Abstract
Costimulation signals have been recognized as critical for optimal T cell responses and result from important interaction between receptors on the surface of T cells and their ligands on antigen presenting cells. Two families of receptors, the CD28 family and the TNFR family have been found to be major players in providing costimulation to CD8+ T cells. Recent studies using viral infection models have highlighted the importance of CD28 costimulation signals during memory responses against viruses. PD-1 another member of the CD28 family may contribute to functional defects of helpless memory CD8+ T cells. Members of the TNFR family such as CD27, 4-1BB, CD40, TRAIL and OX40 have also being implicated in the survival, generation, maintenance and quality of virus-specific memory CD8+ T cells. The delivery of costimulatory molecules such as CD28, 4-1BB and OX40 can help boost the generation and function of virus-specific memory CD8+ T cells. Taken together this suggests that the use of costimulatory molecules as adjuvants along with viral antigens in vaccines may facilitate the generation of effective antigen-specific memory CD8+ T cell responses. Understanding the costimulatory requirements of memory CD8+ T cells therefore may lead to improved vaccines that target anti-viral CD8+ T cell memory.
Keywords: CD28, TNFR, 4-1BB, CD27, OX40, CD40, memory T cell, costimulation
INTRODUCTION
The CD8+ T cell response to a viral infection is characterized by the detection and destruction of virally infected cells and it is mediated by the production of secretory molecules like perforin and granzyme B and cytokines such as IFNγ.1 The clearance of virus is followed by the apoptosis of the vast majority of virus-specific CD8+ T cells, but a small pool of memory virus-specific CD8+ T cells is retained for protection against re-infection.2 Understanding the mechanisms behind the generation and maintenance of function and number of antigen-specific memory CD8+ T cells are of great importance in the design of effective vaccines. Recent experimental studies have shown that costimulatory molecules are important for the generation, maintenance and function of memory CD8+ T cells and in this review we will examine the literature on costimulatory molecules in memory CD8+ T cells responses against viruses.
I. MAJOR COSTIMULATION FAMILIES
A. Early studies in building the concept of costimulation
The idea of the requirement for two signals for the activation of an immune cell was hypothesized based on the observation that B cells may or may not produce antibodies in response to an antigenic stimulus.3 Bretscher and Cohn provided an explanation for this observation by suggesting that the receptors on the surface of an immune cell must interact with more than one antigenic determinant on the surface of the antigen, in order to trigger antibody production.3 Lafferty and his collaborators further hypothesized that a second signal or costimulation,4 apart from that delivered by antigen is required to trigger an allogenic stimulus, and stated that this second signal involved cells of the haematopoietic system.5 Since the initial proposal of the second signal a plethora of costimulatory molecules have been discovered which are stimulatory or inhibitory in their action. Most costimulatory molecules can now be broadly classified into two families, the CD28 family and the TNFR family members.
1. The CD28 Family
Members of the CD28 family are characterized by a variable Ig like extracellular domain and a short cytoplasmic tail. These costimulatory molecules CD28, CTLA-4, ICOS, PD-16 and BTLA7, interact with their respective ligands on APC surface as follows CD28:B7-1 or B7-2, CTLA-4:B7-1 or B7-2,8 ICOS:B7h,9,10 PD-1:B7-H1 or B7-DC11,12 and BTLA:HVEM.7 Two additional molecules: B7-H313 and B7-H414–16 (also known as B7S1 or B7x) belong to the CD28 family. CTLA-4 and ICOS are structural homologs of CD28, yet they function differently from CD28 upon stimulation.17 CTLA-4 competes with CD28 for binding to B7-1 and B7-2 ligands, and it is not expressed on resting or newly activated T cells. Instead, CTLA-4 is expressed by fully activated T cells.18 The affinity of CTLA-4 for B7-1 and B7-2 was estimated to be 10–20 times greater than the affinity of CD28 for the same ligands.19 Binding of CTLA-4 by B7-1 or B7-2 inhibits T cell proliferation20 by disruption of lipid rafts21 and interruption of TCR signaling.22 The inducible costimulator ICOS has a unique ligand, B7-h.23 Signaling through ICOS augments many cellular functions such as proliferation, antibody response and cytokine production.24,25 In vivo studies with viral infections have shown an important role for ICOS signaling for the development of antibody responses and the maintenance of primary CD8+ T cells during LCMV, VSV and Influenza virus.26
More recently, two other members of the CD28 family, Programmed Death-1 and the B and T cell lymphocyte attenuator have been identified and they have demonstrated inhibitory activity. PD-1 has at least two known ligands B7-H1 and B7-DC, and it is expressed on both T and B cells.27 Signaling through PD-1 has been shown to be involved in peripheral tolerance28 and in the regulation of anti-viral CD8+ T cell responses during viral infection.29–32 BTLA has not been extensively characterized yet.33 BTLA however is constitutively expressed at low levels on T cells and can be up-regulated on activated B and T cells.34,35 It has been suggested that BTLA plays an inhibitory role in the development of adaptive immune responses by inhibiting CTL maturation and memory generation36. Two other potential ligands B7-H313 and B7-H4,15 are also believed to interact with the members of the CD28 family, however the identity of their respective receptors is still unknown. B7-H3 stimulates proliferation of CD8+ and CD4+ T cells, enhances the induction of cytotoxic T cells and selectively stimulates IFNγ production in the presence of T cell receptor signaling. In contrast, B7-H4 has an inhibitory effect of T cell stimulation.15,16
2. TNFR Family
Members of the TNF/TNFR super family are type I transmembrane proteins and they all present extracellular domains rich in six cysteine repeats that form disulphide bridges.37 They differ from the CD28 family members by displaying a more complex cytoplasmic tail.38 Members of the TNF/TNFR family can be subdivided into three groups; those containing cytoplasmic death domains, those lacking a death domain but containing decoy receptors and those which lack a death domain but contain a TRAF motif.39 These receptors may be monomers or they may form oligomers but they always form trimeric complexes during a signaling event.40 The ligands for the TNFR family members are trimeric type II transmembrane proteins that carry a TNF homology domain (THD) in the carboxyl terminus which interacts with the receptors.37,41 The members of the TNFR family are expressed on T cells, and they are constitutively present in lower concentrations on naive T cell, but are upregulated upon activation of the T cell as it is seen in the case of OX40, 4-IBB and CD30.42,43
The major receptor/ligand pairs of costimulatory molecules that belong to the TNF/TNFR family are: OX-40:OX40L, CD27:CD70, 4-1BB:4-1BBL, CD30:CD30L, GITR:GITRL and HVEM:Light.44,45 The ligands for these molecules may also be expressed on T cells, suggesting that these molecules may mediate interaction between T cells.40 The members of this family are differentially expressed at different stages of the T cell activation during an immune response. The observation that the ligand for HVEM-LIGHT is expressed on immature dendritic cells suggest that certain molecules of the TNFR family may have a role during the initial activation stage of the T cell,40 whereas other members become more important during the later stages of the T cell activation cycle,46–48 thus helping to shape effector and memory T cell responses. Recent studies have shown that the initial CD28-B7-2 or B7-1 interaction may enhance the expression profile and the subsequent interaction between TNF/TNFR receptors and their ligands suggests an intricate interplay between these two families of costimulatory molecules.49,50
The role played by the TNF/TNFR costimulatory molecules and their ligands during the immune response has been addressed in many studies. HVEM-LIGHT interact at an early stage of T cell activation and induce clonal expansion.40 Studies have shown that the absence of LIGHT on splenocytes led to a reduced cytokine production and CTL activity.51 CD27–CD70 ligation leads to a surge in the initial proliferation of activated T cells by regulating the factors that cause cell death and by affecting the cell cycle to enhance continuous cell division.52–54 Studies with CD27 deficient mice have shown that upon infection, the total number of antigen-specific CD4+ and CD8+ T cell effector pool is low and this in turn translates into a much reduced memory response.53 Studies to date suggest that CD28 signals are important for the initial T cell response during a primary stage of infection, while OX40 and 4-1BB gain importance during the late effector and memory stage of antigen-specific T cells.26,55 Mice deficient in OX40 and OX40L have low number of effector CD4+ T cells during a primary response to a viral infection.56,57 CD8+ T cells that lack OX40 divide normally during the initial antigen encounter but the effector T cells do not expand very well 1–6 days post infection.58 Similarly in the absence of 4-1BBL, the memory CD8+ T cell response is greatly reduced,59–61 as these memory cells undergo apoptosis. CD30 has been shown in vitro to have a costimulatory function.62,63 CD30 may also provide pro-survival signals for effector T cells during the peak of infection in a primary response.64 Therefore an optimal immune response to pathogens is the result of many receptor-ligand interactions, some of them having a stimulatory effect that enhances the immune response, others with an inhibitory function. These signals may dominate different phases of the immune response (early versus late) to ensure optimal expansion and contraction of primary CD8+ T cells and the generation of memory CD8+ T cells.
II. COSTIMULATION AND THE CD8+ T CELL RESPONSES IN VIRAL INFECTIONS
During antiviral CD8+ T cell responses costimulatory signals from the CD28 and TNF family affect different phases of the immune response. It has now become apparent that different members of these families play important roles in the initiation phase, the generation and maintenance of memory, quality of memory and the secondary response.
A. Role of CD28-B7-1/B7-2 interactions during primary CD8+ T cell immune responses to viruses
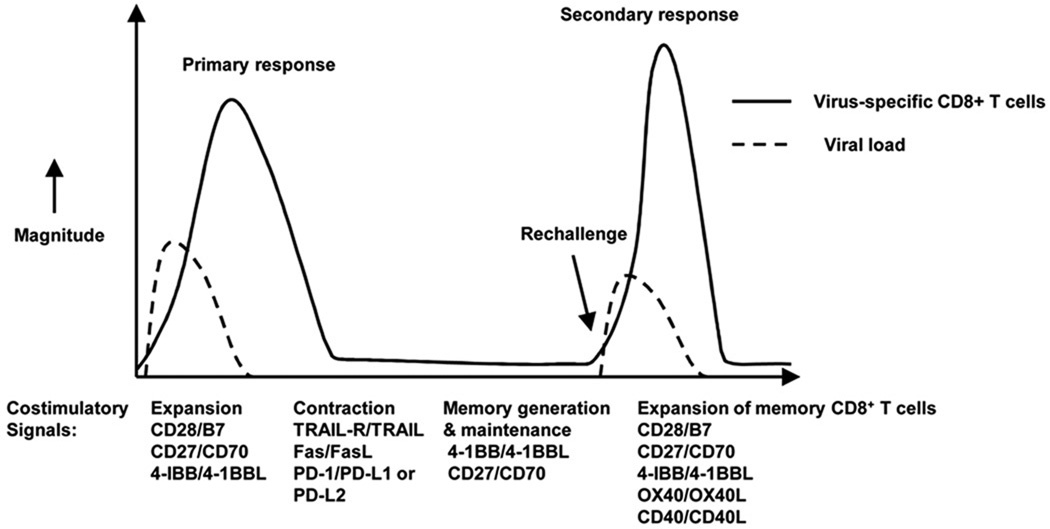
CD8+ T cell responses are indispensable for clearance of viral infections.65,66 Dendritic cells initiate an immune response by presenting viral antigens to CD8+ T cells in the local draining lymph nodes.66 Costimulatory signals delivered by molecules such as CD28 as shown in Figure. 1 will determine whether CD8+ T cells will become activated and expand, or they will be suboptimally activated. In studies regarding costimulation blockade, Lumsden et al. showed that the absence of CD28 signaling negatively impacted both CD4+ and CD8+ T cells.67 Studies in vivo examining infection of mice with viruses such as VSV,68 MHV69 and Influenza type A virus,70,71 indicated that CD28 was required for expansion of antiviral CD8+ T cells during primary immune responses. Lack of CD28 costimulation was also shown to reduce the immune response to subdominant epitopes of Influenza virus.71 In contrast, infection of mice with lymphochoriomeningitis virus (LCMV) showed that, even in the absence of CD28 costimulation, an effective CD8+ T cell response can develop.72,73 CD28 knockout mice (CD28−/−) were infected with LCMV, and despite the absence of CD28 signaling, virus-specific CD8+ T cells expanded and viral burden was eliminated at levels comparable to wild type controls. Similar CD8+ T cell expansion was observed against all measured epitopes of LCMV, including subdominant epitopes.72,73 The reason for the discrepancy in costimulation requirements observed with most virus infections versus LCMV came from studies that showed that under high levels of TCR stimulation, the need for costimulation can be overcome.74,75 Kundig et al. demonstrated that the disparity in requirement for CD28 in primary LCMV infection versus VSV infection was due to differences in TCR signal duration.74 LCMV replicates much more rapidly and extensively than other viruses and therefore antigen presentation persists for a longer period of time and at higher levels, providing a strong and sustained TCR signal which overcomes the need for CD28 costimulation.74 Clearly for most viruses CD28 signaling is indispensable for the optimal primary CD8+ T cell response. The question arises as to whether other phases of the immune response also require such signals.
Figure 1. Involvement of costimulatory molecules during the primary and memory phases of an immune response.
This figure shows the different phases of the anti-viral immune response in which costimulatory molecules play a role. Costimulatory molecules can enhance the generation, maintenance and quality of memory virus-specific CD8+ T cells. They can also affect the re-activation of memory CD8+ T cells and the resulting secondary response.
B. CD28 and B7 interaction and its effect of CD8 memory T cells
After pathogen clearance effector T cells undergo contraction and a memory T cell pool is formed. These memory cells respond faster and more effectively in the event of secondary insult to the host76 because of their higher precursor frequency and higher affinity for antigen that leads to a lower threshold of activation.77,78 As the requirement for costimulation affects the strength of TCR signaling and the threshold of activation it is important to determine whether or not memory T cells have a requirement for CD28 costimulation during re-activation. Early studies relied on in vitro experiments to address this. More recently however, the availability of new reagents and of genetically modified mice have allowed the direct assessment in vivo of the requirement of CD28 signaling by memory CD8+ T cells as shown in Figure 1.
1. Early in vitro studies on memory CD8+ T cell dependence on costimulation
Memory CD8+ T cell immune responses have been considered for a number of years not to require costimulation based on several studies that used in vitro systems of restimulation79,80 or CD28 deficient mice.73,81 In one of the earliest experiments81 OVA-specific OT-I TCR transgenic CD8+ T cells were transferred into wild type C57Bl/6 mice which were subsequently infected with VSV-OVA. Rechallenge of these mice with VSV-OVA was done in the presence of CTLA4-Ig in order to block costimulation signals. Memory cells rechallenged with VSV-OVA presented similar size increase (blastogenesis) and ex-vivo cytolytic activity, irrespective of costimulation blockade. The conclusion was drawn that memory CD8+ T cell responses occurred independently of CD28 signaling. However, blastogenesis of memory cells may not be sufficient to evaluate memory CD8+ T cell function, as we have determined that in the absence of CD28 signaling functionally impaired CD8+ T cell memory showed normal blastogenesis but their cell cycle is arrested.82
Memory LCMV-specific CD8+ T cell responses in CD28 deficient mice also seem to be reactivated independently of CD28 costimulation.73 In this study, Suresh et al.73 showed that in LCMV infected CD28−/− mice, the primary CD8+ T cell responses exhibited a strong activation profile and primary expansion. The memory CD8+ T cell pool was reduced in CD28 deficient mice compared to control mice, yet it represented a sizeable population and exhibited cytotoxic functions. The functionality of the memory CD8+ T cells was tested by rechallenging wild type or CD28−/− mice with a lethal dose of LCMV and in fact, all mice survived infection while all naive controls in the study died. The interpretation of these results was that, during LCMV infection, memory CD8+ T cell cells, just like naïve CD8+ T cells are capable of functioning independently of CD28 costimulation. However as mentioned above, the high levels of TCR signaling induced by LCMV infection may override the requirement for CD28 costimulation by memory CD8+ T cells.74,75 Thus these early studies indicated that memory CD8+ T cells were independent or less dependent on CD28 costimulation compared to naive CD8+ T cells. Recent in vivo studies however, have challenged this notion by demonstrating that memory CD8+ T cells need both APC and CD28 costimulation.
Two recent studies have indicated the in vivo requirement for dendritic cells during secondary immune responses to VSV and LCMV in mice.66,83 The expansion of rechallenged memory VSV-specific CD8+ T cell populations was reduced when CD11c+ cells were depleted.83 This suggested that during rechallenge, reactivation of memory cells is dependent on the presence of dendritic cells (DC).
DC can provide a number of signals to T cells, CD28 costimulation being one of them. In an experimental system using LCMV infections of mice that were reconstituted with bone marrow of mice that had MHC-I deficient antigen presenting cells, there was a reduced expansion of central memory LCMV-specific CD8+ T cells in the spleen by more than 85% and a reduction by more than 55% of memory CD8+ T cells in the bronchioalveolar lavage.66 The requirement for APC during secondary immune responses raised questions regarding the signals provided by APC to memory CD8+ T cells that drive the secondary response and whether one of these signals could be CD28.
2. Memory CD8+ T cells are dependent on CD28 signaling
Recent publications, from our laboratory and others, have demonstrated that CD28 plays a critical role in the secondary CD8+ T cell response,82,84 thus challenging the notion that costimulation is not required by memory CD8+ T cells. To circumvent the problem of generating virus-specific memory cells in CD28 deficient mice that have an impaired primary response, memory CD8+ T cells, were generated in wild-type mice by in vivo viral infections. Following the development of intact primary T cell responses, and the generation of memory CD8+ T cells, the requirement of the memory population for CD28 costimulation during a secondary response was examined by blocking CD28 binding to its ligands with either CTLA4-Ig, anti-B7 or anti-CD28 monoclonal antibodies or transfer of memory cells to CD80/CD86 double deficient mice (B7.1 and B7.2 knockouts).82,84
In our studies, virus-specific memory CD8+ T cells were generated through primary infection of C57Bl/6 mice with influenza type A virus or with Herpes Simplex Virus (HSV-1).82 In this way, we ensured that the primary immune response and the resulting memory population develop in conditions of unimpaired costimulation. During secondary infection, CD28 costimulation blockade was achieved by treating these mice with a non-depleting, blocking anti-CD28 monoclonal antibody82,85 or with an isotype control antibody. Another approach that we used was to adoptively transfer influenza virus-specific memory CD8+ T cells generated in wild type C57Bl/6 mice into CD80/CD86 double deficient mice which were then challenged with influenza virus. In both experimental setups that we employed, we measured the re-expansion of virus-specific CD8+ T cells, the functional properties of these cells and the lung viral loads throughout the secondary immune response. Blocking of CD28 costimulation induced a significant reduction of expansion of memory CD8+ T cells against either influenza virus or HSV rechallenge. In mice treated with anti-CD28 antibody, there was a three fold reduction in the absolute number of pulmonary influenza virus-specific CD8+ T cells at the peak of the secondary response, when compared to untreated or isotype control treated mice. When memory virus-specific CD8+ T cells were transferred into CD80/CD86 deficient mice, the reduction in the magnitude of the immune response was 9-fold.82 In addition to absolute numbers, a significant reduction in the cytolytic function was observed. The reduction of the secondary CD8+ T cell immune response after blocking of costimulation was not limited to influenza virus infections. When HSV-1 specific memory CD8+ T cells were transferred to CD80/CD86 deficient mice and mice were challenged with HSV-1 in the foot pad, we observed a significant 5-fold reduction in the absolute number of virus-specific CD8+ T cells in the local draining lymph node in comparison to controls.82
To examine the mechanism behind the loss of expansion of memory CD8+ T cells in the absence of CD28 costimulation, we examined cellular markers of proliferation and apoptosis. In agreement with previous reports,86,87 we found Bcl-xL to be significantly decreased in anti-CD28 treated memory CD8+ T cells as compared to controls. Surprisingly, a second anti-apoptotic molecule, Bcl-2, which is rapidly downregulated in activated naive CD8+ T cells88 fails to downregulate in CD28 blocked memory CD8+ T cells when compared to controls. In fact, during a normal activation cycle of a cell, this molecule is downregulated and the cell proceeds into cell cycle.88 However, if Bcl-2 fails to downregulate, cell cycle is arrested and the cell fails to proliferate.89–92 Cell cycle analysis of rechallenged memory virus-specific CD8+ T cells in CD80/CD86 deficient mice showed that indeed these cells were selectively arrested in the G1/S phase of the cell cycle. Blastogenesis of these cells was not affected, and this is in agreement with earlier studies.81 Although these findings do not illustrate a direct interaction, our data do suggest a previously unappreciated relationship between signaling through CD28 and downregulation of Bcl-2. Importantly, the reduced expansion of virus-specific CD8+ T cells was accompanied by a decrease in viral clearance. In influenza virus and in HSV infected mice in which costimulation was blocked at the beginning of the secondary immune response, we found significantly increased viral loads when compared to their control or isotype treated counterparts. The finding of increased viral loads reminds us of the critical role that CD8+ T cells play in the elimination of viral infection, and thus the significance of developing efficient CD8+ T cell secondary responses.
Fuse et al.84 brought additional evidence for the role of costimulation for optimal recall CD8+ T cell responses. After infection with Murine Gammaherpes Virus 68 (MHV-68), purified memory CD8+ T cells from either C57Bl/6J or CD28 deficient mice were transferred into naive congenic recipients that were then infected with the same virus. Interestingly, virus-specific memory CD8+ T cells that were generated in the absence of CD28 costimulation expanded approximately 9 times, whereas virus-specific memory CD8+ T cells that were generated in C57Bl/6 mice expanded more than 40 times. These findings were further substantiated when virus-specific memory CD8+ T cells were generated in C57Bl/6 mice and transferred into CD80/CD86 deficient mice or wild-type control mice, challenged and analyzed five days later for expansion of the virus-specific memory population.84 The importance of CD28 signaling during secondary responses was further supported with Vaccinia virus infections where the lack of CD28 signaling impaired the responses of memory virus-specific CD8+ T cells.84
Thus the CD28 requirement of memory CD8+ T cells for expansion has been shown in multiple viral infections such as Influenza type A virus, HSV, Vaccinia Virus and Murine Gamma Herpes Virus 68 and is required for rapid pathogen clearance.82,84 The reduced expansion of virus-specific memory CD8+ T cells in the absence of costimulation challenges the paradigm that memory immune responses occur independently of costimulatory signals. CD28 signaling during primary response may be affecting the quality of memory CD8+ T cells generated84 while the expansion of memory T cells clearly requires CD28 costimulation signals for optimal secondary responses and normal pathogen clearance82,84 This CD28 requirement by memory cells is not restricted to CD8+ T cells, CD4+ T cells have also been shown to require in vivo CD28 costimulation.90 This information has important implications in designing efficient vaccination strategies against pathogens and tumors which can downregulate costimulatory signals. Costimulation blockade for transplantation and autoimmunity may also hamper the ability of the host to mount efficient recall responses. Finally, little is known about other members of the CD28 family and their role in memory CD8+ T cells. In vivo ICOS signaling has been shown not to be required for memory responses,70 while PD-1 signaling in vivo may play a role in the dysfunction of helpless memory CD8+ T cells.91
C. TNFR family members and virus-specific CD8+ T cells
1. Role of TNFR family members during primary immune responses
Members of the TNFR family have been implicated in shaping the primary immune response during viral infections as shown in Figure 1. Experiments with influenza virus have shown that CD27 contributes to the survival of antigen-specific CD8+ T cells during the primary phase of the infection.52,92 Recent studies in our laboratory52 have shown that CD27 signals are needed by virus-specific CD8+ T cells during the late stages of the primary response. The reduction in the response of virus-specific CD8+ T cells was mainly due to their increased sensitivity to CD95/Fas-mediated apoptosis in the absence of CD27 signals, which leads to the deletion of effector cells. This deletion is dependent on the presence of CD4+ T cells. One of the earliest studies done on CD27-CD70 showed that CD27 may be important for the CTL function of virus-specific CD8+ T cells.93 In vitro studies have shown that CD27-CD70 interactions enhance antigen-specific cytotoxic CD8+ T cell activity and enhance perforin expression.93 Studies involving CD27−/−, CD28−/− and CD27/CD28 double knockouts hinted that CD27 may play a role in the primary immune response during viral infections by directly affecting the function of virus-specific CD8+ T cells.94
4-1BB may also be important for the initial response in a viral infection. Although the 4-1BBL−/− mice showed a decrease in the number of primary CD8+ T cells, this is not as reduced as the reduction observed in CD27−/− mice. Studies with LCMV60 and influenza virus have shown that 4-1BBL−/− mice have a decreased primary CD8+ T cell response as compared to wild type mice.60,92 This reduced primary response is most likely due to a decreased proliferation of naïve T cells.60 Blocking both 4-1BB and CD28 signals results in an eight fold reduction of virus-specific CD8+ T cells and the virus could not be cleared in these mice. Thus 4-1BB may work in conjunction with CD28 to initiate and maintain the pool of effector cells generated during a primary response.60 In addition, our studies showed that, 4-1BB costimulation broadens the repertoire of virus-specific CD8+ T cells by increasing the number of CD8+ T cells that recognized subdominant epitopes of influenza virus.71 4-1BB-4-1BBL interactions preferentially affect antigen-specific CD8+ T cells rather than CD4+ T cells during a primary response.95 Studies with HSV-1 have shown that virus-specific CD8+ T cells express more 4-1BB than antigen-specific CD4+ T cells 2 days post infection. Stimulation of infected mice with 4-1BB antibody on day 5 post infection increased the frequency of antigen- specific CD8+ T cells by ten fold as compared to two to three fold in CD4+ T cells.95 It should be mentioned, however, that some primary CD8+ T cell responses to viruses may not need 4-1BB costimulation. An example of this is Murine Gamma Herpes virus (MHV-68) infection of mice, where the total numbers of effector CD8+ T cells and cytokine secretion remain unaffected in the absence of 4-1BB during the primary response to MHV-68.97 Why some viruses require 4-1BB costimulation while others do not, may relate to differences in their antigen load, the costimulatory ligand expression they induce, the pattern recognition receptors they trigger, and/or the inflammatory milieau they induce.
4-1BB-4-1BBL interactions can also rescue antigen-specific CD8+ T cells in the absence of CD2871 and this may be by inducing expression of other members of the CD28 family such as ICOS. Studies with isolated CD8+ T cells from CD28−/− mice showed that stimulation with anti-CD28 and anti-4-1BB leads to an upregulation in the expression of ICOS.96 In the absence of CD28 signaling purified CD8+ T cells show enhanced proliferation when stimulated with anti-4-1BB and anti-ICOS antibodies.96 In vivo experiments with 4-1BB in our laboratory71 have shown that stimulation with agonistic anti-4-1BB antibody could restore primary virus-specific CD8+ T cell responses in the CD28−/− mice infected with influenza virus.71 In contrast to CD27 and 4-1BB, the primary CD8+ T cell response in the OX40L−/− mice does not seem to be affected.92
2.Virus-specific memory CD8+ T cells receive costimulatory signals from TNFR family members
In addition to their role in primary CD8+ T cells response it has become apparent that TNFR members participate at different phases of memory CD8+ T cell responses as shown in Figure 1.
a. The role of 4-1BB and 4-1BBL in memory CD8+ T cell maintenance
TNFR family members and their ligands help memory CD8+ T cells by providing pro-survival signals. A number of studies suggest that 4-1BB–4-1BBL mediated interactions can positively influence memory responses during viral infections.95 Studies done using 4-1BBL deficient mice showed that on re-challenge with the viral pathogen there is a much reduced memory CD8+ T cell response in these mice as compared to wild type controls.59,61,98 The studies done by Pulle et al. showed that 4-1BB-4-1BBL signals are also important for maintaining the number of memory CD8+ T cells. A two to three fold decrease was observed in the total number of virus-specific memory CD8+ T cells recovered from the spleen and bone marrow from 4-1BBL deficient mice as compared to wild type.98 These results indicated that 4-1BBL plays a role in survival of memory CD8+ T cells. IL-15 is known to play an important role in the maintenance of CD8+ T cell memory but the interplay between IL-15 and 4-1BBL was not obvious until it was shown that IL-15 enhances expression of 4-1BB on the surface of CD44hi CD8+ T cells.98 Thus IL-15 induces expression of 4-1BB on memory CD8+ T cells and the subsequent interaction between 4-1BB and 4-1BBL, provides a survival signal to the memory CD8+ T cells for their maintenance. The upregulation of 4-1BB may not be restricted to IL-15, but may also involve other cytokines.99 The stimulation of 4-1BB during the primary response can enhance the secondary response during viral rechallenge,95 but this most likely is due to the augmented memory generated by the heightened primary response.
Fuse et al.97 used a Murine Gamma Herpes virus (MHV-68) model to study the function of 4-1BB-4-1BBL interactions during chronic infections. Their studies have showed that the quantity of memory CD8+ T cell is not affected but the quality of these cells was affected in the absence of 4-1BBL. 4-1BBL deficient mice infected with MHV-68 had 9 to 40 fold more latent viral load than the wild type controls. Cytotoxicity was greatly reduced and the secretion of granzyme B on restimulation was also impaired. Thus the antigen-specific memory CD8+ T cells were impaired in their functional abilities and were unable to clear the virus. The recall response in the absence of 4-1BBL was also affected but the secretion of cytokines such as TNFα and IFNγ by the antigen-specific memory CD8+ T cells remains unaffected. The authors made an interesting observation that in their previous studies with MHV-68 infected CD80/CD86 deficient mice,69 the secretion of pro-inflammatory cytokines was impaired in the virus-specific CD8+ T cells whereas the cytolytic function remained unaffected. However in the absence of 4-1BB-4-1BBL interactions97 the cytolytic function of the virus-specific CD8+ T cells was affected.97 Thus it may appear that signaling by different costimulatory molecules may affect specific functions of memory virus-specific CD8+ T cells.
The reduced quality of memory in 4-1BBL deficient mice is similar to that seen in the case of helpless memory CD8+ T cells,100–103 and chronically antigen stimulated CD8+ T cells.104 Therefore the role of 4-1BB for optimal quantity and quality of memory CD8+ T cell responses suggests a possible use of 4-1BB as an adjuvant. Studies done by Myers et al.105 have shown that the immunization of mice with a peptide and anti-CD137 antibody led to the generation of a robust peptide specific pool of memory CD8+ T cells in the lymphoid and non lymphoid compartments. These antigen-specific memory CD8+ T cells in the vaccinated mice were fully functional and were retained for more than a year.105
The current model on the pro-survival mechanism of action of 4-1BBL involves TRAF signaling. Upon ligand binding, 4-1BB, recruits and interacts with mainly two TRAF molecules, TRAF-1 and TRAF-2.106 Studies done with TRAF deficient transgenic mice showed that memory CD8+ T cells were lower in TRAF-1 deficient mice as compared to wild type hosts.107 The number of memory CD8+ T cells increased by 7–8 fold in the presence of TRAF-1.107 The pro-survival effect mediated by 4-1BB on TRAF-1 is due to the upregulation of Bcl-xL and the down regulation of Bim which in turn is dependant on ERK signaling.106 Thus 4-1BB– 4-1BBL interactions also appear to play a critical role in the survival of memory CD8+ T cells.
4-1BB- 4-1BBL interactions are also important for antigen-specific memory CD8+ T cells in humans. Studies with human memory CD8+ T cells have demonstrated the need for signals from selective costimulatory molecules for their proliferation as shown in studies with Human Cytomegalovirus (HCMV).108 The virus-specific memory CD8+ T cells in HCMV infections are predominantly CD28− CD45RAhi, these cells are cytotoxic but their potential for proliferation has always been questioned. In recent studies, these cells were isolated from the blood of HCMV patients and they were cultured with autologous fibroblasts that selectively expressed CD137L. Limiting dilution analysis showed that in the presence of CD137L, the virus-specific memory CD8+ T population was able to proliferate better and at a rate comparable with other antigen-specific CD8+ T cell subsets. Interestingly the CD28−CD45ROhi population did not proliferate if signals were given by CD80-CD86 alone, or in the absence of CD137L. From these studies the authors drew the conclusion that in the presence of antigenic stimulation and CD137-CD137L costimulation signals, the virus-specific CD28−CD45RAhi subset of memory CD8+ T cells could undergo proliferation in HCMV infections, hence protecting the host.
b. Help provided to memory CD8+ T cells by OX40-OX40L interactions
OX40 has been shown to play a crucial role in the primary immune response to Vaccinia virus (VACV) and in the generation of CD8+ T cell memory to this virus, while in other virus infections such as LCMV and influenza virus, it did not play a role.109,110 In OX40-deficient mice, CD8+ T cells that can protect against Vaccinia virus challenge, do not develop and this defect was found to be intrinsic to CD8+ T cells generated in OX40 deficient mice since naïve OX40−/− CD8+ T cells cannot expand in response to virus challenge when transferred into wild-type hosts.109 The generation of memory CD8+ T cells is also affected in the absence of OX40. In these studies109 splenocytes were isolated 40 days post infection with Vaccinia virus (VACV) and stimulated with VACV epitopes. It was found that irrespective of the route of infection (intraperitoneally or by scarification) the frequency of virus-specific memory CD8+ T cells for all epitopes had decreased by 60–80% in the absence of OX40. This was a direct effect of OX40 on CD8+ T cells. In vitro influenza virus-specific memory CD8+ T cells and HIV-specific CD8+ T cells from chronically infected individuals expand more in the presence of OX40L.111 Delivery of other costimulatory molecules such as B7-1 and 4-1BBL along with OX40L had a synergistic effect on the expansion of virus-specific memory CD8+ T cells.111
Subsequent studies have associated OX40 signaling with the help provided by CD4+ T cells to HIV-specific CD8+ T cells or EBV-specific CD8+ T cells.112 The interaction between OX40-OX40L promotes a significant increase in CTL responses against HIV-1 but this increase was manifested only in the presence of CD4+ T cells.112 The mechanism by which the interactions between OX40 and OX40L increases the cytotoxic capacity of CD8+ T cells seems to be mediated by increasing the proliferative capacity of CD4+ T cells. Interestingly, this effect was independent of IL-2, IFNγ, and TNFα production by CD4+ T cells.112 Increased expansion and survival of memory CD8+ T cells in vivo can be induced by treatment with an agonistic anti-OX40 antibody.113 Treatment with anti-OX40 antibody increases the level of Akt phosphorylation and expression of markers CD62L and CD127, this effect however is dependent on the presence of CD4+ T cells and is not a direct effect of OX40 on CD8+ T cells.113,114 Altogether, the above demonstrate that OX40-OX40L signaling has an important beneficial impact on survival and expansion of memory CD8+ T cells. This effect may be directly mediated through CD8+ T cells or indirectly through CD4+ T cells.
c. Effect of CD27-CD70 interactions on the generation of memory CD8+ T cells
Recent studies have suggested that memory CD8+ T cells have reduced CD27 expression when primed in the absence of CD4+ T cell help.115 Hendriks et al. observed a reduced CD8+ T cell memory pool in the absence of CD27, 4-1BB and OX40 during viral infections.92 Experiments using an LCMV model system have showed that helpless memory CD8+ T cells have a reduced expression of CD27, whereas helped memory CD8+ T cells have upregulated expression of CD27. These studies suggested that CD27 ligation lead to an autocrine secretion of IL-2 that stimulated the expansion of the antigen-specific memory CD8+ T cells.115
Using an OVA protein model for immunization, it was showed that CD27 improves the quality of the CD4+ T cells in terms of secretion of cytokines such as IFNγ and IL-2, and these CD27 primed CD4+ T cells can now help memory CD8+ T cell expansion.116 Thus it appears that expression of CD27 on both CD4+ T cells and CD8+ T cells may be required for an effective antigen-specific memory CD8+ T cell response. A recent study using CD70 transgenic mice showed that the constitutive expression of CD70 on T cells enhanced the primary CD8+ T cell response against influenza virus but adversely affects the generation and maintenance of memory CD8+ T cells.117 The authors suggested that the selective upregulation of markers such as CD70 on different cell types during chronic infections may lead to exhaustion of antigen-specific CD8+ T cells.117 In some instances CD70 signaling is able to directly influence the secondary expansion of CD8+ T cells, thus replacing CD4+ T cell help, as shown by Bullock et al.119 Additionally CD70 expressing antigen presenting cells may directly interact with CD8+ T cells to regulate their initial expansion and secondary responses in the absence of CD4+ T cell help,120 as shown in studies involving CD70 expressing dendritic cells that can trigger effector CD8+ T cell expansion and memory responses against certain tumors.120 CD40 mediated expansion of primary and secondary CD8+ T cells may be mediated by CD27/CD70 interactions. Studies using anti-CD40 agonist antibodies have shown that in the absence of CD27 signaling, CD40 mediated expansion of primary and memory CD8+ T cells is reduced.118 From the studies92,115–117 it can be concluded that CD27-CD70 interaction can help to enhance or reduce the memory CD8+ T cells response. The cellular factors that regulate this effect are still unknown.
3. Quality of virus-specific memory CD8+ T cells
a. Effect of TRAIL on memory CD8+ T cells
Memory CD8+ T cells generated in the absence of CD4+ T cell help have been called helpless memory CD8+ T cells and they are characterized by their inability to respond effectively and expand to a rechallenge.101–103 Studies have shown that CD4+ T cells are required for maintenance of memory CD8+ T cells during acute infection,121 thus CD4+ T cells have been implicated in generation and maintenance of memory during virus infections.
Janssen et al.122 used an in vivo cross priming model and LCMV model system to address this question and they suggested that the antigen-specific helpless memory CD8+ T cells are more prone to TRAIL mediated activation induced cell death. Their experiments showed that the helpless antigen-specific CD8+ T cell expressed TRAIL and that the RNA expression of proapoptotic molecules such as FasL and TRAIL was upregulated in helpless antigen-specific CD8+ T cells while anti-apoptotic Bcl-2 and Bcl-xL were reduced. Moreover the secondary expansion of CD8+ T cells from wild type animals was 5 fold higher than the expansion of helpless CD8+ T cells on re-stimulation. Ex vivo culture of CD8+ T cells in the presence of a pan caspase inhibitor z-vad and the TRAIL receptor fusion protein (DR5-Ig) helped to partially restore the expansion of the helpless CD8+ T cells. However these studies did not clearly explain whether the effect that the CD4+ T cells have on memory CD8+ T cells is dependant solely on secretion of TRAIL. Sacks et al.123 carried out in vivo studies where they traced the fate of antigen-specific memory CD8+ T cells over a period of time prior to re-challenge and post rechallenge using LCMV and Listeria monocytogenes. Their experiments showed that in antigen-specific CD8+ T cells the expression of CD127 and CD27, was similar between wild type and TRAIL deficient mice day 8 and day 90 post infection. The secretion of IFNγ, TNFα, and IL-2 was also comparable in the wild type and TRAIL deficient mice. Rechallenge studies were done where CD4+ T cell depleted and non depleted wild type and TRAIL deficient mice were infected with LCMV intraperitoneally. In both wild type and TRAIL deficient mice the frequency of virus-specific CD8+ T cells was low in the absence of CD4+ T cells. Rechallenge with the virus led to a considerable decrease in the frequency and absolute number of secondary virus-specific CD8+ T cells in the absence of CD4+ T cells in the wild type mice. Curiously this defect in expansion was not restored in the TRAIL deficient mice. Other studies have suggested that the contribution of TRAIL to the helpless phenotype may be transient and that other factors also contribute.100 The above suggest that CD4+ T help is indispensable for effective memory CD8+ T cells, however the role of TRAIL in the dysfunction in the helpless memory CD8+ T cells remains to be fully elucidated.
b. Effect of CD40 on quality of memory CD8+ T cells
Studies involving CD40 have shown that functional memory CD8+ T cells could be generated in the absence of CD40.124 Studies by Fuse et al.91 provided useful hints that may help understand the mechanism of action of CD4+ T cells on the quality of memory CD8+ T cell responses mediated and the role of TRAIL or CD40 in this process. They used Vaccinia virus infections for their in vivo studies and showed that the impairment of helpless CD8+ T cells is independent of TRAIL as shown previously.91 They further reported that the administration of agonistic anti-CD40 antibody at the priming stage could restore the function of the antigen-specific memory CD8+ T cells generated in the absence of CD4+ T cell help.91 Exogenously administered IL-2 could also repair the dysfunctional recall response of helpless memory CD8+ T cells.91 Helpless CD8+ T cells dysfunction could be due to failure to down regulate the expression of PD-1 and to prevent cell death. The signals provided by CD4+ T cells involve CD40-CD40L interactions and IL-2 signaling and in their absence the memory CD8+ T cells become dysfunctional and are more prone to activation induced cell death which may be PD-1 dependent and TRAIL dependent or independent.91 Whether CD4+ T cells directly signal to CD8+ T cells or via some APC is not clear at this stage.
Recent vaccine studies125 suggest that CD40-CD40L interactions between dendritic cells and virus-specific CD8+ T cells can help to enhance the quality of memory CD8+ T cells in the absence of CD4+ T cells. In these studies125 BALB/c mice were immunized with canary pox vector expressing membrane bound CD40L (vCPmCD40L) and this led to an increase in number of antigen-specific CD8+ T cells. Ex-vivo culture of human monocyte derived dendritic cells (MDDC) infected with CD40L expressing canary pox vector led to a greater secretion of IFNγ by the helpless CD8+ T cells. The CTL activity of the virus-specific helpless CD8+ T cells was also partially restored with CD40L stimulation. These results suggest that CD40L on dendritic cells may play a role in shaping an effective memory CD8+ T cell response.
III. IMPLICATIONS FOR THE FUTURE
The need for potent CD8+ T cell eliciting vaccines remains largely unfulfilled. Discovery of adjuvants and strategies that enhance the generation, maintenance and quality of memory CD8+ T cells is important for the development of effective vaccines against viruses, intracellular pathogens and tumors. Costimulatory molecules from the CD28 and the TNFR family augment the immune response during viral infections and contribute to different phases of CD8+ T cell responses as seen in Figure 1. It appears that the CD28 and CD27 are required to initiate a primary CD8+ T cell response.126 For secondary responses of memory CD8+ T cells, costimulatory molecules such as: CD28, 4-1BB, OX40 and CD27 all seem to play a role in shaping the memory responses either by providing pro-survival signals or by enhancing the quality of the memory CD8+ T cells. Secondary expansion of memory CD8+ T cells requires professional APC66,83 and CD28 costimulation.82,84 TNFR family members as costimulatory molecules could also be used as adjuvants. Indeed DNA and adenovirus based vaccines have shown that expression of 4-1BBL, OX40L and CD70 leads to increased T cell expansion, enhanced CTL activity and antibody response.127,128 Agonistic antibodies to TNF family members such as 4-1BB can also provide an adjuvant effect and enhance memory CD8+ T cell generation.105,129 Direct delivery of ligands such as using 4-1BBL to ‘decorate’ tumor cells may provide costimulatory signals that enhance anti-tumor CTL.130–132 An alternative strategy may be the use of oligonucleotide based ligands known as Aptamers, an example being multivalent 4-1BBL Aptamers that act as agonists that can directly trigger CD8+ T cells and inhibit tumors in mice.133 Chronic viral infections are characterized by accumulation of functionally impaired antigen-specific CD8+ T cells, and studies have shown that 4-1BBL in combination with CD80 can induce the expansion of the antigen-specific CD8+ T cells from this impaired pool.134 Therefore such strategies may prove valuable for the design of effective vaccines not only against acute viral infections but also against chronic viral infections.
Concluding remarks
Animal models of viral infections have shown that costimulatory molecules of both the CD28 and the TNFR family, as indicated in Table 1, help in the generation and maintenance of virus-specific memory CD8+ T cells, but are also important for the reactivation of memory CD8+ T cells and secondary responses. These costimulatory molecules may act in a redundant fashion, but they may also provide an opportunity to augment virus-specific memory CD8+ T cell responses and may prove useful in designing effective vaccines against chronic viral infections such as HBV and HIV.134 A number of questions still remain about the function of costimulatory molecules in memory anti-viral CD8+ T cell responses (Box 1). The function of CD28 and TNFR family members has been studied for RNA and DNA viruses, and studies have been done to delineate their functions at the acute and chronic stages of disease. However the redundancy in the function of these molecules questions whether there is a hierarchy in the expression and function of the costimulatory molecules. Although agonistic antibodies may enhance responses when CD28 signaling is absent,135 this does not necessarily suggest a switch in costimulation requirements. It may however suggest that the lack of critical costimulatory molecules may be overcome by targeting alternative receptors with monoclonal antibodies or Aptamers.
Table 1. Effect of costimulatory molecules on virus-specific memory CD8+T cells.
The major costimulatory interactions that affect anti-viral memory CD8+ T cells are shown. The specific actions resulting from these interactions are diverse and can affect many aspects of memory CD8+ T cell responses.
| Receptor | Ligand | Effect on memory CD8+ T cell |
|---|---|---|
| CD28 | B7-1, B7-2 | ↑ expansion and IL-2 production82,84 |
| PD-1 | PD-L1/B7-H1, PD-L2/B7-DC |
↓ proliferation of helpless CD8+ T cells91 |
| CD27 | CD70 | ↑ expansion with CD4+ T cell help and maintenance92,115–117 |
| 4-1BB | 4-1BBL | ↑ generation, maintenance and enhances proliferation95, 97,99,105 –108 |
| OX40 | OX40L | ↑ survival and expansion in presence of CD4+ T cells 109–114 |
| CD40 | CD40L | ↑ quality91,122 |
| TRAIL receptors | TRAIL | ↓ survival of helpless memory CD8+ T cells119,120 |
Unanswered questions
Can one cell provide multiple costimulatory signals?
Do different APC provide different costimulatory signals?
The importance of redundant signalling?
Do central and effector memory CD8+ T cells differ in their requirement for costimulation?
Does persistent viral load alter expression of costimulatory molecules on APC?
Effect of costimulation on tertiary memory?
Does chronic infection change costimulation requirements of CD8+ T cells?
Can costimulatory ligand combination enhance adjuvant activity
The recent discovery of the important role of costimulatory molecules such as CD28 during reactivation of memory CD8+ T cells would raise the danger that therapeutic blocking of costimulation in transplantation and autoimmunity may impair the host’s ability to respond to viral infection. On the other hand, some viral infections manipulate costimulation to evade the immune response. A number of viruses such as Measles136 and HIV137,138 downregulate B7 family members so as to hamper CTL responses. This would suggest that successfully priming CTL vaccines may not be very effective against such viruses. A strategy against such pathogens and tumors may be to ‘add back’ costimulatory ligands to reverse the impairment of CTL responses, or to generate memory, if possible, which is less dependent on CD28 costimulation. One of the hallmarks of chronic viral infections is the occurrence of systemic inflammation; the effect of different pro-inflammatory cytokines on the expression of costimulatory receptors and their ligands during different phases of viral infections is still poorly understood.
The expression of TNFR or CD28 costimulatory molecules or their corresponding ligands on the antigen presenting cells is necessary for an effective antiviral CD8+ T cell response, especially for vaccinations against viral infections. The important interplay between different costimulatory molecules for the generation, maintenance and functionality of memory CD8+ T cells, indicates that the design of preventive vaccines will require we further understand the important contributions of costimulation to effective anti-viral memory CD8+ T cell responses.
Acknowledgements
This work was supported by NIH grants R01 AI66215 and R01 AI46719 to PDK.
References
- 1.Whitmire JK, Ahmed R. Costimulation in antiviral immunity: differential requirements for CD4(+) and CD8(+) T cell responses. Curr Opin Immunol. 2000;12:448–455. doi: 10.1016/s0952-7915(00)00119-9. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 4.Lafferty KJ, Misko IS, Cooley MA. Allogeneic stimulation modulates the in vitro response of T cells to transplantation antigen. Nature. 1974;249:275–276. doi: 10.1038/249275a0. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty KJ, Jones MA. Reactions of the graft versus host (GVH) type. Aust J Exp Biol Med Sci. 1969;47:17–54. doi: 10.1038/icb.1969.3. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 7.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 8.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- 10.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 13.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 14.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 15.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 16.Suh WK, Wang S, Duncan GS, Miyazaki Y, Cates E, Walker T, Gajewska BU, Deenick E, Dawicki W, Okada H, Wakeham A, Itie A, Watts TH, Ohashi PS, Jordana M, Yoshida H, Mak TW. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol. 2006;26:6403–6411. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 18.Linsley PS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 20.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walunas TL, Sperling AI, Khattri R, Thompson CB, Bluestone JA. CD28 expression is not essential for positive and negative selection of thymocytes or peripheral T cell tolerance. J Immunol. 1996;156:1006–1013. [PubMed] [Google Scholar]
- 23.Ling V, Wu PW, Finnerty HF, Bean KM, Spaulding V, Fouser LA, Leonard JP, Hunter SE, Zollner R, Thomas JL, Miyashiro JS, Jacobs KA, Collins M. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- 24.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS costimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 25.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 26.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 27.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 28.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 30.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 31.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 33.Zeng C, Wu T, Zhen Y, Xia XP, Zhao Y. BTLA, a new inhibitory B7 family receptor with a TNFR family ligand. Cell Mol Immunol. 2005;2:427–432. [PubMed] [Google Scholar]
- 34.Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- 35.Krieg C, Han P, Stone R, Goularte OD, Kaye J. Functional analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J Immunol. 2005;175:6420–6427. doi: 10.4049/jimmunol.175.10.6420. [DOI] [PubMed] [Google Scholar]
- 36.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 37.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27:19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 38.Gruss HJ, Dower SK. Tumor necrosis factor ligand superfamily: involvement in the pathology of malignant lymphomas. Blood. 1995;85:3378–3404. [PubMed] [Google Scholar]
- 39.Gravestein LA, Amsen D, Boes M, Calvo CR, Kruisbeek AM, Borst J. The TNF receptor family member CD27 signals to Jun N-terminal kinase via Traf-2. Eur J Immunol. 1998;28:2208–2216. doi: 10.1002/(SICI)1521-4141(199807)28:07<2208::AID-IMMU2208>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 41.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 42.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 43.Ellis TM, Simms PE, Slivnick DJ, Jack HM, Fisher RI. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol. 1993;151:2380–2389. [PubMed] [Google Scholar]
- 44.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 45.Armitage RJ. Tumor necrosis factor receptor superfamily members and their ligands. Curr Opin Immunol. 1994;6:407–413. doi: 10.1016/0952-7915(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 46.De Jong R, Brouwer M, Hooibrink B, Van der Pouw-Kraan T, Miedema F, Van Lier RA. The CD27− subset of peripheral blood memory CD4+ lymphocytes contains functionally differentiated T lymphocytes that develop by persistent antigenic stimulation in vivo. Eur J Immunol. 1992;22:993–999. doi: 10.1002/eji.1830220418. [DOI] [PubMed] [Google Scholar]
- 47.Borst J, Sluyser C, De Vries E, Klein H, Melief CJ, Van Lier RA. Alternative molecular form of human T cell-specific antigen CD27 expressed upon T cell activation. Eur J Immunol. 1989;19:357–364. doi: 10.1002/eji.1830190221. [DOI] [PubMed] [Google Scholar]
- 48.Lens SM, Baars PA, Hooibrink B, van Oers MH, van Lier RA. Antigen-presenting cell-derived signals determine expression levels of CD70 on primed T cells. Immunology. 1997;90:38–45. doi: 10.1046/j.1365-2567.1997.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Jong R, Loenen WA, Brouwer M, van Emmerik L, de Vries EF, Borst J, van Lier RA. Regulation of expression of CD27, a T cell-specific member of a novel family of membrane receptors. J Immunol. 1991;146:2488–2494. [PubMed] [Google Scholar]
- 50.Diehl L, van Mierlo GJ, den Boer AT, van der Voort E, Fransen M, van Bostelen L, Krimpenfort P, Melief CJ, Mittler R, Toes RE, Offringa R. In vivo triggering through 4-1BB enables Th-independent priming of CTL in the presence of an intact CD28 costimulatory pathway. J Immunol. 2002;168:3755–3762. doi: 10.4049/jimmunol.168.8.3755. [DOI] [PubMed] [Google Scholar]
- 51.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 54.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins NA, Gilbert DJ, Copeland NG, Muto T, Yagita H, Okumura K. Characterization of murine CD70 by molecular cloning and mAb. Int Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- 55.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 56.Chen AI, McAdam AJ, Buhlmann JE, Scott S, Lupher ML, Jr, Greenfield EA, Baum PR, Fanslow WC, Calderhead DM, Freeman GJ, Sharpe AH. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 57.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 58.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 59.DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, Peschon JJ, Watts TH. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- 60.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 61.Tan JT, Whitmire JK, Murali-Krishna K, Ahmed R, Altman JD, Mittler RS, Sette A, Pearson TC, Larsen CP. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J Immunol. 2000;164:2320–2325. doi: 10.4049/jimmunol.164.5.2320. [DOI] [PubMed] [Google Scholar]
- 62.Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- 63.Del Prete G, De Carli M, D'Elios MM, Daniel KC, Almerigogna F, Alderson M, Smith CA, Thomas E, Romagnani S. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–1661. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Podack ER, Strbo N, Sotosec V, Muta H. CD30-governor of memory T cells? Ann N Y Acad Sci. 2002;975:101–113. doi: 10.1111/j.1749-6632.2002.tb05945.x. [DOI] [PubMed] [Google Scholar]
- 65.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–277. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 66.Belz GT, Wilson NS, Smith CM, Mount AM, Carbone FR, Heath WR. Bone marrow-derived cells expand memory CD8+ T cells in response to viral infections of the lung and skin. Eur J Immunol. 2006;36:327–335. doi: 10.1002/eji.200535432. [DOI] [PubMed] [Google Scholar]
- 67.Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J Immunol. 2000;164:79–85. doi: 10.4049/jimmunol.164.1.79. [DOI] [PubMed] [Google Scholar]
- 68.McAdam AJ, Farkash EA, Gewurz BE, Sharpe AH. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J Virol. 2000;74:203–208. doi: 10.1128/jvi.74.1.203-208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuse S, Obar JJ, Bellfy S, Leung EK, Zhang W, Usherwood EJ. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J Virol. 2006;80:9159–9170. doi: 10.1128/JVI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertram EM, Tafuri A, Shahinian A, Chan VS, Hunziker L, Recher M, Ohashi PS, Mak TW, Watts TH. Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol. 2002;32:3376–3385. doi: 10.1002/1521-4141(200212)32:12<3376::AID-IMMU3376>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 71.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 72.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 73.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 74.Kundig TM, Shahinian A, Kawai K, Mittrucker HW, Sebzda E, Bachmann MF, Mak TW, Ohashi PS. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 75.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 76.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 77.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 78.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. J Exp Med. 1999;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flynn K, Mullbacher A. Memory alloreactive cytotoxic T cells do not require costimulation for activation in vitro. Immunol Cell Biol. 1996;74:413–420. doi: 10.1038/icb.1996.71. [DOI] [PubMed] [Google Scholar]
- 81.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 82.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 83.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prilliman KR, Lemmens EE, Palioungas G, Wolfe TG, Allison JP, Sharpe AH, Schoenberger SP. Cutting edge: a crucial role for B7-CD28 in transmitting T help from APC to CTL. J Immunol. 2002;169:4094–4097. doi: 10.4049/jimmunol.169.8.4094. [DOI] [PubMed] [Google Scholar]
- 86.Wu LX, La Rose J, Chen L, Neale C, Mak T, Okkenhaug K, Wange R, Rottapel R. CD28 regulates the translation of Bcl-xL via the phosphatidylinositol 3-kinase/mammalian target of rapamycin pathway. J Immunol. 2005;174:180–194. doi: 10.4049/jimmunol.174.1.180. [DOI] [PubMed] [Google Scholar]
- 87.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 88.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 89.Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347:543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 91.Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H, Usherwood EJ. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol. 2009;182:4244–4254. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 93.Yamada S, Shinozaki K, Agematsu K. Involvement of CD27/CD70 interactions in antigen-specific cytotoxic T-lymphocyte (CTL) activity by perforin-mediated cytotoxicity. Clin Exp Immunol. 2002;130:424–430. doi: 10.1046/j.1365-2249.2002.02012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS. 4-1BB costimulation enhances HSV-1-specific CD8+ T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol. 2005;238:76–86. doi: 10.1016/j.cellimm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 96.Vidric M, Suh WK, Dianzani U, Mak TW, Watts TH. Cooperation between 4-1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J Immunol. 2005;175:7288–7296. doi: 10.4049/jimmunol.175.11.7288. [DOI] [PubMed] [Google Scholar]
- 97.Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. J Immunol. 2007;178:5227–5236. doi: 10.4049/jimmunol.178.8.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pulle G, Vidric M, Watts TH. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- 99.Sabbagh L, Snell LM, Watts TH. TNF family ligands define niches for T cell memory. Trends Immunol. 2007;28:333–339. doi: 10.1016/j.it.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of "helpless" memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 101.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 102.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 104.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Myers L, Lee SW, Rossi RJ, Lefrancois L, Kwon BS, Mittler RS, Croft M, Vella AT. Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. Int Immunol. 2006;18:325–333. doi: 10.1093/intimm/dxh371. [DOI] [PubMed] [Google Scholar]
- 106.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180:8093–8101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 107.Sabbagh L, Srokowski CC, Pulle G, Snell LM, Sedgmen BJ, Liu Y, Tsitsikov EN, Watts TH. A critical role for TNF receptor-associated factor 1 and Bim down-regulation in CD8 memory T cell survival. Proc Natl Acad Sci U S A. 2006;103:18703–18708. doi: 10.1073/pnas.0602919103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific "effector memory" (CD28(−) CD45RA(HI)) CD8(+) T cells. Blood. 2007;110:4360–4366. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 109.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 111.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly RP, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 112.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486–2495. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 113.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37:157–166. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 114.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 115.Matter MS, Claus C, Ochsenbein AF. CD4+ T cell help improves CD8+ T cell memory by retained CD27 expression. Eur J Immunol. 2008;38:1847–1856. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]
- 116.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181:1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 117.van Gisbergen KP, van Olffen RW, van Beek J, van der Sluijs KF, Arens R, Nolte MA, van Lier RA. Protective CD8 T cell memory is impaired during chronic CD70-driven costimulation. J Immunol. 2009;182:5352–5362. doi: 10.4049/jimmunol.0802809. [DOI] [PubMed] [Google Scholar]
- 118.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 119.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 120.Keller AM, Xiao Y, Peperzak V, Naik SH, Borst J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood. 2009;113:5167–5175. doi: 10.1182/blood-2008-03-148007. [DOI] [PubMed] [Google Scholar]
- 121.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 123.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 125.Liu J, Yu Q, Stone GW, Yue FY, Ngai N, Jones RB, Kornbluth RS, Ostrowski MA. CD40L expressed from the canarypox vector, ALVAC, can boost immunogenicity of HIV-1 canarypox vaccine in mice and enhance the in vitro expansion of viral specific CD8+ T cell memory responses from HIV-1-infected and HIV-1-uninfected individuals. Vaccine. 2008;26:4062–4072. doi: 10.1016/j.vaccine.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol. 2004;172:981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- 127.Du X, Zheng G, Jin H, Kang Y, Wang J, Xiao C, Zhang S, Zhao L, Chen A, Wang B. The adjuvant effects of co-stimulatory molecules on cellular and memory responses to HBsAg DNA vaccination. J Gene Med. 2007;9:136–146. doi: 10.1002/jgm.1004. [DOI] [PubMed] [Google Scholar]
- 128.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–566. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, Shirwan H. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009;69:4319–4326. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Q, Ai J, Song Z, Liu J, Shan B. 4-1BB (CD137) ligand enhanced anti-tumor immune response against mouse forestomach carcinoma in vivo. Cell Mol Immunol. 2008;5:379–384. doi: 10.1038/cmi.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stockl J, Herndler-Brandstetter D, Grubeck-Loebenstein B, Reipert BM, Pickl WF, Pfistershammer K, Steinberger P. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–2688. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, Sullenger B, Gilboa E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J Immunol. 2007;179:8252–8263. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]
- 135.Van Gool SW, de Boer M, Ceuppens JL. CD28 ligation by monoclonal antibodies or B7/BB1 provides an accessory signal for the cyclosporin A-resistant generation of cytotoxic T cell activity. J Immunol. 1993;150:3254–3263. [PubMed] [Google Scholar]
- 136.Servet-Delprat C, Vidalain PO, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol. 2000;164:1753–1760. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- 137.Chaudhry A, Das SR, Hussain A, Mayor S, George A, Bal V, Jameel S, Rath S. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J Immunol. 2005;175:4566–4574. doi: 10.4049/jimmunol.175.7.4566. [DOI] [PubMed] [Google Scholar]
- 138.Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr, Ayyavoo V. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]