, 632.89 ± 125.6 and experimental, 678.35 ± 101.12. At the time of first challenge, there was a significant statistical difference between the control and experimental groups in mean activity [Fig. 3a Upper: control, 677.38 ± 96.33; experimental, 259.75 ± 115.84; F(1,14) = 6.032, P < 0.028], with a main effect of treatment × time interaction [F(1,8) = 7.943, P < 0.001]. Analysis of simple main effects revealed significant differences between the GNC92H2 and control IgG-treated groups at 10, 70, and 90 min into the session. Stereotyped behavior also differed significantly between groups (Fig. 3a Lower: 2Î = 96.15, df = 1, 9). Both psychomotor measures achieved significance at the time of the second cocaine challenge [locomotor: Fig. 3b Upper: control, 883.5 ± 118.19; experimental, 366.5 ± 94.69; F(1,14) = 25.93, P < 0.0001; with main effects of treatment × time interaction F(1,8) = 13.8, P < 0.001; stereotypy: Fig. 3b Lower: 2Î = 94.53, df = 1, 9; P < 0.01]. According to simple main effects analysis, differences between groups were greater throughout the first 50 min of the testing session. The difference in both measures between the groups persisted into the last cocaine challenge [locomotor: Fig. 3c Upper: control, 873.88 ± 118.03; experimental, 359.75 ± 70.71; F (1,14) = 31.66, P < 0.0001; with significant main effects of treatment × time interaction F(1,8) = 13,97, P < 0.001; stereotypy: Fig. 3c Lower: 2Î = 80.43, df = 1, 9; P < 0.05]. The difference between the locomotor curves was greater throughout the first 40 min of the session.
Figure 3.
Locomotor activity (crossovers; Upper) and stereotyped behavior (sniffing and rearing; Lower) after i.p. injection of cocaine (15 mg/kg) after immunization with GNC92H2. The figure shows the response to postimmunization cocaine challenge on the 3rd (a), 7th (b), and 12th (c) day after the passive immunization treatment. Upper values represent means ± SEM of 16 animals (n = 8). *, P < 0.01; ANOVA, significant difference between groups. Lower data represent the percentage of incidence of the observed behavior. *, P < 0.05.
Discussion
The results demonstrate that immunization with either the second-generation immunoconjugate GND-KLH or the mAb GNC92H2 measurably suppressed the psychomotor effects of cocaine as compared with control rats. Furthermore, this effect was sustained for up to 12 days after immunization in both studies. These findings corroborate and improve on our previous reports (8, 15).
Active immunization with GND-KLH resulted in a dramatic blunted behavioral response to cocaine (Fig. 2). At the time of the first challenge, the vaccinated group showed a 76% reduction in locomotor activity (crossovers) compared with baseline (preimmunization) values, resulting in significant differences in mean activity between groups (Fig. 2a). Also, marked differences in percent of stereotypy displayed between groups achieved significance throughout the entire session (90 min). The abatement in locomotion produced by GND-KLH persisted into the second cocaine challenge, and a maximum decrease in locomotion was obtained upon the last challenge, with an 80% reduction versus baseline values (Fig. 2 b and c). Stereotypy differed significantly between groups across all testing sessions. Notably, the experimental mean activity curve and the total activity counts resembled those after saline injection (data not shown). Moreover, this effect endured across all challenges, a total of 12 days after immunization.
Treatment with GND-KLH also prevented cocaine sensitization, a psychopharmacological phenomenon by which repeated injections of acute peripheral cocaine induce an increased motor stimulant response with each subsequent injection (25, 26). This condition was observed in the control group, where there was a progressive increase in both measures in control animals across all challenges (Fig. 2 Upper). At the third cocaine challenge, a 38% increase in ambulatory behavior was seen in the control group compared with baseline, and the enhanced stereotypic response was sustained throughout the session (Fig. 2c).
These findings are an extension of an earlier study where GNC-KLH was also tested in a rat model of locomotor activity (8). Comparatively, the present results indicate that GND-KLH produced a greater and more enduring suppression of cocaine-induced psychomotor behavior than GNC-KLH. These enhanced behavioral effects could be explained by the design features of the diamide hapten 3, which maintained an effective titer of between 12,800 and 25,600 that persisted throughout the time frame of the experiment (a total of 12 days). Although the absolute value of the highest titer was not greater than that elicited by GNC-KLH, the high-quality and longer-lived GND-KLH titer was apparently responsible for the improvement in the blockade of cocaine-induced psychomotor effects.
The GND hapten incorporates structural modifications hypothesized to be advantageous during immunization. As depicted (Fig. 1), the distinct difference between GND and GNC is the ester–amide interchange, where 3 is devoid of the C-2/C-3 esters found in 1 and mimicked in 2. Although the change in chemical structure appears radical at first glance, it has a sound physicochemical foundation. The intermolecular forces that contribute to the stabilization of an antibody–antigen complex are similar to those involved in the stabilization of the structures of proteins and other macromolecules (27). Hydrogen bonding has held particular interest in this regard because of the central role it plays in molecular recognition (28). Indeed, one of the best hydrogen-bond donor/acceptors in water is the amide functionality (29). The GND hapten displays two amide groups in the correct stereochemical configuration as found in the cocaine framework. Therefore, antibody affinity to cocaine itself could be optimized by taking advantage of interactions that rely on hydrogen bonds established by 3 during the immune response.
Besides the interactions that could be generated by GND, enhanced stability of GND-KLH compared with GNC-KLH during immunization should also be expected. The duration of the primary and secondary antibody responses varies with the dose, mode of administration, and persistence of the antigen (30). GND with its amide functionalities will be more stable than GNC (31). It is expected that this added stability could manifest itself into a higher sustained immunoconjugate concentration for the duration of the vaccination (32, 33). Clearly, a longer-lived antigen, together with a highly specific antibody response to cocaine, is especially beneficial to the success of this immunopharmacological approach.
Animals treated with GNC92H2 (90 mg/kg) showed a 62% decrease in ambulatory behavior after the first cocaine challenge as compared with baseline, and the mean activity curves between groups were significantly different (Fig. 3a Upper). This suppressive effect was also observed to be significant in the stereotypy measure throughout the session (Fig. 3a Lower). The results are consistent with a recent study, where it was found that passive transfer of GNC92H2 dose-dependently affected cocaine self-administration, resulting in a profound suppression in response rate and in a full blockade of cocaine self-administration reinstatement (15).
On two subsequent cocaine challenges, similar but more modest decreases were seen in the experimental group in both measures, with significant differences between groups at the time of the second and third challenges (Fig. 2 a and b). As in the first experiment, control animals showed increases in crossovers of up to 29%. In the experimental group, there was a 46–47% decrease in crossovers as compared with baseline values, and by the third challenge, a noticeable re-emergence of stereotypy early in the session was observed (Fig. 2b Lower). These attenuated effects may be explained by the relatively short half-life of murine mAbs in the rat (∼8 days), resulting in rapid clearance (34).
Similar patterns of behavioral suppression were observed in both GND-KLH- and GNC92H2-treated animals. Hence, the antibody titers elicited by the second-generation vaccine could be viewed to possess the robust and persistent quality of successive mAb passive transfers. This important feature, coupled with the intrinsic benefits of active immunization, namely a protective, endogenously sustained anti-cocaine mechanism, renders this conjugate a powerful immunotherapeutic.
In humans as in laboratory animals, a main determinant of the addictive potential is the mode of ingestion of cocaine (35). The significance of this factor relates to the course of plasma cocaine concentration as a function of route of administration (36), determining the rapidity with which the drug reaches the brain to exert its euphoric effects (37). Therefore, a stable and long-lasting endogenous mechanism that binds peripheral cocaine should provide a safe and reliable means of protection against the drug. The data reported herein describe two immune-mediated cocaine-blocking agents that effectively impede the passage of the drug into the brain as reflected by the dramatic and persistent suppression of cocaine-induced psychomotor activation. These findings are an important addition to the evidence accumulated in recent years that immunopharmacotherapy offers a promising strategy for the treatment of cocaine abuse.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grants DA08590 (to K.D.J.) and DA04398 (to G.F.K.) and by private funding from the Joseph Drown Foundation and Mr. Robert E. Martini.
Abbreviation
- KLH
keyhole limpet hemocyanin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041610998.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041610998
References
- 1.Gawin F F, Ellinwood E H., Jr Annu Rev Med. 1989;40:149–161. doi: 10.1146/annurev.me.40.020189.001053. [DOI] [PubMed] [Google Scholar]
- 2.Hall W C, Talbert R L, Ereshefsky L. Pharmacotherapy. 1990;10:46–65. [PubMed] [Google Scholar]
- 3.Tennant F S, Jr, Sagherian A A. Arch Intern Med. 1987;147:109–112. [PubMed] [Google Scholar]
- 4.Giannini A J, Malone D A, Giannini M C, Price W A, Loiselle R H. J Clin Pharmacol. 1986;26:211–214. doi: 10.1002/j.1552-4604.1986.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 5.Winger G. In: Cocaine Abuse Research: Pharmacology, Behavior, and Clinical Applications. Higgins S T, Katz J L, editors. New York: Academic; 1998. pp. 259–302. [Google Scholar]
- 6.Spector S, Berkowitz B, Flynn E J, Peskar B. Pharmacol Rev. 1973;25:281–291. [PubMed] [Google Scholar]
- 7.Bonese K F, Wainer B H, Fitch F W, Rothberg R M, Schuster C R. Nature (London) 1974;252:708–710. doi: 10.1038/252708a0. [DOI] [PubMed] [Google Scholar]
- 8.Carrera M R A, Ashley J A, Parsons L H, Wirsching P, Koob G F, Janda K D. Nature (London) 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 9.Basagra O, Forman L J, Howeedy A, Whittle P A. Immunopharmacology. 1992;23:173–179. doi: 10.1016/0162-3109(92)90023-6. [DOI] [PubMed] [Google Scholar]
- 10.Fox B S, Kantak K M, Edwards M A, Black K M, Bollinger B K, Botka A J, French T L, Thompson T L, Schad V C, Greenstein J L, et al. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger R H, Ettinger W F, Harless W E. Pharmacol Biochem Behav. 1997;58:215–220. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 12.Gallacher G. Immunopharmacology. 1994;27:79–81. doi: 10.1016/0162-3109(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 13.Self D W. Nature (London) 1995;378:666–667. doi: 10.1038/378666a0. [DOI] [PubMed] [Google Scholar]
- 14.Wise R A, Rinaldi R. Nat Med. 1996;2:1073. doi: 10.1038/nm1096-1073. [DOI] [PubMed] [Google Scholar]
- 15.Carrera M R A, Ashley J, Zhou B, Wirsching P, Koob G F, Janda K D. Proc Natl Acad Sci USA. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakurai M, Wirsching P, Janda K D. Tetrahedron Lett. 1996. 5479–5482. [Google Scholar]
- 17.Scheel-Krüger J, Braestrup C, Nielson M, Golembiowska K, Mogilnicka E. In: Cocaine and Other Stimulants. Ellinwood H Jr, Kilbey M M, editors. New York: Plenum; 1977. pp. 373–408. [Google Scholar]
- 18.Kelly P H, Iversen S D. Eur J Pharmacol. 1976;40:45–47. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- 19.Köhler G, Howe S C, Milstein C. Eur J Immunol. 1976;6:292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- 20.Köhler G, Milstein C. Eur J Immunol. 1976;6:511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 21.Caine S B, Lintz R, Koob G F. In: Behavioral Neuroscience: A Practical Approach. Shagal A, editor. Oxford: Oxford Univ. Press; 1993. pp. 117–143. [Google Scholar]
- 22.Fray P J, Sahakian B J, Robbins T W, Koob G F, Iversen S D. Psychopharmacology. 1980;69:153–539. doi: 10.1007/BF00433091. [DOI] [PubMed] [Google Scholar]
- 23.Robbins T W. In: Handbook of Psychopharmacology. Iversen L, Iversen S, Snyder S, editors. New York: Plenum; 1977. pp. 37–82. [Google Scholar]
- 24.Kullback S. Information Theory and Statistics. New York: Dover; 1968. [Google Scholar]
- 25.Post R M, Rose H. Nature (London) 1976;260:731–734. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- 26.Kalivas P W, Duffy P, DuMars A, Skinner C. J Pharmacol Exp Ther. 1988;245:485–502. [PubMed] [Google Scholar]
- 27.Steward M W. Antibodies: Protein Structure and Function. New York: Chapman and Hall; 1984. [Google Scholar]
- 28.Jeffery G A, Saenger W. Hydrogen Bonding in Biological Structures. New York: Springer; 1991. [Google Scholar]
- 29.Rablen P R, Lockman J W, Jorgensen W L. J Phys Chem. 1998;102:3782–3797. [Google Scholar]
- 30.Eisen H N. In: General Immunology. McAllister L, Winters R, Bellus A, editors. Philadelphia: Lippincott; 1990. pp. 167–189. [Google Scholar]
- 31.Jencks W P. In: Catalysis in Chemistry and Enzymology. Jencks W P, editor. New York: Dover; 1967. pp. 163–182. [Google Scholar]
- 32.Gray D, Skarvall H. Nature (London) 1988;336:70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- 33.Tew J G, Phipps R P, Mandel T E. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 34.Bazin-Redureau M I, Renard C B, Scherrmann J M. J Pharm Pharmacol. 1997;49:277–281. doi: 10.1111/j.2042-7158.1997.tb06795.x. [DOI] [PubMed] [Google Scholar]
- 35.Post R M, Contel N R. In: Stimulants: Neurochemical, Behavioral and Clinical Perspectives. Creese I, editor. New York: Raven; 1983. pp. 169–203. [Google Scholar]
- 36.Jatlow P. Yale J Biol Med. 1988;61:105–113. [PMC free article] [PubMed] [Google Scholar]
- 37.Koob G F, Goeders N E. In: The Neuropharmacological Basis of Reward. Cooper S J, Lieberman J M, editors. Oxford: Clarendon; 1989. pp. 214–263. [Google Scholar]