Abstract
Purpose of review
Globally, tuberculosis (TB) is the commonest opportunistic infection in people living with HIV. Many co-infected patients first present with advanced immunosuppression and require antiretroviral therapy (ART) initiation during TB treatment. The incidence of TB in patients established on ART remains high. Co-treatment presents several management challenges. Recent data on these management issues are reviewed.
Recent findings
Efavirenz concentrations at standard doses are similar with and without concomitant rifampicin-based TB treatment. Nevirapine concentrations are frequently subtherapeutic during lead-in dosing at 200 mg daily in patients on rifampicin-based TB treatment, which may result in inferior virological outcomes. Hepatotoxicity occurred in 3 pharmacokinetic studies (conducted in healthy volunteers) of boosted protease inhibitors initiated in participants on rifampicin. Results of a clinical trial comparing efavirenz and nevirapine-based ART in patients on TB treatment, with no lead-in dosing of nevirapine, are awaited. Concurrent TB treatment increases the need for stavudine substitutions, mainly related to neuropathy. Consensus case definitions for TB immune reconstitution inflammatory syndrome (TB-IRIS) have been published. It is important to exclude TB drug resistance in patients with suspected TB-IRIS. A clinical trial demonstrated benefit of prednisone for treating TB-IRIS, reducing a combined endpoint of days hospitalised and outpatient therapeutic procedures. Starting ART during TB treatment improved survival in patients with CD4 < 500 cells/μL, but the optimal interval between starting TB treatment and starting ART remains to be determined in several ongoing trials.
Summary
ART improves survival in co-infected TB patients, but is complicated by several management challenges that compromise programmatic implementation in resource-limited settings. Recent findings and the findings of ongoing studies will assist clinicians in dealing with these challenges.
Keywords: Tuberculosis, TB, HIV, antiretroviral therapy, ART, immune reconstitution inflammatory syndrome, IRIS
Introduction
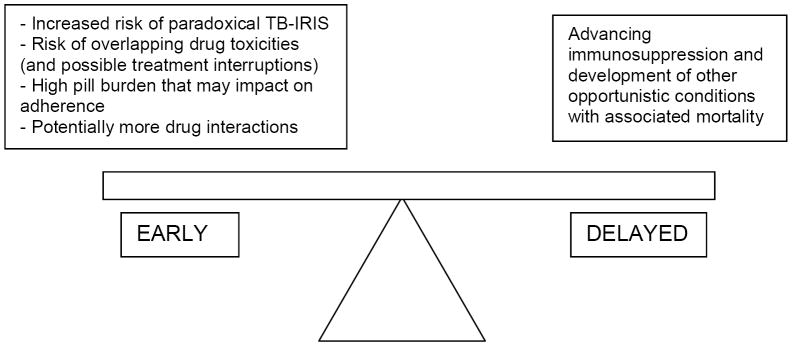
A substantial proportion of patients who enter antiretroviral therapy (ART) programmes with advanced immunosuppression in resource-limited settings do so while on tuberculosis (TB) treatment (up to 25% in one South African cohort [1]) and among patients established on ART many will need to be treated for TB during their lifetime [1-3]. Concurrent treatment for these two conditions creates several management challenges, including drug interactions, shared drug toxicities, TB immune reconstitution inflammatory syndrome (TB-IRIS) and high pill burden, which potentially impacts on adherence (Figure 1). The optimal time to initiate ART in those on TB treatment is not defined. The situation is further complicated by the emergence of drug-resistant TB among HIV-infected people particularly in Southern Africa and Eastern Europe, where additional management issues arise [4, 5]. The management of HIV-TB co-infection has previously been reviewed [6-9]. The focus of the current review is studies relating to adult management issues published since January 2008.
Fig 1. Potential risks associated with early versus delayed ART in patients with HIV-associated TB.
Drug Interactions
Rifampicin is a potent inducer of many genes controlling drug metabolism and transport, including cytochrome P450 isoenzymes and the drug efflux pump p-glycoprotein[10]. Rifampicin may therefore reduce plasma concentrations of concomitantly administered non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs), potentially resulting in inadequate ART plasma concentrations and inferior ART outcomes.
Efavirenz is the NNRTI of choice for use together with rifampicin-based TB treatment [11, 12]. However, nevirapine is also extensively prescribed in patients with TB in resource-limited settings due to its low cost and wide availability in fixed dose combination formulations and due to concerns about the teratogenic potential of efavirenz.
Recent studies in India [13] and South Africa [14] found no significant decrease in efavirenz concentrations in the presence of rifampicin-based TB treatment. The cytochrome P450 2B6 516 G>T polymorphism, which impairs function of the primary route of efavirenz metabolism, 2B6, is common in African populations [14-16]. This polymorphism has a far greater effect on efavirenz concentrations than rifampicin, resulting in high efavirenz concentrations at standard efavirenz doses even in the presence of TB treatment [13, 14]. This may result in increased central nervous system adverse effects [17]. In African patients on concomitant TB treatment, efavirenz concentrations in patients weighing more than 60 kg were similar to those in lighter patients [14]. Efficacy of efavirenz-based therapy at standard doses (600mg daily) is unaffected by concomitant TB treatment [18]. Centers for Disease Control and Prevention [11] and World Health Organisation (WHO) [12] guidelines have previously suggested that increasing efavirenz dose to 800 mg may be necessary in patients weighing more than 60 kg, but we do not recommend this based on these data.
Nevirapine concentrations are decreased by concomitant rifampicin-based TB treatment, and Thai [19] and South African [20] investigators found sub-theraputic nevirapine concentrations in 21-38% of coinfected patients on rifampicin-based TB treatment taking standard nevirapine doses (200mg twice daily). Modeling of data from South African patients indicates that increasing nevirapine doses by 50% to 300 mg twice daily would achieve therapeutic concentrations in the majority [21], but the safety of this strategy has not been adequately explored for recommendation as routine practice, and may result in increased hypersensitivity reactions [22].
Nevirapine is generally initiated at a dose of 200 mg daily for the first 2 weeks (termed the lead-in dose), during which time auto-induction of metabolism takes place. This reduces the rate of early adverse effects [23]. The optimal dose in the first two weeks in patients already on rifampicin, which induces nevirapine metabolism, is debated. In Malawi [24] and Thailand [22] 59-79% of patients had subtherapeutic nevirapine concentrations during the lead-in period when taking concomitant rifampicin-based TB treatment, and South African cohort data suggest that this may result in inferior virological outcomes [18]. Switching without a lead-in period from efavirenz (a less potent inducer of drug metabolism than rifampicin) to full dose nevirapine is recommended [25]. Recruitment into a Thai study comparing standard lead-in and standard dosing of nevirapine to omission of lead-in and a dose increase to 300 mg twice daily at 2 weeks was stopped prematurely because of increased adverse effects in the higher-dosed arm. However, the difference between the 2 arms did not reach statistical significance, with adverse events in 1/16 in the lower and 4/16 in the higher dose groups [22]. Preliminary safety data from the CARINEMO-ANRS 12146 trial are reassuring [26]. In 236 Mozambican patients on rifampicin-based TB treatment randomized to ART containing either efavirenz 600mg, or nevirapine without lead-in dosing, less than 5% of participants developing a significant transaminitis requiring NNRTI discontinuation, and there were no severe skin reactions [26]. Although these data are not broken down by study arm, because the study is still ongoing, these adverse event rates are low even if all occurred in the nevirapine arm. Based on these data, we recommend that the lead-in dose be omitted in patients initiated on nevirapine who have been taking rifampicin-based TB treatment for more than a week.
A cohort study in 310 patients in Botswana [27] found no difference in efficacy of NNRTI-based ART with and without rifampicin-based TB treatment, and no difference between the efficacy of nevirapine (initiated with lead-in dosing) and efavirenz when combined with TB treatment. In a cohort of 188 Thai patients [28], no significant difference in virological suppression at 48 weeks was seen between efavirenz and nevirapine (78% versus 67% suppressed respectively) when combined with TB treatment. However a larger and better powered cohort study including 2035 South Africans found that a higher proportion of patients failed ART virologically at 6 months when nevirapine (with lead-in dosing) was initiated during TB treatment than without TB treatment (16.3% (95% CI 10.6%; 23.5%) vs 8.3% (95% CI 6.7%; 10.0%) [18]. TB treatment did not affect efavirenz efficacy, and nevirapine efficacy was unchanged in those patients who were initiated on TB treatment while taking nevirapine-based ART [18]. It is likely that sub-therapeutic nevirapine concentrations during the lead-in period accounted for inferior outcomes with nevirapine [18]. The only published randomized controlled trial to date, in 142 Thai patients, showed no difference in virological suppression at 48 weeks between efavirenz and nevirapine (with lead-in dosing) administered with concomitant TB treatment (73% and 72% suppressed, respectively) [19]. Results of the CARINEMO-ANRS 12146 randomized controlled trial of efavirenz versus nevirapine (without lead-in dosing) with TB treatment in Mozambique are expected in 2011.
In our opinion, based on current evidence, efavirenz at standard doses remains the preferred NNRTI in patients treated concomitantly with rifampicin-based TB treatment. Nevirapine at standard doses, and without lead-in dosing, should be prescribed where efavirenz is not available or compelling indications for use of nevirapine exist.
Rifampicin causes marked reduction in the concentration of all protease inhibitors (PIs) except ritonavir [11]. To overcome the effect of rifampicin on boosted PIs, the dose of ritonavir used for boosting may be increased to 400 mg 12 hourly (with saquinavir or lopinavir only) [29-31], or the dose of boosted PI may be doubled (for lopinavir in adults only) [29]. Severe hepatotoxicity was observed in three recently reported healthy volunteer studies of rifampicin in combinations with ritonavir-boosted lopinavir [32], atazanavir [33] and saquinavir [34]. In these studies, hepatotoxicity developed predominantly in participants initiated on rifampicin before the boosted PI. It has been theorised that the sequence of drug initiation may be important and that prior induction with rifampicin may result in increased concentrations of a hepatotoxic PI metabolite, or that inhibition by ritonavir may reduce clearance of a hepatotoxic rifampicin metabolite [33]. The implications of these studies for coadministration of boosted PIs with rifampicin-based TB treatment is unclear. Based on the data above, rates of hepatotoxicity may be lower in patients already taking second-line PI-based therapy before tuberculosis is diagnosed and TB treatment initiated. To date, there are no cohort studies documenting efficacy or safety of ritonavir-boosted lopinavir in combination with rifampicin-based TB treatment. With increasing numbers of patients in tuberculosis-endemic areas requiring second-line ART, this is a research priority. Liver function monitoring is recommended in all patients treated with boosted PIs in combination with rifampicin, particularly when the PI must be initiated when the patient is already induced on rifampicin-based TB treatment.
Rifabutin, which is a less potent inducer of drug metabolism, is recommended as an alternative to rifampicin in well resourced countries [11], but is currently unaffordable in resource-limited settings, and logistically difficult to implement in TB control programmes which use rifampicin-based fixed dose combination formulations of TB drugs.
Triple NRTI therapy is associated with increased risk of treatment failure when compared to efavirenz based therapy [35], but may be considered as an option in combination with TB treatment where NNRTIs are not tolerated or an NNRTI-based regimen failure has previously occurred and PIs are unavailable or not tolerated. Our recommendations regarding co-administration of rifampicin-based TB treatment and ART are summarized in Table 1. Potential drug interactions between ART and drugs used to treat drug-resistant TB have recently been reviewed [5].
Table 1.
Antiretroviral interactions with rifampicin-based TB treatment and recommendations for co-administration in adults (References: 11, 13, 14, 24, 29-32, 34)
| Class | Antiretroviral agent | Interaction | Dose of ARV with rifampicin-based TB treatment |
|---|---|---|---|
| NRTI | All in class | No clinically significant pharmacokinetic interactions known. | No dose adjustment required. |
| NNRTI | Efavirenz | Mild reduction in efavirenz concentrations. | No dose adjustment required. 600 mg daily. |
| Nevirapine | Moderate reduction in nevirapine concentrations. Concern about increased risk of virological failure and shared hepatotoxicity. | No dose adjustment required. 200 mg 12 hrly. | |
| Omit lead-in dose (200 mg daily) if patient has been taking rifampicin for >1 week. | |||
| PI | Ritonavir | Moderate reduction in ritonavir concentrations. | No dose adjustment required. 600 mg 12 hrly. |
| This dose of ritonavir is very poorly tolerated (GIT intolerance) | |||
| Lopinavir + ritonavir | Lopinavir plasma concentrations are significantly decreased. | This is the preferred PI option. | |
| Lopinavir /ritonavir (600/150 mg or 800/200 mg, both dosed 12 hrly) caused high rates of hepatitis in healthy volunteers already taking rifampicin. | There are 2 dosing options:
|
||
| Regular liver function monitoring is recommended. | |||
| Caution when initiating lopinavir/ritonavir in patients already taking rifampicin. | |||
| Saquinavir + ritonavir | Saquinavir concentrations are significantly decreased. | Saquinavir 400 mg + ritonavir 400 mg, both 12 hrly (may be poorly tolerated − GIT intolerance). | |
| Saquinavir 1000 mg + ritonavir 100 mg, both 12 hrly caused high rates of hepatitis in healthy volunteers already taking rifampicin. | Regular liver function monitoring is recommended. | ||
| Caution when initiating saquinavir/ritonavir in patients already taking rifampicin. | |||
| All other PI’s | Marked reduction in PI levels. | Do not prescribe concomitantly. | |
NRTI = Nucleoside reverse transcriptase inhibitors, NNRTI = Non-nucleoside reverse transcriptase inhibitors, PI = Protease inhibitors, GIT = gastro-intestinal tract.
Adverse events and co-toxicities
HIV infection itself results in an increased rate of serious adverse events in patients on TB treatment [36] and ART may further increase this. A retrospective cohort study in South African patients on rifampicin-based TB treatment found increased serious adverse events, primarily peripheral neuropathy and increased vomiting, in HIV-infected compared with uninfected patients, but no association with concomitant use of ART [37]. Isoniazid, stauvudine and didanosine are common causes of neuropathy [6]. In another South African study, patients taking ART together with TB treatment were found to be at increased risk of stavudine discontinuation, primarily due to peripheral neuropathy [38]. The risk of stavudine substitution in the first 2 months of ART was nearly 7 fold higher in patients initiating stavudine-containing ART concurrently with TB treatment, when compared to those not receiving TB treatment [38]. Patients treated concomitantly for both HIV and TB infection require routine pyridoxine supplementation (to prevent isoniazid-related neuropathy), and alternatives to stavudine should be considered when available, particularly in those patients with preexisting neuropathy.
Among the first-line TB drugs pyrazinamide, isoniazid and rifampicin have all been associated with hepatotoxicity [6]. There are concerns about increased hepatoxicity when NNRTIs, particularly nevirapine, are prescribed with TB treatment. Study results differ. Increased hepatotoxicity was seen when both efavirenz [27, 39] and nevirapine [27] were administered concomitantly with TB treatment. However, in a large South African cohort, there was no increase in drug substitution due to toxicity in patients on TB treatment who were treated with either efavirenz or nevirapine compared with those not on TB treatment, although rates of substitution for toxicity in the cohort were higher overall for nevirapine [18]. Boosted PIs with rifampicin-based TB treatment may also result in hepatotoxicity (discussed above). Development of deranged liver functions may siginificantly complicate treatment of co-infected patients, and necessitate interruption of all potentially hepatotoxic antiretrovirals and TB drugs [40], and inpatient rechallenge. Such interruptions of therapy for TB and HIV, although essential when severe drug toxicities occur, may worsen prognosis.
Other shared side effects include drug rashes (that may occur due to many of the TB drugs, co-trimoxazole, nevirapine and, less frequently, efavirenz), gastro-intestinal intolerance (especially with zidovudine, didanosine, protease inhibitors, pyrazinamide, ethionamide and para-aminosalicylic acid) and neurospychiatric side effects (especially with efavirenz, isoniazid, ethionamide and cycloserine) [5, 9]. Aminoglycosides (for example amikacin and kanamycin) and capreomycin used in the treatment of drug-resistant TB may result in nephrotoxicity, and co-administration of tenofovir, which may also result in nephrotoxicity, should be avoided if possible [5, 9].
Tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS)
Two forms of TB-IRIS are recognised. Paradoxical TB-IRIS occurs in patients diagnosed with TB and established on TB treatment prior to ART who then manifest with recurrent or new TB symptoms and signs after ART initiation [41-43]. Unmasking TB-IRIS occurs in patients who are not on TB treatment when they start ART, who then have an unusually inflammatory presentation of TB in the first 3 months of ART [42, 44]. There have been several recent case reports of unmasking TB-IRIS [45-47] including one with a fatal outcome [45]. Both forms of TB-IRIS are thought to result from rapid recovery of mycobacterial immune responses resulting in inflammatory reactions to MTB antigen [44, 48-50].
The incidence of paradoxical TB-IRIS is reported to be 8-43% among patients starting ART while on TB treatment in 12 published cohort studies from 5 continents [2, 40, 51-60]. Major risk factors are low CD4 count, disseminated TB and short interval between starting TB treatment and ART [40, 52-54, 56-58, 60]. The most frequently reported features are recurrent TB symptoms, fever, enlargement of lymph nodes, worsening radiographic pulmonary infiltrates and enlargement of pleural effusions (Figure 2) [41, 61]. Patients typically develop TB-IRIS symptoms 2-4 weeks after starting ART [51-53, 57-59].
Fig 2. Illustrative case of paradoxical TB-IRIS.
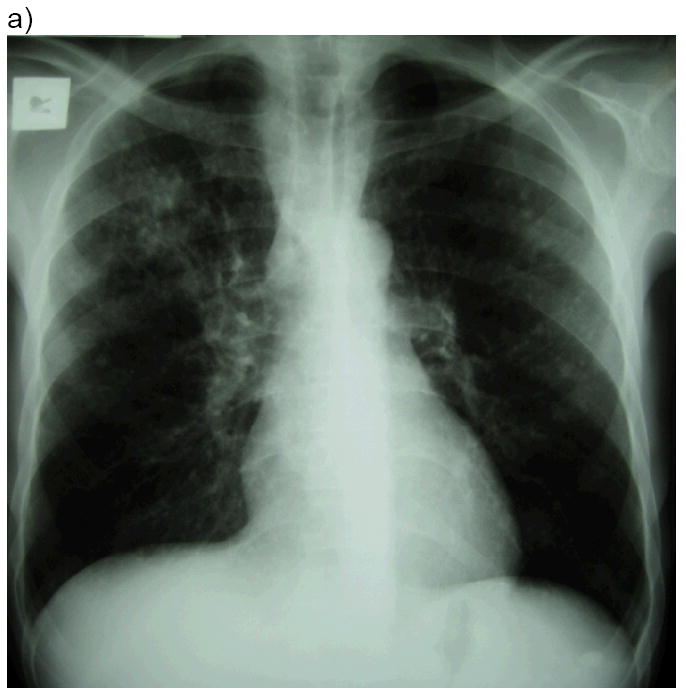
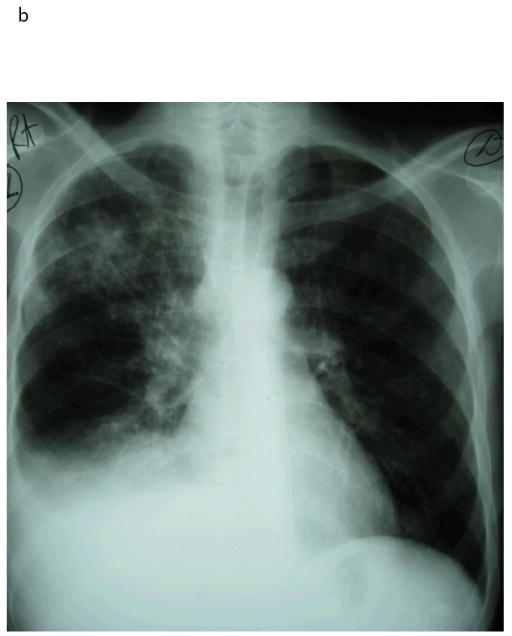
A 49 year-old man was diagnosed with pulmonary TB (sputum cultured Mycobacterium tuberculosis susceptible to rifampicin and isoniazid). Chest radiograph at TB diagnosis (a) showed a right upper lobe infiltrate. His symptoms improved on TB treatment. His CD4 count was 29 cells/μL and HIV viral load 191 000 copies/mL. He was started on antiretroviral therapy 2 weeks after TB treatment and 2 weeks later developed recurrent cough, night sweats and dyspnoea. Chest radiograph (b) showed worsening of the right upper lobe infiltrate and a new right pleural effusion. His CD4 had risen to 51 cells/μL. Repeat TB cultures from sputum and pleural aspirate were negative. His effusion was therapeutically aspirated and he was treated with prednisone for paradoxical TB-IRIS to which he symptomatically responded. His viral load performed 6 months after ART initiation was < 50 copies/mL.
A recent study from Uganda [2] reported paradoxical TB-IRIS in 13 of 45 patients (29%) being treated for TB when starting ART. Several other studies have reported a short interval between starting TB treatment and ART to be a risk factor for paradoxical TB-IRIS [40, 52, 54, 58, 60]. However, in this study, delaying ART until after 2 months of TB treatment did not appear to prevent paradoxical TB-IRIS: 22% of patients starting ART within 2 months developed TB-IRIS and 31% of those starting after 2 months developed TB-IRIS.
Abdominal features of TB-IRIS are increasingly recognised [41, 61, 62]. These include hepatic and splenic involvement, intestinal lesions, peritonitis [63], ascites, intra-abdominal lymphadenopathy and abscesses. Abdominal symptoms are reported in up to 59% of patients [61] and include pain, nausea, vomiting and diarrhoea [57, 61]. Hepatic involvement, which occurs in between 21-56% of TB-IRIS cases, can be difficult to differentiate from drug-induced hepatitis [61, 62]. Hepatic TB-IRIS manifests with tender liver enlargement, cholestatic liver function derangement with or without jaundice and granulomatous hepatitis on liver histology [62].
Mortality associated with paradoxical TB-IRIS is rare, except when there is central nervous system involvement. Neurological deterioration has been reported in 12% of paradoxical TB-IRIS cases [64]. Features include new or recurrent meningitis, enlarging tuberculomas and radiculomyelopathy. In a series of 23 neurologic TB-IRIS cases, only 70% of patients were known to be alive at 6 months; and of the survivors 6 of 16 were left with neurologic disability [64]. Other potentially life threatening manifestations recently reported include splenic rupture [65], acute renal failure [66] and enlargement of pericardial effusions [67, 68].
The diagnosis of paradoxical TB-IRIS may be difficult, as there is no confirmatory diagnostic test. In patients with advanced HIV there is a wide differential diagnosis for clinical deterioration during early ART [69]. Other opportunistic infections, malignancies, drug resistant TB and drug reactions need to be excluded. A high prevalence of rifampicin resistant TB (10%) was recently reported in a cohort of patients presenting with suspected paradoxical TB-IRIS to a referral hospital in South Africa [61]. The clinical presentation of TB-IRIS in these patients was indistinguishable from those with drug susceptible disease, suggesting that patients with undiagnosed drug resistant TB might also develop TB-IRIS after starting ART with resultant acceleration of clinical deterioration [61]. Consensus case definitions for TB-IRIS were recently published by the International Network for the Study of HIV-associated IRIS (INSHI) [42].
Paradoxical TB-IRIS has most frequently been treated with corticosteroids, non-steroidal anti-inflammatory drugs and aspiration or surgical drainage of collections. Evidence for efficacy of treatment is anecdotal apart from one randomized placebo-controlled clinical trial of prednisone in 110 patients with paradoxical TB-IRIS which excluded patients with immediately life threatening TB-IRIS. Prednisone (at a dose of 1.5mg/kg/day for 2 weeks followed by 0.75mg/kg/day for 2 weeks) reduced duration of hospitalization and outpatient therapeutic procedures, the combined primary endpoint of the trial. Participants who received prednisone also experienced more rapid symptom and radiographic improvement [70]. When considering corticosteroids to treat TB-IRIS clinicians need to confidently exclude alternative causes for deterioration and consider potential risks such as herpes reactivations and Kaposi’s sarcoma [71-73].
When to start ART in TB patients?
The decision regarding when to start ART during TB treatment needs to balance several considerations (Figure 1). The two most important variables in the decision are the incidence and mortality associated with TB-IRIS (mortality is reported to be 0-12%, [40, 54, 55, 57-59, 64]) and the excess mortality associated with delaying ART and the development of other opportunistic infections [74, 75]. Studies in South Africa have shown considerable mortality among patients after enrolment into ART programmes before starting ART, particularly in those with advanced HIV and low CD4 counts [76, 77]. Fear of causing TB-IRIS may result in clinicians delaying ART despite this high mortality associated with delay [78, 79].
A retrospective cohort study from Spain of 313 patients with TB and HIV reported lower mortality (9 vs 20%) in patients started on ART within 2 months of TB treatment compared to those started later. This difference remained significant after adjustment for other factors potentially associated with mortality (adjusted HR = 0.37) [80]. Another retrospective study from Iran, in hospitalised HIV-TB patients with CD4 < 100 cells/μL, found that changing guidelines for initiating ART from 8 weeks to 2 weeks after TB treatment was associated with a reduction in mortality and no excess of adverse drug reactions or IRIS [81].
Several randomised-controlled studies are underway that address the question of the optimum time to start ART in TB patients [82]. The SAPIT study [83], being conducted in South Africa, has reported interim results. It is an open-label randomised controlled trial comparing 3 different ART initiation strategies in smear positive TB patients with CD4 count between 0 and 500 cells/μL. ART is started during intensive phase TB treatment in Arm 1, after intensive phase in Arm 2 or after completing TB treatment in Arm 3. At interim analysis, Arm 3 was halted because of excess mortality compared with the Arm 1 and 2; 12.1 versus 5.4 deaths per 100 person years respectively. This mortality increase was significant in both those with CD4 ≤ 200 and > 200 cells/μL. While awaiting results from these trials, WHO guidelines recommend starting ART between 2 and 8 weeks of TB treatment in patients with CD4 < 200 cells/μL and during continuation phase in those with CD4 counts 200-350 cells/μL [12]. In patients with very low CD4 counts there is greater urgency to start ART.
Treatment outcomes
Use of ART in TB patients is associated with improved survival [83-86]. Commencing ART while on TB treatment does not compromise virological and immunological outcomes [1]. The mortality rate in patients starting ART while on TB treatment is, however, higher than for those not on TB treatment [1, 87, 88], although this is likely related to confounding factors. In a recent study [88], despite those on TB treatment having 70% higher mortality in crude analysis, in multivariate analysis there was no association between being on TB treatment and death. The higher mortality rate was explained by confounding factors: low CD4 count, low BMI and WHO stage 4 disease [88]. In a Haitian study, patients diagnosed with incident TB in the first 3 months of ART had a higher mortality rate than controls diagnosed with TB before ART or after 3 months of ART [89]. Unmasking TB-IRIS may have been a factor.
Conclusion
Many patients with HIV-associated TB present to health care services with advanced immunosuppression necessitating concurrent ART and treatment for TB. Concurrent treatment has been demonstrated to improve survival [83-86], but is complicated by drug interactions, high pill burden, shared toxicities and paradoxical TB-IRIS. Adjustments in TB treatment and/or ART regimen may be required to account for drug interactions. Clinicians should be aware of potential toxicities from ART and TB treatment and monitor for these. There is evidence demonstrating the benefit of prednisone for patients who develop significant symptoms related to paradoxical TB-IRIS as long as other diagnoses are confidently excluded. Better TB-HIV service integration remains a priority in order to improve diagnosis and management of co-infected patients and reduce diagnostic and treatment delays [9, 90-92].
Acknowledgments
Graeme Meintjes is supported by a Wellcome Trust Fellowship.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 2*.Baalwa J, Mayanja-Kizza H, Kamya MR, et al. Worsening and unmasking of tuberculosis in HIV-1 infected patients after initiating highly active anti-retroviral therapy in Uganda. Afr Health Sci. 2008;8:190–5. [PMC free article] [PubMed] [Google Scholar]; A study from a Ugandan ART cohort that reports the incidence, timing and clinical manifestations of paradoxical TB-IRIS and incident TB on ART.
- 3.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–33. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Scano F, Vitoria M, Burman W, et al. Management of HIV-infected patients with MDR-and XDR-TB in resource-limited settings. Int J Tuberc Lung Dis. 2008;12:1370–5. [PubMed] [Google Scholar]; A review article of management issues in patients with HIV and drug resistant TB (MDR and XDR) in resource limited settings.
- 5*.Coyne KM, Pozniak AL, Lamorde M, Boffito M. Pharmacology of second-line antituberculosis drugs and potential for interactions with antiretroviral agents. AIDS. 2009;23:437–46. doi: 10.1097/qad.0b013e328326ca50. [DOI] [PubMed] [Google Scholar]; A review of the pharmacology of drugs used for the treatment of drug-resistant TB with specific emphasis on potential drug interactions and shared toxicities with antiretroviral agents.
- 6.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl 1):S63–75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 7.Burman WJ. Issues in the management of HIV-related tuberculosis. Clin Chest Med. 2005;26:283–94. vi–vii. doi: 10.1016/j.ccm.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.de Jong BC, Israelski DM, Corbett EL, Small PM. Clinical management of tuberculosis in the context of HIV infection. Annu Rev Med. 2004;55:283–301. doi: 10.1146/annurev.med.55.091902.103753. [DOI] [PubMed] [Google Scholar]
- 9**.Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis. 2009;13:6–16. [PubMed] [Google Scholar]; This review discusses three major aspects of HIV/TB co-infection management: provider-initiated HIV testing, co-trimoxazole preventive treatment and ART.
- 10.Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin : clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis. [25 August 2009];2007 December; Available from http://www.cdc.gov/tb/publications/guidelines/TB_HIV_Drugs/PDF/tbhiv.pdf.
- 12.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. [11 August 2009];Recommendations for a public health approach. 2006 revision. Available from http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 13*.Ramachandran G, Hemanth Kumar AK, Rajasekaran S, et al. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009;53:863–8. doi: 10.1128/AAC.00899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; A pharmacokinetic study in 72 Indian patients which found that rifampicin-based TB treatment did not change efavirenz concentrations significantly and that the 516G>T polymorphism was associated with high efavirenz concentrations even in the presence of TB treatment.
- 14*.Cohen K, Grant A, Dandara C, et al. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516 G>T polymorphism on efavirenz concentrations in adults in South Africa. Antiviral therapy. 2009;14:687–695. [PMC free article] [PubMed] [Google Scholar]; A cross sectional study of 142 patients in South Africa which found that efavirenz mid-dosing interval concentrations with and without rifampicin-based TB treatment did not differ significantly. Efavirenz concentrations were not reduced in patients on TB treatment weighing more than 60 kgs.
- 15.Gross R, Aplenc R, Tenhave T, et al. Slow efavirenz metabolism genotype is common in Botswana. J Acquir Immune Defic Syndr. 2008;49:336–7. doi: 10.1097/QAI.0b013e31817c1ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwara A, Lartey M, Sagoe KW, et al. CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009;67:427–36. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzolini C, Telenti A, Decosterd LA, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 18**.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–9. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]; A large cohort study including 2035 South African patients on efavirenz or nevirapine-based ART, comparing outcomes in patients initiated on ART with and without concomitant TB treatment. Initiating nevirapine while on TB treatment was found to be associated with poorer virological outcomes, suggesting that sub-therapeutic nevirapine concentrations during lead-in dosing might be the cause.
- 19**.Manosuthi W, Sungkanuparph S, Tantanathip P, et al. A randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R Study. Clin Infect Dis. 2009;48:1752–9. doi: 10.1086/599114. [DOI] [PubMed] [Google Scholar]; The first published randomized controlled trial comparing efavirenz-based and nevirapine-based ART during and after rifampicin-based TB treatment in 142 Thai patients. No difference in response to ART was found between the efavirenz and nevirapine groups.
- 20.Cohen K, van Cutsem G, Boulle A, et al. Effect of rifampicin-based antitubercular therapy on nevirapine plasma concentrations in South African adults with HIV-associated tuberculosis. J Antimicrob Chemother. 2008;61:389–93. doi: 10.1093/jac/dkm484. [DOI] [PubMed] [Google Scholar]
- 21.Elsherbiny D, Cohen K, Jansson B, et al. Population pharmacokinetics of nevirapine in combination with rifampicin-based short course chemotherapy in HIV- and tuberculosis-infected South African patients. Eur J Clin Pharmacol. 2009;65:71–80. doi: 10.1007/s00228-008-0481-y. [DOI] [PubMed] [Google Scholar]
- 22.Avihingsanon A, Manosuthi W, Kantipong P, et al. Pharmacokinetics and 48-week efficacy of nevirapine: 400 mg versus 600 mg per day in HIV-tuberculosis coinfection receiving rifampicin. Antiviral Therapy. 2008;13:529–536. [PubMed] [Google Scholar]
- 23.Pollard RB, Robinson P, Dransfield K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clinical Therapeutics. 1998;20:1071–1092. doi: 10.1016/s0149-2918(98)80105-7. [DOI] [PubMed] [Google Scholar]
- 24.van Oosterhout JJG, Kumwenda JJ, Beadsworth M, et al. Nevirapine-based antiretroviral therapy started early in the course of tuberculosis treatment in adult Malawians. Antiviral Therapy. 2007;12:515–521. [PubMed] [Google Scholar]
- 25*.Laureillard D, Prak N, Fernandez M, et al. Efavirenz replacement by immediate full-dose nevirapine is safe in HIV-1-infected patients in Cambodia. HIV Med. 2008;9:514–8. doi: 10.1111/j.1468-1293.2008.00597.x. [DOI] [PubMed] [Google Scholar]; A retrospective cohort study in 394 patients on ART in Cambodia switched from efavirenz immediately to full dose nevirapine (200mg 12 hourly), and followed up for at least 6 months. This study confirmed that switching directly from efavirenz to full dose nevirapine (without 200mg daily lead-in for 2 weeks) was safe.
- 26.Bhatt N, Baudin E, J IV, et al. Preliminary safety results of co-administration nevirapine (NVP) orefavirenz (EFV), and rifampicin (RMP) in HIV-tuberculosis (TB) coinfected patients in Maputo (Mozambique), ANRS 12146 (CARINEMO). Abstracts of the 5th IAS conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. 2009; abstract number MOPEB032. [Google Scholar]
- 27.Shipton LK, Wester CW, Stock S, et al. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB-HIV co-infection in Botswana. Int J Tuberc Lung Dis. 2009;13:360–6. [PMC free article] [PubMed] [Google Scholar]
- 28.Manosuthi W, Mankatitham W, Lueangniyomkul A, et al. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 2008;9:294–9. doi: 10.1111/j.1468-1293.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- 29.la Porte CJ, Colbers EP, Bertz R, et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:1553–60. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veldkamp AI, Hoetelmans RM, Beijnen JH, et al. Ritonavir enables combined therapy with rifampin and saquinavir. Clin Infect Dis. 1999;29:1586. doi: 10.1086/313548. [DOI] [PubMed] [Google Scholar]
- 31.Rolla VC, da Silva Vieira MA, Pereira Pinto D, et al. Safety, efficacy and pharmacokinetics of ritonavir 400mg/saquinavir 400mg twice daily plus rifampicin combined therapy in HIV patients with tuberculosis. Clin Drug Investig. 2006;26:469–79. doi: 10.2165/00044011-200626080-00005. [DOI] [PubMed] [Google Scholar]
- 32*.Nijland HM, L’Homme RF, Rongen GA, et al. High incidence of adverse events in healthy volunteers receiving rifampicin and adjusted doses of lopinavir/ritonavir tablets. AIDS. 2008;22:931–5. doi: 10.1097/QAD.0b013e3282faa71e. [DOI] [PubMed] [Google Scholar]; A pharmacokinetic study in which HIV seronegative participants were given rifampicin for 5 days, after which lopinavir/ritonavir was added. The study was stopped when the first 11 participants developed deranged liver functions following lopinavir/ritonavir initiation.
- 33*.Haas DW, Koletar SL, Laughlin L, et al. Hepatotoxicity and gastrointestinal intolerance when healthy volunteers taking rifampin add twice-daily atazanavir and ritonavir. J Acquir Immune Defic Syndr. 2009;50:290–3. doi: 10.1097/QAI.0b013e318189a7df. [DOI] [PMC free article] [PubMed] [Google Scholar]; A pharmacokinetic study in which HIV seronegative participants were given rifampicin for 8 days, after which atazanavir/ritonavir was added. After addition of atazanavir/ritonavir, the first 3 participants developed nausea, vomiting and deranged liver functions, and the study was stopped.
- 34*.Schmitt C, Riek M, Winters K, et al. Unexpected Hepatotoxicity of Rifampin and Saquinavir/Ritonavir in Healthy Male Volunteers. Arch Drug Inf. 2009;2:8–16. doi: 10.1111/j.1753-5174.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; A pharmacokinetic study in which HIV seronegative participants were initiated on either saquinavir/ritonavir 1000/100 mg or rifampicin, followed by the combination of all three drugs. Most participants initated on rifampicin developed deranged liver functions when saquinavir and ritonavir were introduced. The study was stopped.
- 35.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 36.Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–7. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 37.Marks DJ, Dheda K, Dawson R, et al. Adverse events to antituberculosis therapy: influence of HIV and antiretroviral drugs. Int J STD AIDS. 2009;20:339–45. doi: 10.1258/ijsa.2008.008361. [DOI] [PubMed] [Google Scholar]
- 38**.Westreich DJ, Sanne I, Maskew M, et al. Tuberculosis treatment and risk of stavudine substitution in first-line antiretroviral therapy. Clin Infect Dis. 2009;48:1617–23. doi: 10.1086/598977. [DOI] [PMC free article] [PubMed] [Google Scholar]; A cohort study of 7066 HIV infected South African patients on stavudine-containing ART, exploring the impact of concomitant TB treatment on rates of stavudine substitution. Stavudine substitution rates were higher than those not on TB treatment, particularly in patients initiated concurrently on ART and TB treatment.
- 39.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–8. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 40.Tansuphasawadikul S, Saito W, Kim J, et al. Outcomes in HIV-infected patients on antiretroviral therapy with tuberculosis. Southeast Asian J Trop Med Public Health. 2007;38:1053–60. [PubMed] [Google Scholar]
- 41.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 42*.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes consensus case definitions for paradoxical and unmasking TB-IRIS. The aim of these case definitions is to promote standardization and comparability of data from research in different settings and to assist clinicians in making the diagnosis of TB-IRIS.
- 43*.Leone S, Nicastri E, Giglio S, et al. Immune reconstitution inflammatory syndrome associated with Mycobacterium tuberculosis infection: a systematic review. Int J Infect Dis. 2009 doi: 10.1016/j.ijid.2009.05.016. [DOI] [PubMed] [Google Scholar]; A systematic review of TB-associated IRIS. Two-hundred and thirty eight cases from the reported literature are included in this review.
- 44.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–5. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23:143–5. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- 46.Chen WL, Lin YF, Tsai WC, Tsao YT. Unveiling tuberculous pyomyositis: an emerging role of immune reconstitution inflammatory syndrome. Am J Emerg Med. 2009;27:251, e1–2. doi: 10.1016/j.ajem.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Tahir M, Sinha S, Sharma SK, Mitsuyasu RT. Immune reconstitution inflammatory syndrome manifesting as disseminated tuberculosis, deep venous thrombosis, encephalopathy and myelopathy. Indian J Chest Dis Allied Sci. 2008;50:363–4. [PubMed] [Google Scholar]
- 48.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. Aids. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 49.Meintjes G, Wilkinson KA, Rangaka MX, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–9. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manabe YC, Breen R, Perti T, et al. Unmasked tuberculosis and tuberculosis immune reconstitution inflammatory disease: a disease spectrum after initiation of antiretroviral therapy. J Infect Dis. 2009;199:437–44. doi: 10.1086/595985. [DOI] [PubMed] [Google Scholar]
- 51.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–61. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 52.Breen RA, Smith CJ, Bettinson H, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59:704–7. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breton G, Duval X, Estellat C, et al. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–12. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 54.Burman W, Weis S, Vernon A, et al. Frequency, severity and duration of immune reconstitution events in HIV-related tuberculosis. Int J Tuberc Lung Dis. 2007;11:1282–9. [PubMed] [Google Scholar]
- 55.Kumarasamy N, Chaguturu S, Mayer KH, et al. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–6. doi: 10.1097/00126334-200412150-00007. [DOI] [PubMed] [Google Scholar]
- 56.Michailidis C, Pozniak AL, Mandalia S, et al. Clinical characteristics of IRIS syndrome in patients with HIV and tuberculosis. Antivir Ther. 2005;10:417–22. doi: 10.1177/135965350501000303. [DOI] [PubMed] [Google Scholar]
- 57.Manosuthi W, Kiertiburanakul S, Phoorisri T, Sungkanuparph S. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect. 2006;53:357–63. doi: 10.1016/j.jinf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. Aids. 2007;21:335–41. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 59.Serra FC, Hadad D, Orofino RL, et al. Immune reconstitution syndrome in patients treated for HIV and tuberculosis in Rio de Janeiro. Braz J Infect Dis. 2007;11:462–5. doi: 10.1590/s1413-86702007000500004. [DOI] [PubMed] [Google Scholar]
- 60.Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 61*.Meintjes G, Rangaka MX, Maartens G, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48:667–76. doi: 10.1086/596764. [DOI] [PMC free article] [PubMed] [Google Scholar]; A case series of 100 patients presenting with suspected paradoxical TB-IRIS. The authors emphasise the importance of excluding TB drug resistance in such patients (10% had undiagnosed rifamipicin resistance in this series).
- 62.Lawn SD, Wood R. Hepatic involvement with tuberculosis-associated immune reconstitution disease. AIDS. 2007;21:2362–3. doi: 10.1097/QAD.0b013e3282f1be39. [DOI] [PubMed] [Google Scholar]
- 63.Wu SW, Chen CJ, Lin TY, Wang NC. Acute peritonitis as presentations of tuberculosis-associated immune reconstitution inflammatory syndrome in an HIV-infected man. Am J Med Sci. 2008;335:387–9. doi: 10.1097/MAJ.0b013e318149e6de. [DOI] [PubMed] [Google Scholar]
- 64*.Pepper DJ, Marais S, Maartens G, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48:e96–107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]; The largest case series of neurological TB-IRIS cases to be reported (23 cases). This is the most severe form of TB-IRIS. Most patients were treated with corticosteroids. Three died, 4 were lost to follow-up, 6 had neurologic disability and only 10 were known to have a favorable outcome.
- 65.Weber E, Gunthard HF, Schertler T, Seebach JD. Spontaneous splenic rupture as manifestation of the immune reconstitution inflammatory syndrome in an HIV type 1 infected patient with tuberculosis. Infection. 2009;37:163–5. doi: 10.1007/s15010-008-8260-3. [DOI] [PubMed] [Google Scholar]
- 66*.Salliot C, Guichard I, Daugas E, et al. Acute kidney disease due to immune reconstitution inflammatory syndrome in an HIV-infected patient with tuberculosis. J Int Assoc Physicians AIDS Care (Chic Ill) 2008;7:178–81. doi: 10.1177/1545109708320683. [DOI] [PubMed] [Google Scholar]; A case report of paradoxical TB-IRIS complicated by acute renal failure. The four other cases of acute renal failure due to IRIS (3 due to TB and 1 due to MAC) reported in the literature are also reviewed. Although rare this is a potentially life threatening manifestation of IRIS.
- 67.Rapose A, Sarvat B, Sarria JC. Immune reconstitution inflammatory syndrome presenting as pericarditis and pericardial effusion. Cardiology. 2008;110:142–4. doi: 10.1159/000110494. [DOI] [PubMed] [Google Scholar]
- 68.Wang SH, Menon A, Hyslop NE. Cardiac tamponade: an unusual complication of simultaneous treatment of tuberculosis and HIV. South Med J. 2008;101:558–60. doi: 10.1097/SMJ.0b013e3181684b92. [DOI] [PubMed] [Google Scholar]
- 69.Pepper DJ, Rebe K, Morroni C, et al. Clinical deterioration during antitubercular treatment at a district hospital in South Africa: the importance of drug resistance and AIDS defining illnesses. PLoS One. 2009;4:e4520. doi: 10.1371/journal.pone.0004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. Randomised placebo-controlled trial of prednisone for the TB immune reconstitution inflammatory syndrome. 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009; Abstract 34. [Google Scholar]
- 71.Elliott AM, Halwiindi B, Bagshawe A, et al. Use of prednisolone in the treatment of HIV-positive tuberculosis patients. Q J Med. 1992;85:855–60. [PubMed] [Google Scholar]
- 72.Elliott AM, Luzze H, Quigley MA, et al. A randomized, double-blind, placebo-controlled trial of the use of prednisolone as an adjunct to treatment in HIV-1-associated pleural tuberculosis. J Infect Dis. 2004;190:869–78. doi: 10.1086/422257. [DOI] [PubMed] [Google Scholar]
- 73.Volkow PF, Cornejo P, Zinser JW, et al. Life-threatening exacerbation of Kaposi’s sarcoma after prednisone treatment for immune reconstitution inflammatory syndrome. AIDS. 2008;22:663–5. doi: 10.1097/QAD.0b013e3282f4f223. [DOI] [PubMed] [Google Scholar]
- 74.Schiffer JT, Sterling TR. Timing of antiretroviral therapy initiation in tuberculosis patients with AIDS: a decision analysis. J Acquir Immune Defic Syndr. 2007;44:229–34. doi: 10.1097/QAI.0b013e31802e2975. [DOI] [PubMed] [Google Scholar]
- 75.Lawn SD, Wood R. Optimum time to initiate antiretroviral therapy in patients with HIV-associated tuberculosis: there may be more than one right answer. J Acquir Immune Defic Syndr. 2007;46:121–3. doi: 10.1097/QAI.0b013e3181398d28. author reply 123. [DOI] [PubMed] [Google Scholar]
- 76.Fairall LR, Bachmann MO, Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 77.Lawn SD, Myer L, Orrell C, et al. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 78.Chilton D, Edwards SG, Pellegrino P, Miller RF. Factors influencing delay in initiating antiretroviral therapy among HIV infected patients coinfected with tuberculosis. Thorax. 2008;63:935–6. doi: 10.1136/thx.2008.104232. [DOI] [PubMed] [Google Scholar]
- 79.Friedland G. Tuberculosis immune reconstitution inflammatory syndrome: drug resistance and the critical need for better diagnostics. Clin Infect Dis. 2009;48:677–9. doi: 10.1086/596765. [DOI] [PubMed] [Google Scholar]
- 80**.Velasco M, Castilla V, Sanz J, et al. Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr. 2009;50:148–52. doi: 10.1097/QAI.0b013e31819367e7. [DOI] [PubMed] [Google Scholar]; A retrospective study from Spain that demonstrated that starting ART within two months of TB treatment in HIV-TB patients, rather than at later time points, was associated with improved survival.
- 81*.Tabarsi P, Tehrani AS, Baghaei P, et al. Early initiation of antiretroviral therapy results in decreased morbidity and mortality among patients with TB and HIV. J Int AIDS Soc. 2009;12:14. doi: 10.1186/1758-2652-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; A retrospective chart review from a hospital in Iran reporting a lower mortality rate in HIV-TB patients with very low CD4 cell counts (< 100 cells/μL) who commenced ART earlier during their TB treatment.
- 82.Blanc FX, Havlir DV, Onyebujoh PC, et al. Treatment strategies for HIV-infected patients with tuberculosis: ongoing and planned clinical trials. J Infect Dis. 2007;196(Suppl 1):S46–51. doi: 10.1086/518658. [DOI] [PubMed] [Google Scholar]
- 83.Karim SA, Naidoo K, Grobler A, Padayatchi N, Nair G, Bamber S, Pienaar J, Friedland G, El-Sadr W, Karim QA. Initiating ART during TB Treatment Significantly Increases Survival: Results of a Randomized Controlled Clinical Trial in TB/HIV-coinfected Patients in South Africa. 16th Conference on Retroviruses and Opportunistic infections; Montreal. 2009; Abstract 36a. [Google Scholar]
- 84.Haar CH, Cobelens FG, Kalisvaart NA, et al. HIV-related mortality among tuberculosis patients in The Netherlands, 1993-2001. Int J Tuberc Lung Dis. 2007;11:1038–41. [PubMed] [Google Scholar]
- 85.Sanguanwongse N, Cain KP, Suriya P, et al. Antiretroviral therapy for HIV-infected tuberculosis patients saves lives but needs to be used more frequently in Thailand. J Acquir Immune Defic Syndr. 2008;48:181–9. doi: 10.1097/QAI.0b013e318177594e. [DOI] [PubMed] [Google Scholar]
- 86.Akksilp S, Karnkawinpong O, Wattanaamornkiat W, et al. Antiretroviral therapy during tuberculosis treatment and marked reduction in death rate of HIV-infected patients, Thailand. Emerg Infect Dis. 2007;13:1001–7. doi: 10.3201/eid1307.061506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–9. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 88*.Westreich D, MacPhail P, Van Rie A, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23:707–15. doi: 10.1097/QAD.0b013e328325d115. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large cohort study from South Africa that reports a higher crude mortality rate in patients starting ART while on treatment for TB. However, in multivariate analysis it was found that this higher rate was attributable to features associated with advanced HIV-related immunosuppression rather than the presence of TB.
- 89.Koenig SP, Riviere C, Leger P, et al. High mortality among patients with AIDS who received a diagnosis of tuberculosis in the first 3 months of antiretroviral therapy. Clin Infect Dis. 2009;48:829–31. doi: 10.1086/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gandhi NR, Moll AP, Lalloo U, et al. Successful integration of tuberculosis and HIV treatment in rural South Africa: the Sizonq’oba study. J Acquir Immune Defic Syndr. 2009;50:37–43. doi: 10.1097/QAI.0b013e31818ce6c4. [DOI] [PubMed] [Google Scholar]
- 91.Harris JB, Hatwiinda SM, Randels KM, et al. Early lessons from the integration of tuberculosis and HIV services in primary care centers in Lusaka, Zambia. Int J Tuberc Lung Dis. 2008;12:773–9. [PubMed] [Google Scholar]
- 92.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA. 2008;300:423–30. doi: 10.1001/jama.300.4.423. [DOI] [PubMed] [Google Scholar]