Abstract
Purpose
The purpose of this study was to determine if providing bony stabilization between the tibia and femur would improve the structural properties of an “enhanced” ACL repair using a collagen-platelet composite when compared to the traditional (Marshall) suture technique.
Methods
Twelve pigs underwent unilateral ACL transection and were treated with sutures connecting the bony femoral ACL attachment site to the distal ACL stump (LIGAMENT group), or to the tibia via a bone tunnel (TIBIA group). A collagen-platelet composite was placed around the sutures to enhance the biologic repair in both groups. Anteroposterior (AP) knee laxity and the graft structural properties were measured after 15 weeks of healing in both the ACL-repaired and contralateral ACL-intact joints.
Results
Enhanced ACL repair with bone-to-bone fixation significantly improved yield load and linear stiffness of the ACL repairs (p<0.05) after 15 weeks of healing. However, laxity values of the knees were similar in both groups of repaired knees (p>0.10).
Conclusions
Using an enhanced ACL suture repair technique that includes bone-to-bone fixation to protect the repair in the initial healing stages resulted in an ACL with improved structural properties after 15 weeks in the porcine model.
Clinical Relevance
The healing response of an ACL suture repair using a collagen-platelet composite can be enhanced by providing bony stabilization between the tibia and femur to protect the graft during the initial healing process in a translational model.
INTRODUCTION
Anterior cruciate ligament (ACL) reconstruction, or replacement of the torn ligament with an autologous graft, remains the current gold standard of treatment for the ACL-deficient knee. However, issues of graft harvest morbidity and post-traumatic arthritis have led to the search for improved surgical methods of treatment of ACL tears. One potential method is “enhanced” suture repair of the ACL using a tissue engineered scaffold containing growth factors to stimulate or accelerate healing. Enhanced repair may have several benefits over ACL reconstruction: it can be performed arthroscopically, does not require graft harvest, it preserves proprioceptive fibers in the ligament, and maintains the complex attachment sites of the native ACL. For skeletally immature patients, this procedure could also be performed with minimal violation of the physes. If normal kinematics could be restored with this procedure, it could also reduce the risk of post -injury OA in ACL-injured patients.
Previous studies of traditional ACL repair in animal models have reported large increases in anterior-posterior (AP) laxity and minimal return of strength.1–4 Likewise human studies of ACL repair, primarily using the Marshall technique (which involved weaving a suture through the tibial stump of the ACL and passing it up through a bone tunnel in the femur), resulted in high rates of abnormal AP laxity and joint instability.5, 6 Because ACL reconstruction provided better stability,7, 8 primary ACL repair was abandoned. However, normal knee kinematics are not restored following ACL reconstruction, and is believed to be responsible, in part, for the high rate of osteoarthritis reported in these patients.9 Improved methods to enhance ACL healing are needed. One option is to place a provisional scaffold (a collagen-platelet composite) in the wound site at the time of suture repair.10
Recently, enhanced suture repair using a collagen-platelet composite has been shown to improve healing in short-term animal studies.10–12 Further, in an ex vivo model, we have shown that normal AP knee laxity can be achieved at time of surgery with placement of a suture from the bony femoral ACL insertion site to the tibia via a bony tunnel. Re-establishing normal laxity was not possible with the traditional Marshall technique when the sutures were placed within the tibial stump.13 The primary aim of the current study was to determine if using a tibial tunnel to achieve bone-to-bone suture fixation in conjunction with enhanced ACL repair as compared to the fixation to the tibial ACL stump would improve the structural properties of the repair and AP knee laxity. We hypothesized that bone-to-bone suture fixation would result in an increase in the graft structural properties and decrease in AP knee laxity when compared to the traditional suturing technique.
METHODS
Experimental Design
Institutional Animal Care and Use Committee approval was obtained prior to initiating the study. Twelve 30 Kg female skeletally immature 4-month-old Yorkshire pigs underwent unilateral ACL transection and enhanced suture repair using a collagen-platelet composite. Six animals underwent enhanced suture repair using the traditional Marshall suture technique (LIGAMENT group), and six animals received enhanced ACL repair in which the sutures were tied directly to the tibia through a tibial tunnel (TIBIA group). The contralateral knee was treated as the ACL-intact control (the INTACT knee). The knee undergoing surgery was block randomized preoperatively to ensure equal distribution of left and right knees in both groups. After surgery, all animals were allowed free activity within standardized housing, and the animals were euthanized after fifteen weeks of healing.
Surgical Procedure
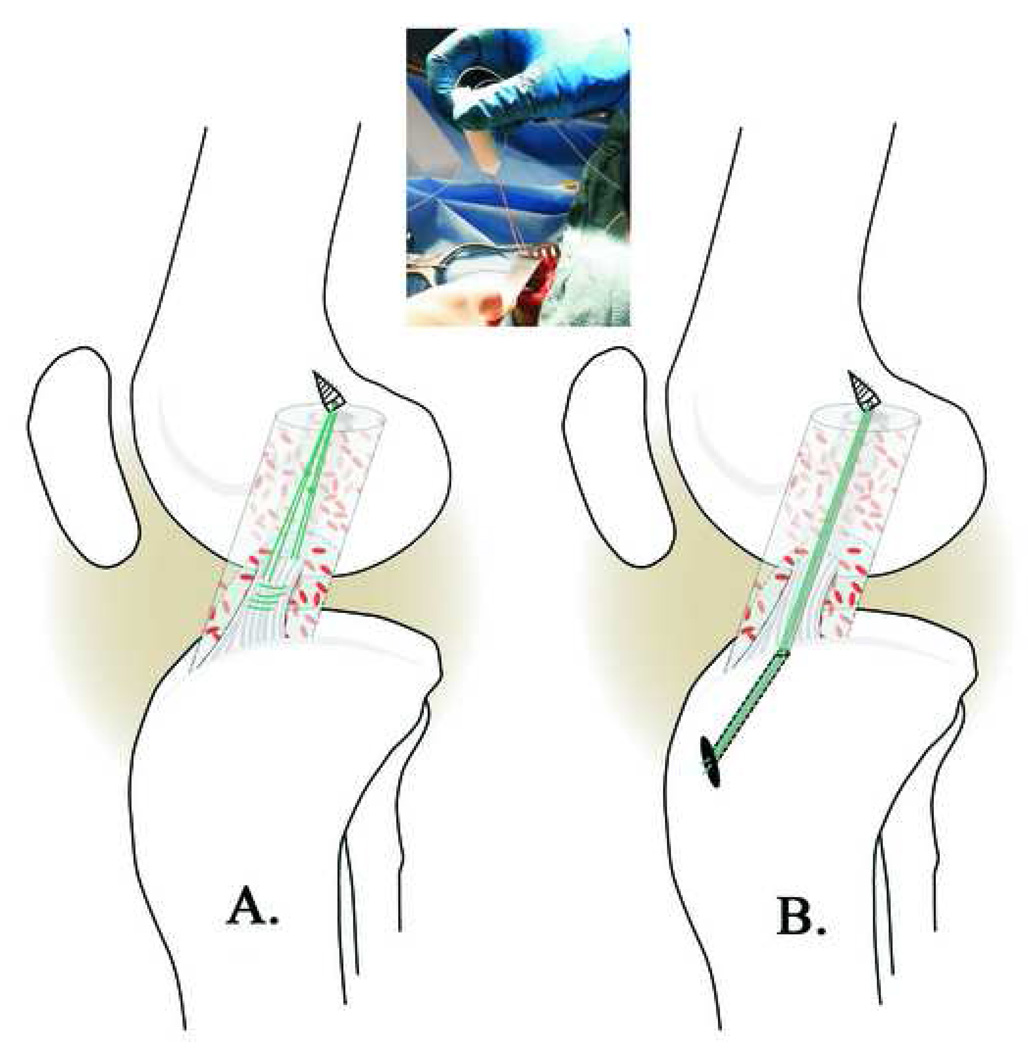
Prior to surgery but after anesthesia was induced, a clinical examination of the knee was performed. The pigs then underwent unilateral ACL transection as previously reported.10 In brief, the joint was opened through a parapatellar incision and the ACL was cut with a scalpel. Complete transection was verified using the Lachman test. All knees were irrigated with sterile saline to remove synovial fluid before suture anchor placement. An absorbable suture anchor (TwinFix AB 5.0 Suture Anchor with DuraBraid Suture (USP#2); Smith and Nephew, Inc, Andover MA) was placed at the back of the femoral notch within the footprint of the native ACL (Fig.1). A collagen sponge soaked in collagen-platelet-rich plasma solution (details below) was threaded onto the sutures attached to the femoral anchor and passed up into the notch. For the pigs in the LIGAMENT group, the two #1 Vicryl sutures (Ethicon Inc., Somerville, New Jersey) were woven through the distal ACL stump using a modified Kessler stitch. The sutures exiting the ligament were tied to those from the suture anchor to form a four-stranded repair (Fig 1a). For the pigs in the TIBIA group, a 2.4 mm guide pin was used to drill a tunnel from the anteromedial tibia to the middle of the ACL tibial footprint using a drill guide (Acufex Elbow Aimer, Smith-Nephew, Andover, MA). The nonabsorbable sutures from the suture anchor were brought down thru the tibia tunnel using a suture passer and tied over a Delrin button (Acufex Elbow Aimer, Smith-Nephew, Andover, MA) at the anteromedial tibial cortex (Fig 1b). The sutures were maximally tensioned with the knee in 60° flexion.13 The incisions for both repair strategies were closed in multiple layers with absorbable sutures. One intramuscular injection of buprenorphine (0.01mg/kg intramuscular) and a fentanyl patch (1–4ug/kg transdermal) provided post-operative analgesia. All animals were weight bearing on their hind limbs within 24 hours after surgery.
Fig. 1.
Schematic of the enhancement of the suture repair using the collagen-platelet composite (CPC) for the LIGAMENT group (A) and the TIBIA group (B). For the pigs in the LIGAMENT group, the two #1 Vicryl sutures (green) were secured in the distal ACL stump using a modified Kessler stitch. A suture anchor was placed into the bony femoral ACL insertion site. The collagen-platelet composite was threaded onto the sutures exiting the anchor and passed into the notch (Insert). The sutures from the anchor were then tied to the Vicryl sutures in the distal ACL stump with maximum manual tension. For the pigs in the TIBIA group, the femoral anchor and collagen-platelet scaffold were placed in the same manner, and after the collagen-platelet composite was threaded onto the femoral sutures, the sutures were passed through the through the tibia tunnel (B) and tied over a delrin button at the anteromedial tibial cortex with the knee in 60° of flexion
Physical Examination Assessments
At time of surgery and euthanasia, the knee was flexed and extended while the flexion and extension limits were measured with a goniometer. Blood was drawn into a 60 cc syringe containing 10% acid-citrate-dextrose at both time points for analysis. The anticoagulated blood samples were evaluated using a VetScan HM5 (Abaxis: Union City, CA) to determine the white blood cell (WBC) counts. Synovial fluid samples were also aspirated from the injured knee at the time of euthanasia to assess synovial white blood cell counts.
Qualitative MRI
After four weeks, the animals underwent another anesthesia and MRI evaluation was performed to qualitatively evaluate the integrity of the repair. At 15 weeks, the animals were euthanized using a pentobarbital solution (Fatal Plus; Vortech Pharmaceuticals, Dearborn MI). The knees were harvested and frozen at −20°C until mechanical testing.
Collagen-Platelet Composite
Bovine collagen was acid-solubilized as previously described,10, 11 neutralized to a pH of 7.4 and lyophilized in a mold resulting in a cylindrical collagen sponge approximately 11mm × 35mm. Additional collagen was acid-solubilized using the same technique and kept in liquid form at a concentration of 8 mg collagen per ml solution until surgery. At the time of surgery, 54 cc of blood was drawn into a syringe containing 6 cc of acid-citrate dextrose and processed into platelet-rich plasma using a commercially available system (SmartPReP, Harvest Technologies, Plymouth, MA). The SmartPReP system resulted in a platelet enrichment that ranged from 2.9X to 6.6X (average 4.3±0.9, mean±SD). At the time of gel placement, the acid-solubilized collagen that had been kept in liquid form was neutralized and mixed with the platelet-rich plasma in a 1:1 collagen to plasma ratio and the resultant collagen-platelet gel added to the suture repair (Fig. 1). Previous studies combining platelet rich plasma and collagen have demonstrated gelation of the combination within 10 minutes when exposed to body temperature (37°C).10, 14
Biomechanical Testing
The specimens were thawed to room temperature approximately 12 hours prior to testing. The muscle tissue was removed while leaving the joint capsule intact. Throughout preparation and testing, the specimens were kept moist with a wrapping of saline-soaked gauze. The long bones were transected below the hip trochanter and above the ankle joint and potted as previously described.13, 15
Mechanical testing consisted of cyclic anteroposterior (AP) laxity loading (total displacement between the AP shear load limits of ±30 N) with the knee flexed at 30°, 60° and 90°.13 Fully-reversed, sinusoidal loads were applied at 0.0833 Hz on an material testing system (MTS 810; MTS Systems Corporation; Eden Prairie MN). AP translations between the AP shear load limits of ±30 N were measured over 12 loading cycles.13 The first three cycles were performed to pre-condition the joint and the laxity values for the remaining cycles were averaged. During the AP laxity tests, axial rotation was locked in the neutral position, while the varus-valgus angulation and coronal plane translations were left unconstrained.13 Load and displacement data were acquired at 20 Hz.
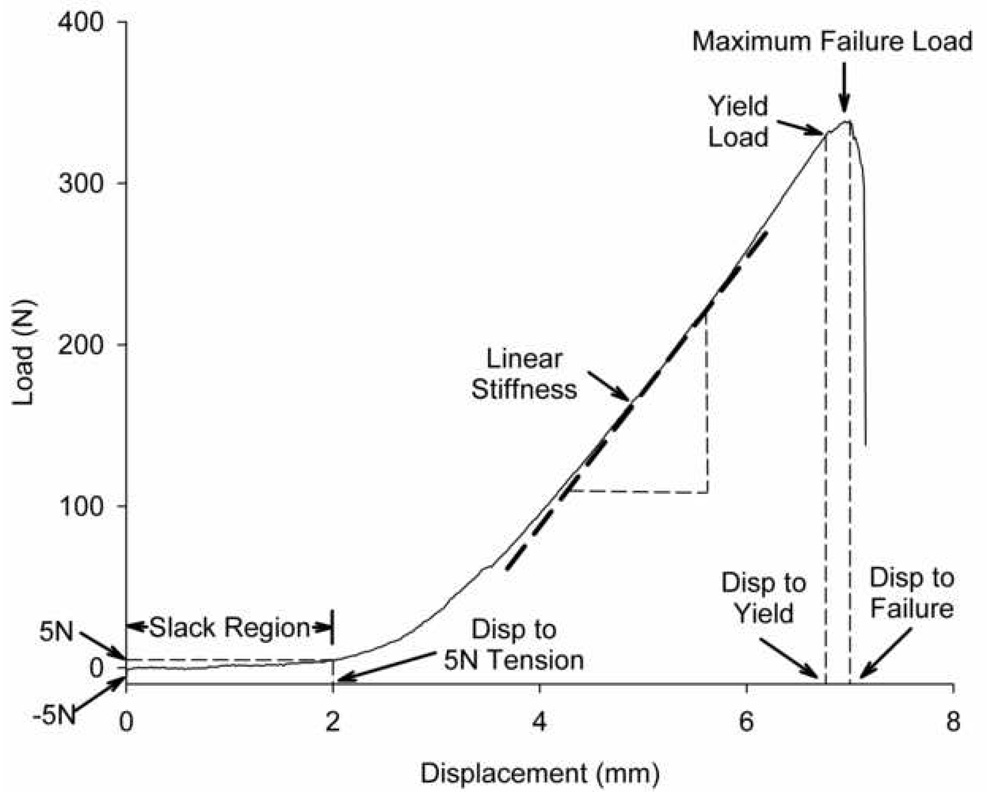
After completion of the AP laxity tests, tensile testing to failure at 20 mm/min was performed as previously reported.15–18 The joint capsule, menisci, collateral ligaments, and PCL were dissected from the joint leaving the ACL repair and integrated scar mass intact. Once the specimen was placed in the jig with the knee at 30° of flexion and the mechanical axis of the ligament oriented with the loading axis of the test system, a −5N compression force was applied to the tibiofemoral joint at which the displacement was zeroed. After testing the knee to failure, the load-displacement tracing of the failure test was used to determine the yield load, maximum failure load, linear stiffness, and energy to failure. Linear stiffness represented the slope of the load-displacement curve between the points corresponding to 20% and 80% of the yield load. Energy to failure was the area under the load displacement curve to maximum failure load. In addition, the amount of initial displacement that occurred from the reference −5 N compressive load to a 5 N tensile load was calculated and labeled as the “slack region” of the curve (Fig. 2).
Fig. 2.
Schematic of the load-displacement curve from the mechanical testing demonstrating the key mechanical parameters used to evaluate the function of the repair groups. The slack region is designated as the displacement from 5N of compression on the joint to 5N of tension. The linear stiffness is calculated as the slope of the load-displacement curve between the points corresponding to 20% and 80% of the yield load. Yield load is defined as the point where the load-displacement curve becomes non-linear and failure load is the maximum load supported by the sample before failure. Figure used with permission.23
Statistical Analysis
A mixed model analysis of variance (ANOVA) was used to compare the dependent variables between treatment groups (TIBIA vs LIGAMENT) and knees (REPAIRED vs INTACT). The animal was treated as an across subject factor while knee was treated as a within subjects factor when comparing the dependent variables.19 All statistical analyses were performed by the statistician (DZ) using the SPSS 16.0 software (SPSS Inc., Chicago, IL). The level of significance was set at a two-tailed α-level of p<0.05.
RESULTS
Animal Exclusions
Upon MRI inspection of all of the knees at 4 weeks, three of the suture anchors in the TIBIA group had pulled out of the femur. It was unknown when the sutures failed within the four week period. Two of these animals were euthanized at four weeks since it was thought that the ligament had failed. However, both of these knees were noted to have a healing ligament with no evidence of synovitis. The third animal was allowed to continue to the 15 week time point with careful monitoring for lameness. The integrity of the repairs looked intact in the remaining knees. Thus, only four animals remained for analysis in the TIBIA group at 15 weeks.
Physical Examination Parameters
After 15 weeks of healing, there was a significant loss of flexion in the group in which the repair fixation was to the tibial bone tunnel (TIBIA group 11.3±2.5°, LIGAMENT −2.5±5.2°; p=0.05; mean±SD), a difference that was also significant when compared to the INTACT knees (2.4±7.9°; p=0.03). There was no significant loss of extension in any of the groups, in fact, all groups gained extension during the period of the study (TIBIA 10.0±8.1°, LIGAMENT 5.0±6.3°, INTACT 5.8±8.9°; p>0.34 for all comparisons).
There was a significant increase in the systemic white count during the 15 week period for the LIGAMENT group (3.8±3.2K/ml) when compared with the TIBIA group (−1.5±0.7K/ml; p=0.02). However, there was no significant change in the synovial fluid white count in either of the repair groups in comparison with the INTACT knees (0±0 in LIGAMENT, 0.0± 0.0 in TIBIA and 0.5±0.8 INTACT; p>0.40 for all comparisons). There was also no significant difference in weight gain between groups (28.5±4.4 kg TIBIA group and 23.3±5.4 kg LIGAMENT, p=0.46).
Gross Appearance
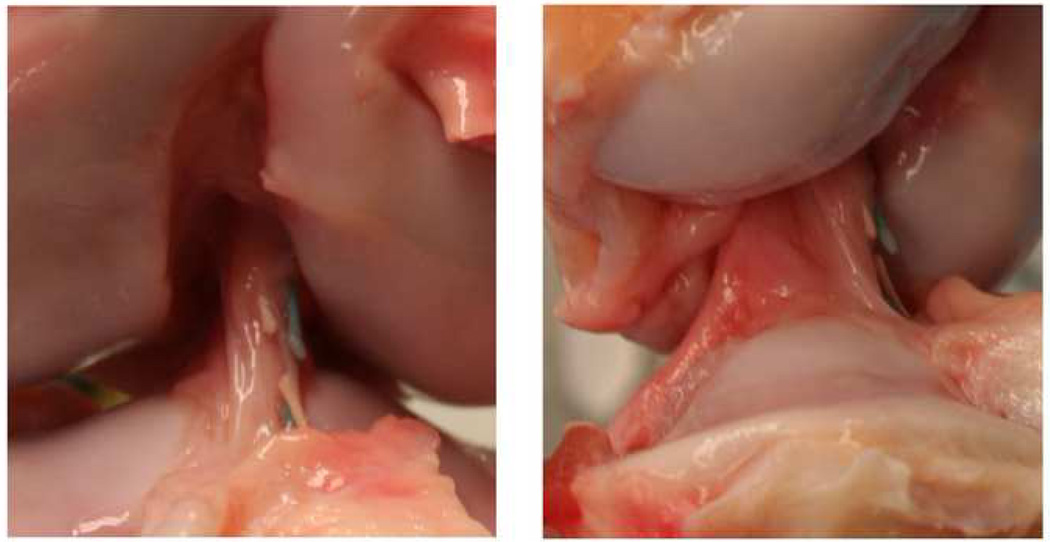
After fifteen weeks of healing, there was no evidence of the repair sutures seen in the knees or ligament stump of the LIGAMENT group. For the repairs in the TIBIA group, some of the sutures were still intact (Fig. 3). The anchor had pulled out of the femur as noted at the four week MRI so there was no suture bridging the knee in one of the four knees. In a second knee, all four suture limbs had broken intra-articularly. In the remaining two knees, two of the four suture limbs were still intact. For all knees in the TIBIA and LIGAMENT groups, there were no gross cartilage defects noted on inspection of the joint surfaces. There was no evidence of infection or significant synovitis on evaluation of the joint fluid, synovium or capsule.
Fig. 3.
Shown is an anterior (left) and sagittal (right) view of a enhanced ACL repair from the TIBIA group after 15 weeks of healing.
AP Laxity
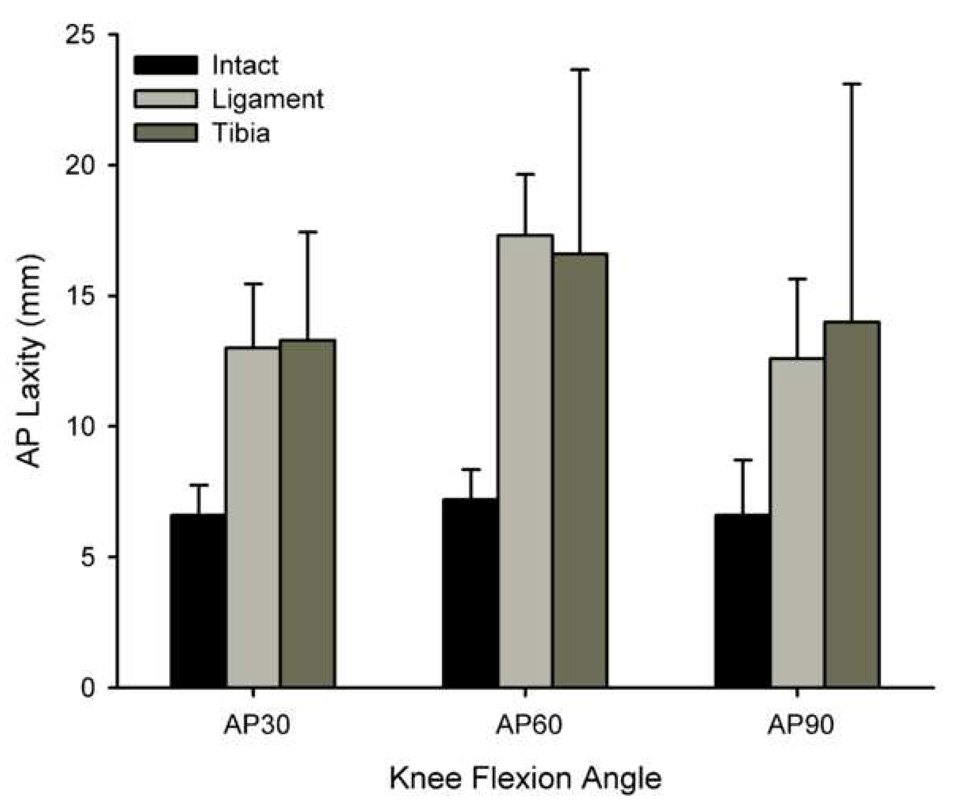
The AP laxity values of the knees (repair – intact laxity values) were similar in both groups of repaired knees at all angles of testing (Table 1). The AP laxity values of all of the treated knees (LIGAMENT and TIBIA) were significantly greater than the contralateral controls (INTACT) at fifteen weeks (p<0.0001) (Table 1). The increase in laxity was similar when the suture repair was performed to the tibial ACL stump (LIGAMENT) or to bone fixation (TIBIA) when tested at 30° (p<0.001 for INTACT vs all other groups), 60° (p<0.001 for INTACT vs all other groups) and 90° (p<0.001 for INTACT vs all other groups) of knee flexion (Fig. 4).
Table 1.
Mechanical data after 15 weeks of healing.
| Normalized Laxity Data (Experimental – Intact) | ||
|---|---|---|
| Outcome | Tibia, mm | Ligament, mm |
| AP Laxity at 30° | 6.8 (0.7–12.8) | 6.8 (4.7–8.9) |
| AP Laxity at 60° | 10.8 (2.4–19.2) | 10.3 (7.1–13.4) |
| AP Laxity at 90° | 9.9 (0.0–21.3) | 6.2 (2.7–9.7) |
| Normalized Failure Data (Experimental/Intact) | ||
| Outcome | Tibia, % | Ligament, % |
| Displ to 5N | 327 (0–1022) | 596 (0–1275) |
| Yield Displ from 5N | 87 (45–129) | 107 (70–143) |
| Max Displ from 5N | 56 (25–87)* | 68 (46–89) |
| Yield Load | 39 (0–96)† | 10 (5–14)* |
| Max Load | 26 (0–62) | 7 (4–9)* |
| Stiffness | 39 (0–90)† | 7 (4–11)* |
| Energy | 11 (0–30) | 4 (2–6)* |
Data represent mean differences (95% confidence intervals).
No significant laxity differences between tibia or ligament groups, all p > 0.10, ANOVA
TIBIA or LIGAMENT repair significantly lower than INTACT, p < 0.05
TIBIA significantly higher than LIGAMENT repair, p < 0.05
Fig. 4.
Bar graph of AP laxity at 30°, 60° and 90° of flexion for both types of repair (LIGAMENT and TIBIA) and the INTACT group. The AP laxities of the repaired knees were similar in both groups (Table 1). However, the AP laxities of all of the treated knees (LIGAMENT and TIBIA) were significantly greater than their intact contralateral controls at fifteen weeks (p<0.0001 for all angles).
Tensile Properties
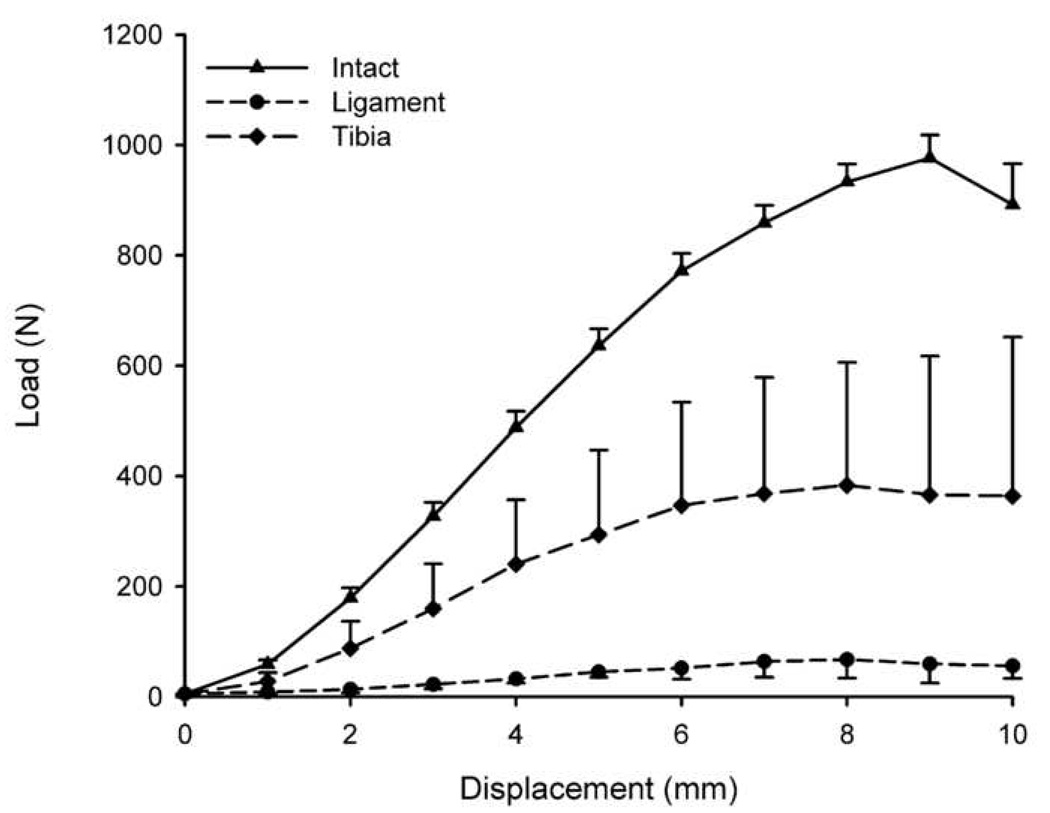
The yield load was significantly higher in the TIBIA group when compared to that of the LIGAMENT group (267±243 N vs 60±34 N; mean ± SD; p =0.01); however, both repair groups were significantly lower than the values of the INTACT knees (708±107 N; p < 0.0001). The normalized yield load (experimental/intact) was also significantly higher in the TIBIA group when compared with the LIGAMENT group (Table 1, p < 0.05). The maximum failure load showed similar findings, with four times higher average loads sustained by the TIBIA group when compared with the LIGAMENT group (342±310 N vs 84±36N; p=0.03); however, the normalized values were not significantly different (Table 1, p > 0.05). The maximum failure load of the INTACT ACL was 1120±264 N.
When the displacement to yield and displacement to maximum load starting at 5N of tension, there was no significant difference between the two groups (p=0.468 and p=0.747 for displacement to yield and displacement to maximum load, respectively; Fig. 5). There was no significant difference in the length of the “slack region” (from −5N of compression to 5N of tension) between the two groups (p=0.382).
Fig. 5.
Mean load-displacement curves for TIBIA, LIGAMENT and INTACT groups where the curve starts at 5N of tension showing the highest linear stiffness and yield load in the INTACT group, with the enhanced repair group with bony fixation (TIBIA) comparing favorably to the LIGAMENT group in terms of replicating the INTACT ligament behavior at low load conditions. Error bars represent one standard error. For statistical analyses, the failure properties were normalized to the contralateral INTACT knee (Table 1).
The ACL stiffness of the TIBIA group was significantly greater than that of the LIGAMENT group (66±53 N/mm vs 12.1±4.5 N/mm; p=0.001). This was also true for the normalized values (Table 1, p < 0.05). Both groups were significantly lower than the INTACT group (163±20 N/mm; p<0.0001 for all comparisons).
DISCUSSION
Enhanced suture repair of the ACL using a collagen-platelet composite has been previously shown to stimulate the healing response of the torn ligament in an animal model.10, 12 In this study, we assessed whether providing initial protection to the healing ligament using the bone-to-bone suture technique would further improve healing. We found that the bone-to-bone method improved the structural properties of the ligament following enhanced ACL repair when compared to traditional suturing to the tibial stump of the ACL (Marshall procedure) after 15 weeks of healing. These data suggest that the concept of providing some protection to the healing ligament using a temporary stint during the initial healing stage may be beneficial. Although significant improvements in graft structural properties were measured, there was no detectable change in the AP laxity of the joint. Thus, long-term translational studies are needed to establish if these initial improvements will affect outcome.
Using the porcine model, we have previously shown that enhanced suture repair with a collagen-platelet composite increases the structural properties of the ligament when compared to traditional suture repair without the collagen-platelet composite after three months of healing.12 When using a collagen-platelet composite to enhance the repair, there was a 59% and 221% improvement in the yield load (126 vs 79 N) and linear stiffness (40.2 vs 12.5 N/mm), respectively.12 The LIGAMENT suture technique, as described in the present study, was used for both the enhanced and traditional ACL repair groups in the previous study.12 When using the TIBIA suture technique, further improvements in the yield load and stiffness were obtained (267 N and 66 N/mm, respectively).
We have also used the porcine model to assess healing following ACL reconstruction with a bone-patellar tendon-bone allograft in age, species and gender matched animals.16 The yield load and stiffness of the reconstruction after 3 months of healing were 279 N and 48.2 N/mm, respectively.16 For the enhanced ACL repairs using the TIBIA suturing technique, these values are comparable to those following ACL reconstruction. Thus, it would appear that if early temporary bony stabilization of the knee is accomplished with a suture technique, improved functional healing may progress to levels that approximate that of ACL reconstruction with bone-patellar tendon-bone allograft.
The failure load of the healing ACL with the collagen-platelet composite when sutures were placed in the tibial stump (LIGAMENT group) was improved from those reported for traditional suture repair in animal models.2–4 With complete transection and no suture repair, it has been shown there was no connection between the two ligament stumps, and no strength returned to the ACL in the rabbit and canine models.1, 4 Although an ACL transected group was not included in the present study, it is likely that the same response would occur in the porcine model given the limited improvement seen in unenhanced repair.12 In long-term canine studies, the strength of a repaired ACL was noted to be less than 25% of the intact control ACL from 1 to 5 years.2, 3 In contrast, the maximum loads of the repairs enhanced with collagen-platelet composite reported here were over 30% of the intact ACL at 3 months from surgery, a significant improvement over the “unenhanced repairs” previously reported.
The optimal surgical technique for enhanced repair is not yet known. Our recent ex vivo work of traditional suture repair to the tibial stump (i.e. LIGAMENT group) (methods previously used in both clinical and animal studies) resulted in knees with over 5 mm greater AP laxity when compared to the ACL-intact knee at the time of surgery whereas those with direct suturing between the femur and tibia restored normal AP laxity.13 However, it remained unknown if these ex vivo results would lead to improved biomechanical healing in-vivo. It seems reasonable to assume that an ACL suture repair technique that restores laxity at the time of surgery would be optimal for healing.
Although the structural properties were different between the two groups, AP laxity values were not. For example, the mean difference in AP laxity between the repaired and intact knees was 10.8 and 10.3 mm for the TIBIA and LIGAMENT groups, respectively, with the porcine knee at 60° of flexion (which is equivalent to 30° of flexion in the human). These differences are also similar to the 10.6 mm difference between the reconstructed and intact knee 15 weeks after ACL reconstruction in the same model.16 It should be noted that the AP laxity values of the ACL deficient porcine knee are greater than 25 mm.16 Thus the repaired ACL does restrain some of the anterior shear applied to the tibia. The large magnitudes of AP laxity following ACL repair are similar to that of other animal models of ACL reconstruction, including the goat.15
The primary mode of suture fixation failure in the three animals assigned to the TIBIA group was pull out of the suture anchors within the first four weeks post-operatively. Unfortunately, two of them were euthanized because we thought the repairs had failed. Upon dissection, it appeared that these ligaments were healing well, thus the third animal was not euthanized. We also inspected the integrity of the sutures used to repair the ACL in both groups of animals at time of harvest. None of the absorbable sutures were present in the LIGAMENT group, and only two of the animals in the TIBIA had partially intact non-absorbable sutures after fifteen weeks of healing. It should be noted that these were released prior to biomechanical testing. It is unknown exactly when or how the sutures failed, but these data suggest that the concept of temporary bony fixation is feasible and effective.
Absorbable sutures were selected for the repairs performed on the animals in the LIGAMENT while non-absorbable sutures were utilized for those in the TIBIA group. Typically absorbable sutures would be used for a traditional repair since they are secured in the soft tissue of the ligament. Non-absorbable sutures for the TIBIA group were selected in hopes that they would remain intact for the 15 week duration of the study. Although this proved not to be the case, they remained intact long enough to improve the healing response of the ACL for the animals in the TIBIA group. Further work will be necessary to determine the optimum suture material, design and the time joint support will be required to prevent failure.
The porcine model was chosen because of its size, because it is anatomically equivalent to other large animal models of ACL reconstruction:20 it is similar knee anatomy to humans, it is functionally dependent on the ACL,21 and because it has baseline coagulation values and platelet characteristics similar to those in humans.22 The latter feature is particularly important when looking at treatments involving platelets and wound healing. Therefore, we feel that the porcine model is superior to other animal models for this study. We have successfully used the porcine model to show that the addition of a collagen-platelet composite to a suture repair of the ACL improves its healing capacity,10, 12 while treatment with either the platelets or collagen sponge alone does not.17, 23 We have also used it to evaluate graft healing following ACL reconstruction.16 Thus, the porcine model represents a translational model of ACL surgery.
One of the weaknesses of the porcine model is the rapid growth of the animals during the healing period. During the three months of this study, the pigs doubled in size, and the maximum failure load of the ACL almost tripled. The effects of rapid growth on the repair strength and knee laxity are unknown. It is also possible that the rapid growth rate could distract the joint or transected ACL during healing damaging the sutures of both treatment groups over a relatively short time frame. Nonetheless, a difference was detected between treatments. For this study the contralateral knee was used as a control to normalize the structural properties and joint laxity and account for changes due to growth. Future studies in adolescent or adult animals using a minipig strain may help shed light on the relative contributions of growth and time on suture repair strength.
The porcine model has other weaknesses that are common to all large animal studies in quadrupeds – predominantly that these animals weight bear on four limbs, there is minimal ability to control the rehabilitation after surgery, and that there are certainly likely to be subtle differences in the wound healing cascade that are not yet appreciated. These factors may explain why all animal models of ACL reconstruction show large increases in post-operative laxity. These are weaknesses that must be considered when interpreting the results of this study.
In this study, both experimental groups experienced loss of flexion. To determine the etiology of this change, the 15 week MR images were examined for evidence of physeal arrest, including narrowing or localized closure. There was no gross evidence of physeal closure or sagittal dimension deformity in the distal femur; however, it is possible that subtle deformities were developing that we were unable to appreciate with this imaging modality. We also did not notice any cyclops lesion formation at the 15 week time point, rather, the ACL scar was remodeled and no extension block due to scar formation was noted either clinically or on gross examination of the retrieved joints. Interestingly, thigh girth measured at 5 cm above the superior pole of the patella increased 25% (from approximately 20 inches to 25 inches) during the period of the study, likely due to the rapid growth of the animals. It is possible that this increased soft tissue in the thigh contributed to the decrease in maximum flexion noted at the 15 week time point.
When dissecting the knee for tensile testing, it was not possible to separate the scar mass from the ACL because the two are integrated. It may be possible that the scar tissue could influence the mechanical testing results but it is part of the repaired ACL that will support the loads applied to the joint. Long-term studies are needed to see how the structural properties changes with the amount of scar tissue that is present.
A limitation of the study was the relatively small sample size. We intended to have six animals in each group but due to failure of some of the sutures within four weeks of surgery, two of the animals in the TIBIA group were euthanized. We found that the AP laxity were comparable after 15 weeks of healing between the two treatment groups. A power analysis suggests that laxity results would not have reached statistical significance by increasing the original sample size three-fold. Given the relatively small difference in mean values, running additional animals would be hard to justify. On the other hand, differences in structural properties (i.e. yield load, linear stiffness) were significantly improved in the TIBIA group compared to the LIGAMENT group even with the small sample size.
CONCLUSION
Using an enhanced ACL suture repair technique that includes bone-to-bone fixation to protect the repair in the initial healing stages resulted in an ACL with improved structural properties after 15 weeks in the porcine model.
Acknowledgements
This study was supported by grants from the National Institutes of Health R01 AR054099 (MMM), K02 AR049346 (MMM) and RO1-AR049199 (BCF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hefti FL, Kress I, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73:373–383. [PubMed] [Google Scholar]
- 2.Cabaud HE, Rodkey WG, Feagin JA. Experimental studies of acute anterior cruciate ligament injury and repair. Am J Sports Med. 1979;7:18–22. doi: 10.1177/036354657900700105. [DOI] [PubMed] [Google Scholar]
- 3.O'Donoghue DH, Frank GR, Jeter GL, Johnson W, Zeiders JW, Kenyon R. Repair and reconstruction of the anterior cruciate ligament in dogs. Factors influencing long-term results. J Bone Joint Surg Am. 1971;53:710–718. [PubMed] [Google Scholar]
- 4.O'Donoghue DH, Rockwood C, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48:503–519. [PubMed] [Google Scholar]
- 5.Feagin JA, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am. 1987;69:1120–1126. [PubMed] [Google Scholar]
- 7.Engebretsen L, Benum P, Fasting O, Molster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18:585–590. doi: 10.1177/036354659001800605. [DOI] [PubMed] [Google Scholar]
- 8.Grontvedt T, Engebretsen L, Benum P, Fasting O, Molster A, Strand T. A prospective, randomized study of three operations for acute rupture of the anterior cruciate ligament. Five-year follow-up of one hundred and thirty-one patients. J Bone Joint Surg Am. 1996;78:159–168. doi: 10.2106/00004623-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 11.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 12.Joshi S, Mastrangelo A, Magarian E, Fleming BC, Murray MM. Collagen-Platelet Composite Enhances Biomechanical and Histologic Healing of the Porcine ACL. Am J Sports Med. 2009;37:2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anterioposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray MM, Forsythe B, Chen F, et al. The effect of thrombin on ACL fibroblast interactions with collagen hydrogels. J Orthop Res. 2006;24:508–515. doi: 10.1002/jor.20054. [DOI] [PubMed] [Google Scholar]
- 15.Spindler KP, Murray MM, Carey JL, Zurakowski D, Fleming BC. The Use of Platelets to Affect Functional Healing of an Anterior Cruciate Ligament (ACL) Autograft in a Caprine ACL Reconstruction Model. J Orthop Res. 2009;27:631–638. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: An in vivo study. J Orthop Res. 2009;27:639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effect of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 19.Vittinghoff E, Glidden D, Shiboski S, McCulloch C, editors. Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York: Springer; 2005. Regression Methods. [Google Scholar]
- 20.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Engin. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]
- 21.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Effect of anterior translation on total knee force in a porcine model; International Symposium of Ligament and Tendon VIII; San Francisco: 2008. [Google Scholar]
- 22.Mueller XM, Tevaearai HT, Jegger D, Tucker O, von Segesser LK. Are standard human coagulation tests suitable in pigs and calves during extracorporeal circulation? Artif Organs. 2001;25:579–584. doi: 10.1046/j.1525-1594.2001.025007579.x. [DOI] [PubMed] [Google Scholar]
- 23.Fleming BC, Magarian EM, Harrison SL, Paller DJ, Murray MM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. Journal of Orthopaedic Research. 2009 doi: 10.1002/jor.21071. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]