Abstract
High-grade astrocytoma (HGA) is an invariably fatal malignancy with a mean survival of 14 months despite surgery, radiation, and chemotherapy. We have found that a restricted receptor for interleukin-13 (IL-13), IL-13 receptor alpha 2 (IL13Rα2), is abundantly overexpressed in the vast majority of HGAs but is not appreciably expressed in normal tissue, with the exception of the testes. Therefore, IL-13Rα2 is a very attractive target for anti-HGA immunotherapy. In order to test protein and genetic vaccines that target IL13Rα2, we developed a G26-IL13Rα2-expressing syngeneic immunocompetent murine glioma model. Using this glioma model, mice were immunized with recombinant extracellular IL13Rα2 protein (IL13Rα2ex) or a DNA expression vector containing the gene for IL13Rα2 and were subsequently challenged with IL13Rα2(+) G26 tumors. Mice immunized with either recombinant or genetic IL13Rα2, but not mock-immunized controls, demonstrated complete protection against IL13Rα2(+) glioma growth and mortality. Of interest, only the recombinant-protein-based vaccines generated detectable anti-IL13Rα2 antibodies. These studies demonstrate the in vivo efficiency of protein- and DNA-based immunotherapy strategies that target IL13Rα2 that may play a clinical role to eradicate the residual microscopic HGA cells that inevitably cause disease recurrence and mortality.
Key words: brain tumors, gliomas, astrocytomas, immunotherapy, IL13Rα2, interleukin-13
Introduction
High-grade astrocytoma (HGA) remains one of the deadliest malignancies, with a mortality rate approaching 100%, despite aggressive therapy.1 Disease recurrence is the primary cause of mortality in HGA and is due to residual microscopic disease that remains even after the most rigorous of therapy combinations. Molecularly targeted immunotherapy may offer a means of eliminating the residual microscopic HGA cells that remain after standard therapy and thus prevent inevitable disease recurrence.
The key to successfully developing anti-HGA immunotherapy is to exploit the differential over-expression of various proteins in cancer cells and activate the immune system to target and ablate cells harboring these cancer-restricted proteins. When a cancer cell acquires the ability to override the normal cellular checkpoints and divide indefinitely, it also begins to present proteins that are not generally expressed in normal tissues. Recent innovations in technologies have allowed for the identification and utilization of these cancer-associated antigens.2–5 Such molecules include mutated cellular proteins (e.g., epidermal growth-factor receptor [EGFR] mutants), viral proteins (e.g., human papiloma virus antigens), and cancer testes antigens (CTAs), which are reactivated embryonic proteins expressed predominantly in cancer and the testes, and reside on chromosome X.3,4,6,7 A plethora of distinct cancer-restricted antigens are currently being explored as targets for cancer immunotherapy. Many diverse methods of activating an anticancer immune response, including whole-cell, protein, peptide, and DNA vaccines, are being examined in a variety of different cancers.
In this work, we demonstrate the viability of using immunotherapy to target and ablate HGA cells expressing interleukin-13 receptor alpha 2 (IL13Rα2), an HGA-restricted IL-13 receptor. Our laboratory and subsequent independent investigations have consistently demonstrated that IL13Rα2 is overexpressed in a vast majority (∼80%) of HGA cells in vitro and in vivo,8–13 making it an attractive target for antiglioma molecular therapy. The potential of utilizing IL13Rα2 as a cancer target has been demonstrated by using a number of distinct delivery strategies. In past work, we have demonstrated the efficiency and safety of IL-13-based cytotoxins,14 using IL13Rα2-targeted mutants of IL13 that were fused to derivatives of Pseudomonas exotoxin to kill HGA cells in vitro and cure IL13Rα2-expressing tumors in vivo.8,14–18 Additionally, we successfully made IL13Rα2-targeted nanoparticles that selectively delivered antineoplastic agents to human HGA orthotopic xenographs.19
In addition to utilizing targeted cytotoxins, others have shown IL13Rα2 to be a robust cancer target. Zhou et al. incorporated a mutant of IL13 at the binding region of the herpes symplex virus (HSV) and demonstrated the ability of these HSV constructs to efficiently target and kill IL13Rα2-expressing cells.20 Kahlon et al. exchanged the T-receptor zeta chain with a mutant of IL-13 and demonstrated the ability of these T-cell constructs to target and kill IL13Rα2 cells and eradicate tumors.21 Okano et al. demonstrated the use of IL13Rα2-peptide activated dendritic cells to eradicate HGA, further validating the use of IL13Rα2 as a target for HGA.22 In this work, we demonstrate the in vivo efficiency of protein- and DNA-based IL13Rα2-based vaccines as proof of concept for the application of IL13Rα2-targeted immunotherapy for potential use against the residual microscopic HGA cells that inevitably cause disease recurrence.23
Materials and Methods
DNA ligase and restriction endonucleases were purchased from New England Biolabs (Beverly, MA). Pfu turbo DNA polymerase was purchased from Stratagene (La Jolla, CA). Oligonucleotide primers were synthesized in the Macromolecular Core Laboratory of the Pennsylvania State University College of Medicine (Hershey, PA). All polymerase chain reaction purifications, DNA extraction from agarose gels, and plasmid minipreps were done by using respective Qiagen kits (Qiagen Inc., Valencia, CA). Tissue-culture equipment was from Corning Glass (Corning, NY). RNA-blotted membranes were purchased from Clontech (Palo Alto, CA). HGA cell lines, U-87 MG, SW-1088, U-373 MG, SNB-19, LGA cell line, H-4, epidermoid carcinoma cell line, A-431, and the Chinese hamster ovary cell line, CHO-K1, were purchased from American Type Culture Collection (ATCC; Manassas, VA) or were primary HGA cultures isolated in this lab (G-48a). The ATCC CHO-K1 cell line was derived as a subclone from the parental CHO cell line initiated from a biopsy of an ovary of an adult Chinese hamster. G26 mouse glioma cells were the gift of Dr. Marzenna Wiranowska. G26-IL13Rα2 cells were produced, as previously described.
Production of Recombinant Proteins
Plasmids and recombinant proteins for IL13 and mutants and IL-4 were prepared, as previously described.24–26
Human IL13Rα2 extracellular domain (IL13Rα2ex) protein purification was performed by using affinity chromatography. Bacterial pellets were directly solublized in 6 M of guanidine-HCl and spun down at 50,000 × g. The protein was purified by passing the supernatant through a Nickel-Agarose column (Qiagen) and eluting IL13Rα2 in elution buffer (250 Mm imidazole, 300 mM NaCl, 50 mM NaH2PO4; pH 8.0). Protein was further purified on an ion-exchange fast protein liquid chromatography, as previously described.27
Autoradiography
Recombinant human IL13.E13K was labeled with [125I] by using Iodo-Gen reagent (Pierce Chemical Co., Rockford, IL), as instructed by the manufacturer. Then, 5 × 104 cells were dotted onto sterile slides and allowed to adhere overnight. Cells were then fixed by using ice-cold ethanol and were then either treated with binding buffer (200 mM sucrose, 5 mM HEPES, 1% bovine serum albumin, 10 mM ethylenediaminetetraacetic acid) alone or with 500 times the excess blocking agent for 1 hour. Cells were subsequently treated with labeled IL-13 for 1 hour. Slides were washed 4 times for 5 minutes each with 0.1% phosphate-buffered saline (PBS) and exposed to film for 1 day to 2 weeks. For autoradiography on tumor sections, the same procedure was used on serial tissue sections, which were cut on a cryostat, thaw-mounted on chromalum slides, and ethanol fixed.
All films from autoradiography were scanned on a transparency scanner at a pixel size of 88 × 88 microns (Molecular Dynamics, Sunnyvale, CA), and the densities were plotted by using Quantity 1 software (Bio-Rad, Hercules, CA). The images were compiled in Paint Shop Pro V 5.0 (Jasc Software Inc., Eden Prairie, MN).
Protein Immunizations
Groups of 5 mice were immunized with IL13Rα2ex/Freund's adjuvant or Freund's adjuvant alone. Next, 60 μg of IL13Rα2ex or PBS was mixed with 40 μL of Freund's complete adjuvant and repeatedly passed through a 22-G syringe until the mixture became pasty. Then, 100 μL of mixture was injected intraperitoneally (i.p.) into every mouse. Mice were immunized every 3 weeks for a total of 3 immunizations. Four (4) weeks after the last injection, the mice were challenged s.c. with 5 × 106 G26-IL13Rα2 cells in a volume of 100 μL.
DNA Immunizations
Groups of 5 mice were vaccinated with pcDNA 3.1 or pcDNA 3.1-human IL13Rα2. DNA was precipitated onto 1.6-diameter gold particles (1 μg DNA/0.5 mg gold) and were coated onto the inner surface of Tefzel tubing, as recommended by the manufacturer (Bio-Rad, Hercules, CA), and vaccinated by using a gene gun. Mice were shaven on the abdomen and bombarded with 3 shots/mouse (1 μg/shot) with gold particles/DNA by a gene gun at 400 lb/in2. Mice were immunized every other week for a total of 3 immunizations. Three (3) weeks after the last injection, mice were challenged s.c. with 5 × 106 G26-IL13Rα2 cells in a volume of 100 μL.
ELISA Assays
First, 1 μg of purified IL13Rα2ex was diluted into 50 μL of sodium carbonate (pH 9.5) and placed in each well of a 96-well plate overnight at 4°C. The following morning, the buffer was removed and the wells were washed 3 times with PBS. Nonspecific binding sites were blocked by using 5% dry milk dissolved in PBS. After 30 minutes of blocking, a 1:10 dilution of appropriate serum was added to each well for 45 minutes while shaking gently at room temperature. Wells were washed 3 times with PBS and a 1:1000 dilution of antimouse polyclonal–alkaline phosphatase (Roche, Indianapolis, IN) was added for 45 minutes at room temperature. Cells were washed 3 times with PBS and p-nitrophenyl phosphate substrate was added (1 mg of substrate per 1 mL) in alkaline phosphatase buffer (100 mM NaCl, 5 mM MgCl2, 100 mM Tris-HCl; pH 9.5) (Invitrogen; Carlsbad, CA). Plates were then read by a microplate reader (Cambridge Technology, Watertown, MA) at an absorbance of 405 nm. Values were obtained by subtracting the background immunoreactivity observed in wells not treated with serum. Each experiment was performed in triplicate and repeated at least twice.
Results
G26-IL13Rα2(+) Cells Express IL-13 Binding Sites
In contrast to human HGA, none of the mouse models of HGA tested overexpressed IL13Rα2 (not shown). We, therefore, stably transfected G26 murine glioma cells, which lack appreciable IL-13 binding sites,28 with IL13Rα2 and confirmed its expression.28 In order to examine if the G26-IL13Rα2(+) cells manifested HGA-restricted IL13Rα2 binding characteristics, we tested their binding affinity to IL13.E13K-PE38QQR, a chimeric recombinant fusion protein consisting of IL13.E13K and a derivative of pseudamonous exotoxin, which has been shown in past work to bind strongly and kill IL13Rα2(+) HGA cells in vitro and in vivo.15,16 Only the G26-IL13Rα2(+) tumors (Fig. 1A) but not G26-IL13Rα2(−) tumors28 demonstrated characteristic functional IL13Rα2 binding sites through autoradiography by binding IL13Rα2-targeted IL-13 mutant derivative IL13.E13K-PE38QQR (Fig. 1A). This strong-affinity IL-13 binding was completely displaced by unlabeled IL13.E13K (Fig. 1A), but not unlabeled IL-4 (not shown) in a competition assay, which are characteristics unique only to the cancer-restricted IL13Rα2 binding sites. Further, only G26-IL13Rα2 cells, but not G26-mock-transfected cells, were efficiently killed by IL-13-mutant-PE1E fusion proteins (Fig. 1B), which are characteristic of IL13Rα2-expressing HGA cells. This receptor-mediated cytotoxicity was successfully ablated by blocking the IL13Rα2 binding sites with IL13.E13K, but not IL-4 (Fig. 1C), again indicating the IL13Rα2 nature of these new IL-13 binding sites. Of significance, 100% of the G26-IL13Rα2(+) transfectants formed subcutaneous (s.c.) tumors in a similar fashion to the parental and mock-transfected G26 control cells, confirming that they remained highly tumorigenic.
FIG. 1.
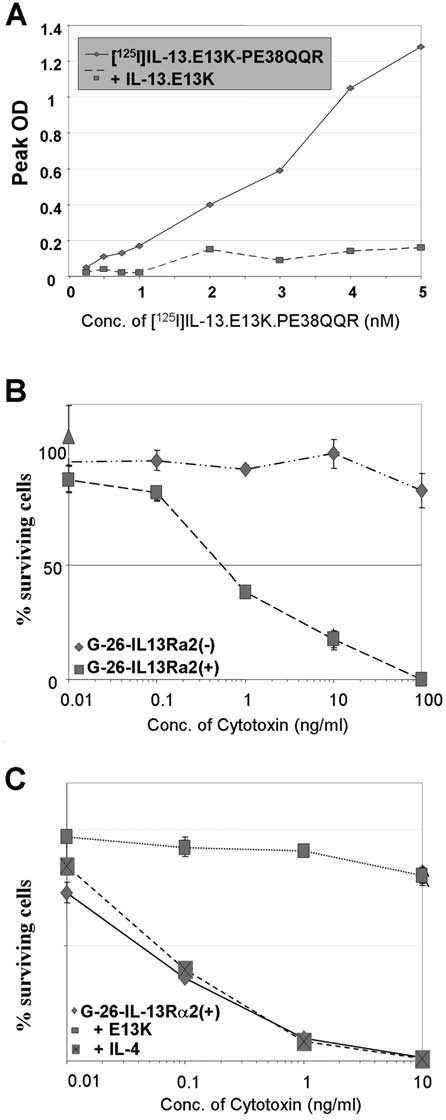
(A)I Autoradiography of G-26-IL-13Rα2(+) tumor sections using various concentrations of [125I]IL-13.E13K.PE38QQR in the absence or in the presence of excess unlabeled IL-13.E13K. (B) Cytotoxicity using IL-13mutant-PE1E on G-26-hIL-13Rα2 (+) cells and parental G-26 cells determined in a colorimetric cell-proliferation assay. (C) Neutralization assay using excess IL-13.E13K and IL-4 in the presence of IL-13mutant-PE1E. Vertical lines represent standard deviation.
Immunization with Recombinant IL13Rα2 Extracellular Domain Prevents Tumor Formation
We used the above-described G26-IL13Rα2(+) tumor model to test the in vivo anti-HGA potential of IL13Rα2 by immunizing syngeneic immunocompetent mice with recombinant IL13Rα2 extracellular domain (IL13Rα2ex) (Fig. 2A). The purity and identity of this protein was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analysis (Fig. 2B).
FIG. 2.
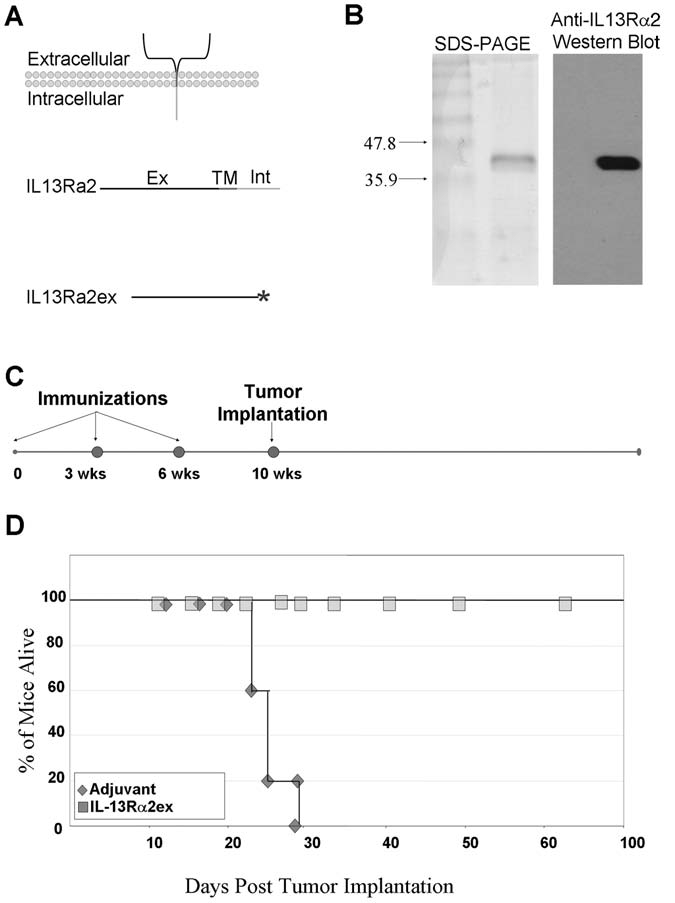
(A) IL13Rα2 consists of a large extracellular domain (ex), a transmembrane domain (TM), and a small intracellular domain (Int). The genetic sequence encoding only the extracellular domain of IL-13Rα2 was cloned into a bacterial expression vector containing an in-frame C-terminus his-tag (*), which was used to affinity purify IL13Rα2ex. (B) Coomassie stained sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analysis, using anti-IL13Rα2 anti-serum of purified recombinant IL13Rα2ex, used to immunize mice. (C) Schedule of IL13Rα2ex immunizations and tumor implantations in syngeneic immunocompetent groups of mice. (D) Post-tumor challenge survival of mice immunized with recombinant IL13Rα2ex protein. Mice were challenged with 5 × 106 tumor cells at day 0. Mice were sacrificed when tumor volumes reached 400 mm3
Syngeneic C57BL/J6 mice were either injected with IL13Rα2ex together with Freund's complete adjuvant or with adjuvant alone every 3 weeks for a total of 3 injections. Four (4) weeks following the final vaccination, G26-IL13Rα2(+) cells were s.c. injected into syngeneic mice (Fig. 2C). Tumors began to form in the control mice immunized with adjuvant alone 13 days after tumor implantation. The tumors grew rapidly in 100% of the control mice and all succumbed to the tumor load within 30 days of tumor implantion (Fig. 2D). In contrast, mice immunized with recombinant IL13Rα2ex were completely protected against G26-IL13Rα2 tumors (Fig. 2D). In a separate experiment, mice were immunized with IL13Rα2ex and subsequently implanted with untransfected parental G26 cells that lacked IL13Rα2. These control mice grew tumors unabated, which was similar to the adjuvant only group (not shown). These data indicate that IL13Rα2ex is effective at eliciting an IL13Rα2-specific antiglioma immune response.
IL13Rα2 DNA Vaccinations Effectively Protect Mice from G26-IL13Rα2(+) Tumors
We separately assessed the efficiency of DNA-based genetic IL13Rα2 vaccinations, using a gene-gun-based strategy. Groups of mice were vaccinated with either a pcDNA3.1 expression vector encoding IL13Rα2 under the cytomegalovirus promoter or with an identical control plasmid that did not encode IL13Rα2. Mice were immunized every 3 weeks for a total of three times. Four (4) weeks following the final injection, mice were implanted with G26-IL13Rα2(+) cells (Fig. 3A). Tumors began to form in the control mice immunized with adjuvant alone 11 days after tumor implantation. The tumors grew rapidly in 100% of the control mice and all succumbed to the tumor load within 30 days of tumor implantation (Fig. 3B). In contrast, mice immunized with pcDNA3.1-IL13Rα2 did not grow G26-IL13Rα2(+) tumors (Fig. 3B), demonstrating the potential of genetic IL13Rα2 vaccines against IL13Rα2-expressing tumors.
FIG. 3.
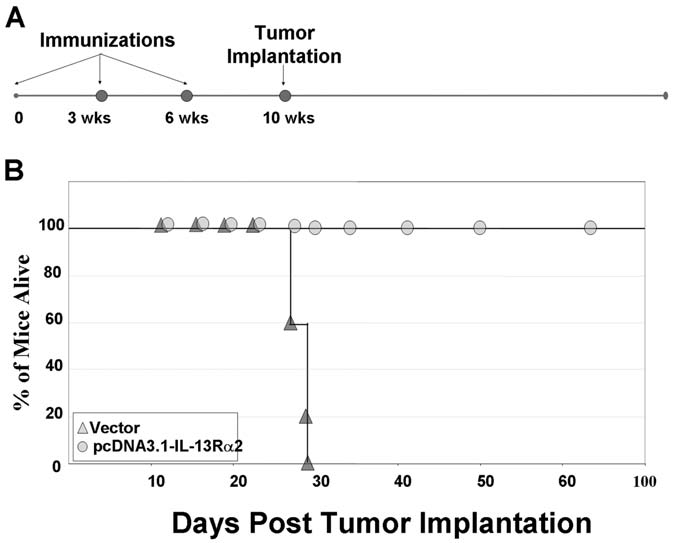
(A) Schedule of genetic immunizations and tumor implantations in syngeneic immunocompetent groups of mice. (B) Survival of mice immunized with DNA encoding IL13Rα2 or vector alone. Mice were challenged with 5 × 106 tumor cells at day 0. Mice were sacrificed when tumor volumes reached 400 mm3
Serum from Mice Immunized with IL13R α 2ex Protein Immunoreacted with IL13Rα2 Protein
Mice were bled through the tail vein either 3 weeks after the final immunization or 3–4 weeks after tumor formation (controls only), and the serum was tested for anti-IL13Rα2 immunoreactivity, using an ELISA assay. Only serum from mice immunized with recombinant IL13Rα2ex significantly immunoreacted with IL13Rα2ex recombinant protein (Fig. 4A). In contrast, serum from mice immunized with pcDNA3.1-IL13Rα2, vector-alone, or tumor-bearing nonimmunized mice did not demonstrate significant immunoreactivity by the ELISA assay toward recombinant IL13Rα2ex (Fig. 4A). To confirm that the anti-IL13Rα2 antibodies generated by the protein-based immunizations reacted to native functional IL13Rα2 found on HGA, immunoflourescence was performed on a frozen IL13Rα2-expressing human HGA specimen. Only serum from mice vaccinated with IL13Rα2ex protein demonstrated immunoactivity toward the IL13Rα2(+) HGA sample (Fig. 4B).
FIG. 4.
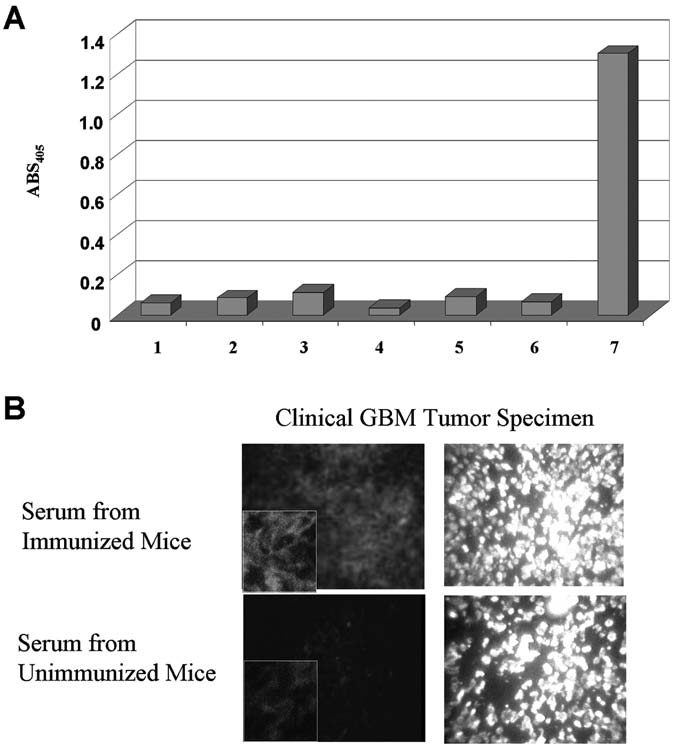
(A) IL13Rα2 reactivity of sera in an enzyme-linked immunosorbent assay of sera from (1) nonimmunized mice; (2) mice immunized with empty vector (no tumor); (3) mice immunized with empty vector (G26-IL13Rα2(+) (tumor bearing); (4) mice immunized with IL13Rα2-expressing eukaryotic vector (no tumor); (5) mice immunized with adjuvant only (no tumor); (6) mice immunized with adjuvant only (G26-IL13Rα2(+) tumor bearing); (7) Mice immunized with recombinant IL13Rα2ex. Serum was obtained 3 weeks after the last immunization for the nontumor samples and at least 4 weeks after tumor formation for the tumor-bearing samples. Mice immunized with recombinant IL13Rα2ex or genetic IL13Rα2 did not grow tumors. (B) IL13Rα2 immunoreactivity in a GBM tumor specimen. Nonimmunized serum was used on a contiguous section of the same tumor as a negative control. Tissues were also stained for nuclear presence, using DAPI.
Discussion
In the current work, we confirmed the proof-of-concept for utilizing anti-IL13Rα2-based immunonotherapies in HGA. Our aim was to use similar strategies that target the cancer-restricted IL13Rα23,29 as adjuvant molecular therapy to eradicate microscopic residual disease in combination with current clinical practices to prevent the inevitable tumor recurrence that is responsible for the majority of HGA mortality. We showed that both protein and genetic vaccination modalities were successful in rejecting IL13Rα2(+) syngeneic tumor cells and protected the mice from rapidly growing IL13Rα2(+) gliomas, which inevitably formed and killed control or unimmunized mice. We are currently further exploring the mechanistic aspect behind the observed anticancer response to IL13Rα2-targeted immunotherapy. In addition, we are also examining some potential molecular events associated with high-grade astrocytoma that may be responsible for the overexpression of the IL13Rα2 biomarker.
IL13Rα2 is a unique CTA because it is a plasma-membrane receptor. Of importance, 360 of 380 amino acids are located extracellularly.30 This location exposes it to the humoral arm of the immune system, a branch that is not regarded as a major factor in anticancer immunity. Evidence has even recently attributed a dominant humoral response to dismal clinical survival rates.31 But the fact that IL13Rα2 is a membrane-associated receptor that is predominantly extracellular provides a viable target for the humoral immune system. The current work demonstrated that mice immunized with a protein-based strategy formed a high titer of anti-IL13Rα2 antibodies that may have played a role in the killing of the G26-IL13Rα2(+) cells and, therefore, preventing tumor growth. We are currently investigating the role that these antibodies play in protecting mice from IL13Rα2(+) tumors by designing antibody-based passive immunization strategies.
In contrast to the protein-based immunization strategy, no significant antibody response was observed by ELISA in mice vaccinated with the IL13Rα2 genetic vaccine. This is consistent with past studies, using DNA vaccines, that reported a dominant T-cell immune response.32 These results open the possibility of the isolation and injection of activated anti-IL13Rα2 T-cells that can be used to kill residual IL13Rα2(+) tumor cells, as has been clinically demonstrated in a number of malignancies, using other cancer-restricted antigens. We are currently investigating the role played by the various T-cell subtypes in the observed anti-IL13Rα2 immune response elicited by the immunization strategies used in this work.
Conclusions
Based on this initial work and previous work done by our laboratory23 and by other independent investigators, we are hopeful that anti-IL13Rα2-based immunotherapy strategies will play a role in eradicating microscopic IL13Rα2(+) HGA and, therefore, prevent inevitable disease recurrence.
Disclosure Statement
No competing financial interests exist.
References
- 1.Davis FG. Freels S. Grutsch J, et al. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on surveillance, epidemiology, and end results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in the development of immunotherapy for the treatment of patients with cancer. J Intern Med. 2001;250:462. doi: 10.1046/j.1365-2796.2001.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mintz A. Debinski W. Cancer genetics/epigenetics and the X chromosome: Possible new links for malignant glioma pathogenesis and immune-based therapies. Crit Rev Oncogen. 2000;11:77. [PubMed] [Google Scholar]
- 4.Ribas A. Butterfield LH. Glaspy JA, et al. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol. 2003;21:2432. doi: 10.1200/JCO.2003.06.041. [DOI] [PubMed] [Google Scholar]
- 5.Boon T. Cerottini JC. Van den Eynde B, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 6.Mocellin S. Rossi CR. Lise M, et al. Adjuvant immunotherapy for solid tumors: From promise to clinical application. Cancer Immunol Immunother. 2002;51:583. doi: 10.1007/s00262-002-0308-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma [see comments] Nat Med. 1998;4:321. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debinski W. An immune regulatory cytokine receptor and glioblastoma multiforme: An unexpected link. Crit Rev Oncogen. 1998;9:255. [PubMed] [Google Scholar]
- 9.Debinski W. Gibo DM. Hulet SW, et al. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985. [PubMed] [Google Scholar]
- 10.Debinski W. Gibo DM. Slagle B, et al. Receptor for interleukin 13 is abundantly and specifically overexpressed in patients with glioblastoma multiforme. Int J Oncol. 1999;15:481. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 11.Debinski W. Obiri NI. Powers SK, et al. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253. [PubMed] [Google Scholar]
- 12.Debinski W. Slagle B. Gibo DM, et al. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neuro-Oncol. 2000;48:103. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 13.Saikali S, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: Interest of EGFRvIII, IL-13Ralpha2, gp100, and TRP-2 for immunotherapy. J Neuro-Oncol. 2007;81:139. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 14.Debinski W. Recombinant cytotoxins specific for cancer cells. Ann NY Acad Sci. 1999;886:297. doi: 10.1111/j.1749-6632.1999.tb09441.x. [DOI] [PubMed] [Google Scholar]
- 15.Debinski W. Gibo DM. Puri RK. Novel way to increase targeting specificity to a human glioblastoma-associated receptor for interleukin 13. Int J Cancer. 1998;76:547. doi: 10.1002/(sici)1097-0215(19980518)76:4<547::aid-ijc17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Mintz A. Gibo DM. MadhanKumar AB, et al. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J Neuro-Oncol. 2003;64:117. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 17.Nash KT. Thompson JP. Debinski W. Molecular targeting of malignant gliomas with novel multiply-mutated interleukin-13-based cytotoxins. Crit Rev Oncogen. 2001;39:87. doi: 10.1016/s1040-8428(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 18.Debinski W. Gibo DM. Obiri NI, et al. Novel antibrain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 19.Madhankumar AB. Slagle-Webb B. Mintz A, et al. Interleukin-13-receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5:3162. doi: 10.1158/1535-7163.MCT-06-0480. [DOI] [PubMed] [Google Scholar]
- 20.Zhou G. Ye G. Debinski W. Engineered herpes simplex virus 1 is dependent on IL13Rα2 receptor for cell entry, independent of glycoprotein D receptor interaction. Proc Natl Acad Sci U S A. 2002;99:15124. doi: 10.1073/pnas.232588699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahlon KS, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 22.Okano F. Storkus WJ. Chambers WH, et al. Identification of a novel HLA-A*0201-restricted, cytotoxic T lymphocyte epitope in a human glioma-associated antigen, interleukin 13 receptor alpha2 chain. Clin Cancer Res. 2002;8:2851. [PubMed] [Google Scholar]
- 23.Mintz A, et al. Effective active immunotherapy against interleukin 13 receptor alpha-2 expressing gliomas [presented at the 7th Annual Scientific Meeting of the Society for Neuro-Oncology, San Diego, CA] Neuro-Oncology. 2002;4:434. [Google Scholar]
- 24.Davis FG. Freels S. Grutsch J. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: An analysis based on surveilance, epidemiology, and end results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 25.Debinski W. Obiri NI. Pastan I, et al. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 26.Madhankumar A. Mintz A. Gibo DM, et al. Alaninescanning mutagenesis of α-helix D degment of onterleukin-13 reveals new functionally important residues of the cytokine. J Biol Chem. 2002;277:43194. doi: 10.1074/jbc.M205047200. [DOI] [PubMed] [Google Scholar]
- 27.Nash KT. Thompson JP. Debinski W. Molecular targeting of malignant gliomas with novel multiply-mutated interleukin-13-based cytotoxins. Crit Rev Oncol Hematol. 2001;39:87. doi: 10.1016/s1040-8428(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 28.Mintz A. Gibo DM. Webb B, et al. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debinski W. Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440. [PMC free article] [PubMed] [Google Scholar]
- 30.Caput D, et al. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor alpha chain. J Biol Chem. 1996;271:16921. doi: 10.1074/jbc.271.28.16921. [DOI] [PubMed] [Google Scholar]
- 31.Dix AR. Brooks WH. Roszman TL, et al. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 32.Yu M. Finn OJ. DNA vaccines for cancer too. Cancer Immunol Immunother. 2006;55:119. doi: 10.1007/s00262-005-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


