Abstract
Objectives. To evaluate the association between improvements in physical function, fatigue and pain and improvements in productivity at work and at home in patients treated with certolizumab pegol (CZP) in combination with MTX.
Methods. Physical function, fatigue and pain were assessed in two CZP clinical trials (Rheumatoid Arthritis PreventIon of structural Damage 1 and 2) using the HAQ-Disability Index (HAQ-DI), Fatigue Assessment Scale (FAS) and Patient Assessment of Pain, with minimal clinically important differences (MCIDs) defined as ≥0.22, ≥1 and ≥10 points, respectively. Work and home productivity were evaluated using the RA-specific Work Productivity Survey (WPS-RA). The odds of achieving an HAQ-DI, FAS or pain ‘response’ at Week 12, defined as improvements ≥MCID, were compared between CZP and control groups. Improvements in productivity at Week 12 were compared between CZP-treated HAQ-DI, FAS or pain responders and non-responders.
Results. The odds of achieving improvements ≥MCID were five times higher for pain, and two to three times higher for physical function and fatigue, in patients receiving CZP vs control. Per month, responders reported significantly greater improvements in productivity at work and reduced interference of RA with their work productivity than non-responders. Responders also reported significantly greater improvements in productivity at home and participation in family, social and leisure activities.
Conclusions. This study demonstrated a clear association between patient-reported improvements in physical function, fatigue and pain, and improvements in productivity both at work and home.
Keywords: Rheumatoid arthritis, Certolizumab pegol, Physical function, Fatigue, Pain, TNF, Work productivity, Household productivity, Daily activities
Introduction
As a consequence of their disease, patients with RA experience pain, fatigue and disability that can significantly impact their everyday lives. Patients have identified pain, associated with the inflammation and joint destruction that characterize the disease, as one of the most important outcomes for RA treatment and one of the main reasons for which they seek medical care [1, 2]. Fatigue, another common symptom of the disease, has been described by patients as overwhelming and more intense compared with the typical tiredness experienced before being diagnosed with RA [1, 3]. The cause of fatigue is multi-factorial and involves the inflammatory process, pain, anaemia, depression and poor sleep quality [4, 5], with pain and sleep disturbance demonstrating the strongest influence on fatigue [4, 5].
Symptoms of both pain and fatigue are associated with impairments in physical function [5–7], which significantly impact patients’ lives as well as society. Limitations in physical function have been shown to affect patients’ ability to attend work and to perform paid and unpaid work (e.g. employment and volunteer work) [8–10] and household activities, and to impair their ability to engage in family, social and leisure activities [11–13]. These limitations often force patients to seek additional support to meet individual role obligations, including assistance from family members or hired household personnel and flexibility and job modifications from employers.
Since the majority of RA patients become work disabled (i.e. unable to participate in paid work) within 10 years of disease onset [14–16], research has focused on determining the predictors of work disability, including functional status and RA signs and symptoms, which can be modified to improve work-related outcomes [16–21]. In addition, research has focused on evaluating the impact of the disease and its treatment on paid work in terms of productivity, including the number of days absent from work (absenteeism) as well as the number of days present at work with reduced efficiency (presenteeism) [9, 22–25].
Few studies have evaluated the impact of RA on household work [11–13, 26], despite the fact that women do the majority of household activities and that the incidence of RA in women is three times that in men. Evaluations of household activity limitations are particularly relevant in established RA, where the majority of sufferers have left the work force but still may be expected to participate in their work roles at home, including cooking, cleaning and child care. In addition, there are few studies on improvement in paid and household work productivity and improvement in the participation in family and social activities following symptom relief with newly available therapies, such as anti-TNF agents, in established RA.
Certolizumab pegol (CZP) is the only PEGylated anti-TNF agent approved for the treatment of RA. In patients with active RA, CZP has been shown to rapidly improve signs and symptoms and physical function, to relieve pain and fatigue and to significantly improve productivity within and outside the home as add-on therapy to MTX [27–31]. The objective of the present analysis was to evaluate the association between clinically meaningful improvements in physical function, fatigue and pain and improvements in productivity at work and at home, as well as participation in family, social and leisure activities in patients with active RA.
Patients and methods
Study design
This analysis used data from two multinational, Phase III, multi-centre, double-blind, placebo-controlled studies comparing the efficacy and safety of two dose regimens of CZP added to MTX in patients with active RA who had incomplete responses to MTX [27, 28]. The Institutional Review Boards and Institutional Ethics Committees at each study centre in both multinational trials approved the studies, the subject information sheets and the informed consent forms. The studies were carried out in accordance with the International Conference on Harmonization (ICH) E6 Note for Guidance on Good Clinical Practice [ICH/Committee for Proprietary Medicinal Products (CPMP) 135/95], the principles that have their origin in the Declaration of Helsinki, and local laws and regulations. All subjects (or their legally acceptable representative) provided written informed consent before taking part in the studies.
In Rheumatoid Arthritis Prevention of Structural Damage (RAPID) 1 and 2, 982 and 619 patients were randomly assigned to treatment, respectively. In each clinical trial, patients were randomly assigned (2 : 2 : 1) to receive s.c. CZP 200 or 400 mg (preceded by three dosages of 400 mg at Weeks 0, 2 and 4) plus MTX, or placebo plus MTX, every 2 weeks for 52 weeks (RAPID 1) or 24 weeks (RAPID 2). Following screening, eligible patients were assessed for efficacy at baseline; Weeks 1, 2, 4, 6, 8 (10, RAPID 1 only), 12, 14 and 16; and every 4 weeks thereafter until Week 52 or 24 (or at withdrawal) for RAPID 1 and RAPID 2, respectively. Patients who were ACR20 non-responders at Week 12 (confirmed at Week 14) were to be withdrawn from study at Week 16 as per the protocol. Patients who withdrew at Week 16 or who successfully completed the trial were offered enrolment in an open-label extension study of CZP 400 mg every 2 weeks plus MTX.
The present analysis assesses the association between clinically meaningful improvements in physical function, fatigue and pain from baseline to Week 12 and improvements in productivity at work and home in these two trials. Data from the two CZP treatment groups (CZP 200 mg plus MTX and CZP 400 mg plus MTX) from both trials were pooled for analysis. Clinically meaningful improvements were defined as changes from baseline greater than or equal to the minimal clinically important difference (MCID) for each outcome measure (physical function, fatigue and pain). Patients reporting a clinically meaningful improvement were considered responders. MCIDs were used in this analysis as they are clinically relevant for patients and have been well studied and documented in the literature. Week 12 was chosen as the assessment time point for this analysis because treatment guidelines suggest switching therapy if a response is not observed within 12 weeks [32].
Health outcome measures
Physical function
Physical function was assessed using the 20-item HAQ-Disability Index (HAQ-DI), which evaluates the degree of difficulty experienced by the patient in eight categories of daily living: dressing and grooming, hygiene, arising, eating, walking, reaching, gripping and outdoor activities. Scores for each domain range from 0 (no difficulty in performing the activity) to 3 (unable to do the activity), with total index scores ranging from 0 to 3 [33]. Patients reporting a decrease of ≥0.22 points in the HAQ-DI, which is defined as the MCID [34, 35] from baseline to Week 12, were considered responders.
Physical function was also assessed using the physical functioning (PF) domain and physical component summary (PCS) scores of the Short Form 36-Item Health Survey (SF-36) [36]. Patients reporting improvements ≥MCID for the SF-36 PCS and PF scores (2.5 and 5 points, respectively) from baseline to Week 12 were considered responders.
Fatigue
Fatigue (tiredness) was assessed by the Fatigue Assessment Scale (FAS) [37, 38]. In this single-item, numeric rating scale, patients are asked, ‘Please rate your fatigue (weariness, tiredness) during the past week on a scale of 0–10, where 0 is “no fatigue”‚ and 10 is “fatigue” as bad as you can imagine’. Patients reporting a ≥1-point decrease in the FAS (which is defined as the MCID based on an internal anchor-based approach) [39] from baseline to Week 12 were considered responders. In addition, fatigue was assessed using the SF-36 vitality (VT) domain [36]; those achieving at least a 5-point increase from baseline to endpoint in VT scores were considered responders [40].
Pain
Patients reported their level of arthritis pain using the Patient’s Assessment of Pain Visual Analogue Scale (VAS), a component of the ACR response criteria in RA [41], by answering the query, ‘My pain at the time is …’ by using a 100-mm VAS, where 0 is ‘no pain’ and 100 is ‘most severe pain’. It is well accepted that MCID for 100-mm VAS scales is an improvement of 10 mm [42]; therefore, patients reporting ≥10-point decrease in pain from baseline to Week 12 were considered responders. Pain was also assessed using the SF-36 bodily pain (BP) domain [36]; patients reporting a ≥5-point decrease from baseline to Week 12 were considered responders [34, 36].
Productivity within and outside the home and daily activities
The RA-specific Work Productivity Survey (WPS-RA) is a novel, validated questionnaire developed to assess the impact of RA on productivity at paid work outside the home and work within the home and on family, social and leisure activities over the preceding month [43]. During the recent OMERACT 9 meeting, based on available evidence supporting their validity, the WPS-RA was one of the five selected instruments for assessing productivity changes in RA [44]. The WPS-RA is based on self-report and is interviewer administered, with a recall period of 1 month. The survey, shown to be valid and responsive to clinical changes [43], consists of nine questions. The first question addresses employment status and provides additional information on job type for employed subjects and the status of those not employed. For employed patients only, three questions assess absenteeism (full days of work missed due to arthritis) and presenteeism (days with work productivity reduced by ≥50% due to arthritis; does not include days counted in the previous question) in the workplace, and rate of interference of RA with work productivity on a scale of 0–10 (0: no interference; 10: complete interference). The last five questions of the survey, which are answered by all patients, are related to productivity limitations at home and participation in family, social and leisure activities over the previous month as follows: number of days of household work missed due to arthritis; days with household productivity reduced by ≥50% (does not include days counted in the previous question); days with outside help hired; rate of interference with household productivity by RA on a scale of 0–10; and days with family, social or leisure activities missed.
The WPS-RA survey was assessed in the RAPID 1 and 2 trials at baseline (Week 0) and every 4 weeks until the end of the study or until study withdrawal. Days missed from work or activities due to scheduled per-protocol study visits were not counted in the assessment.
Statistical methods
Analyses of response rates at Week 12 for physical function, fatigue and pain were conducted on the intent-to-treat (ITT) populations for the RAPID 1 and 2 trials, which were defined as all patients being randomly assigned to receive treatment. Responder status was determined based on the above definitions of MCID for each measure (physical function, fatigue and pain) by categorizing patients as responders if they achieved improvement from baseline to Week 12 ≥MCID or non-responders if they did not. Logistic regression models (with treatment as factor and baseline HAQ-DI, fatigue or pain scores as covariates) were conducted to evaluate the odds of being a responder by physical function, fatigue and/or pain at Week 12. Additional analyses were also performed using other MCID thresholds and using a responder definition based on 20% improvement in HAQ-DI scores from baseline.
Analyses of the relationship between improvements in each health outcome measure and changes in productivity were conducted on observed data at Week 12 [i.e. only on patients with non-missing data at Week 12 in both productivity and outcome measures (physical function, fatigue or pain)] from the pooled CZP dose groups (200 and 400 mg) from the two RAPID studies. Analyses of productivity at home and daily activities were included in all patients (employed plus unemployed) at Week 12, while analyses of productivity outside home were conducted in the employed population only. The relationship between improvements in each health outcome and changes in productivity was examined by comparing differences in WPS-RA changes from baseline to Week 12 in responders vs non-responders by physical function (HAQ-DI, SF-36 PCS and PF), fatigue (FAS and SF-36 VT) and pain (pain VAS and SF-36 BP). The tests of comparison were conducted using a non-parametric bootstrap t-method [45]. As the WPS-RA questions capture lost days or rate of interference, improvement in productivity within and outside the home and daily activities is attained when there is a decrease in the WPS response, which translates to a negative change. Accordingly, a positive change indicates a loss or worsening. In other words, the higher the negative mean change (in absolute value), the better the impact of the therapy on productivity and daily activities.
Results
Population characteristics at baseline
A total of 1601 patients were enrolled in the RAPID studies (982 patients in RAPID 1 and 619 in RAPID 2). Baseline demographics, disease characteristics and health outcomes measures were similar across both trials (Tables 1 and 2). On average, patients were 52 years of age, had experienced RA for ∼6 years and reported moderate to severe impairment in physical function, fatigue and pain (Tables 1 and 2). More than three quarters of patients were female (Table 1).
Table 1.
Baseline demographical characteristics in RAPID trials (ITT population)
| Characteristics | RAPID 1 (N = 982) | RAPID 2 (N = 619) |
|---|---|---|
| Age, mean (s.d.), years | 52.0 (11.6) | 51.9 (11.5) |
| Gender, female, % | 83.2 | 81.6 |
| Disease duration, mean (s.d.), years | 6.1 (4.3) | 6.2 (4.2) |
| Number of previous DMARDS, mean (s.d.) | 1.3 (1.3) | 1.2 (1.3) |
| DAS-28 (ESR), mean (s.d.) | 6.9 (0.8) | 6.8 (0.8) |
| RF (≥14 IU/ml), n (%) | 802 (81.8) | 462 (76.9) |
| Employment statusa, n (%) | ||
| Employed | 370 (37.7) | 245 (39.6) |
| Homemakers | 128 (13.0) | 45 (7.3) |
| Retired | 178 (18.1) | 173 (27.9) |
| Unable to work due to RA | 188 (19.1) | 148 (23.9) |
| Other (not employed) | 25 (2.5) | 5 (0.8) |
aAs captured by the WPS-RA; percentages are computed on the overall ITT population. DAS-28(ESR): Disease Activity Score based on 28-joint count ESR.
Table 2.
Baseline physical function, fatigue, pain and productivity in RAPID trials (ITT population)
| Characteristics | RAPID 1 (N = 982) | RAPID 2 (N = 619) |
|---|---|---|
| HAQ-DI (0–3.0)a, mean (s.d.) | 1.7 (0.6) | 1.6 (0.6) |
| FAS (0–10)b, mean (s.d.) | 6.5 (2.0) | 6.5 (1.9) |
| Pain VAS (0–100)c, mean (s.d.) | 63.1 (18.9) | 60.9 (20.2) |
| SF-36 | ||
| PCS summary score (0–50), mean (s.d.) | 30.8 (6.5) | 30.9 (6.2) |
| Physical function domain score (0–100), mean (s.d.) | 32.9 (21.1) | 32.3 (20.3) |
| VT domain score (0–100), mean (s.d.) | 35.3 (18.2) | 37.0 (17.7) |
| BP domain score (0–100), mean (s.d.) | 29.5 (15.4) | 30.2 (15.0) |
| WPS-RA, mean (s.d.) | ||
| Days of paid work missed due to RA per monthd | 3.9 (8.0) | 3.3 (6.8) |
| Days with productivity at work reduced by ≥50% due to RA per monthe | 7.1 (8.5) | 8.8 (8.6) |
| Rate of RA interference on work productivity per monthf | 5.2 (2.6) | 5.5 (2.3) |
| Days of household work missed due to RA per monthd | 8.1 (8.5) | 6.9 (7.4) |
| Days with household productivity reduced by ≥50% due to RA per monthe | 10.4 (8.4) | 10.8 (8.2) |
| Days of missed family, social or leisure activities due to RA per monthd | 6.1 (8.7) | 5.0 (7.5) |
| Days with outside hired help per monthd | 5.4 (9.0) | 5.0 (8.7) |
| Rate of RA interference on household work productivity per monthf | 6.2 (2.3) | 5.9 (2.2) |
aHAQ-DI scores range from 0 to 3, with lower scores indicating improvements in physical function. bFAS scores range from 0 to 10, with lower scores indicating less fatigue. cScores for patient’s assessment of arthritis pain range from 0 to 100 mm, with lower scores indicating relief in arthritis pain. dRange is 0–30 (in past month). eRange is 0–30 (in past month) and does not include days counted in previous question. fRange is 0–10, where 0 indicates no interference and 10 indicates complete interference.
Full details regarding the baseline employment status of patients in these trials have been previously published [29]. In brief, ∼40% of patients were employed outside the home at baseline (Table 1). These patients reported missing an average of 3–4 full days of paid work and had an additional 7–9 days on average of work with productivity reduced by at least 50% in the previous month due to RA (Table 2). RA also had substantial impact on household work productivity and participation in family, social and leisure activities (regardless of employment status; Table 2). On average, patients missed 7–8 full days of household work and had an additional 10–11 days of household work with productivity reduced by at least 50% due to RA during the previous month. They also reported missing on average 5–6 days of family, social and leisure activities during the previous month.
Impact of treatment
Treatment with CZP 200 or 400 mg plus MTX significantly improved physical function and productivity and reduced pain and fatigue in patients with active RA [27–29]. Response rates based on the MCIDs for physical function, fatigue and pain were higher in patients receiving CZP plus MTX (68–76%) compared with patients receiving placebo plus MTX (41–52%) [odds ratio (OR) 2.9–5.5 for CZP plus MTX vs placebo plus MTX; P≤0.01], and were similar for the two CZP dose groups (Table 3).
Table 3.
Response rates at Week 12 in physical function, fatigue and pain in the pooled RAPID trials (ITT population)
| Response | PBO + MTX (n = 326) | CZP 200 mg + MTX (n = 639) | CZP 400 mg + MTX (n = 636) |
|---|---|---|---|
| HAQ responsea | |||
| n (%) | 125 (43.7) | 395 (68.1) | 416 (71.2) |
| OR vs PBOb | 2.93* | 3.41* | |
| FAS responsea | |||
| n (%) | 148 (51.8) | 439 (75.7) | 435 (74.5) |
| OR vs PBOb | 3.54* | 3.35* | |
| Pain VAS responsea | |||
| n (%) | 117 (40.9) | 430 (74.0) | 445 (76.1) |
| OR vs PBOb | 5.08* | 5.54* |
aResponse is defined as an improvement from baseline to Week 12 ≥MCID (in absolute value); MCID equals 0.22 (HAQ-DI); 10 (pain VAS); 1 (FAS). Response rates are computed on available data at Week 12; bOR and P-value from logistic regression with treatment as factor and baseline HAQ-DI, pain VAS or FAS score as covariates, respectively; *P≤0.001. PBO: placebo.
Associations between productivity within and outside the home and physical function, fatigue and pain in CZP-treated patients
Productivity outside the home
Physical function
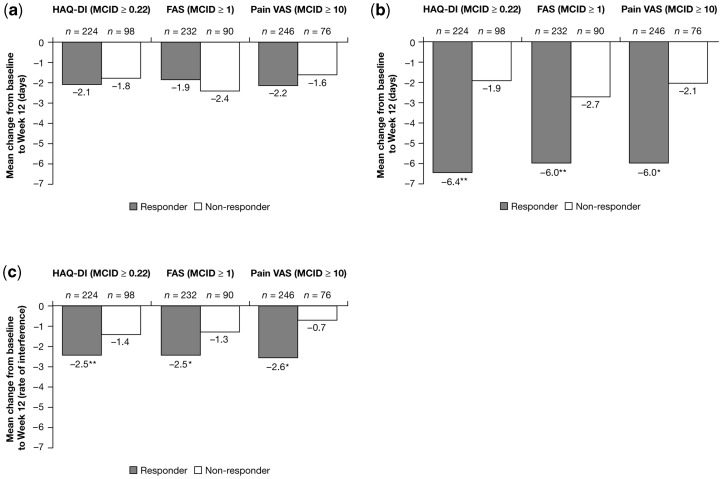
Although HAQ-DI responders and non-responders reported similar reductions in absenteeism by Week 12 (mean decreases of −2.1 vs −1.8 days, respectively; P = 0.622; Fig. 1a), responders reported significantly higher decreases in presenteeism (mean changes of −6.4 vs −1.9 days, respectively; Fig. 1b) and in the rate of RA interference with their work productivity (mean decrease of 2.5 vs 1.4 points on a scale of 0–10; Fig. 1c) compared with non-responders. Comparable associations between work productivity and physical function as reported by SF-36 PF domain scores were evident for all three measures of work productivity (Table 4); associations were also evident between SF-36 PCS and PF domain scores and the rate of interference of RA with work productivity (Table 4).
Fig. 1.
Mean changes in paid work productivity from baseline to Week 12 by responder status (observed data, employed ITT population, pooled RAPID 1 and 2 CZP 200 mg + 400 mg groups). Response is defined as change from baseline to Week 12 ≥MCID (in absolute value), non-response is defined as mean change from baseline to Week 12 <MCID. Recall period for absenteeism (work days missed) is 1 month; recall period for presenteeism (work days with productivity reduced by ≥50%, not including work days missed) is 1 month; range for productivity interference is 0–10, where 0 indicates no interference and 10 indicates complete interference. (a) Reduction in absenteeism (work days missed) due to arthritis. (b) Decrease in presenteeism (days with productivity at paid work reduced by ≥50%) due to RA. *P≤0.001 vs non-responders; **P≤0.01 vs non-responders. (c) Reduction in the rate of RA interference with productivity at paid work. *P≤0.001 vs non-responders; **P≤0.01 vs non-responders; rate of interference 0–10 scale: 0 indicates no interference and 10 indicates complete interference.
Table 4.
Mean changes in productivity from baseline to Week 12 by SF-36 responder status (observed data, pooled RAPID 1 and 2, CZP 200 + 400 mg groups)
| SF-36 PCS (MCID ≥2.5) |
SF-36 PF domain (MCID ≥5.0) |
SF-36 VT domain (MCID ≥5.0) |
SF-36 BP domain (MCID ≥5.0) |
|||||
|---|---|---|---|---|---|---|---|---|
| WPS-RA, mean change from baseline to Week 12 | Responder | Non- responder | Responder | Non- responder | Responder | Non- responder | Responder | Non- responder |
| Employed patients | (n = 201) | (n = 121) | (n = 194) | (n = 128) | (n = 180) | (n = 142) | (n = 245) | (n = 77) |
| Absenteeism (paid work days missed) due to RA | −2.2 | −1.7 | −2.2 | −1.8 | −2.2 | −1.8 | −2.2 | −1.4 |
| Presenteeism (days with productivity at work reduced by ≥50%) due to RA | −5.6 | −4.1 | −5.9* | −3.8 | −6.6** | −3.1 | −5.6* | −3.2 |
| Rate of RA interference on work productivity | −2.5*** | −1.5 | −2.6*** | −1.4 | −2.8*** | −1.3 | −2.4** | −1.3 |
| All patients | (n = 469) | (n = 332) | (n = 436) | (n = 365) | (n = 455) | (n = 346) | (n = 584) | (n = 217) |
| Days of household work missed due to RA | −4.6 | −3.8 | −5.1*** | −3.3 | −5.2*** | −3.1 | −5.0*** | −2.2 |
| Days with household productivity reduced by ≥50% due to RA | −6.0** | −4.2 | −6.1** | −4.3 | −5.9* | −4.5 | −5.6 | −4.4 |
| Days of missed family, social or leisure activities due to RA | −3.6 | −3.3 | −4.1* | −2.8 | −4.2** | −2.5 | −3.8* | −2.5 |
| Days with outside hired help | −3.0 | −2.2 | −3.2* | −2.1 | −3.4** | −1.7 | −3.2*** | −1.3 |
| Rate of RA interference on household work productivity | −2.6*** | −1.4 | −2.6*** | −1.4 | −2.6*** | −1.3 | −2.5*** | −1.0 |
Response is defined as change from baseline to Week 12 ≥MCID (in absolute value), non-response is defined as mean change from baseline to Week 12 <MCID. n represents number of subjects with non-missing WPS-RA, considered in the analysis.
*P≤0.05 vs non-responders; **P≤0.01 vs non-responders; ***P≤0.001 vs non-responders.
Fatigue
FAS responders reported significantly higher decreases in presenteeism by Week 12 (mean changes of −6.0 vs −2.7 days, respectively; Fig. 1b) and significantly higher reductions in the rate of RA interference with their productivity at work (average decrease of 2.5 vs 1.3 on a 0–10 scale; Fig. 1c) compared with non-responders. No significant differences were reported between responders and non-responders for reductions in absenteeism (Fig. 1a). Similar associations between work productivity and fatigue, as evaluated by the SF-36 VT domain, were evident for all three measures of work productivity (Table 4).
Pain
Although responders and non-responders by pain VAS reported similar reductions in absenteeism at paid work at Week 12 (mean changes of −2.2 vs −1.6 days, respectively; Fig. 1a), responders reported significantly higher reductions in presenteeism at work (average change of −6.0 vs −2.1 days, respectively; Fig. 1b) and had significantly higher reductions in RA interference with productivity at work (mean decrease of 2.6 vs 0.7 points on a scale of 0–10; Fig. 1c). Similar associations between work productivity and pain, when evaluated by the SF-36 BP domain, were evident for all three measures of work productivity (Table 4).
Productivity at home and daily activities
Physical function
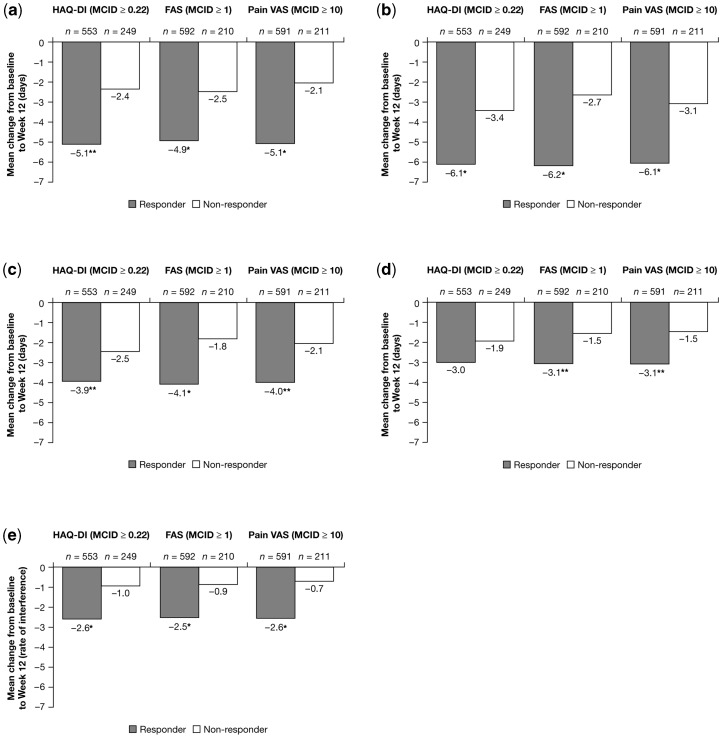
HAQ-DI responders reported significantly higher decreases in the number of household work days missed by Week 12 (mean decreases of −5.1 vs −2.4 days, respectively; Fig. 2a) and in the number of days with productivity in the home reduced by ≥50% (mean change of −6.1 vs −3.4 days, respectively; Fig. 2b) compared with non-responders. Responders also reported significantly higher reductions than non-responders in days of missed family, social and leisure activities (mean changes of −3.9 vs −2.5 days, respectively; Fig. 2c); in days with hired outside help per month (mean changes of −3.0 vs −1.9 days, respectively; Fig. 2d) and the interference of RA with their household work productivity (mean decreases of 2.6 vs 1.0 points on a scale of 0–10; Fig. 2e). Similar trends in improvements were evident when response was defined based on SF-36 PCS and PF domain scores (Table 4).
Fig. 2.
Mean changes in household work productivity and daily activities from baseline to Week 12 by responder status (observed data, ITT population, pooled RAPID 1 and 2 CZP 200 mg + 400 mg groups). Response is defined as change from baseline to Week 12 ≥MCID (in absolute value), non-response is defined as mean change from baseline to Week 12 <MCID. Recall period for household work days missed, household work days with productivity reduced by ≥50% (not including household work days missed), leisure days missed and days with hired help is 1 month; range for productivity interference is 0–10, where 0 indicates no interference and 10 indicates complete interference. (a) Reduction in days of household work missed due to arthritis. *P≤0.001 vs non-responders; **P≤0.01 vs non-responders. (b) Decrease in days with household productivity reduced by ≥50% due to RA. *P≤0.001 vs non-responders. (c) Reduction in days lost of family, social or leisure activities due to arthritis. *P≤0.001 vs non-responders; **P≤0.01 vs non-responders. (d) Reduction in days with hired outside help. **P≤0.01 vs non-responders. (e) Reduction in the rate of RA interference with household work productivity. *P≤0.001 vs non-responders; rate of interference 0–10 scale: 0 indicates no interference and 10 indicates complete interference.
Fatigue
By Week 12, responders by FAS had significantly higher reductions than non-responders in the number of days missed of household work due to RA (mean changes of −4.9 vs −2.5 mean days, respectively; Fig. 2a); in days of household work with productivity reduced by ≥50% (mean changes of −6.2 vs −2.7 days, respectively; Fig. 2b); and in days of missed family, social and leisure activities (mean changes of −4.1 vs −1.8 days; Fig. 2c). Responders also reported significantly higher reductions in days with hired outside help per month (mean changes of −3.1 vs −1.5 days; Fig. 2d) and in the interference of RA with household productivity (2.5 vs 0.9 average decrease on a scale of 0–10; Fig. 2e) than non-responders. Similar results for responders defined based on improvements in the SF-36 VT domain were also evident (Table 4).
Pain
Responders by pain VAS had significantly higher decreases in days missed of household work by Week 12 (mean changes of −5.1 vs −2.1 days, respectively; Fig. 2a); in days with household productivity reduced by 50% (mean changes of −6.1 vs −3.1 days; Fig. 2b); in days lost of family, social and leisure activities (−4.0 vs −2.1; Fig. 2c); in days with hired outside help per month (−3.1 vs −1.5; Fig. 2d); and RA interference with household work (2.6 vs 0.7 average decrease on a scale of 0–10; Fig. 2e) compared with non-responders. Similar findings were evident in responders defined by improvements in the SF-36 BP domain (Table 4).
Sensitivity analysis
A sensitivity analysis was conducted to determine the impact of different thresholds to define clinically meaningful improvements in physical function, fatigue and pain on reported outcomes for work outside and within the home as well as participation in family, social and leisure activities. For each WPS-RA question, responder status was determined for three levels of clinical improvement: HAQ-DI (MCID and sensitivity thresholds assessed = 0.22, 0.50 or 0.70), FAS (1, 2 or 3) and pain VAS (10, 20 or 30). In general, associations between productivity and physical function, fatigue or pain responder status tended to increase when thresholds higher than the MCID were selected to define response (data not shown). An additional sensitivity analysis was conducted using a responder definition of 20% improvement from baseline in HAQ-DI, which also showed comparable associations between productivity and physical function (data not shown).
Discussion
These analyses indicate that RA patients who experienced clinically meaningful improvements in physical function, fatigue and pain following treatment with CZP plus MTX reported significantly greater improvements in work, both within and outside the home. Findings were consistent, regardless of which patient-reported outcome was used to determine a treatment response, which threshold was used to define MCID and when response was defined based on 20% improvement in HAQ-DI from baseline (instead of MCID). The use of MCIDs to categorize patients as responders or non-responders for our primary analysis was a valid approach as they are clinically relevant for patients, capturing a level of benefit that can be perceived by them, and have been well documented in the literature.
Decreased work productivity, as well as decreased participation in family, social and leisure activities, reflects the important impact of RA on patients’ lives. Importantly, findings presented here indicate that productivity both within the workplace and at home can be positively influenced by improvements in physical function and relief of pain and fatigue. In addition, patients treated with CZP in combination with MTX reported significant relief of arthritis-associated pain and fatigue as well as improvements in physical function after 3 months of treatment. Results were particularly striking regarding pain relief, with five times as many CZP-treated patients reporting clinically meaningful improvement, and two to three times as many reporting improvements in physical function and fatigue compared with patients receiving placebo plus MTX.
None of the outcome measures was associated with a statistically significant reduction in absenteeism at paid work due to RA. Although this could be interpreted to indicate that employed patients with and without clinically meaningful improvements in physical function or relief from pain or fatigue have comparable absences from work, responders in all three categories (i.e. those with changes ≥MCID) reported significant improvements in productive days at work compared with non-responders and significantly less interference of RA on productivity at work. One explanation for this observation may be that work-leave policies regarding absenteeism (i.e. the time missed from work due to health reasons) may prevent patients from staying home even when they are experiencing symptoms [46]. Indeed, our study demonstrates that although deficits in physical function, pain and fatigue do not prevent patients from going to work, they do interfere with their productivity while at work. Findings also indicate there may be a continuum of disability that progresses from an initial loss of productivity at work to fewer days worked to ultimately stopping work altogether. Effective treatment strategies that improve signs and symptoms of RA would help to stop or reverse this continuum.
Clinically meaningful improvements in physical function, fatigue and pain were also associated with statistically significant improvements in productivity within the home, including significantly fewer days lost of household work due to RA. These findings differ from those reported for work outside the home, and likely reflect two important points: (i) only 37–40% of patients enrolled in these trials were gainfully employed; and (ii) there are differences between the varying types of work activities (requiring differing levels of physical performance and energy) performed outside the home and those done within the home. While the demands of paid employment at work tend to be ‘fixed’ and highly structured based on work (and societal) policies, those of housework are more flexible and more easily delayed or rescheduled and may be more ambiguous based on societal status, family cycle, private negotiations and personal preferences. In addition, unlike paid employment, household work may be more easily delegated to others, either within the home or done for payment.
Findings in these analyses are consistent with previously reported improvements in patient-reported outcomes following treatment with CZP plus MTX [47] and complement the significant improvements in disease activity that have also been observed following CZP plus MTX treatment [27, 28]. Additional analyses have also demonstrated that the improvements in patient-reported outcomes (physical function, pain and fatigue) are associated among each other (Pearson’s coefficients ranging between 0.54 and 0.77) and that these improvements correlate well with clinical improvements in disease activity following treatment with CZP plus MTX [47]. Findings in these analyses are also consistent with those in a study evaluating the relationship between hiring outside help and achieving clinical responses to treatment—where patients with clinically meaningful improvements in pain, fatigue and physical function reported greater reductions in the number of days they hired help to perform household work [48]. In addition to improvement in productivity inside and outside the home, responders reported significantly less interference of RA on the participation in family, social and leisure activities. This improvement was significantly associated with reduction in fatigue and pain, further confirming the inter-related matrix of the ancillary burdens of disease.
Strengths of this study include the robust findings from two large well-controlled trials in meeting primary endpoints of ACR responses as well as radiographic benefit at Weeks 24 and 52, with large effect sizes compared with placebo plus MTX. In addition, statistically significant and meaningful improvements in work and household productivity as well as participation in family, social and leisure activities were reported after only 12 weeks of treatment. Future studies are needed to determine whether these observed improvements are maintained or even increased with longer exposure to effective treatment. Limitations include patients being asked to recall events that occurred in the proceeding month that may introduce bias in reporting. However, previous research has indicated that individuals are able to accurately report work days lost over the past 30 days [49].
In summary, results of these analyses from two large multinational, Phase III clinical trials demonstrate that CZP significantly improves physical function, fatigue and pain in patients with active RA, and that there is a clear association between clinically meaningful improvements in pain, fatigue and physical function and increased productivity within and outside the home, and less interference of RA with work, household activities and participation in family, social and leisure activities. These findings have important societal implications in the context of direct and indirect costs attributed to active RA.

Acknowledgements
The authors wish to thank Mrs Laurence Boquia, Mr Yves Brabant, Mr Christophe Gervasoni and Dr Lucian Ionescu for their support in developing this manuscript; and Linda Wychowski and Karen Munro of PAREXEL for writing and editorial assistance, which was funded by UCB, Inc.
Funding: This paper was funded by UCB, which sponsored the clinical trials in which the data were collected. Funding to pay the Open Access publication charges for this article was provided by UCB, Inc.
Disclosure statement: P.T. serves as a consultant to UCB, Inc., and has received honoraria from UCB, Inc. for speaking. O.P. and G.C. are employees of UCB, Inc. V.S. serves as a consultant to Abbott Immunology, Alder, Almiral, Amgen Corporation, AstraZeneca, Biogen Idec, CanFite, Centocor, Chelsea, Crescendo, Cypress Biosciences, Inc., Eurodiagnostica, Fibrogen, Forest Laboratories, Genentech, Human Genome Sciences, Idera, Incyte, Jazz Pharmacetucials, Lexicon Genetics, Logical Therapeutics, Lux Bioschiences, Novartis Pharmaceticals, NovoNordisk, Nuon, Ono Pharmaceuticals, Pfizer, Rigel, Roche, Sanofi-Aventis, Savient, Schering Plough, SKK and UCB, Inc. G.C. is an employee and owns shares of UCB, Inc. J.M.H. serves as a consultant to UCB, Inc., and has received honoraria from UCB, Inc. for speaking. P.M. serves as a consultant to UCB, Inc. and has received research funding and honoraria for speaking from UCB, Inc.
References
- 1.Carr A, Hewlett S, Hughes R, et al. Rheumatology outcomes: the patient’s perspective. J Rheumatol. 2003;30:880–3. [PubMed] [Google Scholar]
- 2.Sakalys JA. Illness behavior in rheumatoid arthritis. Arthritis Care Res. 1997;10:229–37. doi: 10.1002/art.1790100404. [DOI] [PubMed] [Google Scholar]
- 3.Hewlett S, Cockshott Z, Byron M, et al. Patients' perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis Rheum. 2005;53:697–702. doi: 10.1002/art.21450. [DOI] [PubMed] [Google Scholar]
- 4.Belza BL, Henke CJ, Yelin EH, Epstein WV, Gilliss CL. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res. 1993;42:93–9. [PubMed] [Google Scholar]
- 5.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407–17. [PubMed] [Google Scholar]
- 6.Plant MJ, O’Sullivan MM, Lewis PA, Camilleri JP, Coles EC, Jessop JD. What factors influence functional ability in patients with rheumatoid arthritis. Do they alter over time? Rheumatology. 2005;44:1181–5. doi: 10.1093/rheumatology/keh707. [DOI] [PubMed] [Google Scholar]
- 7.Sokka T, Kankainen A, Hannonen P. Scores for functional disability in patients with rheumatoid arthritis are correlated at higher levels with pain scores than with radiographic scores. Arthritis Rheum. 2000;43:386–9. doi: 10.1002/1529-0131(200002)43:2<386::AID-ANR19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Backman CL, Kennedy SM, Chalmers A, Singer J. Participation in paid and unpaid work by adults with rheumatoid arthritis. J Rheumatol. 2004;31:47–56. [PubMed] [Google Scholar]
- 9.Burton W, Morrison A, Maclean R, Ruderman E. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup Med. 2006;56:18–27. doi: 10.1093/occmed/kqi171. [DOI] [PubMed] [Google Scholar]
- 10.Young A, Dixey J, Kulinskaya E, et al. Which patients stop working because of rheumatoid arthritis? Results of five years' follow up in 732 patients from the early RA study (ERAS) Ann Rheum Dis. 2002;61:335–40. doi: 10.1136/ard.61.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allaire SH, Meenan RF, Anderson JJ. The impact of rheumatoid arthritis on the household work performance of women. Arthritis Rheum. 1991;34:669–78. doi: 10.1002/art.1780340607. [DOI] [PubMed] [Google Scholar]
- 12.Habib G, Artul S, Ratson N, Froom P. Household work disability of Arab housewives with rheumatoid arthritis. Clin Rheumatol. 2007;26:759–63. doi: 10.1007/s10067-007-0554-9. [DOI] [PubMed] [Google Scholar]
- 13.Katz PP, Morris A, Yelin EH. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis. 2006;65:763–9. doi: 10.1136/ard.2005.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacaille D. Arthritis and employment research: where are we? Where do we need to go? J Rheumatol. 2005;32(Suppl. 72):42–45. [PubMed] [Google Scholar]
- 15.Sokka T, Kautiainen H, Möttönen T, Hannonen P. Work disability in rheumatoid arthritis 10 year after the diagnosis. J Rheumatol. 1999;26:1681–5. [PubMed] [Google Scholar]
- 16.Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–17. [PubMed] [Google Scholar]
- 17.Callahan LF, Bloch DA, Pincus T. Identification of work disability in rheumatoid arthritis: physical, radiographic and laboratory variables do not add explanatory power to demographic and functional variables. J Clin Epidemiol. 1992;45:127–38. doi: 10.1016/0895-4356(92)90005-8. [DOI] [PubMed] [Google Scholar]
- 18.Mau W, Bornmann M, Weber H, Weidemann HF, Hecker H, Raspe HH. Prediction of permanent work disability in a follow-up study of early rheumatoid arthritis: results of a tree structured analysis using RECPAM. Br J Rheumatol. 1996;35:652–9. doi: 10.1093/rheumatology/35.7.652. [DOI] [PubMed] [Google Scholar]
- 19.Reisine S, McQuillan J, Fifield J. Predictors of work disability in rheumatoid arthritis patients. A five-year followup. Arthritis Rheum. 1995;38:1630–7. doi: 10.1002/art.1780381115. [DOI] [PubMed] [Google Scholar]
- 20.Verstappen SM, Bijlsma JW, Verkleij H, et al. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheum. 2004;51:488–97. doi: 10.1002/art.20419. [DOI] [PubMed] [Google Scholar]
- 21.Yelin E, Henke C, Epstein W. The work dynamics of the person with rheumatoid arthritis. Arthritis Rheum. 1987;30:507–12. doi: 10.1002/art.1780300504. [DOI] [PubMed] [Google Scholar]
- 22.Mittendorf T, Dietz B, Sterz R, Cifaldi MA, Kupper H, von der Schulenburg JM. Personal and economic burden of late-stage rheumatoid arthritis among patients treated with adalimumab: an evaluation from a patient's perspective. Rheumatology. 2008;47:188–93. doi: 10.1093/rheumatology/kem317. [DOI] [PubMed] [Google Scholar]
- 23.Puolakka K, Kautiainen H, Mottonen T, et al. Early suppression of disease activity is essential for maintenance of work capacity in patients with recent-onset rheumatoid arthritis: five-year experience from the FIN-RACo trial. Arthritis Rheum. 2005;52:36–41. doi: 10.1002/art.20716. [DOI] [PubMed] [Google Scholar]
- 24.Puolakka K, Kautiainen H, Mottonen T, et al. Impact of initial aggressive drug treatment with a combination of disease-modifying antirheumatic drugs on the development of work disability in early rheumatoid arthritis: a five-year randomized followup trial. Arthritis Rheum. 2004;50:55–62. doi: 10.1002/art.11436. [DOI] [PubMed] [Google Scholar]
- 25.Smolen JS, Han C, van der Heijde D, et al. Infliximab treatment maintains employability in patients with early rheumatoid arthritis. Arthritis Rheum. 2006;54:716–22. doi: 10.1002/art.21661. [DOI] [PubMed] [Google Scholar]
- 26.Reisine ST, Goodenow C, Grady KE. The impact of rheumatoid arthritis on the homemaker. Soc Sci Med. 1987;25:89–95. doi: 10.1016/0277-9536(87)90210-3. [DOI] [PubMed] [Google Scholar]
- 27.Keystone E, van der Heijde D, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis. Arthritis Rheum. 2008;58:3319–29. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 28.Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. Ann Rheum Dis. 2009;68:797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanaugh A, Smolen J, Emery P, Purcaru O, Richard L, van Vollenhoven R. Certolizumab pegol with methotrexate improves home and workplace productivity and social activities in patients with active rheumatoid arthritis. Arthritis Care Res. 2009;61:1592–600. doi: 10.1002/art.24828. [DOI] [PubMed] [Google Scholar]
- 30.Strand V, Keininger D, Tahiri-Fitzgerald E. Certolizumab pegol therapy added to methotrexate (MTX) produces clinically meaningful improvements in health-related quality of life and relieves fatigue in patients with rheumatoid arthritis with an incomplete response to MTX: results from RAPID 2 study. Ann Rheum Dis. 2007;66(Suppl. 2):601. [Google Scholar]
- 31.Schiff M, Keininger DL, Tahiri-Fitzgerald E. Certolizumab pegol added onto methotrexate improves physical functioning and reduces pain in patient with rheumatoid arthritis who have an incomplete response to methotrexate: data from RAPID 2. Ann Rheum Dis. 2007;66(Suppl. II):187. [Google Scholar]
- 32.Furst DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on biological agents, specifically tumour necrosis factor α (TNFα) blocking agents and interleukin-1 receptor antagonist (IL-1ra), for the treatment of rheumatic diseases, 2005. Ann Rheum Dis. 2005;64(Suppl. 4):iv2–14. doi: 10.1136/ard.2005.044941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fries JF, Ramey DR. “Arthritis specific” global health analog scales assess “generic” health related quality-of-life in patients with rheumatoid arthritis. J Rheumatol. 1997;24:1697–702. [PubMed] [Google Scholar]
- 34.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43:1478–87. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. J Rheumatol. 1993;20:557–60. [PubMed] [Google Scholar]
- 36.Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2008;14:234–54. [PubMed] [Google Scholar]
- 37.Belza B. Self-reported fatigue in rheumatoid arthritis – a pilot study. Arthritis Care Res. 1990;3:154–7. [PubMed] [Google Scholar]
- 38.Hewlett S, Hehir M, Kirwan JR. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum. 2007;57:429–39. doi: 10.1002/art.22611. [DOI] [PubMed] [Google Scholar]
- 39.Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007;34:280–9. [PubMed] [Google Scholar]
- 40.Ware JE, Kosinski M, Gandek B. The SF-36 health survey: a manual and interpretation guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 41.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36:729–40. doi: 10.1002/art.1780360601. [DOI] [PubMed] [Google Scholar]
- 42.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 43.Osterhaus JT, Purcaru O, Richard L. Discriminant validity, responsiveness and reliability of the rheumatoid arthritis specific work productivity survey (WPS-RA) Arthritis Research and Therapy. 2009;11:R73. doi: 10.1186/ar2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaton D, Bombardier C, Escorpizo R, et al. Measuring worker productivity: frameworks and measures. J Rheum. 2006;36:2100–9. doi: 10.3899/jrheum.090366. [DOI] [PubMed] [Google Scholar]
- 45.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19:3219–36. doi: 10.1002/1097-0258(20001215)19:23<3219::aid-sim623>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 46.Escorpizo R, Bombardier C, Boonen A, et al. Worker productivity outcome measures in arthritis. J Rheumatol. 2007;34:1372–80. [PubMed] [Google Scholar]
- 47.Strand V, Mease P, Burmester GR, et al. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther. 2009;11:R170. doi: 10.1186/ar2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T, Gignac M, Wells G, Shen S, Westhovens R. Decreased external home help use with improved clinical status in rheumatoid arthritis: an exploratory analysis of the abatacept in inadequate responders to methotrexate (AIM) trial. Clin Ther. 2008;30:734–48. doi: 10.1016/j.clinthera.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Revicki DA, Irwin D, Reblando J, Simon GE. The accuracy of self-reported disability days. Med Care. 1994;32:401–4. doi: 10.1097/00005650-199404000-00008. [DOI] [PubMed] [Google Scholar]