Abstract
To mimic the molecular specificity and cell selectivity of monoclonal antibody (mAb) binding while decreasing size, nanomolecules (selective high-affinity ligands; SHALs), based on in silico modeling, have been created to bind to human leukocyte antigen-DR (HLA-DR10), a signaling receptor protein upregulated on the malignant B-lymphocytes of non-Hodgkin's lymphoma and chronic lymphocytic leukemia. SHALs were synthesized with a biotin or DOTA chelate (1,4,7,10-tetraazacyclododecane-N,N′,N″,N″′-tetraacetic acid), using a solid-phase lysine-polyethyleneglycol backbone to link sets of ligands shown previously to bind to HLA-DR10. Using cell-binding and death assays and confocal microscopy, SHAL uptake, residualization, and cytocidal activity were evaluated in HLA-DR10 expressing and nonexpressing live, human lymphoma cell lines. All of the SHALs tested were selective for, and accumulated in, expressing cells. Reflecting binding to HLA-DR10 inside the cells, SHALs having the Ct ligand (3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid) residualized in expressing cells greater than 179 times more than accountable by cell-surface membrane HLA-DR10. Confocal microscopy confirmed the intracellular residualization of these SHALs. Importantly, SHALs with a Ct ligand had direct cytocidal activity, similar in potency to that of Lym-1 mAb and rituximab, selectively for HLA-DR10 expressing lymphoma cells and xenografts. The results show that SHALs containing the Ct ligand residualize intracellularly and have cytocidal effects mediated by HLA-DR10. These SHALs have extraordinary potential as novel molecules for the selective targeting of lymphoma and leukemia for molecular therapy and imaging. Further, these SHALs can be used to transport and residualize cytotoxic agents near critical sites inside these malignant cells.
Key words: antibodies, nanomolecules, ligands, HLA-DR, lymphoma, therapy, imaging
Introduction
“Nothing is too wonderful to be true if it be consistent with the laws of nature.”
—Michael Faraday
Most chemotherapeutic drugs penetrate and, as readily, exit cells and tissues. Because cell uptake is not selective, the effectiveness of these drugs is limited by their toxicity. Other drugs of small size (e.g., fluorine-18 [18F] deoxyglucose and iodine-131 [131I] sodium iodide), are routinely used for imaging because they also bind to the cancer cells. Because 131I rapidly accumulates in thyroid cancer (in the absence of normal thyroid tissue), this drug has a major role in the management of metastatic thyroid cancer and is a paradigm for molecular-targeted systemic radiotherapy.
Larger molecules, such as monoclonal antibodies (mAbs), have also been used for these purposes. Rituximab (Rituxan®; Biogen Idec Inc., Cambridge, MA, and Genentech Inc., South San Francisco, CA), a chimeric anti-CD20 mAb, induces cells, to which it binds, to die. To increase these effects and to permit imaging, radionuclides have been conjugated to anti-CD20 mAbs. Ibritumomab (Zevalin®; Biogen Idec Inc.), the mouse parent of rituximab, labeled with yttrium-90 (90Y), induced higher response rates than rituximab in a randomized trial in patients with non-Hodgkin's lymphoma (NHL).1 Similarly, the anti-CD20 mAb, tositumomab (Bexxar®; GlaxoSmithKline, Research Triangle Park, NC), labeled with 131I, induced more favorable therapeutic outcomes than chemotherapy.2 Another mAb, Lym-1, has also been shown to be an effective carrier of radionuclides for systemic radiotherapy in patients3 and to have antilymphoma activity greater than that of rituximab after ligation of HLA-DR because of cell-death signaling.4–6
Using in silico modeling, novel nanomolecules were designed to serve as carriers of cell toxins, such as radionuclides, by mimicking the specific binding of Lym-1 mAb to the β-subunit of human leukocyte antigen-DR (HLA-DR) in the region of residues shown critical for Lym-1 binding and cytotoxicity in lymphoma cell lines of B-cell genotypes.7,8 Binding of these selective high-affinity ligands (SHALs) mimics that of mAbs because multiple contacts between residues on the surface of the SHAL and its target protein provide high specificity and affinity.9,10 Contrarywise, SHALs mimic the pharmacokinetic behavior of sodium iodide, because they are small and rapidly trapped by HLA-DR10-expressing lymphoma tissue or excreted in the urine.
Although all of the SHALs have discriminated HLA-DR10 expressing from nonexpressing malignant cells, mimicking Lym-1,11–13 and exhibited small-molecule pharmacokinetic behavior,11,14 earlier SHALs tested showed no antilymphoma activity.12 To increase binding and selectivity and, therefore, SHAL residence time in NHL tissue, SHALs having a Ct ligand (3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid) for a third docking site on HLA-DR10 were synthesized.14,15
In this paper, we characterize the cellular fates and effects of both a tridentate and a dimeric, tridentate SHAL, each containing the Ct ligand, compare their behavior with those of other bidentate SHALs containing and lacking the Ct ligand, and show that the Ct ligand SHALs residualize in HLA-DR10-expressing human lymphoma cells. Although intended to be cell-specific carriers for molecular therapy and imaging, SHALs containing the Ct ligand exhibited direct antilymphoma (i.e., cytocidal) activity in the absence of a radionuclide. Because these SHALs readily pass through cell membranes, they also have enormous potential for selective intracellular delivery of a variety of cytotoxic agents.
Materials and Methods
Reagents and Cell Lines
Murine Lym-1 (Peregrine Pharmaceuticals, Tustin, CA) was generated by using Raji malignant lymphocytes as the immunogen. Murine and chimeric (A. Epstein, Los Angeles, CA) Lym-1 bind to an epitope in the beta-subunit of HLA-DR10 and related HLA-DR proteins expressed on malignant B-cells.7,8,16 HLA-DR10 protein that is expressed by antigen-presenting cells was isolated from Raji Burkitt's human lymphoma B-cells and purified on a Lym-1 affinity column, as described previously.13 Two HLA-DR10-expressing human B-cell lymphoma lines, Raji (American Type Culture Collection, Manassas, VA) and SU-DHL4 (A. Epstein), and two nonexpressing human T-cell lymphoma/leukemia lines, Jurkat's, and CEM (American Type Culture Collection), grown as recommended, were used for the experiments.
Drug Design and Chemistry
Using homology modeling, residues critical for Lym-1 binding were mapped on a three-dimensional (3D) model of the HLA-DR10 beta-subunit.13 Cavities within the Lym-1 epitope of the protein were identified by using SPHGEN.17,18 After identifying ligands predicted to bind to the cavities by using computational docking, a combination of nuclear magnetic resonance (NMR) spectroscopy, surface plasmon resonance (BIA-core 3000; Biacore, Piscataway, NJ), and competitive binding experiments were used to confirm that the ligands bound to different sites on HLA-DR10 protein. To create SHALs, ligands were conjugated to the ends of polyethylene glycol (PEG) monomers through the alpha and epsilon amines of the N-terminal lysine, using Fmoc solid-phase chemistry, as previously described.15 The same process was used to synthesize the tridentate SHAL by linking together the three ligands, dabsylvaline (Dv), 4-[4-(4-chlorobenzyl) piperazino]-3-nitrobenzenecarboxylic acid (Cb), and Ct. The Dv ligand was attached to the α-amine of the second lysine residue spaced with a PEG, and the Cb ligand was attached to the α-amine of the third lysine, again spaced with a PEG. Last, the Ct ligand was directly attached to the ɛ-amine of the third lysine from the ɛ-amine of the second lysine residue. For the dimeric, tridentate motif, one additional lysine residue, linked to the α-amine of the first lysine, was used to construct two equivalent branching “SHALs” by inserting three consecutive PEG units at the alpha and epsilon amines. The ɛ-amino group of the first lysine was used to attach a biotin or DOTA chelate (1,4,7,10-tetraazacyclododecane-N,N′,N″,N″′-tetraacetic acid). The reaction solution was purified by using reverse-phase high-performance liquid chromatography (RP-HPLC). Analytic electrospray ionization-mass spectrometry (Agilent 1100 instrument, Waters Symmetry C18 column, Agilent, Santa Clara, CA) confirmed the elemental and mass composition of each of the SHALs (Fig. 1). The SHALs studied herein and their corresponding acronyms are shown in Table 1, and the chemical structures in two dimensions and the acronyms for the Ct-containing tridentate and dimeric, tridentate SHALs are shown in Figure 2. Using surface plasmon resonance (BIAcore 3000), SHAL binding to isolated or recombinant HLA-DR10 protein was observed to be in the nanomolar range, as previously described.13,19 SHALs no longer bound after the addition of Lym-1.
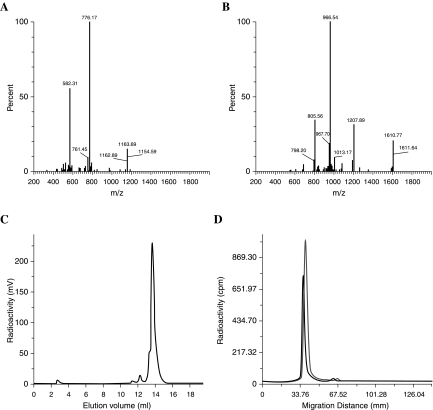
Figure 1.
Purity and stability of selective high-affinity ligands (SHALs), as described in the Materials and Methods section. (A and B) Electrospray ionization ESI mass spectroscopy confirmed that the mass and elemental compositions of the Ct-containing tridentate (A) and dimeric tridentate (B) SHALs corresponded to what was expected. (C) Reverse-phase high-performance liquid chromatography. 111In-DOTA-SHAL ((DvLPLLCtPCbPPP)2LLDo) eluted at 14 mL (>95%), and 111In-EDTA (<5%), eluted at 3 mL. (D) Stability. Radioactive scanning (Fuji Imager BAS1800II, Kanagawa, Japan) of strips electrophoresed for 45 minutes. Traces correspond to the 111In-DOTA SHAL, (DvPLLCtPCbPPP)2LLDo, before and after incubation in plasma for 2 and 4 hours. The SHAL was neutral in the electrical field and did not bind to the plasma proteins.
Table 1.
Multidentate and Dimeric, Multidentate SHALsa Compared and Their Molecular Weight (MW) in Daltons
| Acronym | Identity | MW |
|---|---|---|
| Multidentate | ||
| LeacPLD | Acetylated 5-leuenkephalin PEG lysine deoxycholate | 1505 |
| ItPLD | Triiodothyronine PEG lysine deoxycholate | 1559 |
| DvLPBaPL | Dabsyl-l-valine lysine PEG N-benzoyl-l-arginyl-4-amino-benzoic acid PEG lysine | 1317 |
| CtLPTPL | 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid lysine PEG l-thyronine PEG lysinea | 1163 |
| CtLPBaPL | 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid lysine PEG N-benzoyl-l-arginyl-4-amino-benzoic acid PEG lysine | 1287 |
| DvPLLCtPCbL | Dabsyl-l-valine PEG lysine lysine 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid PEG 4-[4-(4-chlorobenzyl)piperazino]-3-nitrobenzenecarboxylic acid lysine | 1765 |
| Dimeric, multidentate | ||
| (LeacPLD)2LP | (Acetylated 5-leuenkephalin PEG lysine deoxycholate)2 lysine PEG | 3006 |
| (ItPDP)2LL | (Triiodothyronine PEG deoxycholate PEG)2 lysine lysine | 3113 |
| (DvLPBaP)2LL | (Dabsyl-l-valine lysine PEG N-benzoyl-l-arginyl-4-aminobenzoic acid PEG)2 lysine lysine | 2626 |
| (DvLPBaPP)2LL | (Dabsyl-l-valine lysine PEG N-benzoyl-l-arginyl-4-aminobenzoic acid PEG PEG)2 lysine lysine | 2921 |
| (DvLPBaPPP)2LL | (Dabsyl-l-valine lysine PEG N-benzoyl-l-arginyl-4-aminobenzoic acid PEG PEG PEG)2 lysine lysine | 3210 |
| (DvLPBaPPPP)2LL | (Dabsyl-l-valine lysine PEG N-benzoyl-l-arginyl-4-aminobenzoic acid PEG PEG PEG PEG)2 lysine lysine | 3501 |
| (DvLCsPBaPPP)2CsLL | (Dabsyl-l-valine lysine cysteic acid PEG N-benzoyl-l-arginyl-4-aminobenzoic acid PEG PEG PEG)2 cysteic acid lysine lysine | 3666 |
| (DvPLLCtPCbPPP)2LL | (Dabsyl-l-valine PEG lysine lysine 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid PEG 4-[4-(4-chlorobenzyl)piperazino]-3-nitrobenzenecarboxylic acid PEG PEG PEG)2 lysine lysine | 4267 |
SHALs containing the “Ct” ligand are in bold. DOTA on the SHAL contributes an additional 400 (or 244 for biotin) Daltons to the MW.
Figure 2.
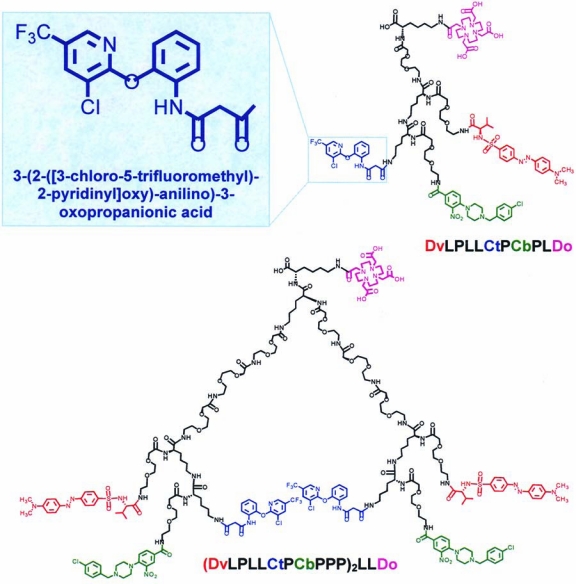
Chemical structures and acronyms for the tridentate SHAL (upper right) DvLPLLCtPCbPLDo; (dabsyl-l-valine PEG lysine lysine 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid PEG 4-[4-(4-chlorobenzyl)piperazino]-3-nitrobenzenecarboxylic acid lysine-DOTA having the Ct ligand (upper left insert); and the dimeric, tridentate SHAL (lower) (DvLPLLCtPCbPPP)2LLDo; ((dabsyl-l-valine PEG lysine lysine 3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl]oxy)-anilino)-3-oxopropanionic acid PEG 4-[4-(4-chlorobenzyl)piperazino]-3-nitrobenzenecarboxylic acid PEG PEG PEG)2 lysine lysine-DOTA. (Analog-biotinylated SHALs differ only with respect to substitution of biotin for the DOTA chelate, upper right of each SHAL.)
DOTA-SHALs were labeled with 111InCl3 in 0.05M HCl (MDS Nordion, Vancouver, Canada), as previously described.11,15 Briefly, the reaction mixture was adjusted to pH 6–7 by adding 4 M NH4OAc, incubated for 1 hour at 37°C, then 0.1 M of ethylenediaminetetraacetic acid (EDTA) was added to sequester free 111In3+. The reaction mixture was purified by using RP-HPLC. The purity of the products ranged from 90% to 95%.
The stability of each 111In-DOTA-SHAL in plasma was assessed, as previously described, by using cellulose acetate electrophoresis for 45 minutes in tris-barbital-sodium buffer (pH 8.6).11,14 Strips were imaged by using a phosphor imager (Fuji BAS1800II, Kanagawa, Japan), and SHAL electromobility was compared with those of the plasma proteins. Plasma aliquots were also mixed with goat antihuman transferrin, antihuman albumin, antihuman IgG, or antihuman ceruloplasmin (Sigma, St. Louis, MO) in excess, then centrifuged at 12,000× g for 10 minutes. The activity of the supernatants and precipitates were measured by counting in a gamma-well counter (Pharmacia 1282 CompuGamma; Pharmacia, Inc., Piscataway, NJ). The 111In-DOTA-SHALs did not bind to plasma proteins and were stable in plasma over 7 days (Fig. 1).
In summary, biotinylated and DOTA-SHALs were synthesized at high purity in multimilligram amounts. DOTA-SHALs were stably labeled with indium-111 (111In) at high efficiency, specific activity, and purity; median-specific activities were 3.8 MBq/μg (102 μCi/μg).
Cell-Binding Assays
To assess SHAL binding to live cells, enzyme-linked immunosorbent assay (ELISA) assays were performed, as previously described.13 Cells were incubated with biotinylated SHALs for 1 hour at RT or 4°C, washed, and transferred to a fresh plate. SA-HRP was added for 30 minutes, the cells pelleted, and 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulphonic acid substrate (ABTS) added for 20 minutes. HLA-DR-expressing cells bound more of the SHAL/SA-HRP complex, giving a greater signal.
The number of HLA-DR10 receptors on Raji cells was determined, as previously described.20 Briefly, Lym-1, trace labeled with iodine-125 (125I), was added to varying amounts of unlabeled Lym-1 and incubated with 1 × 106 cells in 5% bovine serum albumin and phosphate-buffered saline (BSA/PBS) for 1 hour at room temperature (RT). After separation by centrifugation, the cell pellet and supernatant were counted in a calibrated gamma well counter (Pharmacia 1282 CompuGamma, Piscataway, NJ). Scatchard analysis was used to estimate the number of receptors per cell.20 The average for 10 assays was 1.2 ± 0.3 × 106 receptors per cell, or 2.3 pmoles per million cells.
Live Cell Uptake and Residualization
To quantitate uptake and residualization of SHALs in live cells, an assay was performed by using serially diluted SHAL, as previously described.12 Each SHAL labeled with 111In, to which varying amounts of unlabeled SHAL had been added, was incubated with 1 × 106 cells for 1 hour at RT. Cells were pelleted by centrifugation at 300g for 10 minutes. Cell pellet (bound) and supernatant were counted in a gamma-well counter. To determine the extent of SHAL residualization, an identical assay was performed, except that the cells were washed twice with PBS, washes pooled, and the cell pellet and pooled washes counted. Results represent the mean and standard deviation (SD) of replicate samples.
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded NHL tissue sections from a patient after deparaffinization, as previously described.13 Before incubation with Lym-1, antigen retrieval was accomplished by using high temperature. Slides were counterstained in Mayer's hematoxylin.
Confocal Microscopy
Live cells were incubated with biotinylated chLym-1 or tridentate or dimeric, tridentate Ct-containing SHAL, then washed and fixed on slides before incubation with Streptavidin Alexa Fluor® (Invitrogen, Carlsbad, CA) 610 (red) to localize the SHAL or the chLym-1. The mounting medium contained DAPI, a nuclear stain. Laser scanning at 405 and 554 nm was performed by using an Olympus FV 100 confocal microscope (Center Valley, PA). Images were collected at focal planes 1 u apart.
Cytotoxicity Assay
Cells in log-growth phase were suspended in fresh media and counted after adding 10% trypan blue to distinguish nonviable from viable cells, as previously described.12 In untreated samples, the cells multiplied and nonviable cells, initially less than 5% of total cells, remained at this level over the course of the assays. The assays were designed to detect antilymphoma activity from the Auger electrons of 111In and to determine whether the observed effects could be blocked by treatment with 1000 ng of unlabeled SHAL. Tubes containing cells were incubated for 1 hour at RT with 100 ng of 111In-labeled SHAL, 100 ng of unlabeled SHAL as a control, or 100 ng of 111In-labeled SHAL after preincubation with 1000 ng of unlabeled SHAL. To remove unbound SHAL and 111In at the end of incubation, each tube was centrifuged at 300g for 10 minutes, the supernatant was removed, and then cells were washed twice in PBS (0.5 mL) for 15 minutes at RT. The cell pellet resuspended in fresh media and an aliquot of each pooled supernatant were counted to determine the amount of SHAL and 111In subsequently introduced into each of the triplicate wells. Cells from each tube were diluted to 0.5 × 106/mL in media with 10% fetal calf serum, aliquoted (200 uL) into sterile, flat-bottom wells and incubated at 37°C, 5% CO2. Measurements of 111In activity indicated that the maximum amounts of 111In and SHAL were 39 nCi and 3 ng of tridentate SHAL, and 18 nCi and 1.4 ng of dimeric, tridentate SHAL for each well containing expressing cells, and less than 7 nCi and 0.5 ng of either SHAL for each well containing nonexpressing cells. After 1, 2, and 3 days, cells were resuspended and 90 uL from each of the triplicate wells were added to 10 uL of filtered trypan blue, and the refractile live and blue-stained dead cells were counted by using a microscope and hemocytometer.
Radiation-Absorbed Dose
The radiation-absorbed dose to the cells was estimated, using published methods and absorbed fractions.21 Briefly, a measured mean cell diameter of 12 μm, an estimated nuclear diameter of 10 μm, and an S-value that assumed the 111In to be in the cytoplasm and the nucleus to be the radiation target were used. Calculations were based on the highest 111In activity measured for the wells. Radiation exposure received while the cells were incubating before washing was not included; it added less than 10% over 1 day and less than 5% over longer exposures. Because of their short range and the assay conditions, Auger electron exposure to neighboring cells was not considered. Despite these assumptions, the calculations represent an upper limit of exposure.22
Biostatistical Analysis
The data are representative of replicate studies that showed reproducible results. For cell-uptake assays, the % binding was multiplied by the SHAL added to each well and reported as the mean ± SD for each group of triplicates. When least mean squared linear regression was performed over the range of SHAL added, there was a good fit.
To assess the cytocidal effect of the SHALs, a series of regression models, including a nonparametric Wilcoxon test, were fitted to the fractional percentages and to the absolute numbers of nonviable cells as the outcomes, separately in HLA-DR10-expressing and -nonexpressing cells within an experiment. Predictors were included to estimate the effect of radiation dose, both as a linear dose response and—to check linearity—as indicators for the effects of additional 111In above the next lower levels, the effect of adding SHAL, the effect of dimeric, tridentate beyond tridentate SHAL, and the possible interaction of SHAL with radiation. Differences were deemed significant if p ≤ 0.05.
Results
Although the SHALs differed with respect to the nature of the ligands and their number, and the number of PEG monomers and lysines, all of the SHALs were electrically neutral and bound selectively to HLA-DR10 protein and expressing live cells.13,15 In contrast to Lym-1, SHALs accumulated in the cells over an extended range because of internalization. Four SHALs, two bidentate, one tridentate, and one dimeric, tridentate, all containing the Ct ligand, exhibited even greater lymphoma cell uptake. To further assess the influence of the Ct ligand, additional studies were performed on the tridentate and the dimeric, tridentate SHAL in expressing and nonexpressing live lymphoma cells. These included studies of cell uptake and residualization (after washing) of both DOTA and biotinylated motifs at RT and at 4°C, confocal microscopy, and cytocidal assays, using unlabeled and 111In-labeled SHALs.
Cell Binding, Uptake, and Residualization
The tridentate and dimeric, tridentate SHALs discriminated HLA-DR10 expressing from nonexpressing cells in enzyme-linked immunosorbent assay (ELISA) conducted at both 20° and at 4°C (Fig. 3). In assays in which incremental amounts of SHAL were added to cells, each SHAL accumulated at a fixed, fractional mass and number of moles (molecules) over a range greater than 1000 ng of added SHAL (Fig. 4). The affinities of the SHALs could not be measured under these equilibrium conditions.
Figure 3.
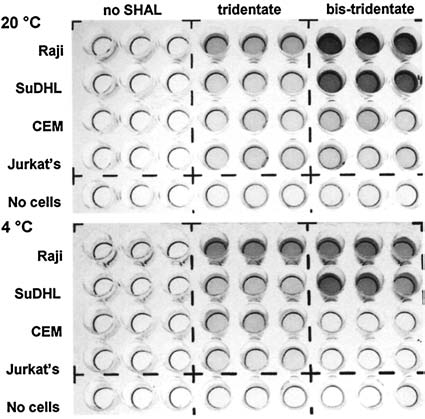
Binding of SHALs to HLA-DR10-expressing and -nonexpressing live human cells. Enzyme-linked imunosorbent assay shows that tridentate, DvLPLLCtPCb-PLB, and dimeric (bis) tridentate, DvLPLLCtPCb-PPP)2LLB, SHALs bind to expressing cells, Raji and SuDHL4, but not to nonexpressing cells, CEM and Jurkat's. Experiments were conducted at RT (20°C) (upper) and, to inhibit endocytosis, at 4°C (lower). Cells were incubated with biotinylated SHALs, as described in Materials and Methods.
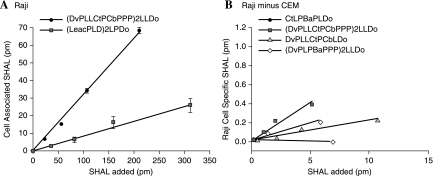
Figure 4.
Cell-associated SHAL uptake increased as SHAL in the media was increased, even over 1000 ng (A). SHALs containing the Ct ligand residualized in expressing cells (B), and SHAL uptake was greater than the surface-membrane HLA-DR10 of Raji cells. As manifested by the differing behaviors of SHALs having and lacking Ct ligand in expressing and nonexpressing cells, residualization was mediated by HLA-DR10 and the Ct ligand. Data are shown for Raji cells after subtracting that for CEM cells (B).
SHALs containing the Ct ligand behaved differently from the other SHALs. When compared to SHALs lacking the Ct ligand, molar uptakes of SHALs having the Ct ligand were about 3-fold greater in expressing cells before washing (Fig. 4A). After washing, residual SHAL in expressing cells was greater than 179 times more than cell-surface membrane HLA-DR10 (>412 vs. 2.3 pmoles/106 cells; > 108 vs. 106 copies per Raji cell). Then, 3D confocal microscopy of Raji cells confirmed the intracellular location of these SHALs and the cell surface membrane location of Lym-1 (Fig. 5).
Figure 5.
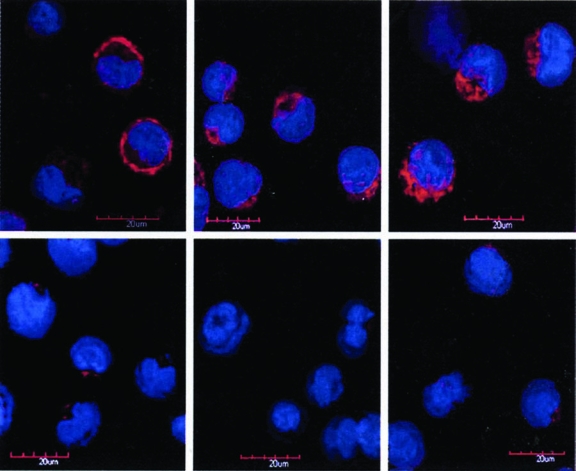
Confocal micrographs of washed live cells after incubation with chLym-1, tridentate (DvPLLCtPCbLB) or dimeric, tridentate ((DvPLLCtPCbPPP)2LLB) SHAL (left to right) in Raji (expressing; top row), and Jurkat's (nonexpressing; bottom row) cells. Lym-1 (left) showed Raji cell-surface membrane binding, whereas the tridentate (middle) and the dimeric, tridentate SHALs (right) showed intracellular Raji binding. There was no binding in Jurkat's cells. DAPI (blue) was used as the nuclear stain, and Alexa Fluor® (Invitrogen, Carlsbad, CA) (red) was used for the location of SHAL or Lym-1 in these merged laser images.
Cytotoxicity
Both tridentate and dimeric, tridentate SHALs having the Ct ligand showed substantial antilymphoma (cytocidal) effects on HLA-DR10-expressing cells at nanomolar concentrations (picomoles/mL), but not on nonexpressing cells over the 3-day interval, even in the absence of 111In (Figs. 6 and 7). Although the effect was not titrated, the fraction and absolute number of dead cells increased more than 5-fold, when comparing SHAL treated with untreated expressing cells or with treated or untreated nonexpressing cells. Raji, treated with tridentate or dimeric, tridentate SHALs, showed an increased number of dead cells after 2 days of exposure (p < 0.01), whereas Jurkat's did not (p = 0.10–0.82), when compared to untreated cells. In regression models, both the tridentate and the dimeric, tridentate SHAL significantly increased dead cells in expressing cell lines (p < 0.001). In models with a dose-response effect for 111In, but no dose-response effect for SHAL nor for 111In and SHAL interaction, the percent nonviable expressing cells increased linearly by about 0.5% per 5 nCi of 111In in the well. Based on the maximum 111In activity measured for the wells, the radiation-absorbed dose to the nucleus of the cells ranged from 0.11 Gy after 1 day to a maximum of 0.55 Gy after 3 days. Neither SHAL nor 111In had a significant effect on the nonexpressing Jurkat's or CEM cells.
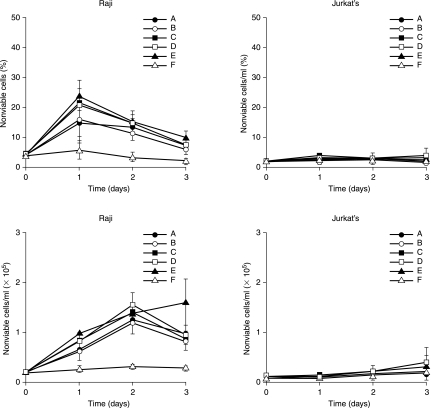
Figure 6.
Temporal effects of tridentate, DvPLLCtPCbLDo, SHAL on cells. Cells in each well were treated with 111In 8 (A), 18 (B), or 39 nCi (C) on 3 ng of SHAL and 39 nCi and 30 ng of SHAL (D), 3 ng of SHAL alone (E), or untreated (F). Treated Raji cells showed more than a 5-fold increased cell death over 3 days, whereas Jurkat's and untreated cells did not. There was little difference in the cytocidal effect if treated with SHAL alone or 111In-SHAL. Data represent means and standard deviations for triplicate samples and are representative of replicate studies that showed reproducible results.
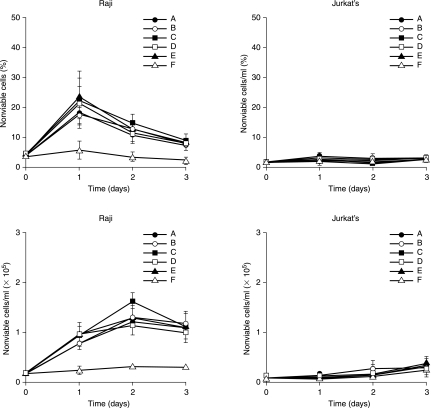
Figure 7.
Temporal effects of dimeric, tridentate, (DvPLLCtPCbPPP)2LLDo, SHAL on cells. Cells in each well were treated with 111In 5 (A), 10 (B), or 18 nCi (C) on 1.4 ng of SHAL and 18 nCi and 30 ng of SHAL (D), 3 ng of SHAL alone (E), or untreated (F). Treated Raji cells showed increased cell death over 3 days, whereas Jurkat's and untreated cells did not. Data represent means and standard deviations for triplicate samples and are representative of replicate studies that showed reproducible results.
Discussion
Although SHALs readily diffused into cells, they distinguished HLA-DR10 expressing from nonexpressing human, live lymphoma cells in assays.12–14 Additionally, SHALs containing the Ct (3-(2-([3-chloro-5-trifluoromethyl)-2-pyridinyl] oxy)-anilino)-3-oxopropanionic acid) ligand residualized in HLA-DR10-expressing cells over a large range of added SHAL, both at RT and at 4°C, so that the HLA-DR10 copy number per expressing cell identified by the SHALs was more than 179 times the cell surface copy number identified by Lym-1 mAb. Confocal micrographs of Raji cells confirmed intracellular localization of the tridentate and dimeric, tridentate SHAL motifs, whereas Lym-1 was restricted to the cell surface (Fig. 5). Further, these SHALs had substantial cytocidal activity on HLA-DR10-expressing Raji cells in culture, and also in xenografts in mice (preliminary data, not shown), such as that of Lym-1 and at SHAL concentrations readily achievable in patients. SHALs lacking the Ct ligand, SHAL diluents, and nonspecific mAbs have not shown cytocidal effects, whereas Lym-1 has shown greater cytocidal apoptotic effects in cell culture and in xenografts in mice than rituximab.5,6,12 The antilymphoma effects of the SHALs on Raji cells and xenografts were similar to those of Lym-1 and greater than those of rituximab.
As part of the immune mechanism, histocompatibility proteins bind peptides and present them on the surface of expressing cells.23 These proteins are also important receptors that have been shown to participate in transmembrane and cytoplasmic signaling.24–26 Although upregulated on the surface membrane of human malignant B-cells, HLA proteins are even more abundant inside these cells (Fig. 8), where they are manufactured and assembled on the endoplasmic reticulum. Some cell-surface proteins internalize by their nature or after ligation.27 HLA-DR-receptor proteins internalize and the SHALs containing the Ct ligand internalized and residualized, functioning like a “Trojan horse,” in HLA-DR10-expressing cells. This likely accounts for our observation that SHALs containing the Ct ligand identified more than 179 times the number of HLA-DR-binding sites (copies) than Lym-1 identified cell-surface HLA-DR protein on Raji cells. Additionally, internalized SHALs were positioned to deposit radiation and other toxins near the DNA, where their effects are more potent and selective.
Figure 8.
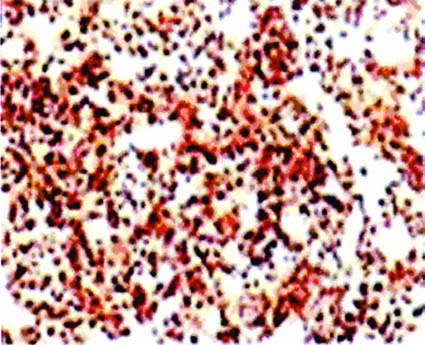
Binding of Lym-1 to non-Hodgkin's lymphoma tissue from a patient. Extensive staining occurred because the preparative method made intracellular HLA-DR10 accessible to Lym-1, detected with biotinylated antimouse monoclonal antibody, followed by HRP-SA and 3,3′-diaminobenzidine, as described in Materials and Methods. SHAL showed similar staining. (Modified with permission from Balhorn R, Clin Cancer Res 2007;13:5621.14 [ref. 14]).
Lym-1 mAb is a useful radionuclide carrier for therapy and imaging and binds with lymphoma tissue from about 90% of patients with B-cell NHL.16,28 Although not modulated when ligated to HLA-DR protein,16 some Lym-1 internalization in malignant B-lymphocytes has been reported.29 Most mAbs, but not Lym-1, when internalized, are promptly catabolized and released.29
Our SHALs bind within the Lym-1 epitopic region of HLA-DR protein, have a molecular weight less than 5 kDa, and are rapidly excreted.11,14 To further increase cell selectivity and binding, tridentate SHALs, having a third ligand, and dimeric SHALs, emulating multivalent mAb binding, have been generated. SHALs have been readily produced, which included a chelator to which metals and lanthanides can be attached for magnetic resonance and nuclear imaging and for radiotherapy. Additionally, the lysine residues in the linkers can also be used to attach other radionuclides and functionalities.30 All of these mAb mimetics have exhibited selectivity for the target protein, have been labeled with radionuclides in high yield and purity, and are expected to have a long shelf-life and potential for oral administration. Gram amounts can be synthesized for a fraction of the cost of biologicals.
The Ct ligand imparted several important properties on the SHALs. The mechanisms underlying these properties are of interest. Differences in the cellular behavior of SHALs having, and lacking, the Ct ligand were not accounted for by differences in SHAL size or dimerism. Presently, we interpret the observations as follows. After binding to HLA-DR10 proteins on the surface membrane of expressing cells, the protein and associated SHAL were endocytosed and carried to sites where the SHAL was unavailable for exchange with media outside the cell. Possible explanations for the special characteristics of Ct ligand SHALs include: 1) the Ct ligand in the SHAL bound to a site on HLA-DR10 protein that increased cell signaling; 2) the Ct ligand improved SHAL ability to penetrate the cell-surface membrane after HLA-DR10 binding; 3) the Ct ligand improved binding to HLA-DR10 on the cell surface and/or inside the cell; and 4) the Ct ligand exhibited pH effects when intracellular. The properties exhibited by SHALs containing the Ct ligand and their mechanisms are deserving of further study, including the ligand's cytocidal effects and potential for transporting agents to critical cell locations. Ct analogs have been shown by others to inhibit fatty-acid synthesis via acetylCoA carboxylase,31 and to have antifungal32 and herbicidal activity.33 This is a fertile area for additional investigation, particularly because other small molecules have been shown to have antagonist or agonist functions useful for treating cancer.34
Irradiating DNA is also an effective way to kill cells. The use of mAbs as radionuclide carriers has improved response rates and patient acceptance, when compared to immunotherapy and chemotherapy, respectively.1,3 Lym-1-induced cell death is increased by the addition of a radionuclide.4 Further, a radionuclide localized inside the cell has improved efficacy and specificity, if its emission ranges are short; Auger electrons inside the cell are efficient for inducing lethal effects.30 If radionuclides that emit both photon and Auger radiations are used, both imaging and radiotherapy can be achieved. The 111In, used in this study, is such a radionuclide and, like the Ct containing SHALs, is also capable of residualizing inside the cell.35
Conclusions
SHALs containing the Ct ligand selectively bound to, residualized inside, and were cytocidal for HLA-DR10-expressing human lymphoma cells. The ability of these SHALs to directly and selectively induce cell death suggests that SHAL binding to HLA-DR10 triggered cell signaling. The tridentate and dimeric, tridentate SHALs described in this paper provide extraordinary opportunities for molecular therapy and imaging. The technology established to target HLA-DR10, and related HLA-DRs, can be modified to transport and residualize toxic agents near critical sites inside other malignant cells, using other proteins as targets.
Acknowledgments
This work was supported by National Cancer Institute PO1-CA47829 and Lawrence Livermore National Laboratory Awards 01-ERD-111, 01-ERD-046, and 01-SI-012. The authors thank B. Petitt for manuscript preparation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Witzig TE. Gordon LI. Cabanillas F, et al. Randomized, controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 2.Kaminski MS. Zelenetz AD. Press OW, et al. Pivotal study of iodine I 131tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 3.DeNardo GL. Sysko VV. DeNardo SJ. Cure of incurable lymphoma. Int J Radiat Oncol Biol Phys. 2006;66(Suppl 2):s46. doi: 10.1016/j.ijrobp.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo GL. Tobin E. Chan K, et al. Direct antilymphoma effects on human lymphoma cells of monotherapy and combination therapy with CD20 and HLA-DR antibodies and yttrium-90 HLA-DR antibodies. Clin Cancer Res. 2005;11:7075. doi: 10.1158/1078-0432.CCR-1004-0008. [DOI] [PubMed] [Google Scholar]
- 5.Tobin E. DeNardo GL. Zhang N, et al. Combination immunotherapy with anti-CD20 and anti-HLA-DR monoclonal antibodies induces synergistic antilymphoma effects in human lymphoma cell lines. Leuk Lymph. 2007;48:944. doi: 10.1080/10428190701272272. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N. Khawli LA. Hu P, et al. Lym-1-induced apoptosis of non-Hodgkin's lymphomas produces regression of transplanted tumors. Cancer Biother Radiopharm. 2007;22:342. doi: 10.1089/cbr.2007.359.A. [DOI] [PubMed] [Google Scholar]
- 7.Rose LM. Gunasekera AH. DeNardo SJ, et al. Lymphoma-selective antibody Lym-1 recognizes a discontinuous epitope on the light chain of HLA-DR10. Cancer Immunol Immunother. 1996;43:26. doi: 10.1007/s002620050299. [DOI] [PubMed] [Google Scholar]
- 8.Rose LM. Deng CT. Scott S. Critical Lym-1 binding residues on polymorphic HLA-DR molecules. Mol Immunol. 1999;36:789. doi: 10.1016/s0161-5890(99)00083-8. [DOI] [PubMed] [Google Scholar]
- 9.Kramer RH. Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature. 1998;395:710. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- 10.Hajduk PJ. Meadows RP. Fesik SW. Discovering high-affinity ligands for proteins. Science. 1997;278:497. doi: 10.1126/science.278.5337.497. [DOI] [PubMed] [Google Scholar]
- 11.DeNardo GL. Natarajan A. Hok S, et al. Pharmacokinetic characterization in xenografted mice of a series of first generation mimics for HLA-DR antibody, Lym-1, as carrier molecules to image and treat lymphoma. J Nucl Med. 2007;48:1338. doi: 10.2967/jnumed.107.041095. [DOI] [PubMed] [Google Scholar]
- 12.West J. Perkins J. Hok S, et al. Direct antilymphoma activity of novel, first-generation “antibody mimics” that bind HLA-DR10-positive non-Hodgkin's lymphoma cells. Cancer Biother Radiopharm. 2006;21:645. doi: 10.1089/cbr.2006.21.645. [DOI] [PubMed] [Google Scholar]
- 13.Balhorn R. Hok S. Burke P, et al. Selective high-affinity ligand antibody mimics for cancer diagnosis and therapy: Initial application to lymphoma/leukemia. Clin Cancer Res. 2007;13(Suppl 18):5621s. doi: 10.1158/1078-0432.CCR-07-1128. [DOI] [PubMed] [Google Scholar]
- 14.DeNardo GL. Hok S. Natarajan A, et al. Characteristics of dimeric (bis) bidentate selective high-affinity ligands as HLA-DR10 beta antibody mimics targeting non-Hodgkin's lymphoma. Int J Oncol. 2007;31:729. [PubMed] [Google Scholar]
- 15.Hok S. Natarajan A. Balhorn R, et al. Synthesis and radiolabeling of selective high-affinity ligands designed to target non-Hodgkin's lymphoma and leukemia. Bioconjug Chem. 2007;18:912. doi: 10.1021/bc060305o. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AL. Marder RJ. Winter JN, et al. Two new monoclonal antibodies, Lym-1 and Lym-2, reactive with human-B-lymphocytes and derived tumors, with immunodiagnostic and immunotherapeutic potential. Cancer Res. 1987;47:830. [PubMed] [Google Scholar]
- 17.Kuntz ID. Blaney JM. Oatley SJ, et al. A geometric approach to macromolecule-ligand interactions. J Mol Biol. 1982;161:269. doi: 10.1016/0022-2836(82)90153-x. [DOI] [PubMed] [Google Scholar]
- 18.Desjarlais RL. Sheridan RP. Seibel GL, et al. Using shape complementarity as an initial screen in designing ligands for a receptor binding site of known three-dimensional structure. J Med Chem. 1988;31:722. doi: 10.1021/jm00399a006. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht H. Cosman M. Ngu-Schwemlein M, et al. Recombinant expression of the b subunit of HLA-DR10 in the selection of novel lymphoma targeting molecules. Cancer Biother Radiopharm. 2007;22:531. doi: 10.1089/cbr.2007.375A. [DOI] [PubMed] [Google Scholar]
- 20.Scatchard G. The attraction of proteins for small molecules and ions. Ann N Y Acad Sci. 1947;51:660. [Google Scholar]
- 21.Goddu SM. Howell RW. Rao DV. Cellular dosimerty: Absorbed fractions for monoenrgetic electron and alpha particle sources and S-values for radionuclides uniformly distributed in different cell compartment. J Nucl Med. 1994;35:303. [PubMed] [Google Scholar]
- 22.Ong GL. Elsamra SE. Goldenberg DM, et al. Single-cell cytotoxicity with radiolabeled antibodies. Clin Cancer Res. 2001;7:192. [PubMed] [Google Scholar]
- 23.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Ann Rev Immunol. 1997;15:821. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 24.Leveille C. Cataigne JG. Charron D, et al. MHC class II isotype-specific signaling complex on human B-cells. Eur J Immunol. 2002;32:2282. doi: 10.1002/1521-4141(200208)32:8<2282::AID-IMMU2282>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Klemm JD. Schreiber SL. Crabtree GR. Dimerization as a regulatory mechanism in signal transduction. Annu Rev Immunol. 1998;16:569. doi: 10.1146/annurev.immunol.16.1.569. [DOI] [PubMed] [Google Scholar]
- 26.Lane PJ. McConnell FM. Schieven GL, et al. The role of class II molecules in human B-cell activation. J Immunol. 1990;144:3684. [PubMed] [Google Scholar]
- 27.Cescato R. Schulz S. Waser B, et al. Internalization of sst2, sst3, and sst5 receptors: Effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47:502. [PubMed] [Google Scholar]
- 28.DeNardo GL. DeNardo SJ. Goldstein DS, et al. Maximum tolerated dose, toxicity, and efficacy of 131I-Lym-1 antibody for fractionated radioimmunotherapy of non-Hodgkin's lymphoma. J Clin Oncol. 1998;16:3246. doi: 10.1200/JCO.1998.16.10.3246. [DOI] [PubMed] [Google Scholar]
- 29.Ong GL. Mattes MJ. Processing of antibodies to the MHC class II antigen by B-cell lymphomas: Release of Fab-like fragments into the medium. Mol Immunol. 1999;36:777. doi: 10.1016/s0161-5890(99)00084-x. [DOI] [PubMed] [Google Scholar]
- 30.Costantini DL. Chan C. Cai Z, et al. 111In-labeled trastuzumab (herceptin) modified with nuclear localization sequences (NLS): An Auguer electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48:1357. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H. Tweel B. Tong L. Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop. Proc Natl Acad Sci U S A. 2004;101:5910. doi: 10.1073/pnas.0400891101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu C-R. Xu L-H. Tu S, et al. Sythesis and bioactivity of novel (3-chloro-5-(trifluoromethyl)pyridin-2-yloxy) phenyl containing acrylate and acrylonitrile derivatives. J Flourine Chem. 2006;127:1540. [Google Scholar]
- 33.Zagnitko O. Jelenska J. Tevzadze G, et al. An isoleucine/leucine residue in the carboxyltransferase domain of acety-CoA carboxylase is critical for interaction with aryloxyphenoxypropionate and cyclohexanedione inhibitors. Proc Natl Acad Sci U S A. 2001;98:6617. doi: 10.1073/pnas.121172798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arkin MR. Wells JA. Small-molecule inhibitors of protein-protein interactions: Progressing towards the dream. Nat Rev Cancer. 2004;3:301. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 35.Press OW. Howell-Clark J. Anderson S, et al. Retention of B-cell-specific monoclonal antibodies by human lymphoma cells. Blood. 1994;83:1390. [PubMed] [Google Scholar]