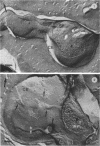
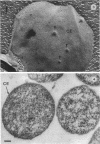
Abstract
An investigation of Proteus mirabilis wild-type strains and their various derived L-forms shows that the enterobacterial common antigen (ECA) is localized in the outer membrane of the cell envelope of these strains. In strains where the outer membrane is lacking (stable protoplast L-forms) or where its amount is reduced (spheroplast UL19) no ECA or only reduced amounts of it are detected by serological tests or by ferritin-labeling techniques.
Full text
PDF

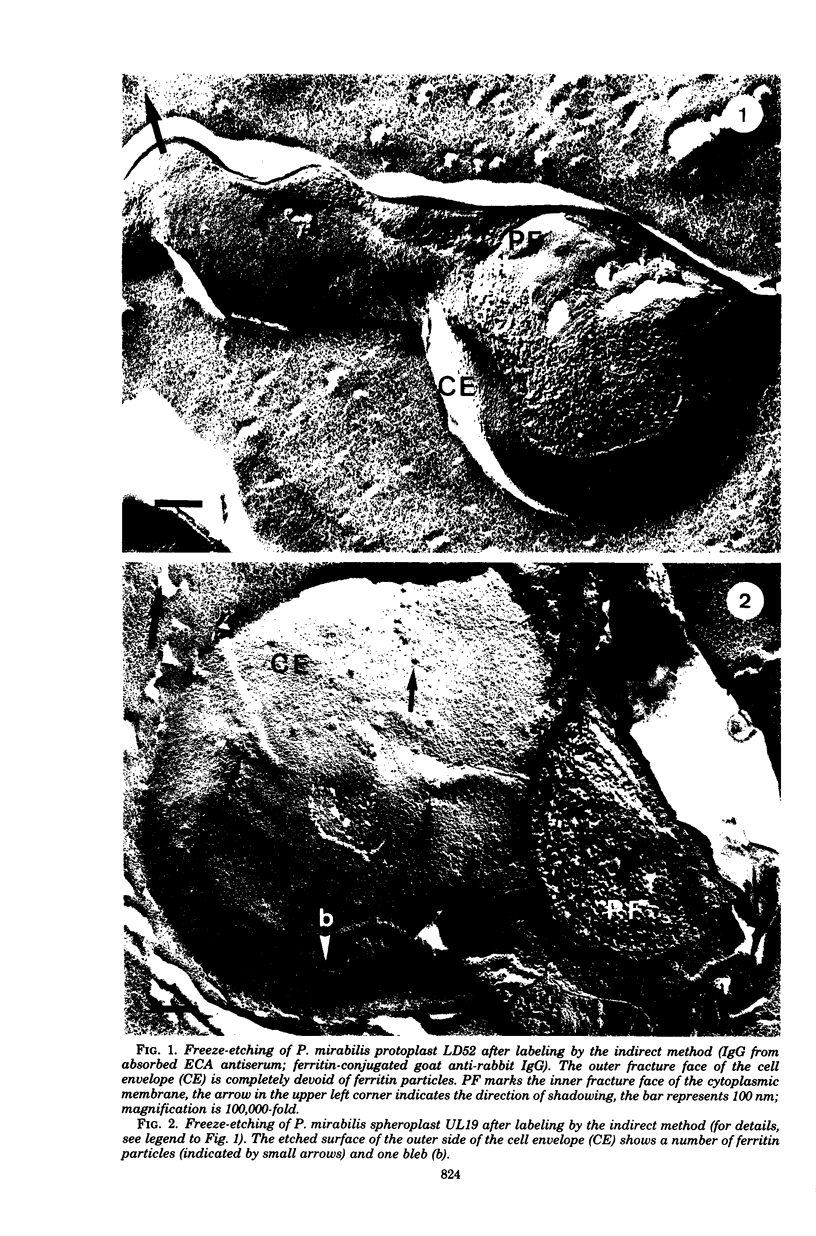
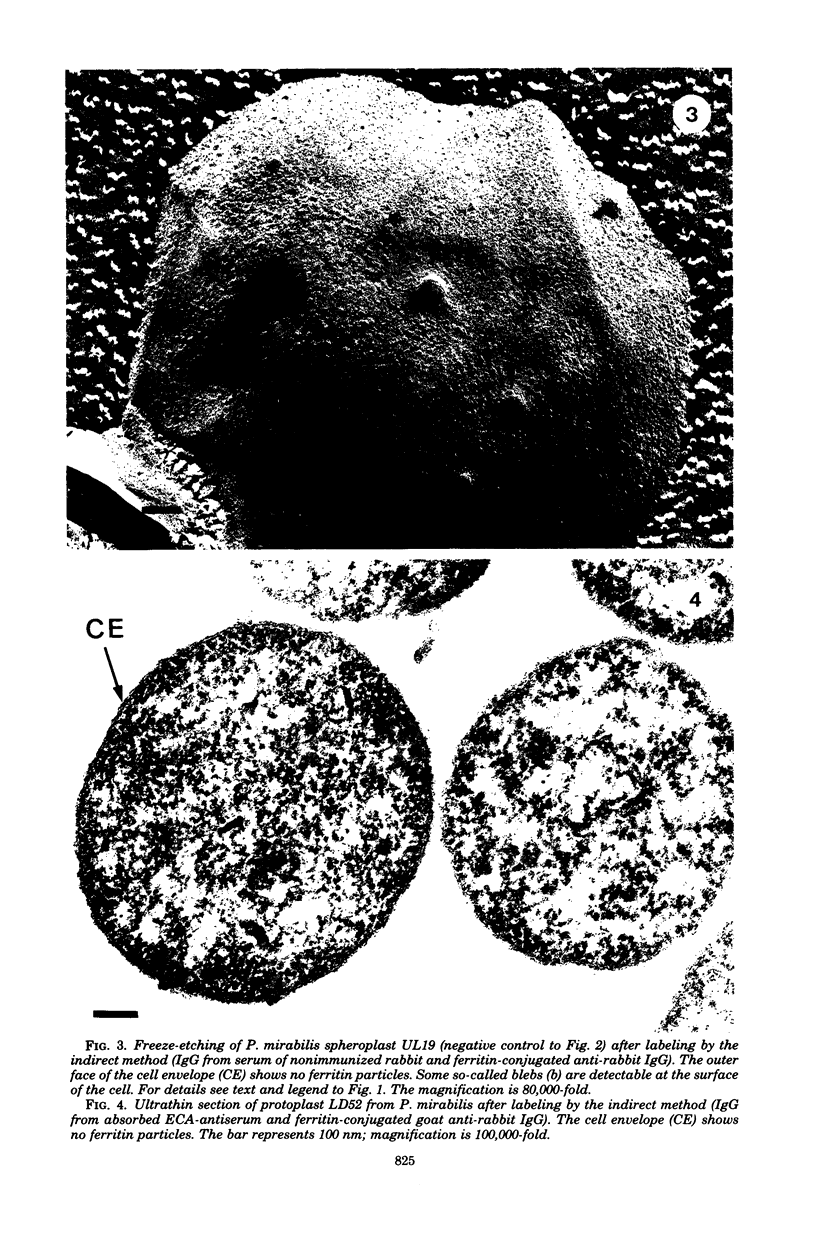


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Domingue G., Johnson E. Isolation of subcellular fractions containing immunogenic enterobacterial common antigen. Z Immunitatsforsch Exp Klin Immunol. 1974 Nov;148(1):23–38. [PubMed] [Google Scholar]
- Geyer R., Galanos C., Westphal O., Golecki J. R. A lipopolysaccharide-binding cell-surface protein from Salmonella minnesota. Isolation, partial characterization and occurrence in different Enterobacteriaceae. Eur J Biochem. 1979 Jul;98(1):27–38. doi: 10.1111/j.1432-1033.1979.tb13156.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Martin H. H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form. Difference in content and fatty acid composition. Eur J Biochem. 1976 Aug 16;67(2):487–494. doi: 10.1111/j.1432-1033.1976.tb10714.x. [DOI] [PubMed] [Google Scholar]
- Hofschneider P. H., Martin H. H. Diversity of surface layers in L-forms of Proteus mirabilis. J Gen Microbiol. 1968 Apr;51(1):23–33. doi: 10.1099/00221287-51-1-23. [DOI] [PubMed] [Google Scholar]
- Johnson E. J., Domingue G. J., Klein G. D. Anatomical locus of the common enterobacterial antigen. Z Immunitatsforsch Immunobiol. 1976 Sep;152(1):15–26. [PubMed] [Google Scholar]
- KANDLER O., HUND A., ZEHENDER C. Cell wall composition in bacterial and L forms of Proteus vulgaris. Nature. 1958 Feb 22;181(4608):572–573. doi: 10.1038/181572a0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN H. H. COMPOSITION OF THE MUCOPOLYMER IN CELL WALLS OF THE UNSTABLE AND STABLE L-FORM OF PROTEUS MIRABILIS. J Gen Microbiol. 1964 Sep;36:441–450. doi: 10.1099/00221287-36-3-441. [DOI] [PubMed] [Google Scholar]
- Martin H. H., Gmeiner J. Modification of peptidoglycan structure by penicillin action in cell walls of Proteus mirabilis. Eur J Biochem. 1979 Apr;95(3):487–495. doi: 10.1111/j.1432-1033.1979.tb12988.x. [DOI] [PubMed] [Google Scholar]
- Mayer H., Schmidt G. Hämagglutinine gegen ein gemeinsames Enterobacteriaceen-Antigen in E. coli R1-Antiseren. Zentralbl Bakteriol Orig. 1971;216(3):299–313. [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976 Sep;40(3):591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D., Mayer H. Isolation and chemical characterization of the enterobacterial common antigen. Eur J Biochem. 1978 May 16;86(2):361–370. doi: 10.1111/j.1432-1033.1978.tb12318.x. [DOI] [PubMed] [Google Scholar]
- Männel D., Mayer H. Serological and immunological properties of isolated enterobacterial common antigen. Eur J Biochem. 1978 May 16;86(2):371–379. doi: 10.1111/j.1432-1033.1978.tb12319.x. [DOI] [PubMed] [Google Scholar]
- Neter E. Indirect bacterial hemagglutination, and its application to the study of bacterial antigens and serologic diagnosis. Pathol Microbiol (Basel) 1965;28(6):859–877. doi: 10.1159/000161853. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHANG H. Y., NETER E. DEMONSTRATION OF COMMON ANTIGEN IN SALMONELLA TYPHI SPHEROPLASTS. Can J Microbiol. 1965 Jun;11:598–601. doi: 10.1139/m65-078. [DOI] [PubMed] [Google Scholar]
- WHANG H. Y., NETER E. Immunological studies of a heterogenetic enterobacterial antigen (Kunin). J Bacteriol. 1962 Dec;84:1245–1250. doi: 10.1128/jb.84.6.1245-1250.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]